Putative Immunodominant Human Immunodeficiency Virus-Specific CD8+ T-Cell Responses Cannot Be Predicted by Major Histocompatibility Complex Class I Haplotype (original) (raw)
Abstract
Recent studies of human immunodeficiency virus (HIV)-specific CD8+ T cells have focused on responses to single, usually HLA-A2-restricted epitopes as surrogate measures of the overall response to HIV. However, the assumption that a response to one epitope is representative of the total response is unconfirmed. Here we assess epitope immunodominance and HIV-specific CD8+ T-cell response complexity using cytokine flow cytometry to examine CD8+ T-cell responses in 11 HLA-A2+ HIV+ individuals. Initial studies demonstrated that only 4 of 11 patients recognized the putative immunodominant HLA-A2-restricted p17 epitope SLYNTVATL, suggesting that the remaining subjects might lack significant HIV-specific CD8+ T-cell responses. However, five of six SLYNTVATL nonresponders recognized other HIV epitopes, and two of four SLYNTVATL responders had greater responses to HIV peptides restricted by other class I alleles. In several individuals, no HLA-A2-restricted epitopes were recognized, but CD8+ T-cell responses were detected to epitopes restricted by other HLA class I alleles. These data indicate that an individual's overall CD8+ T-cell response to HIV is not adequately represented by the response to a single epitope and that individual major histocompatibility complex class I alleles do not predict an immunodominant response restricted by that allele. Accurate quantification of total HIV-specific CD8+ T-cell responses will require assessment of the response to all possible epitopes.
CD8+ T-cell responses are an integral part of the total immune response to lentiviruses. The effect of lentivirus-specific CD8+ T-cell responses has best been demonstrated in primate studies, wherein the removal of CD8+ T cells from simian immunodeficiency virus (SIV)-infected monkeys leads to increased viral replication (11, 27). SIV-specific CD8+ T-cell responses inhibit viral replication after primary infection (8) and have been shown to select for cytotoxic T-lymphocyte (CTL) escape mutations within recognized epitopes (7). Additionally, the induction of strong SIV-specific CD8+ T-cell responses has, in some cases, correlated with protection from infection after challenge (12). These findings and others support the hypothesis that CD8+ T-cell responses are a correlate of protection in SIV infection. Many lines of evidence also suggest that CD8+ T-cell responses are involved in protection from infection and progression in human immunodeficiency virus (HIV) infection. The appearance of HIV-specific CD8+ T cells is concomitant with the suppression of viral load during primary infection (2, 18), and the loss of HIV-specific CD8+ T-cell activity is often associated with rapid progression to AIDS (16). Escape mutations in CD8+ T-cell epitopes occur in many infected individuals, suggesting that HIV-specific CD8+ T-cell surveillance exerts considerable selective pressure on the virus (3, 9, 23). Finally, HIV-specific CD8+ T-cell responses have been identified in multiply exposed uninfected individuals (25, 26). Despite these findings, however, we still do not have a full understanding of the correlates of protection from infection or progression in HIV infection.
With the development of major histocompatibility complex (MHC) class I tetramer technology (1), studies have quantified the CD8+ T-cell populations specific for individual HIV peptides and correlated these findings with various HIV disease parameters (21, 22). The very nature of MHC class I tetramers, however, imposes a critical limitation on the conclusions that can be drawn from these studies. Over 100 different HIV type 1 (HIV-1) peptides recognized by HIV-specific CD8+ T cells have been identified, likely representing only a fraction of the total number of potential epitopes within the virus itself (17). In most infected individuals the CD8+ T-cell response to HIV is broad (6, 10, 24), with multiple epitopes restricted by HLA-A, -B, or -C alleles being recognized. This suggests that the use of MHC class I tetramers to examine responses to single peptides could dramatically underestimate the total or most relevant response in any tested individual. Because a unique tetramer molecule must be produced for every single HIV peptide, it remains difficult to quantify accurately the responses to multiple peptides in an individual and to develop a hierarchy of responses in order to identify potentially immunodominant peptides. Comparison of peptide responses between individuals using MHC class I tetramers depends on immunodominance of those peptides and assumes that those responses are representative of the total CD8+ T-cell response in each individual. It remains to be determined if putative immunodominant epitopes are dominant compared to all other epitopes or only those epitopes restricted by the same MHC class I protein.
To begin to address these issues, we assessed intracellular gamma interferon (IFN-γ) production by CD8+ T cells from HLA-A2+ donors in response to 95 optimally defined HLA class I-restricted HIV-derived epitopes, using peptide mixes and a peptide matrix system. Peptide-specific CD8+ T-cell responses quantified by intracellular IFN-γ production are directly comparable to the responses observed when MHC class I tetramers bearing the same peptide are used and are superior to peptide-specific responses quantified by enzyme-linked spot analysis (20). Intracellular IFN-γ production has a considerable advantage over tetramer analysis in that peptides alone are required to assess CD8+ T-cell responses, rather than separate MHC class I-peptide complexes for each peptide to be examined. Our results indicate that the CD8+ T-cell response to a single HIV peptide is rarely representative of the total HIV-specific CD8+ T-cell response. Additionally, responses to a putative immunodominant HIV epitope, if present at all, may be lower than the response to other recognized HIV epitopes. Definition of immunodominant HIV epitopes therefore requires a complete analysis of all HIV peptides recognized within an HIV-infected individual. Furthermore, given the diversity of MHC class I haplotypes within the human population and our lack of understanding of the relationships between them, an extensive analysis of HIV-specific responses in large numbers of individuals will be required to identify truly immunodominant HIV epitopes. The identification and characterization of the role of immunodominant CD8+ T-cell epitopes are critical in analyzing the immune response to HIV and other human pathogens and developing appropriate vaccine strategies.
MATERIALS AND METHODS
Subjects.
