Induction and Prevention of Apoptosis in Human HEp-2 Cells by Herpes Simplex Virus Type 1 (original) (raw)
Abstract
Cultured human epithelial cells infected with an ICP27 deletion strain of herpes simplex virus type 1 (HSV-1) show characteristic features of apoptotic cells including cell shrinkage, nuclear condensation, and DNA fragmentation. These cells do not show such apoptotic features when infected with a wild-type virus unless the infections are performed in the presence of a protein synthesis inhibitor. Thus, both types of virus induce apoptosis, but the ICP27-null virus is unable to prevent this process from killing the cells. In this report, we show that this ICP27-deficient virus induced apoptosis in human HEp-2 cells through a pathway which involved the activation of caspase-3 and the processing of the death substrates DNA fragmentation factor and poly(ADP-ribose) polymerase. The induction of apoptosis by wild-type HSV-1 occurred prior to 6 h postinfection (hpi), and de novo viral protein synthesis was not required to induce the process. The ability of the virus to inhibit apoptosis was shown to be effective between 3 to 6 hpi. Wild-type HSV-1 infection was also able to block the apoptosis induced in cells by the addition of cycloheximide, staurosporine, and sorbitol. While US3- and ICP22-deficient viruses showed a partial prevention of apoptosis, deletion of either the UL13 or vhs gene products did not affect the ability of HSV-1 to prevent apoptosis in infected cells. Finally, we demonstrate that in UV-inactivated viruses, viral binding and entry were not sufficient to induce apoptosis. Taken together, these results suggest that either gene expression or another RNA metabolic event likely plays a role in the induction of apoptosis in HSV-1-infected human cells.
Herpes simplex virus type 1 (HSV-1) releases viral progeny during productive infection in cultured cells, leading to lytic cell death. The expression of HSV-1 genes precedes infected cell lysis, and it occurs through a highly regulated cascade (28, 29) that begins with the production of the α (immediate-early [IE]) proteins. The α proteins, infected cell protein 0 (ICP0), ICP4, ICP22, and ICP27, have regulatory functions and cooperatively act to regulate the expression of all classes of viral genes (reviewed in reference 55). The β (early [E]) gene products, such as the viral thymidine kinase (TK), are synthesized next and are the proteins principally involved in viral DNA synthesis (reviewed in reference 11). The last set of viral proteins produced are the γ (late [L]) proteins, which are mainly associated with virion structure and assembly, such as VP16 and vhs (7, 18, 51). The completion of the HSV-1 replication cycle leads ultimately to the destruction of the cells in culture, and this process is generally believed to occur through a necrotic route. Consequently, productive HSV-1 replication induces major biochemical alterations in the infected cells, including the loss of matrix binding proteins on the cell surface, membrane modifications, cytoskeletal destabilization, nucleolar alterations, chromatin margination, aggregation or damage, and a decrease in cellular macromolecular synthesis (6, 23, 26, 53–55). In addition, these morphological features observed with HSV-infected cells appear to be different from those associated with cells dying from apoptosis. In this case, the cells are characterized by morphological and biochemical changes that include cell shrinkage, membrane blebbing, nuclear condensation, and fragmentation of chromosomal DNA into nucleosomal oligomers (reviewed in reference 33). Therefore, it appears that a distinction exists between cytolysis due to viral replication and the apoptosis of cells.
Apoptosis, or programmed cell death, is a highly regulated process of cell suicide. It is activated during normal development and by various stimuli that disturb cell metabolism and physiology (73, 75). Apoptotic signals received through a death receptor pathway or a mitochondrial route converge to a central pathway involving a family of aspartate-specific cysteinyl proteases (cysteine aspartases, or caspases) which are activated by proteolytic cleavage during the process of cell death (2, 14, 22, 57, 69). Caspase activation leads to the processing of various cytoplasmic and nuclear targets by a subclass of caspases, called executioners, such as caspase-3, caspase-6, and caspase-7 (57, 74). Among the cleavage targets are the DNA repair enzyme poly(ADP-ribose) polymerase (PARP), the DNA fragmentation factor (DFF), and scaffolding proteins such as lamins and actin (43, 57, 74). Thus, the process of apoptosis generally involves the processing of caspase-3, DFF, and PARP.
Due to the cell’s innate ability to self-destruct, it is likely that apoptosis is also an important mechanism of host cell defense against viral infections. Accordingly, several distinct viruses appear to have developed mechanisms to block the premature apoptosis of infected cells (44, 66, 71). The most likely reason for this is to prevent the cellular apoptosis that occurs as a result of viral infection in order to prolong cell survival so that the production of the viral progeny can be maximized. Among the viral strategies to respond to programmed cell death pathways is the production of (i) antiapoptotic factors such as CrmA of cowpox virus (67, 68) and p35 and inhibitors of apoptosis from baculovirus (12, 13), which are viral inhibitors of caspases, and (ii) viral homologues of the cellular antiapoptotic protein Bcl-2, encoded by either Epstein-Barr virus (27), African swine fever virus (1), or herpesvirus saimiri (16). Recent reports (5, 19, 20, 30, 32, 35, 39, 70) showed that HSV-1 is also able to interfere with the process of apoptosis in infected cells. HSV-1 was able to prevent apoptosis which was externally induced by various stimuli including treatment with cycloheximide (CHX) (5, 20), ceramide, tumor necrosis factor, and anti-Fas antibody (20), osmotic shock using sorbitol (36) or ethanol (32), and hypothermia and thermal shock (19, 38, 39). It was also demonstrated that HSV-1 infection itself could induce apoptosis in cells. This conclusion was based on specific experiments in which cells were infected with mutant viruses (3, 5, 20, 39) or the infections occurred in the presence of the protein synthesis inhibitor CHX (5, 34). While the process of apoptosis in infected cells has now been clearly demonstrated, the specific mechanisms of its induction and prevention by the virus are not known.
In a previous report, we demonstrated that cultured epithelial cells of human origin infected with an ICP27 deletion strain of HSV-1 presented the characteristic features of apoptotic cells, including cell shrinkage, nuclear condensation, and DNA fragmentation (5). When the same cells were infected with a wild-type virus, they did not show these apoptotic features unless the infections were performed in the presence of CHX (5, 34). These results led us to conclude that both the wild-type and the ICP27-null viruses likely induce an apoptotic event in infected human cells, but the virus which lacks ICP27 protein is unable to prevent this process from killing the cells. To further investigate these findings, we used the ICP27-deficient virus (vBSΔ27) as a tool for the study of the induction and inhibition of HSV-1-initiated apoptosis. In this report, we show that vBSΔ27 induced apoptosis in HEp-2 cells through a pathway which involved the activation of the caspase-3 protease and the processing of the death substrates DFF and PARP. The induction of apoptosis by HSV-1 occurred prior to 6 h postinfection (hpi), and experiments involving temporal addition of CHX indicated that de novo viral protein synthesis was not required. The inhibition of apoptosis in HSV-1-infected HEp-2 cells was shown to be effective between 3 to 6 hpi, which corresponds to the transition period from the IE to E phase of viral replication. In addition, wild-type HSV-1 infection was able to block the apoptosis induced in cells by the addition of CHX staurosporine, and sorbitol. Both US3- and ICP22-deficient viruses showed a partial prevention of apoptosis, suggesting that these proteins may play a role in the inhibition process. In contrast, deletion of either the UL13 or vhs gene product does not appear to affect the ability of HSV-1 to prevent apoptosis in infected cells. Finally, using UV-inactivated viruses, we demonstrated that binding and entry of the virus were not sufficient to induce apoptosis following HSV-1 infection. From these results, we conclude that either gene expression or other RNA metabolism processes likely play a role in the induction of apoptosis in HSV-1-infected cells.
MATERIALS AND METHODS
Cell lines and viruses.
All cells were maintained in Dulbecco’s modified Eagle’s medium containing 5% fetal bovine serum. HEp-2 and Vero cells were obtained from the American Type culture Collection (Rockville, Md.). HSV-1(F) was obtained from Bernard Roizman (University of Chicago). Vero 2.2 cells, the HSV-1 (KOS1.1) virus, and vBSΔ27 were generously provided by Saul Silverstein (Columbia University). Vero 2.2 is a derivative Vero cell line expressing ICP27 under its own promoter (62). F and KOS1.1 are the strains of wild-type HSV-1 used in this study. vBSΔ27 (64) is the ICP27-null mutant virus used in this analysis; it contains a replacement of the α27 gene with the Escherichia coli lacZ gene and therefore must be propagated on an ICP27-complementing cell line, such as Vero 2.2 (62). The recombinant viruses R7041, R7356, and R325 [derivatives of HSV-1(F)] were obtained from Bernard Roizman; they contain, respectively, deletions of the genes encoding the protein kinase US3 (47), the kinase UL13 (48), and the regulatory protein ICP22 (46). The _vhs_-ΔSma mutant virus, generously provided by G. Sullivan Read (University of Missouri, Kansas City), is a derivative of KOS1.1 carrying a deletion in the vhs gene (UL41) (51). In all cases, the cell monolayers were infected at a multiplicity of infection of 10 PFU/cell and the infections were maintained at 37°C in Dulbecco’s modified Eagle’s medium with 5% newborn calf serum (NBCS) for the times indicated in the text. All tissue culture reagents were purchased from Life Technologies.
Biochemical and osmotic induction of apoptosis.
Cell apoptosis was induced by the addition of staurosporine (Calbiochem, San Diego, Calif.) to the medium at a final concentration of 1 μM. Cells were maintained in the presence of staurosporine for 24 h. To inhibit protein synthesis in infected cells, and therefore also induce apoptosis (5, 34), CHX (Sigma) was added to the medium at a final concentration of 10 μg/ml for 24 h. This concentration of CHX was previously shown to be sufficient to completely block viral protein synthesis in HSV-1-infected HEp-2 cells (5). Osmotic shock by sorbitol treatment was used to induce apoptosis (34) as follows. At the times (hours postinfection) indicated, infected cells were incubated for 1 h with 5% NBCS containing 1 M sorbitol (Sigma) and then for an additional 3 h with sorbitol-free 5% NBCS before the protein extraction procedures. Confirmation of the induction of apoptosis by staurosporine and sorbitol was made by light microscopy to detect morphological changes as described previously (5).
Inhibition of caspases.
The caspase-3 inhibitory peptide Z-Asp-Glu-Val-Asp-fluoromethyl ketone (Z-DEVD-fmk) and the caspase-1 inhibitory peptide _N_-acetyl-Tyr-Val-Ala-Asp-aldehyde (Ac-YVAD-CHO) were obtained from Calbiochem (San Diego) and used at final concentrations of 50 μM. Each inhibitor was added to the cell culture medium 1 h prior to infection and was present during the entire infection period (24 h).
Preparation of infected cell extracts, denaturing gel electrophoresis, and transblotting.
