Bactericidal/Permeability-Increasing Protein Prevents Mucosal Damage in an Experimental Rat Model of Chronic Otitis Media with Effusion (original) (raw)
Abstract
In this study, the efficacy of bactericidal/permeability-increasing protein (BPI) was assessed in a rat model of chronic otitis media with effusion. BPI injection prevented disturbance of the mucociliary clearance system of the middle ear. Hence, it is postulated that BPI can be a new therapy for chronic otitis media with effusion.
Chronic otitis media with effusion (OME) is a frequent disease during childhood, and its complications and sequelae often persist into the adult years (7). Two important factors in the development of OME are obstruction or dysfunction of the eustachian tube and bacterial infection. In chronic OME, the majority of cases are caused by gram-negative bacteria (GNB), whereas in acute OME gram-positive bacteria are also frequently isolated (1). Lipopolysaccharide (LPS) is a component of GNB. LPS alone has been shown to induce mucosal inflammation with accumulation of effusion in the middle ears of chinchillas (2) and guinea pigs (14). Furthermore, LPS has been detected in human middle ear effusions (3), and the level of it was found to be significantly higher in children with chronic OME than in children with acute OME (13). Finally, LPS is also thought to be cytotoxic to ciliated epithelial cells (10).
We recently developed an animal model of chronic OME using a combination of eustachian tube obstruction (ETO) and LPS injection (12). This procedure induces an increase in secretory cells of the epithelium and degeneration of cilia, which results in a disturbance of the mucociliary clearance system (MCS) of the middle ear. Comparable mucosal changes have been observed in humans with chronic OME (9, 15). The MCS is considered to be an important defense system of the middle ear cavity, and disturbance of this system is suspected to be an important factor in the development of chronic OME.
Bactericidal/permeability-increasing protein (BPI), a 55-kDa cationic protein present in the granules of polymorphonuclear neutrophils (PMNs), is an antimicrobial protein that has been implicated in the host defensive response to GNB infection (5). In addition to being bactericidal against GNB, BPI binds to the highly conserved lipid A portion of LPS with high affinity and can inhibit its actions (11). Previous investigations have shown that a 21-kDa recombinant amino-terminal fragment of BPI (rBPI21) protects animals against the effects of GNB and LPS (6). Furthermore, in humans, rBPI21 appears to be safe and non-immunogenic and is in phase II/III clinical trials with apparent therapeutic benefit (4, 6, 8). In the present study, we aimed to assess the in vivo capacity of rBPI21 to prevent mucosal damage in chronic OME.
Induction of chronic OME.
During anesthesia with nitrous oxide, the eustachian tube was reached by a ventral approach, medially to the posterior belly of the digastric muscle, and obstructed by plugging a small piece of Gelfoam (Upjohn Co.) into the tube. Moreover, a drop of tissue glue (Historesin; Braun, Melsungen, Germany) was used to keep the Gelfoam in the tube. In addition, 50 μl of LPS solution (2 μg/ml) in phosphate-buffered saline (PBS) prepared from Salmonella typhimurium (L-6511; Sigma, Zwijndrecht, The Netherlands) was injected through the tympanic membrane. As a control, the other ear was injected with 50 μl of PBS.
After 1, 2, 4, and 12 weeks, the animals were killed with CO2 gas and subsequently decapitated. The middle ear was dissected from the skull, denuded of adhering tissues, and further processed for light microscopy (LM) and scanning electron microscopy (SEM). For LM the specimens were fixed, decalcified, subsequently dehydrated in a graded series of ethanol, and embedded in glycol methacrylate (JB4; Brunschwig Chemie, Amsterdam, The Netherlands). Sections were stained with toluidine blue for histological studies and with alcian blue–periodic acid-Schiff for glycogen histochemistry. Specimens for SEM analysis were fixed, dehydrated in graded series of ethanol, and critical-point dried with liquid CO2. The distribution of the epithelial cells was studied with a Philips 525M scanning electron microscope after specimens were mounted and coated with gold in a Balzers MED010 Sputtercoater. The absolute numbers of ciliated and secretory cells were counted in duplicate in each ear in two standardized areas of the same size in the tympanic orifice. Statistical comparisons were made by the Tukey highest significant difference (HSD) test, with P < 0.05 considered significant, using the Statistical Package for the Social Sciences.