Eleven HIV-1-infected HLA-A2+ individuals (as determined by molecular typing for 10 subjects or serology for 1) with detectable viral load (≥400 copies/ml) and CD4 T-cell counts greater than 200/μl were recruited into this study. These individuals ranged from acute seroconverters to long-term nonprogressors, and antiretroviral therapy within this cohort varied between untreated, triple-drug therapy (highly active antiretroviral therapy), and salvage regimens. Molecular subtyping of the HLA-A2 allele in 10 of the patients was performed as previously described (4).
Peptides.
HIV peptides corresponding to the 95 optimally defined HIV epitopes as described in the HIV Molecular Immunology Database (17) were used; these included peptides from the HIV gag, pol, env, and nef gene products. The peptides were synthesized as free acids, and the purity was greater than 80% in all cases. Lyophilized peptides were resuspended in dimethyl sulfoxide at stock concentrations of 100 mg/ml for peptide mixes and 10 mg/ml for peptide matrix and single-peptide experiments. Peptide mixes contained the optimally defined epitopes from a particular HIV protein (37 Gag, 18 Pol, 20 Env, and 20 Nef), while the peptide matrix consisted of 20 pools containing up to 10 peptides. The matrix pools were arranged such that any particular peptide could be found in only two pools, as described by Kern et al. (15). The final concentration of each individual peptide was 2 μg/106 cells in all experiments described.
Cell stimulation.
Peripheral blood mononuclear cells (PBMC) were obtained by standard Ficoll-Hypaque density centrifugation (Pharmacia, Uppsala, Sweden). Stimulation was performed as described elsewhere (14). Freshly isolated PBMC (106 in 1 ml of complete RPMI 1640 medium containing 10% fetal calf serum) were incubated with 1 μg each of the costimulatory CD28 and CD49d monoclonal antibodies and 2 μg of each peptide to be tested. To control for spontaneous production of cytokine, cells incubated with only costimulatory antibodies were included in every experiment. The cultures were incubated at 37°C in a 5% CO2 incubator for 1 h, followed by an additional 5-h incubation with the secretion inhibitor brefeldin A (10 μg/ml; Sigma, St. Louis, Mo.).
Immunofluorescent staining.
Peptide-stimulated and control cultures were washed (1,200 rpm, 8 min) in cold Dulbecco's phosphate-buffered saline containing 1% bovine serum albumin and 0.1% sodium azide and transferred into 5-ml Falcon polystyrene tubes for staining. After an additional wash, the cells were surface stained with directly conjugated CD3 and CD8 antibodies for 30 min on ice. The cells were washed once, resuspended in 750 μl of 2× concentration fixation/permeabilization solution (Becton Dickinson Immunocytometry Systems, San Jose, Calif.), and incubated for 10 min in the dark at room temperature. Permeabilized cells were washed twice and stained with fluorochrome-conjugated IFN-γ and CD69 antibodies for 30 min on ice. After a final wash, the cells were resuspended in Dulbecco's phosphate-buffered saline containing 1% paraformaldehyde (Electron Microscopy Systems, Fort Washington, Pa.) prior to analysis.
Flow cytometric analysis.
Six-parameter flow cytometric analysis was performed on a FACScalibur flow cytometer (Becton Dickinson Immunocytometry Systems), using fluorescein isothiocyanate (FITC), phycoerythrin (PE), peridinin-chlorophyll protein (PerCP), and allophycocyanin (APC) as the fluorescent parameters. Between 100,000 and 130,000 live events were acquired, gated on small viable lymphocytes and CD3 and CD8 expression. List mode data files were analyzed using PAINT-A-GATEPlus software (Becton Dickinson Immunocytometry Systems). In all experiments, responses were rated positive when a population of IFN-γ+ and CD69+ events ≥0.05% (above background) of CD3+ CD8+ lymphocytes was observed.
Antibodies.
Unconjugated mouse anti-human CD28, unconjugated mouse anti-human CD49d, FITC-conjugated mouse anti-human IFN-γ, PE-conjugated mouse anti-human CD69, PerCP-conjugated mouse anti-human CD3 and CD8, and APC-conjugated mouse anti-human CD3 and CD8 monoclonal antibodies, along with immunoglobulin G1 and G2 isotype-matched controls, were obtained from Becton Dickinson Immunocytometry Systems.
RESULTS AND DISCUSSION
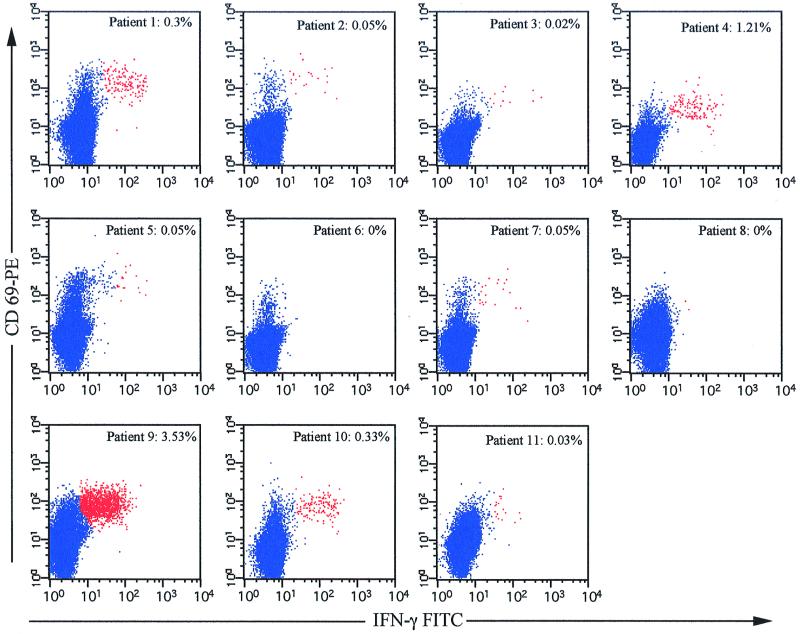
In our initial experiments, we examined the response to the HLA-A*0201-restricted HIV-1 Gag epitope p17 77–85 (SLYNTVATL) in 11 HLA-A2+ HIV-infected subjects. This epitope has been reported to be an immunodominant epitope (4, 5), suggesting that responses to this epitope should account for the majority of Gag-specific responses in our HLA-A2+ HIV-infected cohort. Surprisingly, we found that only 4 of 11 patients (patients 1, 4, 9, and 10) had significant responses to this epitope (Fig. 1). Three individuals (patients 2, 5, and 7) demonstrated very weak responses (∼0.05%) to SLYNTVATL. One of the nonresponders is HLA-A*0205/08 (patient 3 [Table 1]), which, although unlikely, may explain why this individual failed to recognize SLYNTVATL. High levels of SLYNTVATL-specific CD8+ T cells were found in patient 9 (3.53%), a moderate response was found in patient 4 (1.21%), and low responses were found in patients 1 and 10 (0.3 and 0.33%, respectively). Indeed, if responses to SLYNTVATL were assumed to accurately reflect the total HIV-specific CTL response in these individuals, the majority of them would be considered nonresponders. However, since most HIV-infected individuals elicit broad HIV-specific CD8+ T-cell responses, it is likely that these individuals would recognize other HIV-derived peptides, restricted to HLA-A2 or other MHC class I molecules.