Whole extracts of infected cells were obtained as previously described (5). Protein concentrations were measured using a modified Bradford assay (Bio-Rad) as recommended by the vendor. Equal amounts of infected cell proteins (50 μg) were separated in denaturing 12% N,_N_′-diallyltartardiamide–acrylamide gels (9) and electrically transferred to nitrocellulose membranes in a tank apparatus (Bio-Rad) prior to immunoblotting with various primary antibodies. Unless otherwise noted in the text, all biochemical reagents were obtained from Sigma. Nitrocellulose was obtained directly from Schleicher & Schuell. Prestained protein molecular weight markers were purchased from Life Technologies.
Immunological reagents.
The following primary antibodies were used to detect viral proteins: (i) RGST22, rabbit polyclonal antibody specific for full-length ICP22 (10); (ii) 1113, mouse anti-ICP27 monoclonal antibody (Goodwin Institute for Cancer Research, Plantation, Fla.); (iii) 1114, mouse anti-ICP4 monoclonal antibody (Goodwin); (iv) 1112, mouse anti-ICP0 monoclonal antibody (Goodwin); (v) rabbit anti-thymidine kinase (TK) polyclonal antibody (gift of Bernard Roizman); and (vi) VP16 (1-21) mouse anti-VP16 monoclonal antibody (Santa Cruz Biotechnology, Inc.). Immunoblotting experiments were performed to detect cellular apoptotic proteins by using mouse anti-caspase-3 monoclonal antibody (Transduction Laboratories Inc.), mouse anti-PARP monoclonal antibody (Pharmingen), and goat anti-DFF polyclonal antibody (Santa Cruz Biotechnology). Secondary goat anti-rabbit, goat anti-mouse, and rabbit anti-goat antibodies conjugated with alkaline phosphatase were purchased from Southern Biotechnology (Birmingham, Ala.). Tetramethylrhodamine isothiocyanate (Texas red)-conjugated anti-rabbit immunoglobulin G (heavy plus light chain) [IgG (H+L) was purchased from Vector Laboratories (Santa Cruz, Calif.) and used at a dilution of 1:100 in 1% bovine serum albumin (BSA). Fluorescein isothiocyanate-conjugated anti-mouse IgG (H+L) was purchased from Boehringer Mannheim (Indianapolis, Ind.) and used at a dilution of 1:500 in 1% BSA.
UV inactivation of virus.
Virus stocks (107 to 108 PFU) in 2 ml of 199V medium (Life Technologies) containing 2% newborn calf serum were placed in a 10-mm-diameter dish (Falcon) on ice at a distance of 10 cm from a germicidal lamp (model MR-4, 60 Hz; George W. Gates and Company, Franklin Square, N.Y.). Virus particles were exposed to UV light for 10 min with mixing every 2 min. Virus titers after UV treatment were determined on Vero cells for KOS1.1 or Vero 2.2 cells for vBSΔ27.
Indirect immunofluorescence, microscopy, and computer graphics.
The fixation and permeabilization of infected cells for indirect immunofluorescence studies were performed as described previously (45). Briefly, infections were terminated by fixing in 2% methanol-free formaldehyde (Polysciences, Inc.) for 20 min at room temperature. Cells were permeabilized with 100% acetone at −20°C for 3 to 5 min, rinsed twice in phosphate-buffered saline, and then blocked in 1% BSA containing 10 μg of pooled human immunoglobulin (mainly IgG) (Sigma) per ml at 4°C. Each primary antibody was added for 1 h. After extensive rinsing with phosphate-buffered saline, the appropriate secondary antibody was added and incubated for an additional 45 min. Finally, the cells were preserved in a 0.1% solution of Mowoil (Sigma) with 2.5% DABCO (Sigma) used as an antibleaching agent under a fresh coverslip and sealed with nail polish. Cells were visualized on a Zeiss Axiophot fluorescence microscope. Infected cell phenotypes were documented by phase-contrast light microscopy using an Olympus CK2/PM-10AK3 system with an attached 35-mm camera. Immunoblots and 35-mm slides were digitized at a resolution of 600 to 1,200 dots per inch, using an AGFA Arcus II scanner linked to a Macintosh G3 PowerPC workstation. Raw digital images, saved as tagged image files in Adobe Photoshop version 5.0, were organized into figures by Adobe Illustrator version 7.1. Grey-scale and RGB prints of figures were obtained with a Codonics dye sublimation printer.
RESULTS
HSV-1-induced apoptosis involves a caspase-3-dependent pathway.
The goal of this study was to analyze HSV-1-induced apoptosis in cells in tissue culture. In a previous report (5), we showed that a recombinant strain of HSV-1 possessing a deletion in the gene encoding the viral ICP27 regulatory protein (vBSΔ27) induced apoptosis during the infection of human epithelial cells. In addition, no apoptotic features were seen during infection of the same cells with a wild-type virus strain (i.e., KOS1.1) unless the infections were performed in the presence of the protein synthesis inhibitor CHX. These results led to the conclusion that while both types of viruses were likely inducing apoptosis, the HSV-1 ICP27 deletion virus was incapable of preventing this process from killing the cells. In this study, we have expanded our system to discern the induction and inhibition of apoptosis during HSV-1 infections in human tissue culture cells.
Apoptotic signals are received through either of two major pathways, a death receptor or a mitochondrial route, which converge to a central pathway involving a family of aspartate-specific cysteinyl proteases, or caspases (14, 22). These enzymes are activated by proteolytic cleavage during the process of cell death. Caspase activation, particularly that of caspase-3, leads to the processing of various cytoplasmic and nuclear targets. Among the cleavage targets are the DNA repair enzyme PARP and DFF (57, 73). The DFF protein is specifically cleaved by caspase-3 to generate an active factor that induces DNA fragmentation (40). We have now attempted to develop a strategy to follow the induction of apoptosis in HSV-1-infected human cells by determining whether caspase-3, PARP, and DFF become processed.
Whole cell extracts from HEp-2 cells infected with either vBSΔ27 or wild-type KOS1.1 virus were made at 6, 11, 12, 15, 24, and 48 hpi. Infected cell polypeptides were electrophoretically separated in a denaturing gel, electrically transferred to nitrocellulose, and reacted with anti-PARP, anti-DFF, and anti-caspase-3 antibodies as described in Materials and Methods. The processing of PARP, a 116,000-molecular-weight protein, generates an 85,000-molecular-weight product which will be detected by the anti-PARP antibody used in this study (67, 68). Apoptosis-induced processing of DFF (molecular weight of 45,000) and caspase-3 (molecular weight of 32,000) results in the loss of reactivity with the anti-DFF and anti-caspase-3 antibodies. In our first experiment, we focused on infection times prior to 24 hpi because we originally observed the morphological and biochemical features characteristic of apoptosis in vBSΔ27-infected HEp-2 cells as early as 12 hpi (5). The results (Fig. 1A) from these early infection times were as follows.
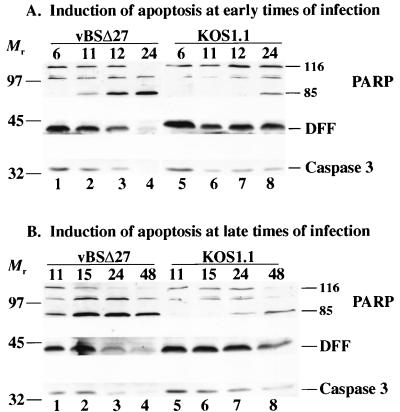
FIG. 1.
Detection of PARP, DFF, and caspase-3 processing in infected HEp-2 cells at early times from 6 to 24 hpi (A) or late times from 11 to 48 hpi (B). Whole cell extracts prepared at various times postinfection from vBSΔ27- or KOS1.1-infected cells were separated in a denaturing gel, transferred to nitrocellulose, and reacted with anti-PARP, anti-DFF, or anti-caspase-3 antibodies as described in Materials and Methods. “116” and “85” denote full-length (116,000-molecular-weight) and processed (85,000-molecular-weight) PARP, respectively. The locations of prestained molecular weight markers are indicated on the left.
In vBSΔ27-infected cells (lane 1 to 4), we detected a decrease of the full-length PARP protein and a concomitant increase of the 85,000-molecular-weight PARP cleavage product at 11 and 12 hpi (lanes 2 and 3). At 24 hpi, no full-length PARP was detected and there was an accumulation of the cleaved product (lane 4). Little to no cleavage of PARP was observed at 6 hpi (lane 1). The DFF protein decreased during vBSΔ27 infection such that it essentially disappeared at 24 hpi. A similar phenomenon was observed for caspase-3. These results indicate that PARP processing can be detected as early as 11 hpi in vBSΔ27-infected cells and at 24 hpi in wild-type virus-infected cells. DFF and caspase-3 were completely processed by 24 hpi only in the vBSΔ27-infected cells. When control infections were performed with the wild-type KOS1.1 virus (lanes 5 to 8), a low level of the processed form of PARP was detected only at 24 hpi (lane 8). In addition, the levels of DFF and caspase-3 did not change substantially between 11 and 24 hpi (lane 6–8). However, a small decrease in DFF and caspase-3 was observed at these later time points compared to the amounts of the proteins present at 6 hpi. It should be noted that the intensities of the 85,000-molecular-weight PARP bands are greater than those for the 116,000-molecular-weight bands because of the differences in electrical transfer efficiencies between the two fragments (9).
It was unexpected that evidence of PARP processing could be detected in wild-type-infected cells. To further investigate this observation, we performed a similar infection experiment in which the extracts were prepared at 11, 15, 24, and 48 hpi. The results (Fig. 1B) from these late infection times showed that in wild-type virus-infected cells (lanes 5 to 8), the processed form of PARP was seen at 24 hpi and it accumulated to a much higher level at 48 hpi (lanes 7 and 8). While the levels of DFF and caspase-3 remained very similar from 11 to 24 hpi (lane 5 to 7), a substantial decrease in the levels of these two proteins was observed at 48 hpi (lane 8). However, the processing of these three proteins in the KOS1.1-infected cells was not complete, and full-length proteins were still detectable at 48 hpi (lane 8). In the vBSΔ27-infected cells, almost no unprocessed PARP, DFF, or caspase-3 could be detected at 48 hpi (lane 4).
From these results, we conclude that the vBSΔ27-infected HEp-2 cells were undergoing apoptosis, as previously described (5), and that this process involves the activation of the caspase-3 protease as well as the proteolysis of the caspase substrates DFF and PARP. We also conclude that wild-type HSV-1 infection alone is capable of inducing apoptosis in HEp-2 cells, consistent with our earlier findings (5). With KOS1.1-infected cells, these features of apoptosis could be detected only starting at 24 hpi, and they always occurred to a much lesser extent than in vBSΔ27-infected cells. Taken together, these results suggest that in infected cells, wild-type HSV-1 is capable of inhibiting or delaying these apoptotic events and vBSΔ27 is missing this inhibitory or delaying function.
