ODA16p, a Chlamydomonas Flagellar Protein Needed for Dynein Assembly (original) (raw)
Abstract
Dynein motors of cilia and flagella function in the context of the axoneme, a very large network of microtubules and associated proteins. To understand how dyneins assemble and attach to this network, we characterized two Chlamydomonas outer arm dynein assembly (oda) mutants at a new locus, ODA16. Both oda16 mutants display a reduced beat frequency and altered swimming behavior, similar to previously characterized oda mutants, but only a partial loss of axonemal dyneins as shown by both electron microscopy and immunoblots. Motility studies suggest that the remaining outer arm dyneins on oda16 axonemes are functional. The ODA16 locus encodes a 49-kDa WD-repeat domain protein. Homologues were found in mammalian and fly databases, but not in yeast or nematode databases, implying that this protein is only needed in organisms with motile cilia or flagella. The Chlamydomonas ODA16 protein shares 62% identity with its human homologue. Western blot analysis localizes more than 90% of ODA16p to the flagellar matrix. Because wild-type axonemes retain little ODA16p but can be reactivated to a normal beat in vitro, we hypothesize that ODA16p is not an essential dynein subunit, but a protein necessary for dynein transport into the flagellar compartment or assembly onto the axoneme.
INTRODUCTION
Motile cilia and flagella are highly organized microtubule-based structures comprised of an estimated 250 proteins (Dutcher, 1995). These ciliary and flagellar components are evolutionarily conserved and are shared between vertebrates and other eukaryotic organisms of diverse lineages (Mitchell, 2004). In humans a failure to correctly assemble motile cilia or flagella causes diseases that affect the respiratory, reproductive, and central nervous systems (Milisav, 1998).
The process of flagellar assembly is not well understood, but is thought to involve partial assembly of proteins into complexes in the cytoplasm, active movement of complexes into the flagellar compartment, and attachment of complexes at specific locations on the flagellar microtubule scaffold. Studies using Chlamydomonas reinhardtii, a biflagellate single-celled alga, have shown that many flagellar proteins assemble into complexes in the cytoplasm. Some flagellar components known to preassemble include outer arm dynein (Fowkes and Mitchell, 1998), inner arm dyneins (Piperno and Mead, 1997) and radial spokes (Qin et al., 2004). Here we focus on outer arm dynein assembly by characterizing a new mutation that disrupts the assembly process.
One current model for how both membrane and nonmembrane bound flagellar proteins shuttle between the flagellar and cytoplasmic compartments involves intraflagellar transport (IFT; Rosenbaum and Witman, 2002; Scholey, 2003). Proteins destined for flagella accumulate around basal bodies in the apical cytoplasm where they come into contact with IFT motors (kinesin for anterograde transport, dynein for retrograde transport) and/or motor-associated particles. The loss of an IFT kinesin results in cells with stubby flagella, suggesting that IFT is essential for transport and assembly of every axonemal component including tubulin (Walther et al., 1994). The role of IFT particles is not clearly understood but may include recognition of an appropriate cargo for transportation and regulation of transport direction (Iomini et al., 2001; Rosenbaum and Witman, 2002)
After entering the flagellar compartment, proteins must undergo final assembly. For the outer arm dynein motor this process must include detachment from the IFT complex and binding to tubulin (King et al., 1995), to a docking complex (Wakabayashi et al., 2001), and to an accessory complex (Wirschell et al., 2004) on the flagellar scaffold. The location and normal order of these assembly events are not known, although analysis of mutants suggests that the microtubule scaffold assembles first, the docking and accessory complexes assemble onto this scaffold independently of each other, and finally the motor attaches.
As a means of understanding the mechanism of dynein assembly in flagella, we have characterized new Chlamydomonas outer arm dynein assembly mutants. Here we describe two mutant alleles at a new locus that encodes a novel, evolutionarily conserved protein. This protein does not resemble previously characterized dynein subunits and fractionates with the soluble flagellar matrix rather than the flagellar axoneme. We hypothesize that this new protein is a factor needed for assembly of a functional dynein motor.