FIG. 1.
Assessment of p17 SLYNTVATL-specific IFN-γ production in 11 HLA-A2+ HIV-positive patients. PBMC from each patient were stimulated with 2 μg of SLYNTVATL peptide per ml and costimulatory antibodies as described in Materials and Methods. The number shown in each plot represents the percentage of CD3+ CD8+ CD69+ IFN-γ+ events, with background IFN-γ production subtracted. The background IFN-γ production was ≤0.02% in all patients except patients 1 (0.03%), 4 (0.15%), and 11 (0.07%). Responses equal to or greater than 0.05% above background are considered positive.
TABLE 1.
Patient MHC class I type and HIV- or CMV-derived peptide-specific IFN-γ productiona
| Patient no. | MHC class I type | IFN-γ response (%) | Other HIV peptides recognized | MHC class I restriction | % IFN-γ production | |
|---|---|---|---|---|---|---|
| A2-restricted CMV | p17 77–85 | |||||
| Group 1 | ||||||
| 2 | A*0201, A30, | 0.28 | 0.05 | 3 (RT 476–484) | A2 | 0.44 |
| B13 | 11 (p17 20–29) | A30 | 0.29 | |||
| 3 | A*0205/08, A29, | 1.99 | 0.02 | 30 (gp120 419–427) | A29 | 0.07 |
| B27, B45 | 54 (p24 263–272) | B27 | 0.60 | |||
| 71 (Nef 120–128) | B37 | 0.23 | ||||
| 94 (Nef 117–127) | Bw62 | 0.06 | ||||
| 5 | A*0201, A3 | 0.11 | 0.05 | 11 (p17 20–29) | A3.1 | 0.13 |
| B7, B44 | 12 (RT 325–333) | A3.1 | 0.05 | |||
| 15 (Nef 73–82) | A3.1 | 0.26 | ||||
| 6 | A*0201, A32, | 1.45 | 0 | 3 (RT 476–484) | A2 | 0.05 |
| B60, B78 | 79 (gp41 557–565) | B51 | 0.07 | |||
| 7 | A2 | 0.38 | 0.05 | 51 (gp120 818–827) | A2 | 0.08 |
| 20 (Nef 84–92) | A11 | 0.31 | ||||
| 93 (Nef 84–91) | Bw62 | 0.50 | ||||
| 27 (p24 167–175) | A26 | 0.12 | ||||
| 95 (p24 168–175) | Cw01,02 | 0.12 | ||||
| 71 (Nef 120–128) | B37 | 0.15 | ||||
| 94 (Nef 117–127) | Bw62 | 0.12 | ||||
| 8 | A*0201, | 1.48 | 0 | |||
| B35, B56 | ||||||
| 11 | A*0201, A1 | 0.22 | 0.03 | 25 (p24 145–155) | A25 | 1.43 |
| B57 | 83 (p24 147–155) | B57 | 2.14 | |||
| 32 (gp120 419–427) | A32 | 0.07 | ||||
| 84 (p24 140–149) | B57 | 0.72 | ||||
| 85 (p24 162–172) | B57 | 0.05 | ||||
| 88 (Nef 116–125) | B57 | 0.26 | ||||
| Group 2 | ||||||
| 1 | A*0201, A31, | 1.13 | 0.30 | 10 (p17 20–28) | A3.1 | 0.23 |
| B51, B58w4 | 78 (RT 295–302) | B51 | 1.17 | |||
| 4 | A*0201, | 1.58 | 1.21 | |||
| B44, B70 | ||||||
| 9 | A*0201 | 0.13 | 3.53 | 77 (p24 325–333) | B51 | 1.75 |
| B51 | 78 (RT 295–302) | B51 | 2.01 | |||
| 10 | A*0201, A31, | 2.89 | 0.33 | 14 (gp41 775–785) | A3.1 | 0.13 |
| B8, B51 | 43 (p24 259–267) | B8 | 0.05 | |||
| 45 (Nef 13–20) | B8 | 0.10 | ||||
| 46 (Nef 90–97) | B8 | 0.08 | ||||
| 77 (p24 325–333) | B51 | 0.09 | ||||
| 78 (RT 295–302) | B51 | 0.28 |
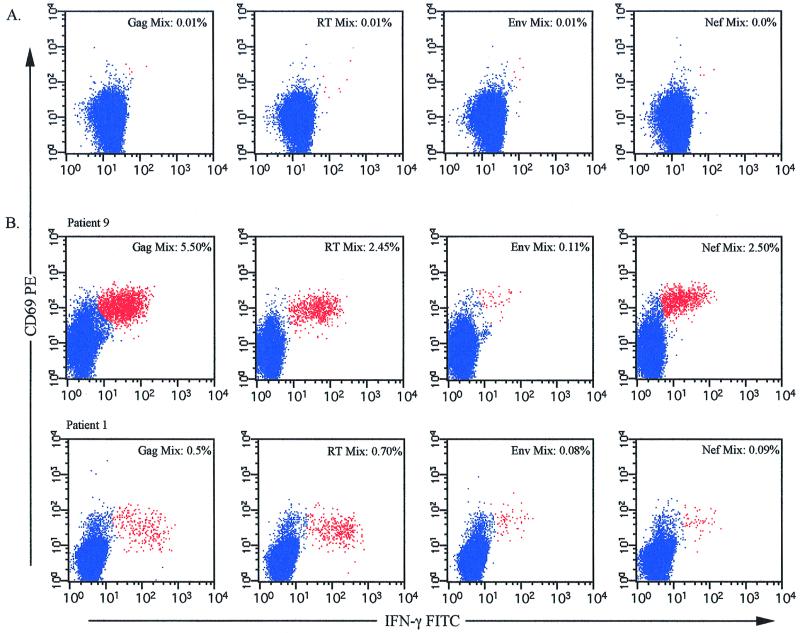
To test HIV-specific CD8+ T-cell responses to 95 optimally defined CTL epitopes in these patients, we made four mixtures of HIV epitopes such that each mixture contained all defined epitopes from a particular HIV protein. These mixtures contained as many as 37 separate epitopes (Gag) to as few as 18 (reverse transcriptase [RT]), with the envelope and Nef mixtures containing 20 epitopes each. Little to no appreciable IFN-γ (<0.05%) was produced in response to these