Inhibition of caspase-3 processing restores IE synthesis in vBSΔ27-infected HEp-2 cells.
Previously, we showed that following vBSΔ27 infection of human cells at 24 h, both IE and L viral proteins accumulated to levels lower than those observed with either human cells infected with the wild-type KOS1.1 virus or vBSΔ27-infected Vero cells (5). In the latter two cases, no obvious morphological features of apoptosis were observed during infection. The goal of this experiment was to determine whether specific inhibition of caspase processing during infection would influence the accumulation of viral proteins in vBSΔ27-infected human cells. HEp-2 cells were mock infected and infected with either the wild-type KOS1.1 virus or vBSΔ27 in the presence and absence of (i) protein synthesis (CHX), (ii) caspase-1 (Ac-YVAD-CHO), or (iii) caspase-3 (Z-DEVD-fmk) inhibitor as described in Materials and Methods. The caspase-3 inhibitor prevents caspase-3 from digesting its substrates and proteolyzing itself. Each caspase inhibitor was used separately or in combination with CHX since we previously detected apoptotic features in HEp-2 cells when CHX was present throughout the course of wild-type virus infection (5). Following the preparation of whole cell extracts at 24 hpi, immunoblotting analyses were performed with anti-PARP, anti-DFF, anti-caspase-3, anti-ICP4, anti-ICP22, and anti-ICP27 antibodies. ICP4, ICP22, and ICP27 were representative IE proteins. The results from this experiment are presented in Fig. 2.
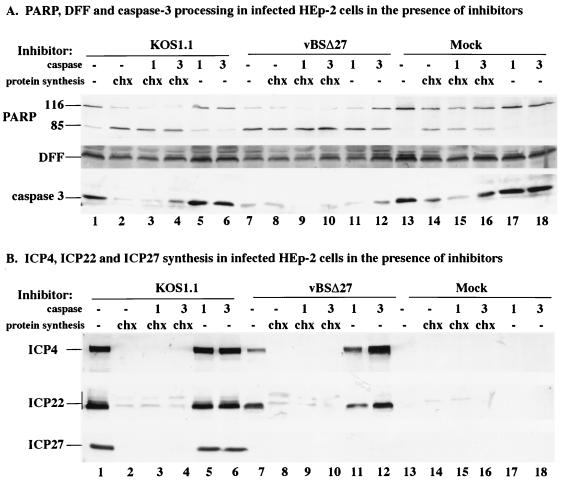
FIG. 2.
Detection of PARP, DFF, and caspase-3 (A) and ICP4, ICP22, and ICP27 (B) in infected HEp-2 cells. Total cell extracts were prepared from mock-infected cells or cells infected with KOS1.1 or vBSΔ27 in the absence (−) and presence of the protein synthesis inhibitor CHX (10 μg/ml) or in the absence (−) and presence of caspase-1 or caspase-3 (1 or 3; 50 μg/ml) as described in Materials and Methods. Immunoblot analyses were done with the anti-PARP, anti-DFF, and anti-caspase-3 antibodies (A) or anti-ICP4 (1114), anti-ICP22 (RGST22), and anti-ICP27 (1113) antibodies (B). Bar at the left mark the distribution of the modified forms of ICP22. Minor background bands due to the anti-ICP22 antibody were observed in all of the CHX-treated cells.
In the first portion of this study (Fig. 2A), the processing of PARP, DFF, and caspase-3 in the infected cells was analyzed. Treatment of mock-, KOS1.1-, or vBSΔ27-infected cells with CHX resulted in (i) lower levels of DFF and caspase-3 and (ii) higher levels of the processed form of PARP than observed with untreated cells (compare lane 2, 8, and 14 with lane 13). This finding confirms our earlier observation (5) that apoptosis occurs when wild-type HSV-1 infections proceed in the absence of de novo viral protein synthesis. When only the caspase-1 or only the caspase-3 inhibitor was present, there was little to no effect on the amounts of PARP, DFF, and caspase-3 observed in mock (compare lane 13 with lane 17, 18)- and KOS1.1 (compare lane 1 with lane 5, 6)-infected cells. However, when cells were infected with vBSΔ27 in the presence of the caspase-3 inhibitor, more DFF, caspase-3, and unprocessed PARP were observed than in vBSΔ27-infected cells in the absence of treatment (compare lanes 7 and 12). The combination of the caspase-3 inhibitor with CHX in the mock (lane 16)- and KOS1.1- (lane 4)-infected cells resulted in greater amounts of DFF, caspase-3, and unprocessed PARP than found with CHX treatment only (compare lanes 4 and 16 with lanes 2 and 14). In contrast, when the caspase-1 inhibitor was combined with CHX, we did not detect a similar increase in the amounts of these proteins compared to that with CHX alone (compare lanes 2 and 3, lanes 8 and 9, and lanes 14 and 15). These findings indicate that under appropriate infection conditions, the addition of the caspase-3 inhibitor results in a higher amount of unprocessed PARP, DFF, and caspase-3 detected in the infected cells.
In the second part of this experiment (Fig. 2B), the effect of the various treatments on the accumulation of three viral IE proteins, ICP4, ICP22, and ICP27, was studied. As expected (5), high levels of ICP4, ICP22, and ICP27 were detected in the KOS1.1-infected cells (lane 1), while lower amounts of ICP4 and ICP22 were detected in vBSΔ27-infected cells (lane 7). Consistent with our earlier findings, only the fastest-migrating forms of ICP22 were observed with the vBSΔ27-infected cells (5). In the KOS1.1- and vBSΔ27-infected cells treated with CHX, in either the absence (lane 2 and 8) or the presence of caspase-1 (lane 3 and 9) and caspase-3 (lane 4 and 10) inhibitors, the IE proteins were not detected, as expected since CHX blocks their synthesis. Minor background bands due to the anti-ICP22 antibody were observed in all of the CHX-treated cells (lane 2 to 4, 8 to 10, and 14 to 16). However, when the vBSΔ27-infected cells were treated with the caspase-3 inhibitor alone, the amounts of ICP4 and ICP22 were substantially greater than those observed following infection in the absence of any treatment (compare lanes 12 and 7). In fact, the levels of ICP4 and ICP22 observed in vBSΔ27-infected cells treated only with the caspase-3 inhibitor were the same as if not greater than those in KOS1.1-infected cells (compare lane 12 with lanes 1, 5, 6). It should be noted that we and others have detected high levels of IE proteins in vBSΔ27-infected Vero cells (5, 64), and this correlates with an absence of apoptosis in these cells (5). Although the accumulation of ICP4 in vBSΔ27-infected cells incubated with the caspase-1 inhibitor was slightly greater than but comparable to that in untreated vBSΔ27-infected cells (compare lanes 7 and 11), it was still lower than the amount detected in KOS1.1-infected cells (lane 5).
Based on these results, we conclude that the lower levels of viral IE proteins detected in vBSΔ27-infected HEp-2 cells, described in our earlier report (5), result from premature cell death which follows a pathway involving the caspase-3 proteolytic activity. This conclusion is based on our findings that vBSΔ27-infected cells incubated with a caspase-3 inhibitor (Z-DEVD-fmk) showed reduced PARP, DFF, and caspase-3 processing that correlated with an increase in the amounts of ICP4 and ICP22 protein detected. In addition, our results suggest that the mechanism by which wild-type HSV-1 induces apoptosis also involves the caspase-3 activity since the presence of the caspase-3 inhibitor resulted in a lower level of caspase-3 processing in the CHX-treated KOS1.1-infected cells.
Induction and inhibition of apoptosis by HSV-1 occurs prior to 6 hpi.
The results presented in Fig. 1 and 2 demonstrate two things. First, HSV-1 infection induces apoptosis in cells. Second, following infection with wild-type HSV-1 but not vBSΔ27, proteins which block apoptosis are synthesized. To determine the time period at which the protein-dependent inhibition of apoptosis was effective in HSV-1-infected cells, we designed the following strategy (Fig. 3A) based on a temporal addition of the protein synthesis inhibitor CHX. HEp-2 cell monolayers were infected with the wild-type KOS1.1 virus; at 1, 3, 6, 8, 12 hpi, CHX was added to the medium and maintained for 24 h. The 24-h CHX treatment was shown by cell morphology and chromatin degradation assays to be sufficient to enable the detection of apoptosis in infected cells (5). The morphologies associated with the induction of apoptosis included small, irregularly shaped cells suggestive of cell shrinkage and membrane blebbing (5). Each infection was performed in duplicate. One set was used to make cell extracts at the time of CHX addition, these extracts corresponded to 1, 3, 6, 8, and 12 hpi without treatment. In the second set, CHX was added at each designated time point postinfection and kept for 24 h before the extracts were prepared. Thus, viral protein synthesis was inhibited at different stages of the HSV-1 replication cycle. Three types of experiments were performed to characterize the apoptotic process in these infected cells. In the first series, phase-contrast microscopy was used to follow cell morphology changes; these results (Fig. 3B) were as follows.
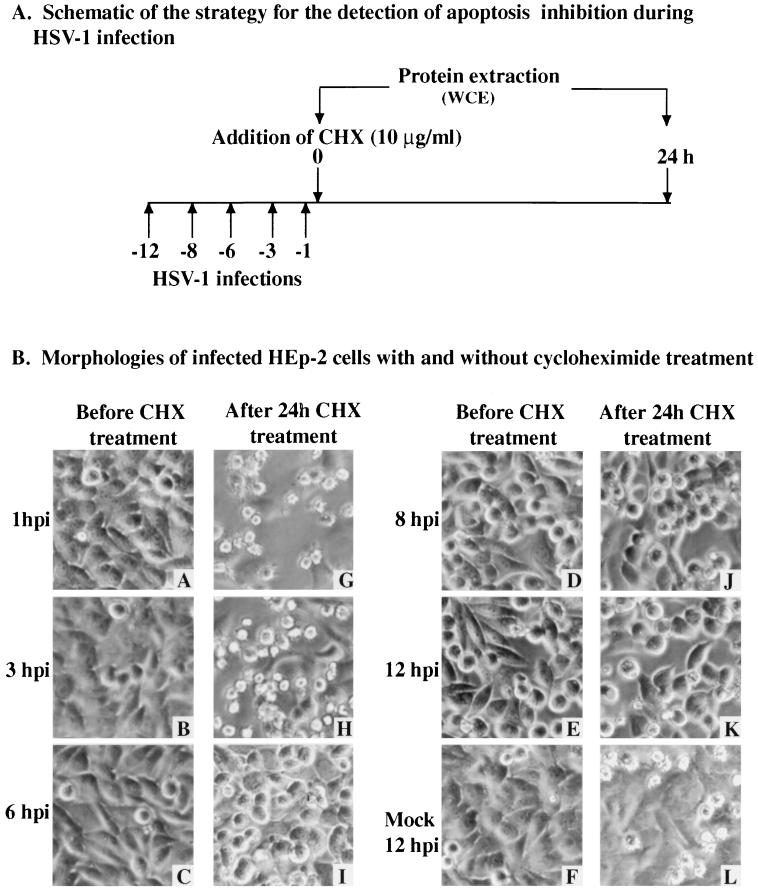
FIG. 3.