MATERIALS AND METHODS
Chlamydomonas Strains and Genetic Analysis
137c (used as a wild-type strain), arg2, and arg7 were obtained from the Chlamydomonas Genetics Center (Duke University, Durham, NC). Strains _oda1_-oda12 were obtained from Dr. Ritsu Kamiya (University of Tokyo). The pf28 allele of the oda2 locus was described previously (Mitchell and Rosenbaum, 1985). The nit1-305cw15 strain used for mutagenesis was provided by Paul Lefebvre (University of Minnesota, St. Paul, MN). oda16-1 (14E2) and oda16-2 (19F11) were produced during this study as described below. All strains were maintained by standard procedures (Harris, 1989) and grown in either M (minimal) medium, MII (acetate supplemented) medium, M-NH4 medium (minimal medium without a reduced nitrogen source), or M medium supplemented with l-arginine (100 mg per liter medium for liquid cultures and 50 mg per liter for plates and slants). Diploids generated from a cross between oda16-1arg2 and oda16-2arg7 were selected by growth on minimal medium. To test for recombination between the two new oda16 mutations and between oda16 and previously characterized oda strains, zygotes were allowed to germinate and resulting colonies were transferred to M medium in 96-well plates. Swimming speed was scored visually in each well using an Olympus SZ60 stereoscope with substage darkfield illumination (Melville, NY).
Mutagenesis
14E2 and 19F11 were created by transforming nitrate reductase-deficient strain nit1-305cw15 with plasmid pMN24 (which contains a nitrate reductase gene) linearized with _Eco_RI, as previously described (Tam and Lefebvre, 1993). Transformants were selected by growth on M-NH4 plates and then screened for a slow-swimming phenotype (Mitchell and Sale, 1999). Each new mutant was back-crossed to a wild-type strain at least three times before further genetic analysis. Evidence presented in this article supports the hypothesis that 14E2 and 19F11 are alleles at the ODA16 locus; therefore 14E2 has been renamed oda16-1 and 19F11 has been renamed oda16-2.
Motility Analysis
Beat frequency was measured on free-swimming cells grown in M for 18 h with aeration under a 10 h dark, 14 h light cycle and as previously described (Mitchell and Kang, 1991). A photophobic stimulus was applied by removing a 625-nm cutoff red filter from a white light source, after allowing cells to acclimate in dim red light for at least 5 min. Visual assessments were made of whether the organisms were induced to stop, change waveform, and/or alter swimming direction. Beat frequency measurements and photophobic observations were made using a Zeiss Axioskop (Thornwood, NY) with a 20× objective under stroboscopic dark-field illumination.
Genomic, BAC and cDNA Clones
The p19F11-Sal plasmid was rescued by digesting oda16-2 (19F11) genomic DNA with _Sal_I and cloning the pUC119 vector and adjacent genomic DNA into Escherichia coli DH5α MCR cells (Invitrogen, Carlsbad, CA), following described methods (Tam and Lefebvre, 1995). A 0.5-kB fragment created by digesting p19F11-Sal with _Sal_I and _Xma_I was used as a hybridization probe to screen a Chlamydomonas genomic BAC (bacterial artificial chromosome) library filter (Incyte Genomics, Palo Alto, CA) and four BACs were selected (26i15, 27c18, 39j20, 5b19). The p19F11-Sal sequence was used to BLAST search the Chlamydomonas genomic sequence V1.0 (DOE Joint Genome Institute; http://genome.jgi-psf.org/chlre1/chlre1.home.html) and the pUC119 insertion break point was found to be at the junction of the second exon and intron of a predicted gene (genie.1022.2).