peptide mixtures in HIV-negative controls (Fig. 2A). In contrast, nearly all (10 of 11) of the HLA-A2+ HIV-infected subjects demonstrated IFN-γ production by CD8+ T cells in response to the HIV epitope mixes. Two representative examples shown in Fig. 2B indicate the wide range of responses and specificities present within the cohort. Patient 9 had strong responses to the Gag, RT, and Nef epitope mixtures and a weak response to the Env mixture. Patient 1 had moderate responses to the Gag and RT epitope mixes and a weak response to the Env and Nef mixtures. All patients except patient 8 showed recognition of at least one epitope mixture (data not shown), and all 11 recognized an HLA-A2-restricted control peptide (cytomegalovirus [CMV] pp65 NLVPMVATV [Table 1]). Comparison of the responding populations to the Gag epitope mixtures and the individual peptide responses to p17 SLYNTVATL indicated that only in patient 4 were the Gag epitope mixture and p17 SLYNTVATL peptide responses equivalent (Gag mix, 1.20%; p17 SLYNTVATL, 1.21%). This indicates that in the majority of these patients, responses to the HLA-A2-restricted p17 SLYNTVATL epitope are not representative of either the total Gag-specific CD8+ T-cell response or the overall HIV-specific response. These results call into question whether the HLA-A2-restricted p17 SLYNTVATL epitope is immunodominant in the context of other HLA-A2-restricted HIV-1 epitopes or of the total array of HIV-1 epitopes restricted by other HLA class I alleles.
FIG. 2.
Intracellular IFN-γ production from CD8+ T cells in response to HIV-1 peptide mixes. PBMC from HIV-seronegative (A) or HIV-seropositive (B) individuals were incubated with HIV-1 peptide mixes for 6 h in the presence of the costimulatory anti-CD28 and anti-CD49d antibodies and brefeldin A (final 5 h only). After incubation, the cells were surface stained as described in Materials and Methods. The number shown in the upper right corner of each plot represents the percentage of CD69+ IFN-γ+ CD3+ CD8+ cells responding to the indicated peptide mixture.
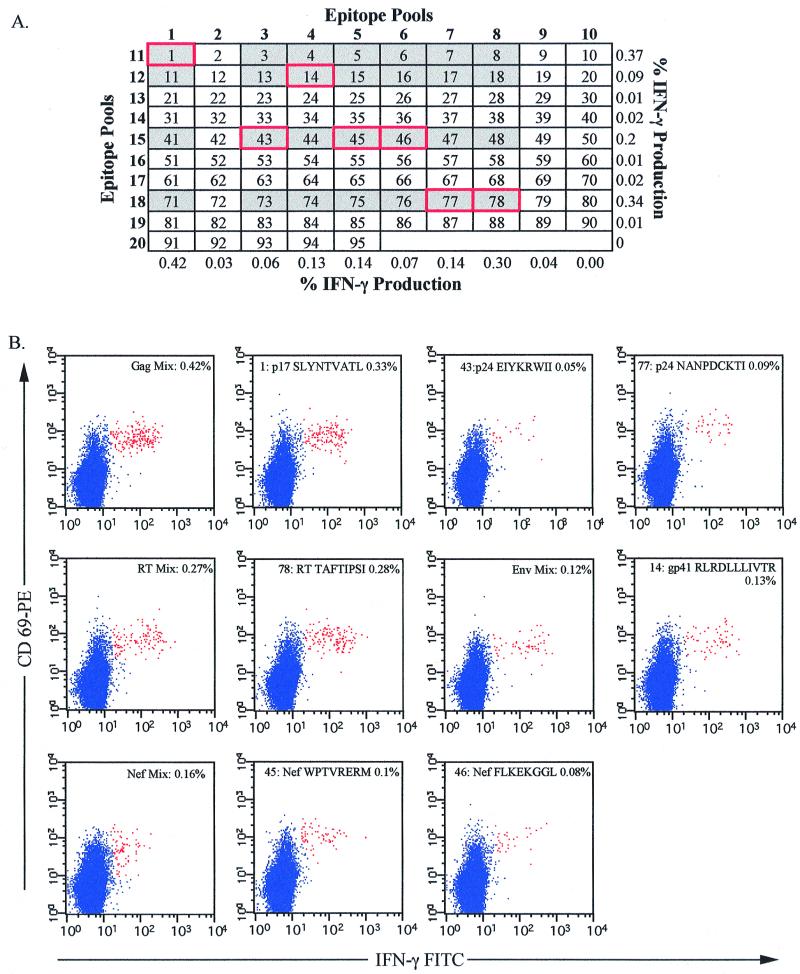
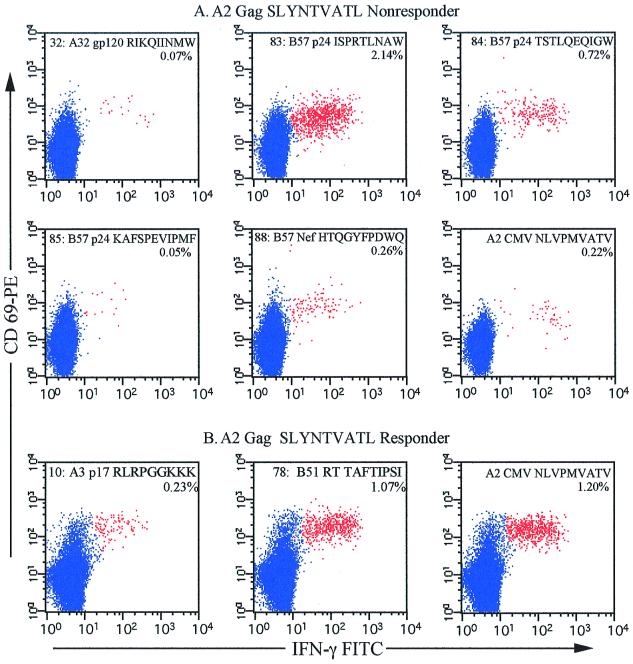
To identify candidate peptides to which each individual responded, we used an epitope pool matrix system (Fig. 3A). Twenty pools of peptides were made, such that each pool contained up to 10 individual epitopes. The peptides were arranged so that each peptide was found in two pools, allowing identification of candidate peptides by combining the results of responding pools. The complete results of these analyses are shown in Table 1. As an example, Fig. 3 shows the complete matrix analysis of patient 10. Eleven of the 20 epitope pools stimulated IFN-γ production from CD8+ T cells, suggesting that up to 28 separate peptides were potentially recognized. Analysis of these 28 peptides identified 7 recognized by CD8+ T cells in this patient (peptides 1, 14, 43, 45, 46, 77, and 78 shown in Fig. 3B). The majority of the patients recognized multiple peptides, and only patient 4 exhibited a monospecific response to p17 SLYNTVATL. With the exception of patient 8, who failed to respond to