Schematic of infections (A) and morphologies (B) of KOS1.1-infected HEp-2 cells with and without CHX treatment. A to L, phase-contrast images of mock-infected cells at 12 hpi (F) and KOS1.1-infected cells at 1, 3, 6, 8, and 12 hpi (A to E) and of mock-infected cells 24 h after the addition of CHX at 12 hpi (L) and KOS1.1-infected cells 24 h after the addition of CHX at 1, 3, 6, 8 and 12 hpi (G to K). Magnification, ×60. WCE, whole cell extract.
In mock-infected cells at 12 hpi or HSV-1-infected cells at 1 to 6 hpi, monolayers of flat confluent HEp-2 cells were observed (Fig. 3B, images A to C and F). At 8 and 12 hpi, the infected cells showed characteristic cytopathic effects (CPE) represented by large rounded cells which result from viral replication (images D and E). When the cells were treated for 24 h with CHX, similar signs of CPE were observed only when CHX was added at 6, 8, or 12 hpi (images I to K). However, when the cells were treated with CHX at 1 or 3 hpi, small, irregularly shaped cells were observed (images G and H). Smaller cells were also seen with the mock-infected cells treated with CHX at 12 hpi but to a lesser extent (image L). These results indicate that morphologies characteristic of apoptotic cells (5) were seen only in infected cells when protein synthesis inhibition was begun at either 1 or 3 h after infection. This finding suggests that at these times postinfection, the virus was not able to prevent the cells from dying of apoptosis.
In the second series of experiments (Fig. 4A and B), we used an immunoblotting assay to monitor the processing of PARP, DFF, and caspase-3 (Fig. 1). Whole cell extracts of infected cells were obtained as described above (Fig. 3) before the addition of CHX (Fig. 4A) and after 24 h of CHX treatment (Fig. 4B). No processing of the PARP, DFF, and caspase-3 proteins was detected in KOS1.1-infected cells (Fig. 4A) from 1 to 12 hpi in the absence of CHX treatment. In contrast, no full-length (116,000-molecular-weight) PARP was detected and very low levels of DFF and caspase-3 proteins were observed when CHX was added at 1 h of infection (Fig. 4B, lane 5). When CHX was added at 3 hpi, PARP was still almost entirely processed (Fig. 4B, lane 4). While the levels of unprocessed DFF and caspase-3 (Fig. 4B, lane 4) were lower than those seen at 3 hpi without the addition of CHX (Fig. 4A, lane 5), the decrease was not as high as when CHX was added at 1 hpi (Fig. 4B, lane 5). Finally, in the cell extracts obtained from KOS1.1-infected cells treated with CHX at 6, 8, or 12 hpi (Fig. 4B, lane 1 to 3), the levels of unprocessed PARP, and DFF or caspase-3 were similar to those levels detected in untreated KOS1.1-infected cells (Fig. 4B, lane 6 to 7). These results suggest that KOS1.1-infected HEp-2 cells became resistant to CHX-induced apoptosis between 3 and 6 hpi. Since CHX inhibits total (both viral and cellular) protein synthesis, the addition of CHX induced some apoptosis in mock-infected cells (Fig. 4B, lane 8), but it was at a much lower level than in the infected cells when CHX was added at 1 or 3 hpi (Fig. 4B, lanes 4 and 5). From these results, we conclude that KOS1.1 infection induces cell apoptosis prior to 6 hpi and that protein(s) synthesized after 3 h but before 6 h of infection prevent the process of cell death.
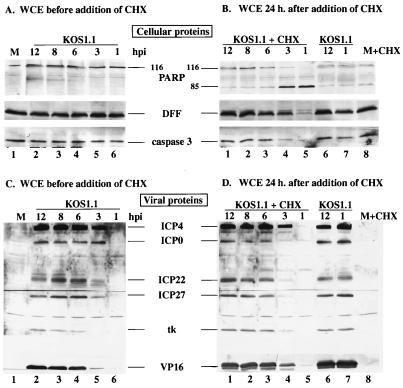
FIG. 4.
Detection of cellular (A and B) and viral (C and D) protein accumulation in KOS1.1-infected HEp-2 cells with and without CHX treatment. Whole cell extracts (WCE) prepared at 1, 3, 6, 8, or 12 hpi or at 24 h after the addition of CHX (10 μg/ml) at 1, 3, 6, 8, or 12 hpi from KOS1.1-infected cells were used for immunoblot analyses with anti-PARP, anti-DFF, and anti-caspase-3 antibodies (A and B) or with anti-ICP4, anti-ICP0, anti-ICP22, anti-ICP27 (IE proteins), anti-TK (E protein), and anti-VP16 (L protein) antibodies (C and D). M, mock-infected cells at 12 hpi; + CHX, addition of CHX for 24 h. In lanes 6 and 7 of panels B and D, the infected cells were incubated for an additional 24 h in the absence of CHX. “116” and “85” denote full-length (116,000-molecular-weight) and processed (85,000-molecular-weight) PARP, respectively.
In the third part of this study (Fig. 4C and D), the levels of several viral proteins were measured to determine the phase of viral replication, using antibodies specific for ICP4, ICP0, ICP22, ICP27, TK, and VP16 as described in Materials and Methods. The levels of protein synthesized from 1 to 12 hpi without the addition of CHX are shown in Fig. 4C. At 1 hpi, no viral proteins were detected (Fig. 4C, lane 6). At 3 hpi, significant levels of the IE proteins ICP0, ICP4, ICP22, and ICP27 were observed (lane 5), confirming the entry into the first phase of viral replication. The TK protein, which belongs in the category of the E proteins, was detected at 6 hpi, as was a large amount of VP16, an L protein (lane 4). From 6 to 12 hpi, no further changes in protein levels were detected. The levels of the same viral proteins detected after the additional 24 h of CHX treatment are shown in Fig. 4D. No viral protein was detected at 1 hpi (Fig. 4D, lane 5). While the amounts of ICP4 observed at 3 hpi with (lane 4) and without (lane 5) CHX treatment were identical, only low levels of ICP0, ICP22, and ICP27 were seen with CHX (lane 4). However, slight amounts of VP16 and TK could be seen with CHX at 3 hpi. All of the viral proteins were detected at 6 hpi, and their levels remained constant to 12 hpi. The slight variances between the levels of the proteins in Fig. 4C and D are likely consequences of the brief lag time required for CHX to act.
Based on the results presented in Fig. 3 and 4, we conclude that protein synthesis prior to 6 hpi is required to prevent apoptosis from killing the cells and it is likely that the viral ICP4 protein is not sufficient to prevent apoptosis. These conclusions are based on our findings that morphological features and cellular protein processing which are hallmarks of apoptosis were detected only in infected cells that were treated with CHX at 1 or 3 hpi. The prevention of apoptosis in infected HEp-2 cells was observed after 6 hpi. The time period prior to 6 hpi corresponds approximately to the transition from the IE to the E phase of viral gene expression, with 3 hpi corresponding to the onset of the E phase (28, 29). In untreated infected cells, we clearly observed all IE proteins at 3 hpi. However, when CHX was added at 3 hpi, a reduction in the amount of ICP0, ICP22, and ICP27 was observed and the cells showed manifestations of apoptosis. These findings are consistent with our earlier hypothesis that ICP27 is required for the prevention of apoptosis in infected human cells (5). We cannot exclude the possibilities that (i) the apoptosis-inducing activity is actually produced very early in infection but it is unstable and (ii) the addition of CHX results in the stabilization of this activity.
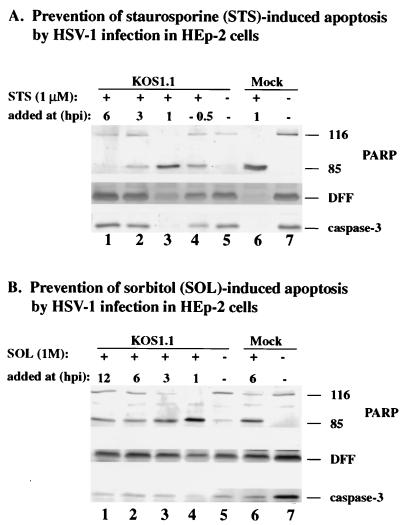
Prevention of staurosporine- and sorbitol-induced apoptosis by HSV-1 occurs prior to 6 hpi.
The previous experiments (Fig. 1 to 4) demonstrated that infection with the wild-type KOS1.1 virus was able to induce as well as prevent apoptosis. Our findings suggest that both induction and prevention occur prior to 6 hpi. The goal of this experiment was to determine whether HSV-1 infection was also effective in preventing apoptosis induced by other stimuli such as staurosporine, a protein kinase inhibitor (8) which induces apoptosis through a caspase-3 pathway (31), and sorbitol, which causes osmotic shock (34). KOS1.1-infected HEp-2 cells were treated with staurosporine or sorbitol at 1, 3, 6, or 12 hpi as described in Materials and Methods. To determine whether HSV-1 infected cells respond to these compounds in a manner similar to that for CHX, immunoblotting analyses were performed to detect PARP, DFF, and caspase-3 processing. As a control, staurosporine was also added to cells 30 min prior to infection.
The results (Fig. 5A) showed that caspase-3, PARP, and DFF were not processed when staurosporine was added at 6 hpi (compare lanes 1 and 7). In contrast, the cleavage of all three proteins was detected in KOS1.1-infected cells treated with the drug at 1 hpi or in treated uninfected cells (lanes 3 and 6). In addition, some processing of these proteins was also seen when the cells were treated at 3 hpi with the drug (lane 2). These results indicate that HSV-1 infection can prevent staurosporine-dependent apoptosis only if the inducer is added at 6 hpi. Surprisingly, we observed a partial inhibition of apoptosis in a control experiment in which staurosporine was added to cells prior to infection (lane 4). In this case, the levels of PARP, DFF, and caspase-3 more closely resembled the result for 3-hpi addition (lane 2) rather than the 1-hpi data (lane 3). The basis of this is not known, but one possibility could be that pretreatment of the cells with staurosporine makes them more susceptible to HSV-1-dependent blocking of apoptosis.
FIG. 5.
Detection of PARP, DFF, and caspase-3 processing in KOS1.1-infected HEp-2 cells treated with staurosporine (A) or sorbitol (B). Whole cell extracts prepared from mock- or KOS1.1 (K)-infected cells untreated (−) or treated with staurosporine or sorbitol at 1, 3, 6, or 12 hpi were used for immunoblotting analyses with anti-PARP, anti-DFF, and anti-caspase-3 antibodies as described in Materials and Methods. “116” and “85” denote full-length (116,000-molecular-weight) and processed (85,000-molecular-weight) PARP, respectively.