Histopathological findings.
By LM and SEM, control middle ears remained apparently normal during the whole period. The hypotympanum of the middle ear consisted of thin, one-layered squamous epithelium, containing very few ciliated cells (Fig. 1A). In the tympanic orifice of the eustachian tube, a more pseudostratified, cuboidal, or cylindrical epithelium was observed, which contained an abundant number of ciliated cells and few secretory cells (Fig. 2A). This part represents the mucociliary clearance system of the middle ear.
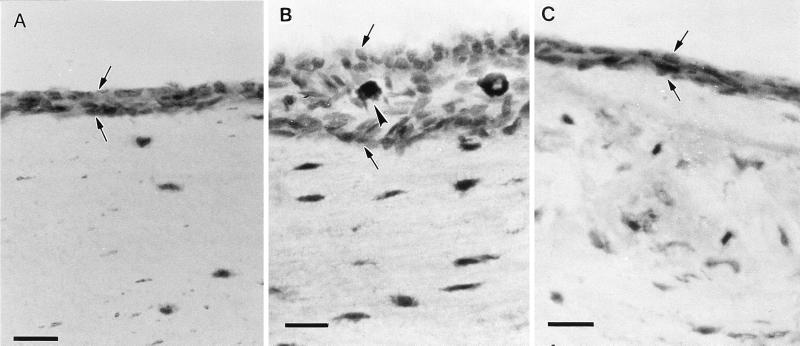
FIG. 1.
Light micrographs of the hypotympanum at 12 weeks after PBS injection (A), ETO and LPS injection (B), and ETO plus LPS with rBPI21 injection after 2 days (C). OME induction caused thickening of the mucosa (between arrows) and infiltration of inflammatory cells (arrowheads). This was not observed after rBPI21 injection. Magnification, ×200; bar, 10 μm.
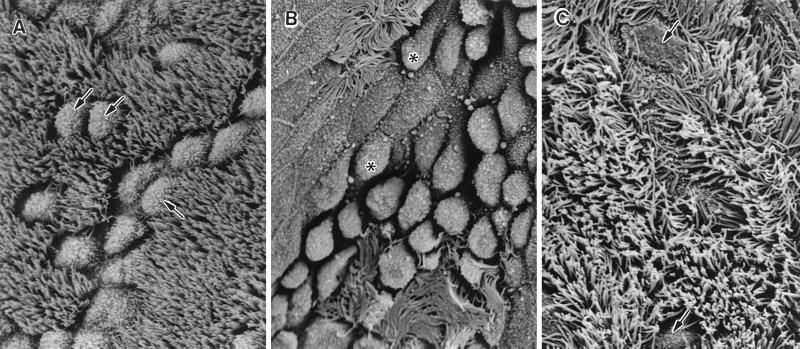
FIG. 2.
SEM of the tympanic orifice at 12 weeks after (A) PBS injection, (B) ETO and LPS injection, and (C) ETO plus LPS with rBPI21 injection after 2 days. Abundant goblet cells (arrows) and ciliated cells are present. OME induction caused degeneration of cilia and swollen epithelial cells (asterisks). This was not observed after rBPI21 injection, and the cilia had a normal appearance. Magnification, ×1,250; bar, 10 μm.
The combination of ETO and LPS injection induced thickening of the middle ear mucosa due to vasodilatation, edema, and infiltration by PMNs, macrophages, and lymphocytes in the hypotympanum (Fig. 1B). Compared with control ears after PBS injection, significantly fewer ciliated cells were observed in the tympanic orifice of ETO plus LPS-treated ears (Fig. 3), and severely swollen squamous epithelium was observed by SEM after 3 months (Fig. 2B). Furthermore, a significant increase in secretory cells was observed (Fig. 4). It is clear from these histopathological findings that the MCS was disturbed. Due to hyperproliferation of secretory cells, increased mucus production induced mucoid middle ear effusion. Dysfunction or degeneration of ciliated epithelial cells was responsible for accumulation of surplus fluid in the tympanic cavity. Therefore, clearance of the middle ear was seriously impaired.