any HIV epitopes tested, all SLYNTVATL nonresponders recognized at least two HIV-1 peptides (Table 1, group 1), restricted to either HLA-A2 or other HLA alleles. Three of the seven SLYNTVATL nonresponders recognized other HLA-A2-restricted HIV-1 peptides, patients 2 and 6 recognizing RT 476–484 (ILKEPVHGV) peptide and patient 7 recognizing gp120 818–827. In four of the SLYNTVATL nonresponders, there was no response to any HLA-A2-restricted HIV epitope, although all the subjects recognized the HLA-A2-restricted CMV epitope. For example, Fig. 4A shows the peptides recognized by CD8+ T cells in subject 11. This patient recognizes five different peptides (and one additional overlapping peptide [Table 1]), restricted to HLA-A32 or HLA-B57. The response to peptide 83 (p24 147–155 [ISPRTLNAW]), restricted by HLA-B57, is quite strong (2.14% of CD3+ CD8+ lymphocytes) compared to the other responses. Thus, in these HLA-A2+ individuals, examination of responses to the p17 SLYNTVATL epitope alone would severely underestimate the total CD8+ T-cell response to HIV, and most would be inappropriately classified as having low or absent HIV-specific CD8+ T-cell responses.
FIG. 3.
Identification of individual peptide responses using a peptide pool matrix. PBMC from patient 10 were incubated in the presence of costimulatory antibodies and brefeldin A as described in Materials and Methods with 20 epitope pools (in bold), each containing up to 10 HIV-1 peptides (A). The percentage of IFN-γ production to each pool from CD3+ CD8+ CD69+ small lymphocytes is shown at the right and along the bottom. Those peptides that could potentially be recognized, as determined by the pool responses, are in gray boxes. The actual peptides recognized (B) are in red-bordered gray boxes. The plots in panel B show the CD8+ T-cell response to the peptide mixes and individual peptides (as identified through the matrix analysis) in patient 10, where the number shown in each box represents the percentage of CD3+ CD8+ CD69+ IFN-γ+ events. The response to each peptide is shown in the top of each plot, as is the peptide source (i.e., HIV protein) and sequence of the peptide. Background IFN-γ production in patient 10 was less than 0.01%.
FIG. 4.
Additional HIV epitopes recognized in a p17 SLYNTVATL nonresponder and responder. Potentially recognized epitopes were identified by peptide matrix analysis in all 11 subjects. Single-peptide analysis was performed to determine the peptides recognized in each subject. Shown are those responses identified in a representative SLYNTVATL nonresponder (A) and SLYNTVATL responder (B). As in the previous figures, all events shown are CD3+ and CD8+ gated events. The percentages represent those events that are CD69+ and IFN-γ+ within the CD8+ T-cell subset. Peptide numbers correspond to those shown in Fig. 3A. Each plot also shows the HLA restriction, source of peptide, and sequence, consecutively, of the peptide tested. The HLA-A2-restricted CMV peptide pp65 NLVPMVATV was included in all experiments as a positive control to demonstrate that all HLA-A2+ patients recognized an HLA-A2-restricted peptide.