When a similar experiment was performed with sorbitol to induce apoptosis (Fig. 5B), we found that HSV-1 infection reduced the processing of PARP, DFF, and caspase-3 only at 6 and 12 hpi (lanes 1 and 2). Processing of these proteins was observed with KOS1.1-infected cells treated at 1 and 3 hpi (lane 3 and 4) and with mock-infected cells treated at 6 hpi (lane 6). In each of these cases, PARP processing was almost complete. As expected, no processing was observed with untreated mock-infected cells (lane 7). Minimal processing was observed with the untreated infected cells (lane 5), probably because these cells were infected for longer than 24 h (Fig. 1). From these results, we conclude that HSV-1 can interfere with both sorbitol- and staurosporine-induced apoptosis and that the prevention process requires virus-dependent factors which are present prior to 6 hpi. While HSV-1 infection almost completely prevented the cell death in staurosporine-treated cells, under our conditions the virus could only partially inhibit the sorbitol-initiated process.
Apoptosis occurs in cells infected with HSV-1 strains deleted for either ICP27, US3, or ICP22 but not with UL13- or _vhs_-deleted virus and wild-type viruses.
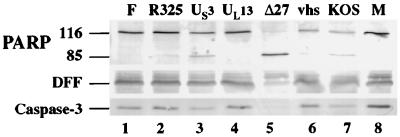
At the very early stage of HSV-1 infection, the virion host shutoff protein (vhs polypeptide) induces degradation of host mRNAs and the shutoff of most protein synthesis (17, 18, 37, 50, 51, 60, 65). This effect of the vhs protein on cellular mRNA stability and protein synthesis could be one of the mechanisms used by HSV-1 to prevent the infected cells from inducing the pathway of apoptosis. It was recently reported that the US3 gene of HSV-1 is involved in protecting cells from apoptosis during infection (39). The US3 protein kinase, along with the UL13 gene product, was implicated in the phosphorylation of the ICP22 protein (48, 49). Since ICP22 was one of the IE proteins whose reduction correlated with increased apoptosis in Fig. 4, the goal of this experiment was to determine whether viruses possessing deletions in each of these genes could prevent cell death during infection. HEp-2 cells were mock infected or infected with vBSΔ27, _vhs_-ΔSma, KOS1.1, R7041, R7356, R325, and HSV-1(F) for 24 hpi prior to performance of immunoblot experiments. HSV-1(F) and KOS1.1 were both used as wild-type controls as they are the parental strains of the recombinant viruses R7041, R7356, and R325 and the _vhs_-ΔSma and vBSΔ27 viruses, respectively. vBSΔ27-infected cells were used as a positive control for the presence of apoptotic features in infected cells (5).
The results (Fig. 6) showed that no processing of the caspase-3, DFF, or PARP protein was observed in either the HSV-1(F)- or mock-infected cells (lanes 1 and 8). When the infection was done with the virus carrying a deletion in the kinase encoded by UL13, results were similar to those for HSV-1(F)-infected cells (lane 4). As expected (5), the vBSΔ27-infected cells presented (i) no detectable level of the intact caspase-3, (ii) a reduction in the level of DFF, and (iii) a large accumulation of the processed form of PARP (lane 5). When the cells were infected with R325, an ICP22 deletion virus, a low level of PARP processing was observed without a significant decrease of DFF or caspase-3 compared to mock-infected cells (compare lane 2 and 8). In contrast, infection with the US3 protein kinase deletion virus showed a low level of PARP processing as well as a decrease in the level of DFF or caspase-3 (lane 3), which is consistent with earlier studies (39). It should be emphasized that although infections with viruses possessing disruptions in either the ICP22 or US3 genes showed features consistent with the induction of cell death, the extent of these apoptotic features was substantially below that observed with the ICP27-deletion virus infection (compare lanes 2 and 3 with lane 5). KOS1.1-infected cells showed low levels of PARP, DFF and caspase-3 processing (lane 7), as expected (Fig. 1). In _vhs_-ΔSma-infected cells, the levels of DFF and caspase-3 were similar to those in mock-infected cells (compare lanes 6 and 8), while some processed PARP was observed (lane 6). However, the extent of PARP cleavage was lower than that detected in KOS1.1-infected cells (compare lane 6 and 7). This latter finding suggests that the vhs polypeptide might play a role in the induction of apoptosis in infected cells rather than in the prevention process. It should be noted that while the minor amounts of PARP, DFF, and caspase-3 processing seen with the KOS1.1 virus are consistent with our earlier results (Fig. 1), no processing of these proteins could be detected with HSV-1(F) at 24 hpi (Fig. 6, lane 1). This observation indicates that HSV-1 strain differences are also likely to influence the induction and prevention of apoptosis in human cells.
FIG. 6.
Detection of PARP, DFF, and caspase-3 processing in HEp-2 cells infected with wild-type and mutant HSV-1. Whole cell extracts prepared from mock-infected cells (M) or cells infected with HSV-1 (F), vBSΔ27 (Δ27), R7041 (US3 deletion), R325 (ICP22 deletion), R7356 (UL13 deletion), _vhs_-ΔSma (vhs deletion), or KOS1.1 at 24 hpi were used for immunoblot analyses with anti-PARP, anti-DFF, and anti-caspase-3 antibodies as described in Materials and Methods. “116” and “85” denote full-length (116,000-molecular-weight) and processed (85,000-molecular-weight) PARP, respectively.
From these results, we conclude the following. (i) The vhs protein might contribute to the induction of apoptosis since the _vhs_-ΔSma virus appears to be less capable of inducing the processing of PARP than its parental KOS1.1 virus. (ii) The UL13 gene product does not play any significant role in the prevention of apoptosis in HSV-1-infected cells. Our results indicate that the UL13-infected cells were as effective as the wild-type HSV-1(F)-infected cells in blocking apoptosis. (iii) The ICP22 and US3 proteins may be necessary but not sufficient for optimal prevention of apoptosis in HSV-1-infected cells. This conclusion is based on the finding that while PARP, DFF, and caspase-3 processing was maximum in vBSΔ27-infected cells, only partial processing was detected in the US3- and R325-infected cells, thus indicating that other factors play a dominant role in the blocking of apoptosis. Perhaps the most significant finding from this experiment is that while ICP27 clearly plays a major role in the prevention of apoptosis in infected cells, at least two other viral proteins, ICP22 and US3, are likely to also be involved in the process. Based on these results, we cannot exclude the possibility that even more viral factors are necessary for efficient inhibition of apoptosis in HSV-1-infected cells.
Binding and entry of HSV-1 are not sufficient to induce apoptosis.
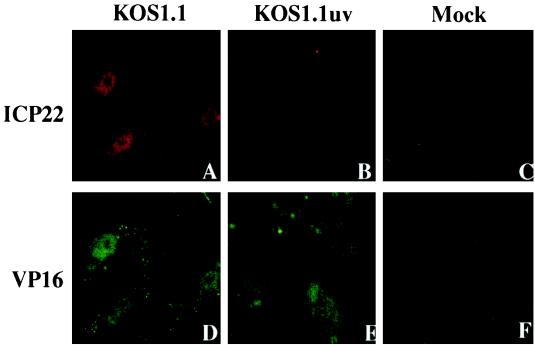
In Fig. 3 and 4, we showed that the KOS1.1 virus could induce apoptosis in the absence of detectable levels of viral proteins, suggesting that events prior to IE protein synthesis are sufficient to trigger cell apoptosis. The goal of this study was to confirm or eliminate the hypothesis that either the binding to or entry of the virion particle into the cell might facilitate the induction process. To focus on the first steps of viral infection, specifically virion binding, envelope fusion, and tegument/nucleocapsid entry into the cell, we prepared viruses which were inactivated by exposure to UV light. Since UV treatment damages the viral DNA genome in the virion, it was expected that viral transcription would be prevented and no IE gene products would be synthesized. In control experiments, KOS1.1 virus was UV inactivated as described in Materials and Methods. Under these conditions, we were able to reproducibly decrease the titer of the UV-treated virus (Kuv) by greater than 5 logs compared to untreated controls (data not shown). To confirm that the UV exposure did not perturb the ability of the treated virions to bind and enter the cells, we performed immunofluorescence experiments using anti-VP16 (tegument protein) and anti-ICP22 (IE protein) antibodies at 2 hpi in Vero cells as described in Materials and Methods. In this system, the ability of VP16 to translocate to the nucleus is used as a positive control for virion binding, fusion, and entry of the capsid and tegument proteins into the cytoplasm. ICP22 was chosen as a representative marker for IE protein synthesis. The results of this study (Fig. 7) indicated that only VP16 and not ICP22 could be detected in the nuclei of cells infected with Kuv whereas both proteins were observed with untreated virus. Based on these findings, we conclude that our UV exposure technique is efficient and that the inactivated viruses are able to enter cells since VP16 was translocated to the nucleus.
FIG. 7.
Indirect immunofluorescence of infected cells double labeled with antibodies specific for ICP22 (A to E) and VP16 (D to F). Vero cells were mock infected or infected (50 PFU/cell) with KOS1.1 or UV-inactivated KOS1.1 (KOS1.1uv) for 2 h and then subjected to formaldehyde-acetone fixation followed by immunostaining with anti-ICP22 (rabbit) and anti-VP16 (mouse) antibodies as described in Materials and Methods.
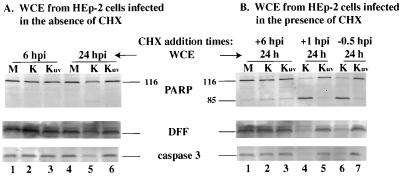
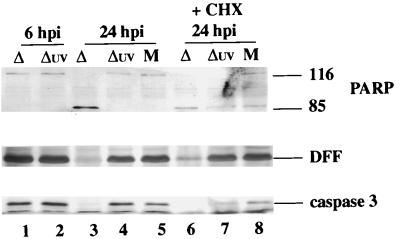
Three sets of experiments were performed to analyze the induction of apoptosis in UV-inactivated viruses. In each case, the induction of apoptosis was followed by immunoblotting for the detection of the processing of PARP, DFF, and caspase-3. In the first series, HEp-2 cells were mock-infected or infected with untreated KOS1.1 virus and Kuv, and whole cell extracts were prepared at 6 and 24 hpi. The results (Fig. 8A) at 6 hpi showed no processing of PARP, DFF, and caspase-3 protein in the mock- or virus-infected cells (lanes 1 to 3). At 24 hpi, a low level of processing of the three proteins was detected for KOS1.1-infected cells (lane 5) as expected (Fig. 1) but not in mock- or Kuv-infected cells (lane 4 and 6). Based on this result, we conclude that Kuv differs from the untreated virus in that it is unable to induce apoptosis at late times of infection. Since VP16 of Kuv was detected in the nuclei of cells, consistent with its ability to translocate, we must assume that vhs polypeptide also entered the cell. Thus, these results suggest that the vhs protein does not play a role in the induction of apoptosis. However, since we have not directly measured the vhs activity, we cannot eliminate the possibility that UV treatment affects vhs function.