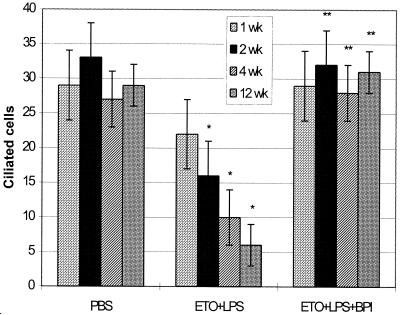
FIG. 3.
Numbers of ciliated cells ± standard deviations (n = 4) counted in the tympanic orifice after PBS injection, ETO with LPS injection (ETO+LPS), and ETO plus LPS with rBPI21 injection after 2 days (ETO+LPS+BPI). Statistical comparisons were made by the Tukey HSD test. ∗, significantly different from PBS; ∗∗, significantly different from ETO+LPS of the same week. Injection of rBPI21 after 2 days prevented the decrease in the numbers of ciliated cells caused by OME induction.
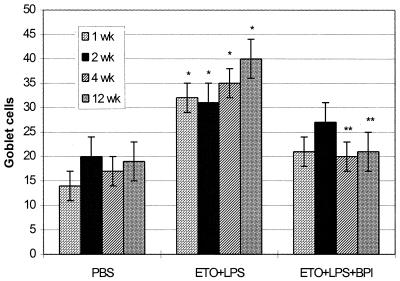
FIG. 4.
Numbers of goblet cells ± standard deviations (n = 4) counted in the tympanic orifice after PBS injection, ETO with LPS injection (ETO+LPS), and ETO plus LPS with rBPI21 injection after 2 days (ETO+LPS+BPI). Statistical comparisons were made by the Tukey HSD test. ∗, significantly different from PBS; ∗∗, significantly different from ETO+LPS of the same week. Application of rBPI21 after OME induction significantly inhibited the increase in the numbers of goblet cells.
Treatment with BPI.
Two days after induction of chronic OME, 50 μl of 2-mg/ml rBPI21, a recombinant 21-kDa amino-terminal fragment of BPI (Xoma LLC., Berkeley, Calif.), was injected into the middle ear cavity. To ensure complete neutralization of LPS, an amount of rBPI21 equal to 1,000 times the LPS concentration was used (11). It was decided to inject the BPI after 2 days to investigate the effect of BPI during an acute inflammation reaction for prevention of chronic OME. Injection of BPI prevented the thickening of the middle ear mucosa in the hypotympanum seen after ETO in combination with LPS injection. No infiltration of inflammatory cells in the subepithelial layer was observed (Fig. 1C). In the tympanic orifice, an abundance of ciliated cells were present and no significant increase in secretory cells was seen (Fig. 3 and 4). BPI prevented the induction of mucosal changes in the rat middle ear (Fig. 2C) seen after ETO and LPS injection.
In cases of ear infections complicated by ETO, bacterial products, including LPS, can be trapped in the middle ear. The uncleared bacterial products can perpetuate the inflammation, even after viable bacteria have been killed with antibiotics, further compromising the MCS. In these situations, continued treatment with antibiotics is not likely to be effective. Moreover, widespread use of antibiotics is unwise due to growing bacterial resistance. Therefore, agents that can inhibit the inflammatory activity of LPS could help break the inflammatory cycle and reestablish an effective MCS. Our results suggest that treatment with rBPI21 is a potential therapy for preventing the occurrence of chronic OME.
Acknowledgments
This work was supported by the Netherlands Organization for Scientific Research, Technology Foundation, grant LGN 33.3249, and partly by a grant from the Heinsius Houbolt Foundation.