Similarly, the SLYNTVATL responders also recognized other HIV-1 epitopes (Table 1, group 2). Three of four SLYNTVATL responders (patients 1, 9, and 10) recognized two or more additional peptides. Only patient 4 showed recognition of SLYNTVATL alone. However, only two peptides restricted to HLA-B44 were included in the epitope matrix, and no peptides restricted to HLA-B70 were included. Thus, it is quite possible that patient 4 responds to presently undefined HIV epitopes restricted to either of these alleles. Figure 4B shows the additional CD8+ T-cell responses identified in patient 1. This patient has responses to two additional epitopes, peptide 10 (A3-restricted p17 RLRPGGKKK) and peptide 78 (B51-restricted RT TAFTIPSI). In this patient, the CD8+ response to the TAFTIPSI epitope was, in fact, higher than the response to the SLYNTVATL epitope. Thus, patients that respond to SLYNTVATL are likely to respond to other HIV epitopes restricted to HLA alleles, and in some SLYNTVATL responders, more potent CD8+ T-cell activity may exist to other epitopes restricted by other HLA alleles.
To use single-peptide responses as a predictor for overall T-cell responses, it is first necessary to identify truly immunodominant epitopes. Immunodominance of CD8+ T-cell epitopes has been well documented in the relatively controlled environment of the inbred mouse (28, 30), but in these inbred populations, heterogeneity in the MHC and non-MHC genes influencing T-cell receptor repertoire, epitope processing, and presentation is at a minimum. Predicting immunodominant CD8+ T-cell responses in humans, however, is more complicated, given the high diversity of MHC class I haplotypes found within the general population and the likely similar heterogeneity among non-MHC class I genes influencing this process. Several putative immunodominant peptides have been identified for some human MHC class I haplotypes, including the HLA-A2-restricted epitopes influenza virus M1 58–66 (19) and CMV pp65 495–503 (29). It is unclear, however, whether these peptides are immunodominant for their particular MHC class I allele or for the entire response specific for the virus. Furthermore, response hierarchies resulting from different combinations of the many available MHC class I genes are virtually unexplored. These issues apply to HIV-specific CD8+ T-cell responses as well, where most studies have focused on relatively few peptides, in particular the HLA-A2-restricted epitopes p17 77–85 (SLYNTVATL) and Pol 476–484 (ILKEPVHGV) (21, 22). These epitopes are often referred to as immunodominant epitopes (4, 5, 13), yet it remains unclear whether they are immunodominant compared to other HLA-A2-restricted epitopes only or compared to all recognized HIV epitopes within an infected individual. Our results suggest that SLYNTVATL may be a common HLA-A2-restricted HIV epitope (if a HLA-A2-restricted response is present) but is not necessarily the immunodominant epitope compared to other HIV-derived epitopes.
These results call into question the validity of using the response to a single HIV epitope as a surrogate measure of the total HIV-specific CD8+ T-cell response. As we have clearly shown, SLYNTVATL responses, often used as a predictor of the total immune response in HLA-A2+ HIV-infected patients (21, 22), are not comparable to the total HIV-specific CD8+ T-cell responses in most individuals. This suggests that it may be difficult to interpret relationships between viral load and single-epitope responses to define the functional antiviral activity of HIV-specific CD8+ T-cells in vivo. Recently, these relationships have been examined using MHC class I tetramers containing single HIV-1 peptides, typically p17 SLYNTVATL and RT ILKEPVHGV (21), where a negative correlation between SLYNTVATL- and ILKEPVHGV-specific CD8+ T-cell numbers and viral load was found. As we have demonstrated, most HIV-infected patients recognize multiple HIV-1 peptides, and rarely is a monotypic response identified. It would be prudent to reexamine the relationship between CD8+ T-cell responses and viral load using a more representative panel of peptides to obtain an accurate assessment of the total HIV-specific CD8+ T-cell response. Certainly, many HLA-A2+ individuals identified in previous studies as having poor SLYNTVATL recognition could have potent CD8+ T-cell responses specific for other HIV-1 peptides restricted by other HLA alleles.
It should be noted that even multiple-peptide analysis described here may still underestimate the total CD8+ T-cell response in these individuals, since there are likely to be more than 95 epitopes, many of which remain to be identified. These could be rapidly identified using peptide matrices containing overlapping peptides comprising the entire amino acid sequence of the viral protein of interest. Using epitope mixes and pools, it is clear that the response obtained to the individual peptides is representative of the responses obtained to the pools containing those same peptides. Additionally, the response obtained with the epitope mixes (Fig. 3B, top four panels) is approximately equal to the sum of the responses to the individual peptides (Fig. 3B, bottom six panels). Through the use of epitope matrices, large numbers of peptides can be screened to identify the individual epitopes being recognized. Importantly, the techniques described here are not constrained by the availability of unique peptide-MHC tetramer combinations.
Precise identification of immunodominant HIV epitopes will require extensive characterization of the CD8+ T-cell responses in a large number of infected individuals of various MHC class I haplotypes. Our results suggest that SLYNTVATL alone should not be used as a predictor of an individual's CD8+ T-cell response to HIV, and potential vaccines should not be dismissed solely on the basis of an inability to stimulate a SLYNTVATL response in HLA-A2+ subjects. Until truly immunodominant epitopes have been identified, assessment of candidate vaccine regimens, as well as analysis of immune reconstitution during viral therapy, should be performed without the assumption that the response to a single peptide represents the total HIV-specific response.