FIG. 8.
Detection of PARP, DFF, and caspase-3 processing in KOS1.1-infected HEp-2 cells untreated (A) or treated (B) with CHX. Whole cell extracts (WCE) were prepared from mock-infected cells (M) or cells infected with KOS1.1 (K) or UV-inactivated KOS1.1 (Kuv) virus at 6 or 24 hpi in the absence of CHX (A) and at 24 h after the addition of CHX (B) at either 1 hpi, 6 hpi, or 30 min before infection (−0.5 hpi). Immunoblotting analyses were performed with anti-PARP, anti-DFF, and anti-caspase-3 antibodies. “116” and “85” are full-length (116,000-molecular-weight) and processed (85,000-molecular-weight) PARP, respectively.
The second series of experiments (Fig. 8B) involves a variation of our temporal addition of CHX protocol (Fig. 3 and 4) in which CHX was added to the infections at 1 hpi, 6 hpi, or 30 min prior to infection, and the cells were maintained in CHX for 24 h. When CHX was added at 6 hpi, a low level of protein processing was detected in mock-, or KOS1.1-, and Kuv-infected cells (lane 1 to 3). When CHX was added at either 1 hpi or 30 min before infection, high levels of PARP, DFF, and caspase-3 processing were observed in KOS1.1-infected cells (lane 4 and 6), while the levels detected in Kuv-infected cells were similar to those in treated mock-infected cells (compare lanes 5 and 7 with lane 1). From these results (Fig. 8), we conclude that the UV-inactivated KOS1.1 virus is unable to induce apoptosis.
The third series of experiments (Fig. 9) was performed similarly to those in Fig. 8, using vBSΔ27 and UV-inactivated vBSΔ27 viruses. At 6 hpi, no processing of PARP, DFF, or caspase-3 was detected (Fig. 9, lanes 1 and 2). At 24 hpi, only the processed form of PARP and almost no DFF and caspase-3 were detected in vBSΔ27-infected cells (lane 3). However, when the infection was done with UV-inactivated vBSΔ27, the results were identical to those for mock-infected cells in that no processing of PARP, DFF, or caspase-3 protein was detected (compare lanes 4 and 5). Equivalent results were obtained when the infections were performed in the presence of CHX for 24 h in that increased processing of caspase-3, PARP, and DFF was seen in vBSΔ27-infected cells (lane 6) and not in UV-treated vBSΔ27-infected cells, since the levels of these proteins were similar to those for mock infection (compare lanes 7 and 8). Together, these results (Fig. 8 and 9) show that both UV-inactivated wild-type and UV-inactivated vBSΔ27 viruses could not induce apoptosis in HEp-2 cells. Even though the presence of CHX could induce minor levels of apoptosis in uninfected cells, infection with UV-treated virus did not increase the extent of apoptosis by any measurable amount. Thus, we conclude that the processes of binding and entry of HSV-1 are not responsible for the induction of apoptosis in infected human cells.
FIG. 9.
Detection of PARP, DFF, and caspase-3 processing in vBSΔ27-infected HEp-2 cells. vBSΔ27 was inactivated by exposure to UV light as described in Materials and Methods. Whole cell extracts (WCE) prepared from mock-infected cells (M) or cells infected with vBSΔ27 (Δ) and UV-inactivated vBSΔ27 (Δuv) at 6 and 24 hpi in the absence of or at 24 hpi in the presence (+) of CHX were used for immunoblot analyses with anti-PARP, anti-DFF, and anti-caspase-3 antibodies. “116” and “85” denote full-length (116,000-molecular-weight) and processed (85,000-molecular-weight) PARP, respectively.
DISCUSSION
Previously, we showed that human cells died by apoptosis after infection with an HSV-1 ICP27 deletion virus and that the apoptotic features of these cells were identical to those of human cells infected with wild-type virus when total protein synthesis has been inhibited (5). From these results we concluded that both viruses likely induced apoptosis in human cells, but the mutant virus was not capable of preventing the apoptotic process from killing the cells (5). In this study, we used the ICP27 deletion virus vBSΔ27 as a tool to further investigate the process of induction and prevention of apoptosis in HSV-1-infected human cells. The significant findings of our study can be summarized as follows.
(i) We showed that HSV-1-infected HEp-2 cells underwent apoptosis through a pathway which involved the activation and proteolysis of caspase-3, resulting in the processing of PARP and DFF. In the absence of any other treatment, these processing events were detected in the infected cells as early as 11 hpi in vBSΔ27-infected cells. This corresponds to the time at which we originally observed the morphological and DNA laddering features associated with apoptosis in vBSΔ27-infected HEp-2 cells (5). Although wild-type HSV-1 does not show obvious signs of apoptosis at 11 hpi, by 24 and 48 hpi, significant amounts of PARP, DFF, and caspase-3 processing could be detected.
One of the more intriguing findings of this study using the wild-type virus (Fig. 1B) is that although PARP and DFF processing was detected at 24 and 48 hpi, the caspase-3 level dropped between 6 and 11 hpi. This seems to infer the following model. Viral infection induces apoptosis via a pathway which activates and processes caspase-3. The virus also causes the synthesis of proteins which appear to prevent the processing of DFF and PARP until later in infection. Thus, the absence of ICP27 enables these two proteins to become processed much earlier, presumably because either ICP27 itself or another function dependent on ICP27 is the blocking activity. Taken together, these findings suggest that the inhibition of apoptosis by HSV-1 is only temporary rather than absolute. Accordingly, the CPE due to lytic viral replication in cells, traditionally assumed to proceed through a necrotic route, likely has an apoptotic component as well. While these studies focused on transformed human HEp-2 cells, we have observed similar apoptotic features following infection of primary human fibroblasts (4).
(ii) Furthermore, we demonstrated that the accumulation of the IE proteins in vBSΔ27-infected HEp-2 cells, previously shown to be lower than in wild-type-infected cells (5), could be restored to wild-type levels when the infections were performed in the presence of a caspase-3 inhibitor. This result suggests that the inability of vBSΔ27 virus to produce IE proteins is due to premature cell death involving the activation of the caspase-3. Therefore, possibly HSV-1 blocks apoptosis because of an increase of the amount of viral, and particularly IE, protein synthesized. The obvious consequence of this would be that the virus is then able to produce higher levels of infectious progeny. Consistent with this model is the fact that Vero cells, which seem unable to undergo apoptosis (5), yield higher-titer stocks of HSV-1 than do HEp-2 cells (21).
Our findings are consistent with recent studies which indicate that sorbitol induction of apoptosis involves caspase-3 and that wild-type HSV-1 is capable of blocking this process and preventing apoptosis which proceeds through the mitochondrial pathway (19). It is of interest that these researchers also observed (19) DNA fragmentation following infection of human neuroblastoma (SK-N-SH) cells with the _d_120 virus (15, 39), and they conclude that caspase-3 was not involved in _d_120-induced apoptosis.
(iii) The virus-dependent inhibitory function is effective in preventing apoptosis which is induced by exposure of cells to CHX, staurosporine, and sorbitol. These results suggest that the types of apoptosis induced by these treatments all proceed through a caspase-3 pathway. Therefore, it is quite likely that the viral blocking function acts at the same location in the pathway. Staurosporine is capable of inhibiting cytoplasmic and receptor tyrosine kinases (76). Recently, we demonstrated that several HSV-1 proteins, including ICP22, were tyrosine phosphorylated in infected cells. It will be of interest to determine whether viral protein phosphorylation might plays a role in the induction or prevention of apoptosis.
(iv) The HSV-1 ICP22 and US3 gene products are necessary but not sufficient for optimal prevention of apoptosis. The conclusion was based on the finding that partial PARP, DFF, and caspase-3 processing was observed during infections using viruses with deletions of either of these two genes. However, the role that these two proteins play in the prevention of apoptosis in cultured cells appears limited inasmuch as this level of caspase-3-dependent processing was negligible compared to that observed during infection with the ICP27 deletion virus. As ICP22 has been implicated in the neurogrowth of HSV-1 in mice (61), it is possible that ICP22’s inhibitory potential is cell type dependent and therefore may be realized only in studies using whole animal models. ICP27 is a multifunctional regulatory phosphoprotein which can associate with other viral regulatory proteins and is required for optimal DNA synthesis and the expression of some late viral genes (41, 52, 56, 72). Recently, it was shown that ICP27 inhibits host cell splicing, redistributes splicing components throughout the nucleus, and aids in the export of RNA from the nucleus (24, 25, 42, 58, 59, 63, 64). Our current data do not indicate whether one of these known functions of ICP27 is also involved in the prevention of apoptosis. The fact that apoptosis is strongly induced when ICP27 is absent during HSV-1 infection suggests that these functions of ICP27 are not essential for the induction of apoptosis. In addition, we do not know whether ICP27 itself is directly involved in blocking apoptosis or whether another viral component, whose production or activity is dependent on ICP27, plays a role.
(v) The induction and prevention of apoptosis by wild-type HSV-1 infection occur prior to 6 hpi. This conclusion was based on results obtained with a protocol developed for this study involving temporal addition of CHX. This time period includes the transition from the IE to the E phase of replication. What was most intriguing was the fact that our detection of increased caspase-3-dependent processing correlated with reductions of the ICP0, ICP27, and ICP22 proteins. This is consistent with our findings described above for both the ICP27 and ICP22 deletion viruses which suggested that these two proteins are involved in the prevention of apoptosis. From our current data we cannot conclude whether ICP0 is directly required in the process. Also still unclear are the actual molecular basis for the virus-dependent inhibition of apoptosis as well as how many proteins are involved. For example, we cannot exclude the possibility that the virus can recruit cellular proteins to participate in the prevention process as well.
(vi) Virion binding and entry are not sufficient to induce apoptosis in HSV-1-infected cells. Neither the wild-type virus in the presence of CHX nor the ICP27 deletion virus could induce apoptosis following UV exposure. Control indirect immunofluorescence experiments confirmed that the UV treatment did not destroy the virus particles since virion-derived VP16 was detected in the nuclei of infected cells at 2 hpi.
Our earlier findings indicated that de novo viral protein synthesis is not required to induce apoptosis in infected cells (5). We now show that viral entry is also not sufficient to stimulate the apoptotic process. Taken together, the data lead us to conclude that either gene expression, presumably viral, or some other RNA processing event likely plays a role in the induction of apoptosis in HSV-1-infected cells. One possibility is that the decrease in RNA stability associated with the vhs activity (17, 50) acts as a stimulus of apoptosis. Consistent with this view was our initial observation that the _vhs_-ΔSma virus induced slightly less PARP processing than that seen with its parental KOS1.1 virus. However, since we were unable to detect PARP, DFF, or caspase-3 processing with UV-treated KOS1.1 and vBSΔ27 viruses, both of which possess wild-type vhs protein, it appears that the vhs function may contribute little to the induction of cell death. Additional studies focusing on the activity of the vhs protein itself should help clarify this point.