REFERENCES
- 1.Bluestone C D, Stephenson J S, Martin L M. Ten-year review of otitis media pathogenesis. Pediatr Infect Dis J. 1992;11:S7–S11. doi: 10.1097/00006454-199208001-00002. [DOI] [PubMed] [Google Scholar]
- 2.DeMaria T F, Briggs B R, Lim D J, Okazaki N. Experimental otitis media with effusion following middle ear inoculation of nonviable H. influenzae. Ann Otol Rhinol Laryngol. 1984;93:52–56. doi: 10.1177/000348948409300113. [DOI] [PubMed] [Google Scholar]
- 3.DeMaria T F, Prior R B, Briggs B R, Lim D J, Birck H G. Endotoxin in middle-ear effusions from patients with chronic otitis media with effusion. J Clin Microbiol. 1984;20:15–17. doi: 10.1128/jcm.20.1.15-17.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Demetriades D, Smith S, Jacobson L E, Moncure M, Minei J, Nelson B J, Scannon P J. Bactericidal/permeability-increasing protein (rBPI21) in patients with hemorrhage due to trauma: results of a multicenter phase II clinical trial. J Trauma Inj Infect Crit Care. 1999;46:667–677. doi: 10.1097/00005373-199904000-00018. [DOI] [PubMed] [Google Scholar]
- 5.Elsbach P, Weiss J. The bactericidal/permeability-increasing protein (BPI), a potent element in host-defense against gram-negative bacteria and lipopolysaccharide. Immunobiology. 1993;187:417–429. doi: 10.1016/S0171-2985(11)80354-2. [DOI] [PubMed] [Google Scholar]
- 6.Elsbach P, Weiss J. Role of the bactericidal/permeability-increasing protein in host defence. Curr Opin Immunol. 1998;10:45–49. doi: 10.1016/s0952-7915(98)80030-7. [DOI] [PubMed] [Google Scholar]
- 7.Giebink G S. Otitis media update: pathogenesis and treatment. Ann Otol Rhinol Laryngol. 1992;101:21–23. doi: 10.1177/00034894921010s105. [DOI] [PubMed] [Google Scholar]
- 8.Giroir B P, Quint P A, Barton P, Kirsch E A, Kitchen L, Goldstein B, Nelson B J, Wedel N I, Carroll S F, Scannon P J. Preliminary evaluation of recombinant amino-terminal fragment of human bactericidal/permeability-increasing protein in children with severe meningococcal sepsis. Lancet. 1997;350:1439–1443. doi: 10.1016/s0140-6736(97)06468-4. [DOI] [PubMed] [Google Scholar]
- 9.Hentzer E. Ultrastructure of the middle ear mucosa in secretory otitis media. II. Mucous effusion. Acta Otolaryngol. 1972;73:467–475. doi: 10.3109/00016487209138967. [DOI] [PubMed] [Google Scholar]
- 10.Johnson A P, Inzana T J. Loss of ciliary activity in organ cultures of rat trachea treated with lipo-oligosaccharide from Haemophilus influenzae. J Med Microbiol. 1986;22:265–268. doi: 10.1099/00222615-22-3-265. [DOI] [PubMed] [Google Scholar]
- 11.Marra M N, Thornton M B, Snable J L, Wilde C G, Scott R W. Endotoxin-binding and neutralizing properties of recombinant bactericidal/permeability-increasing protein and monoclonal antibodies HA-1A and E5. Crit Care Med. 1994;22:559–565. doi: 10.1097/00003246-199404000-00009. [DOI] [PubMed] [Google Scholar]
- 12.Nell M J, Grote J J. Structural changes in the rat middle ear mucosa due to endotoxin and eustachian tube obstruction. Eur Arch Otorhinolaryngol. 1999;256:167–172. doi: 10.1007/s004050050134. [DOI] [PubMed] [Google Scholar]
- 13.Nell M J, Grote J J. Endotoxin and TNF-alpha in middle ear effusions: in relation with upper airway infection. Laryngoscope. 1999;109:1815–1819. doi: 10.1097/00005537-199911000-00017. [DOI] [PubMed] [Google Scholar]
- 14.Nonomura N, Nakano Y, Satoh Y, Fujioka O, Niijima H, Fujita M. Otitis media with effusion following inoculation of Haemophilus influenzae type b endotoxin. Arch Otorhinolaryngol. 1986;243:31–35. doi: 10.1007/BF00457904. [DOI] [PubMed] [Google Scholar]
- 15.Shimooka R, Yajin K, Harada Y. SEM studies of the inflammatory changes of the middle ear mucosa. Auris Nasus Larynx. 1983;10:79–86. doi: 10.1016/s0385-8146(83)80032-7. [DOI] [PubMed] [Google Scholar]