ACKNOWLEDGMENTS
We thank Daniel C. Douek for helpful conversations and review of the manuscript.
This work was supported by grants AI35522 and AI47603 to R.A.K. from the National Institutes of Health. R.A.K. is an Elizabeth Glaser Scientist of the Elizabeth Glaser Pediatric AIDS Foundation.
REFERENCES
- 1.Altman J D, Moss P A H, Goulder P J R, Barouch D H, McHeyzer-Williams M G, Bell J I, McMichael A J, Davis M M. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274:94–96. [PubMed] [Google Scholar]
- 2.Borrow P, Lewicki H, Hahn B H, Shaw G M, Oldstone M B. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J Virol. 1994;68:6103–6110. doi: 10.1128/jvi.68.9.6103-6110.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borrow P, Shaw G M. Cytotoxic T-lymphocyte escape viral variants: how important are they in viral evasion of immune clearance in vivo? Immunol Rev. 1998;164:37–51. doi: 10.1111/j.1600-065X.1998.tb01206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brander C, Hartman K E, Trocha A K, Jones N G, Johnson R P, Korber B, Wentworth P, Buchbinder S P, Wolinsky S, Walker B D, Kalams S A. Lack of strong immune selection pressure by the immunodominant, HLA- A*0201-restricted cytotoxic T lymphocyte response in chronic human immunodeficiency virus-1 infection. J Clin Investig. 1998;101:2559–2566. doi: 10.1172/JCI2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brander C, Yang O O, Jones N G, Lee Y, Goulder P, Johnson R P, Trocha A, Colbert D, Hay C, Buchbinder S, Bergmann C C, Zweerink H J, Wolinsky S, Blattner W A, Kalams S A, Walker B D. Efficient processing of the immunodominant, HLA-A*0201-restricted human immunodeficiency virus type 1 cytotoxic T-lymphocyte epitope despite multiple variations in the epitope flanking sequences. J Virol. 1999;73:10191–10198. doi: 10.1128/jvi.73.12.10191-10198.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dalod M, Dupuis M, Deschemin J C, Sicard D, Salmon D, Delfraissy J F, Venet A, Sinet M, Guillet J G. Broad, intense anti-human immunodeficiency virus (HIV) ex vivo CD8+ responses in HIV type 1-infected patients: comparison with anti-Epstein-Barr virus responses and changes during antiretroviral therapy. J Virol. 1999;73:7108–7116. doi: 10.1128/jvi.73.9.7108-7116.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Evans D T, O'Connor D H, Jing P, Dzuris J L, Sidney J, da Silva J, Allen T M, Horton H, Venham J E, Rudersdorf R A, Vogel T, Pauza C D, Bontrop R E, DeMars R, Sette A, Hughes A L, Watkins D I. Virus-specific cytotoxic T-lymphocyte responses select for amino-acid variation in simian immunodeficiency virus Env and Nef. Nat Med. 1999;5:1270–1276. doi: 10.1038/15224. [DOI] [PubMed] [Google Scholar]
- 8.Gallimore A, Cranage M, Cook N, Almond N N, Bootman J, Rud E, Silvera P, Dennis M, Corcoran T, Stott J, McMichael A, Gotch F. Early suppression of SIV replication by CD8+ nef-specific cytotoxic T cells in vaccinated macaques. Nat Med. 1995;1:1067–1073. doi: 10.1038/nm1195-1167. [DOI] [PubMed] [Google Scholar]
- 9.Goulder P J, Phillips R E, Colbert R A, McAdam S, Ogg G, Nowak M A, Giangrande P, Luzzi G, Morgan B, Edwards A, McMichael A J, Rowland-Jones S. Late escape from an immunodominant cytotoxic T-lymphocyte response associated with progression to AIDS. Nat Med. 1997;3:212–217. doi: 10.1038/nm0297-212. [DOI] [PubMed] [Google Scholar]
- 10.Harrer E, Harrer T, Buchbinder S, Mann D L, Feinberg M, Yilma T, Johnson R P, Walker B D. HIV-1-specific cytotoxic T lymphocyte response in healthy, long-term nonprogressing seropositive persons. AIDS Res Hum Retroviruses. 1994;10:S77–S78. [PubMed] [Google Scholar]
- 11.Jin X, Bauer D E, Tuttleton S E, Lewin S, Gettie A, Blanchard J, Irwin C E, Safrit J T, Mittler J, Weinberger L, Kostrikis L G, Zhang L, Perelson A S, Ho D D. Dramatic rise in plasma viremia after CD8(+) T cell depletion in simian immunodeficiency virus-infected macaques. J Exp Med. 1999;189:991–998. doi: 10.1084/jem.189.6.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson R P, Desrosiers R C. Protective immunity induced by live attenuated simian immunodeficiency virus. Curr Opin Immunol. 1998;10:436–443. doi: 10.1016/s0952-7915(98)80118-0. [DOI] [PubMed] [Google Scholar]
- 13.Johnson R P, Trocha A, Yang L, Mazzara G P, Panicali D L, Buchanan T M, Walker B D. HIV-1 gag-specific cytotoxic T lymphocytes recognize multiple highly conserved epitopes: fine specificity of the gag-specific response defined by using unstimulated peripheral blood mononuclear cells and cloned effector cells. J Immunol. 1991;147:1512–1521. [PubMed] [Google Scholar]
- 14.Kern F, Surel I P, Brock C, Freistedt B, Radtke H, Scheffold A, Blasczyk R, Reinke P, Schneider-Mergener J, Radbruch A, Walden P, Volk H D. T-cell epitope mapping by flow cytometry. Nat Med. 1998;4:975–978. doi: 10.1038/nm0898-975. [DOI] [PubMed] [Google Scholar]