If the general stability of transcripts in the infected cells does not play a role in the induction of apoptosis, other potential viral stimuli might involve any one of the steps associated with the expression of the IE genes. These potential induction mechanisms include transcription of the IE genes, splicing of the α22/47 and α0 genes, export of the IE RNAs from the nucleus, and the initial interactions of these RNAs with the translational machinery. The development of additional molecular genetic and biochemical systems is required to define the molecular trigger(s) for the induction of apoptosis in HSV-1-infected human cells.
ACKNOWLEDGMENTS
We thank Saul Silverstein and Bob Soliman (Columbia University) for graciously providing the HSV-1(KOS1.1) and vBSΔ27 isolates and Vero 2-2 cells used in this study; Bernard Roizman (University of Chicago) for the R7041, R7356, R325, and HSV-1(F) isolates and the anti-TK antibody; Sullivan Read (University of Missouri, Kansas City) for the _vhs_-ΔSma virus; and Lisa Pomeranz (Mount Sinai School of Medicine) for discussions and expert advice regarding the fluorescence microscopy experiments.
This study was supported in part by grants from the U.S. Public Health Service (AI38873) and the American Cancer Society (JFRA 634) and by an unrestricted grant from the National Foundation for Infectious Diseases. J.A.B. is a Markey Research Fellow and thanks the Lucille P. Markey Charitable Trust for their support.
REFERENCES
- 1.Alfonso C L, Neilan J G, Kutish G F, Rock D L. An African swine fever virus Bcl-2 homolog, 5-HL, suppresses apoptotic cell death. J Virol. 1996;70:4858–4863. doi: 10.1128/jvi.70.7.4858-4863.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alnemri E S, Livingston D J, Nicholson D W, Salvesen G, Thornberry N A, Wong W W, Yuan J. Human ICE/CED-3 protease nomenclature. Cell. 1996;87:171. doi: 10.1016/s0092-8674(00)81334-3. . (Letter.) [DOI] [PubMed] [Google Scholar]
- 3.Asano S, Honda T, Goshima F, Watanabe D, Miyake Y, Sugiura Y, Nishiyama Y. US3 protein kinase of herpes simplex virus type 2 plays a role in protecting corneal epithelial cells from apoptosis in infected mice. J Gen Virol. 1999;80:51–56. doi: 10.1099/0022-1317-80-1-51. [DOI] [PubMed] [Google Scholar]
- 4.Aubert, M., and J. A. Blaho. Unpublished results.
- 5.Aubert M, Blaho J A. The herpes simplex virus type 1 regulatory protein ICP27 is required for the prevention of apoptosis in infected human cells. J Virol. 1999;73:2803–2813. doi: 10.1128/jvi.73.4.2803-2813.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Avitabile E, Di Gaeta S, Torrisi M R, Ward P L, Roizman B, Campadelli-Fiume G. Redistribution of microtubules and Golgi apparatus in herpes simplex virus-infected cells and their role in viral exocytosis. J Virol. 1995;69:7472–7482. doi: 10.1128/jvi.69.12.7472-7482.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Batterson W, Roizman B. Characterization of the herpes simplex virion-associated factor responsible for the induction of alpha genes. J Virol. 1983;46:371–377. doi: 10.1128/jvi.46.2.371-377.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bertrand R, Solary E, O’Connor P, Kohn K W, Pommier Y. Induction of a common pathway of apoptosis by staurosporine. Exp Cell Res. 1994;211:314–321. doi: 10.1006/excr.1994.1093. [DOI] [PubMed] [Google Scholar]
- 9.Blaho J A, Roizman B. Analyses of HSV proteins for posttranslational modifications and enzyme functions. In: Brown S M, Maclean A R, editors. Methods in molecular medicine: herpes simplex virus protocols. Vol. 10. Totowa, N.J: Human Press Inc.; 1998. pp. 237–256. [DOI] [PubMed] [Google Scholar]
- 10.Blaho J A, Zong C S, Mortimer K A. Tyrosine phosphorylation of the herpes simplex virus type 1 regulatory protein ICP22 and a cellular protein which shares antigenic determinants with ICP22. J Virol. 1997;71:9828–9232. doi: 10.1128/jvi.71.12.9828-9832.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boehmer P E, Lehman I R. Herpes simplex virus DNA replication. Annu Rev Biochem. 1997;66:347–384. doi: 10.1146/annurev.biochem.66.1.347. [DOI] [PubMed] [Google Scholar]
- 12.Clem R J, Fechheimer M, Miller L K. Prevention of apoptosis by a baculovirus gene during infection of insect cells. Science. 1991;254:1388–1390. doi: 10.1126/science.1962198. [DOI] [PubMed] [Google Scholar]
- 13.Crook N E, Clem R J, Miller L K. An apoptosis-inhibiting baculovirus gene with a zinc finger-like motif. J Virol. 1993;67:2168–2174. doi: 10.1128/jvi.67.4.2168-2174.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cryns V, Yuan J. Proteases to die for. Genes Dev. 1998;12:1551–1570. doi: 10.1101/gad.12.11.1551. [DOI] [PubMed] [Google Scholar]
- 15.DeLuca N A, McCarthy A M, Schaffer P A. Isolation and characterization of deletion mutants of herpes simplex virus type 1 in the gene encoding immediate-early regulatory protein ICP4. J Virol. 1985;56:558–570. doi: 10.1128/jvi.56.2.558-570.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Derfuss T, Fickenscher H, Kraft M S, Henning G, Lengenfelder D, Fleckenstein B, Meinl E. Antiapoptotic activity of the herpesvirus saimiri-encoded Bcl-2 homolog: stabilization of mitochondria and inhibition of caspase-3-like activity. J Virol. 1998;72:5897–5904. doi: 10.1128/jvi.72.7.5897-5904.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fenwick M L, McMenamin M M. Early virion-associated suppression of cellular protein synthesis by herpes simplex virus is accompanied by inactivation of mRNA. J Gen Virol. 1984;65:1225–1228. doi: 10.1099/0022-1317-65-7-1225. [DOI] [PubMed] [Google Scholar]
- 18.Fenwick M L, Walker M J. Suppression of the synthesis of cellular macromolecules by herpes simplex virus. J Gen Virol. 1978;41:37–51. doi: 10.1099/0022-1317-41-1-37. [DOI] [PubMed] [Google Scholar]
- 19.Galvan V, Brandimarti R, Roizman B. Herpes simplex virus 1 blocks caspase-3-independent and caspase-dependent pathways to cell death. J Virol. 1999;73:3219–3226. doi: 10.1128/jvi.73.4.3219-3226.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Galvan V, Roizman B. Herpes simplex virus 1 induces and blocks apoptosis at multiple steps during infection and protects cells from exogenous inducers in a cell-type-dependent manner. Proc Natl Acad Sci USA. 1998;95:3931–3936. doi: 10.1073/pnas.95.7.3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goodkin, M., and J. A. Blaho. Unpublished results.
- 22.Green D R. Apoptotic pathways: the roads to ruin. Cell. 1998;94:695–698. doi: 10.1016/s0092-8674(00)81728-6. [DOI] [PubMed] [Google Scholar]
- 23.Hampar B, Elison S A. Chromosomal aberrations induced by an animal virus. Nature. 1961;192:145–147. doi: 10.1038/192145a0. [DOI] [PubMed] [Google Scholar]
- 24.Hardwicke M A, Sandri-Goldin R M. The herpes simplex virus regulatory protein ICP27 contributes to the decrease in cellular mRNA levels during infection. J Virol. 1994;68:4797–4810. doi: 10.1128/jvi.68.8.4797-4810.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hardy W R, Sandri-Goldin R M. Herpes simplex virus inhibits host cell splicing, and regulatory protein ICP27 is required for this effect. J Virol. 1994;68:7790–7799. doi: 10.1128/jvi.68.12.7790-7799.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heeg U, Dienes H P, Muller S, Falke D. Involvement of actin-containing microfilaments in HSV-induced cytopathology and the influence of inhibitors of glycosylation. Arch Virol. 1986;91:257–270. doi: 10.1007/BF01314285. [DOI] [PubMed] [Google Scholar]
- 27.Henderson S, Huen D, Rowe M, Dawson C, Johnson G, Rickinson A. Epstein-Barr virus-coded BHRF1 protein, a viral homologue of Bcl-2, protects human B cells from programmed cell death. Proc Natl Acad Sci USA. 1993;90:8479–8483. doi: 10.1073/pnas.90.18.8479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Honess R W, Roizman B. Regulation of herpesvirus macromolecular synthesis. I. Cascade regulation of the synthesis of three groups of viral proteins. J Virol. 1974;14:8–19. doi: 10.1128/jvi.14.1.8-19.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Honess R W, Roizman B. Regulation of herpesvirus macromolecular synthesis: sequential transition of polypeptide synthesis requires functional viral polypeptides. Proc Natl Acad Sci USA. 1975;72:1276–1280. doi: 10.1073/pnas.72.4.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Irie H, Koyama H, Kubo H, Fukuda A, Aita K, Koike T, Yoshimura A, Yoshida T, Shiga J, Hill T. Herpes simplex virus hepatitis in macrophage-depleted mice: the role of massive, apoptotic cell death in pathogenesis. J Gen Virol. 1998;79:1225–1231. doi: 10.1099/0022-1317-79-5-1225. [DOI] [PubMed] [Google Scholar]
- 31.Jacobsen M D, Weil M, Raff M C. Role of Ced-3/ICE-family proteases in staurosporine-induced programmed cell death. J Cell Biol. 1996;133:1041–1051. doi: 10.1083/jcb.133.5.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jerome K R, Tait J F, Koelle D M, Corey L. Herpes simplex virus type 1 renders infected cells resistant to cytotoxic T-lymphocyte-induced apoptosis. J Virol. 1998;72:436–441. doi: 10.1128/jvi.72.1.436-441.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kerr F R, Harmon B V. Definition and incidence of apoptosis: an historical perspective. In: Tomei L D, Cope F O, editors. Apoptosis: the molecular basis of cell death. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1991. pp. 5–29. [Google Scholar]
- 34.Koyama A H, Adachi A. Induction of apoptosis by herpes simplex virus type 1. J Gen Virol. 1997;78:2909–2912. doi: 10.1099/0022-1317-78-11-2909. [DOI] [PubMed] [Google Scholar]
- 35.Koyama A H, Akari H, Adachi A, Goshima F, Nishiyama Y. Induction of apoptosis in HEp-2 cells by infection with herpes simplex virus type 2. Arch Virol. 1998;143:2435–2441. doi: 10.1007/s007050050473. [DOI] [PubMed] [Google Scholar]