- 15.Kern F, Surel I P, Faulhaber N, Frommel C, Schneider-Mergener J, Schonemann C, Reinke P, Volk H D. Target structures of the CD8+-T-cell response to human cytomegalovirus: the 72-kilodalton major immediate-early protein revisited. J Virol. 1999;73:8179–8184. doi: 10.1128/jvi.73.10.8179-8184.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klein M R, van Baalen C A, Holwerda A M, Kerkhof-Garde S R, Bende R J, Keet I P M, Eeftinck-Schattenkerk J-K M, Osterhaus A D M E, Schuitemaker H, Miedema F. Kinetics of gag-specific cytotoxic T lymphocyte responses during the clinical course of HIV-1 infection: a longitudinal analysis of rapid progressors and long-term asymptomatics. J Exp Med. 1995;181:1365–1372. doi: 10.1084/jem.181.4.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Korber B T M, Brander C, Walker B D, Koup R A, Moore J P, Haynes B F, Myers G. HIV Molecular Immunology Database. Los Alamos, N. Mex: Los Alamos National Laboratory; 1998. [Google Scholar]
- 18.Koup R A, Safrit J T, Cao Y, Andrews C A, Wu Y, McLeod G, Borkowsky W, Farthing C, Ho D D. Temporal association of cellular immune response with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J Virol. 1994;68:4650–4655. doi: 10.1128/jvi.68.7.4650-4655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lalvani A, Brookes R, Hambleton S, Britton W J, Hill A V, McMichael A J. Rapid effector function in CD8+ memory T cells. J Exp Med. 1997;186:859–865. doi: 10.1084/jem.186.6.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murali-Krishna K, Altman J D, Suresh M, Sourdive D J, Zajac A J, Miller J D, Slansky J, Ahmed R. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity. 1998;8:177–187. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
- 21.Ogg G S, Jin X, Bonhoeffer S, Dunbar P R, Nowak M A, Monard S, Segal J P, Cao Y, Rowland-Jones S L, Cerundolo V, Hurley A, Markowitz M, Ho D D, Nixon D F, McMichael A J. Quantitation of HIV-1-specific cytotoxic T lymphocytes and plasma load of viral RNA. Science. 1998;279:2103–2106. doi: 10.1126/science.279.5359.2103. [DOI] [PubMed] [Google Scholar]
- 22.Ogg G S, Jin X, Bonhoeffer S, Moss P, Nowak M A, Monard S, Segal J P, Cao Y, Rowland-Jones S L, Hurley A, Markowitz M, Ho D D, McMichael A J, Nixon D F. Decay kinetics of human immunodeficiency virus-specific effector cytotoxic T lymphocytes after combination antiretroviral therapy. J Virol. 1999;73:797–800. doi: 10.1128/jvi.73.1.797-800.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Price D A, Goulder P J, Klenerman P, Sewell A K, Easterbrook P J, Troop M, Bangham C R, Phillips R E. Positive selection of HIV-1 cytotoxic T lymphocyte escape variants during primary infection. Proc Natl Acad Sci USA. 1997;94:1890–1895. doi: 10.1073/pnas.94.5.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rinaldo C, Huang X-L, Fan Z, Ding M, Beltz L, Logar A, Panicali D, Mazzara G, Liebmann J, Cottrill M, Gupta P. High levels of anti-human immunodeficiency virus type 1 (HIV-1) memory cytotoxic T-lymphocyte activity and low viral load are associated with lack of disease in HIV-1-infected long-term nonprogressors. J Virol. 1995;69:5838–5842. doi: 10.1128/jvi.69.9.5838-5842.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rowland-Jones S, Sutton J, Ariyoshi K, Dong T, Gotch F, McAdam S, Whitby D, Sabally S, Gallimore A, Corrah T, Takiguchi M, Schultz T, McMichael A, Whittle H. HIV-specific cytotoxic T-cells in HIV-exposed but uninfected Gambian women. Nat Med. 1995;1:59–64. doi: 10.1038/nm0195-59. [DOI] [PubMed] [Google Scholar]
- 26.Rowland-Jones S L, Nixon D F, Gotch F, Hallam N, Froebel K, Aldhous M C, Ariyoshi K, Kroll J S, McMichael A. HIV-specific cytotoxic T-cell activity in an HIV-exposed but uninfected infant. Lancet. 1993;341:860–861. doi: 10.1016/0140-6736(93)93063-7. [DOI] [PubMed] [Google Scholar]
- 27.Schmitz J E, Kuroda M J, Santra S, Sasseville V G, Simon M A, Lifton M A, Racz P, Tenner-Racz K, Dalesandro M, Scallon B J, Ghrayeb J, Forman M A, Montefiori D C, Rieber E P, Letvin N L, Reimann K A. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science. 1999;283:857–860. doi: 10.1126/science.283.5403.857. [DOI] [PubMed] [Google Scholar]
- 28.van der Most R G, Sette A, Oseroff C, Alexander J, Murali-Krishna K, Lau L L, Southwood S, Sidney J, Chesnut R W, Matloubian M, Ahmed R. Analysis of cytotoxic T cell responses to dominant and subdominant epitopes during acute and chronic lymphocytic choriomeningitis virus infection. J Immunol. 1996;157:5543–5554. [PubMed] [Google Scholar]
- 29.Wills M R, Carmichael A J, Mynard K, Jin X, Weekes M P, Plachter B, Sissons J G P. The human cytotoxic T-lymphocyte (CTL) response to cytomegalovirus is dominated by structural protein pp65: frequency, specificity, and T-cell receptor usage of pp65-specific CTL. J Virol. 1996;70:7569–7579. doi: 10.1128/jvi.70.11.7569-7579.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yewdell J W, Bennink J R. Immunodominance in major histocompatibility complex class I-restricted T lymphocyte responses. Annu Rev Immunol. 1999;17:51–88. doi: 10.1146/annurev.immunol.17.1.51. [DOI] [PubMed] [Google Scholar]