- 36.Koyama A H, Miwa Y. Suppression of apoptotic DNA fragmentation in herpes simplex virus type 1-infected cells. J Virol. 1997;71:2567–2571. doi: 10.1128/jvi.71.3.2567-2571.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kwong A D, Frenkel N. Herpes simplex virus-infected cells contain a function(s) that destabilizes both host and viral mRNAs. Proc Natl Acad Sci USA. 1987;84:1926–1930. doi: 10.1073/pnas.84.7.1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leopardi R, Roizman B. The herpes simplex virus major regulatory protein ICP4 blocks apoptosis induced by the virus or by hyperthermia. Proc Natl Acad Sci USA. 1996;93:9583–9587. doi: 10.1073/pnas.93.18.9583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leopardi R, Van Sant C, Roizman B. The herpes simplex virus 1 protein kinase US3 is required for protection from apoptosis induced by the virus. Proc Natl Acad Sci USA. 1997;94:7891–7896. doi: 10.1073/pnas.94.15.7891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu X, Zou H, Slaughter C, Wang X. DFF, a heterodimeric protein that functions downstream of caspase-3 to trigger DNA fragmentation during apoptosis. Cell. 1997;89:175–184. doi: 10.1016/s0092-8674(00)80197-x. [DOI] [PubMed] [Google Scholar]
- 41.McCarthy A M, McMahan L, Schaffer P A. Herpes simplex virus type 1 ICP27 deletion mutants exhibit altered patterns of transcription and are DNA deficient. J Virol. 1989;63:18–27. doi: 10.1128/jvi.63.1.18-27.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McLauchlan J, Phelan A, Loney C, Sandri-Goldin R M, Clements J B. Herpes simplex virus IE63 acts at the posttranscriptional level to stimulate viral mRNA 3′ processing. J Virol. 1992;66:6939–6945. doi: 10.1128/jvi.66.12.6939-6945.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nicholson D W, Thornberry N A. Caspases: killer proteases. Trends Biochem Sci. 1997;22:299–306. doi: 10.1016/s0968-0004(97)01085-2. [DOI] [PubMed] [Google Scholar]
- 44.O’Brien V. Viruses and apoptosis. J Gen Virol. 1998;79:1833–1845. doi: 10.1099/0022-1317-79-8-1833. [DOI] [PubMed] [Google Scholar]
- 45.Pomeranz L E, Blaho J A. Modified VP22 localized to the cell nucleus during synchronized herpes simplex virus type 1 infection. J Virol. 1999;73:6769–6781. doi: 10.1128/jvi.73.8.6769-6781.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Post L E, Roizman B. A generalized technique for deletion of specific genes in large genomes: alpha gene 22 of herpes simplex virus 1 is not essential for growth. Cell. 1981;25:227–232. doi: 10.1016/0092-8674(81)90247-6. [DOI] [PubMed] [Google Scholar]
- 47.Purves F C, Longnecker R M, Leader D P, Roizman B. Herpes simplex virus 1 protein kinase is encoded by open reading frame US3 which is not essential for virus growth in cell culture. J Virol. 1987;61:2896–2901. doi: 10.1128/jvi.61.9.2896-2901.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Purves F C, Ogle W O, Roizman B. Processing of the herpes simplex virus regulatory protein alpha 22 mediated by the UL13 protein kinase determines the accumulation of a subset of alpha and gamma mRNAs and proteins in infected cells. Proc Natl Acad Sci USA. 1993;90:6701–6705. doi: 10.1073/pnas.90.14.6701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Purves F C, Spector D, Roizman B. The herpes simplex virus 1 protein kinase encoded by the US3 gene mediates posttranslational modification of the phosphoprotein encoded by the UL34 gene. J Virol. 1991;65:5757–5764. doi: 10.1128/jvi.65.11.5757-5764.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Read G S, Frenkel N. Herpes simplex virus mutants defective in the virion-associated shutoff of host polypeptide synthesis and exhibiting abnormal synthesis of alpha (immediate early) viral polypeptides. J Virol. 1983;46:498–512. doi: 10.1128/jvi.46.2.498-512.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Read G S, Karr B M, Knight K. Isolation of a herpes simplex virus type 1 mutant with a deletion in the virion host shutoff gene and identification of multiple forms of the vhs (UL41) polypeptide. J Virol. 1993;67:7149–7160. doi: 10.1128/jvi.67.12.7149-7160.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rice S A, Knipe D M. Genetic evidence for two distinct transactivation functions of the herpes simplex virus alpha protein ICP27. J Virol. 1990;64:1704–1715. doi: 10.1128/jvi.64.4.1704-1715.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Roizman B. Polykaryocytosis induced by viruses. Proc Natl Acad Sci USA. 1962;48:228–234. doi: 10.1073/pnas.48.2.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Roizman B, Roanne P R. Multiplication of herpes simplex virus. II. The relationship between protein synthesis and the duplication of viral DNA in infected HEp-2 cells. Virology. 1964;22:262–269. doi: 10.1016/0042-6822(64)90011-x. [DOI] [PubMed] [Google Scholar]
- 55.Roizman B, Sears A. Herpes simplex viruses and their replication. In: Fields B N, Knipe D M, editors. Virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 2231–2295. [Google Scholar]
- 56.Sacks W R, Greene C C, Aschman D P, Schaffer P A. Herpes simplex virus type 1 ICP27 is an essential regulatory protein. J Virol. 1985;55:796–805. doi: 10.1128/jvi.55.3.796-805.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Salvesen G S, Dixit V M. Caspases: intracellular signaling by proteolysis. Cell. 1997;91:443–446. doi: 10.1016/s0092-8674(00)80430-4. [DOI] [PubMed] [Google Scholar]
- 58.Sandri-Goldin R M. ICP27 mediates HSV RNA export by shuttling through a leucine-rich nuclear export signal and binding viral intronless RNAs through an RGG motif. Genes Dev. 1998;12:868–879. doi: 10.1101/gad.12.6.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sandri-Goldin R M, Mendoza G E. A herpesvirus regulatory protein appears to act post-transcriptionally by affecting mRNA processing. Genes Dev. 1992;6:848–863. doi: 10.1101/gad.6.5.848. [DOI] [PubMed] [Google Scholar]
- 60.Schek N, Bachenheimer S L. Degradation of cellular mRNAs induced by a virion-associated factor during herpes simplex virus infection of Vero cells. J Virol. 1985;55:601–610. doi: 10.1128/jvi.55.3.601-610.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sears A E, Halliburton I W, Meignier B, Silver S, Roizman B. Herpes simplex virus 1 mutant deleted in the alpha 22 gene: growth and gene expression in permissive and restrictive cells and establishment of latency in mice. J Virol. 1985;55:338–346. doi: 10.1128/jvi.55.2.338-346.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sekulovich R E, Leary K, Sandri-Goldin R M. The herpes simplex virus type 1 alpha protein ICP27 can act as a trans-repressor or a trans-activator in combination with ICP4 and ICP0. J Virol. 1988;62:4510–4522. doi: 10.1128/jvi.62.12.4510-4522.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Smith I L, Hardwicke M A, Sandri-Goldin R M. Evidence that the herpes simplex virus immediate early protein ICP27 acts post-transcriptionally during infection to regulate gene expression. Virology. 1992;186:74–86. doi: 10.1016/0042-6822(92)90062-t. [DOI] [PubMed] [Google Scholar]
- 64.Soliman T M, Sandri-Goldin R M, Silverstein S J. Shuttling of the herpes simplex virus type 1 regulatory protein ICP27 between the nucleus and cytoplasm mediates the expression of late proteins. J Virol. 1997;71:9188–9197. doi: 10.1128/jvi.71.12.9188-9197.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Strom T, Frenkel N. Effects of herpes simplex virus on mRNA stability. J Virol. 1987;61:2198–2207. doi: 10.1128/jvi.61.7.2198-2207.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Teodoro J G, Branton P E. Regulation of apoptosis by viral gene products. J Virol. 1997;71:1739–1746. doi: 10.1128/jvi.71.3.1739-1746.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tewari M, Beidler D R, Dixit V M. CrmA-inhibitable cleavage of the 70-kDa protein component of the U1 small nuclear ribonucleoprotein during Fas- and tumor necrosis factor-induced apoptosis. J Biol Chem. 1995;270:18738–18741. doi: 10.1074/jbc.270.32.18738. [DOI] [PubMed] [Google Scholar]
- 68.Tewari M, Telford W G, Miller R A, Dixit V M. CrmA, a poxvirus-encoded serpin, inhibits cytotoxic T-lymphocyte-mediated apoptosis. J Biol Chem. 1995;270:22705–22708. doi: 10.1074/jbc.270.39.22705. [DOI] [PubMed] [Google Scholar]
- 69.Thornberry N A, Lazebnik Y. Caspases: enemies within. Science. 1998;281:1312–1316. doi: 10.1126/science.281.5381.1312. [DOI] [PubMed] [Google Scholar]
- 70.Tropea F, Troiano L, Monti D, Lovato E, Malorni W, Rainaldi G, Mattana P, Viscomi G, Ingletti M C, Portolani M, et al. Sendai virus and herpes virus type 1 induce apoptosis in human peripheral blood mononuclear cells. Exp Cell Res. 1995;218:63–70. doi: 10.1006/excr.1995.1131. [DOI] [PubMed] [Google Scholar]
- 71.Tschopp J, Thome M, Hofmann K, Meinl E. The fight of viruses against apoptosis. Curr Opin Genet Dev. 1998;8:82–87. doi: 10.1016/s0959-437x(98)80066-x. [DOI] [PubMed] [Google Scholar]
- 72.Uprichard S L, Knipe D M. Herpes simplex ICP27 mutant viruses exhibit reduced expression of specific DNA replication genes. J Virol. 1996;70:1969–1980. doi: 10.1128/jvi.70.3.1969-1980.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vaux D L, Strasser A. The molecular biology of apoptosis. Proc Natl Acad Sci USA. 1996;93:2239–2244. doi: 10.1073/pnas.93.6.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Villa P, Kaufmann S H, Earnshaw W C. Caspases and caspase inhibitors. Trends Biochem Sci. 1997;22:388–393. doi: 10.1016/s0968-0004(97)01107-9. [DOI] [PubMed] [Google Scholar]
- 75.White E. Life, death, and the pursuit of apoptosis. Genes Dev. 1996;10:1–15. doi: 10.1101/gad.10.1.1. [DOI] [PubMed] [Google Scholar]
- 76.Wilde A, Beattie E C, Lem L, Riethof D A, Liu S H, Mobley W C, Soriano P, Brodsky F M. EGF receptor signaling stimulates SRC kinase phosphorylation of clathrin, influencing clathrin redistribution and EGF uptake. Cell. 1999;96:677–687. doi: 10.1016/s0092-8674(00)80578-4. [DOI] [PubMed] [Google Scholar]