One EST (accession number AV642493) whose sequence matched predicted exons of genie.1022.2 corresponded to the 5′ end of cDNA clone HCL05g07 (referred to as pOda16-cDNA in this article). This clone was obtained from the Kazusa DNA Research Institute (Japan) and completely sequenced by primer walking. Sequences were assembled, translated, and aligned with Vector NTI 9.0 (Invitrogen). The sequence has been deposited in GenBank (Accession number DQ151642).
Cotransformation and Rescue of ODA16 Mutants
The pGenD-oda16 construct consists of the ODA16 cDNA coding region with addition of the first ODA16 intron, under the control of the PsaD promoter and poly-A signal regions and was created as follows. pCRI(256) was created by amplification of pOda16-cDNA with primers AV64t-B-f (5′-GGGACTGCGACACCGGGGATCCCCTGC) and AV64t-E-r (5′-GCCTCCTCCTCCAGGAATTCCCACCCG), which introduces an _Eco_RI site at the 3′ end of the coding region. The product was cloned into pCR-2.1 vector (Invitrogen). pCRII(280) was created by amplification of the region surrounding intron 1 of genie.1022.2 from BAC 26i15 with AV64-B/N-f (5′-CGCGAGCGGATCCCTGCATATGGCTGC) and AV64-A-r (5′-CCTCCACACATCGACGTCCGGCGTCA), which introduces an _Nde_I site at the predicted translation initiation codon (ATG in bold). pCRI(256) was cloned as an _Eco_RI and _Bgl_II fragment into pOda16-cDNA, and pCRII(280) sequences were then added to the pOda16-cDNA(256) construct between _Bam_HI and _Aat_II sites. The resulting construct, pCRII(256/280) contained a _Nde_I restriction site spanning the ATG, the first intron of the ODA16 gene and an _Eco_RI site at the end of the cDNA sequence. pCRII(256/280) was cloned into _Nde_I and _Eco_RI sites in the PsaD expression vector pGenD-ble (Fischer and Rochaix, 2001) in place of the ble gene. The resulting construct, pGenD-Oda16, was cotransformed with the _ARG_-containing plasmid pJD67 into oda16-1arg2 and random transformant colonies were transferred to liquid and visually scored for motility rescue to wild-type swimming speed.
Genomic DNA Preparation and Southern Blots
Chlamydomonas genomic DNA was digested with the indicated enzymes, separated on 0.8% gels in TAE buffer and transferred to NytranN (Schleicher & Schuell, Keene, NH) membrane. Southern blots were probed with either a 0.5-kB _Sal_I + _Xma_I fragment from p19F11-Sal or a 1.2-kB coding region fragment released by _Bam_HI + _Sma_I from pOda16-cDNA-B/A (see Antibodies section). Probes were labeled with Digoxigenin (Roche Applied Science, Indianapolis, IN) and detected using CSPD (Tropix, Bedford, MA) and exposure to Biomax Light film (Eastman Kodak, Rochester, NY).
Protein Preparation and Extraction
Flagellar proteins were prepared as previously described (Witman et al., 1978; Fowkes and Mitchell, 1998). Isolated flagella were resuspended in 2 ml of HMDEK-A (10 mM HEPES, pH 7.4, 5 mM MgSO4, 1 mM dithiothreitol, 0.5 mM EDTA, 25 mM KCl, and 0.6 μM aprotinin [Sigma, St. Louis, MO; bovine lung, powder]). For a whole flagellar protein preparation, 1 ml was spun in a microcentrifuge for 5 min at 16,000 × g at 4°C, and the pellet prepared for SDS-PAGE. For axoneme and membrane/matrix fractions, 1 ml HMDEK-NP40 (HMDEK-A supplemented with 0.2% NP40 [vol/vol], a Nonidet P40 substitute; Fluka, Ronkonkoma, NY; 74385) was added to 1 ml flagella, triturated several times, and spun for 10 min at 9600 × g at 4°C to pellet the axonemes, which were washed by resuspension in 1 ml HMDEK-A and spun in a microcentrifuge for 5 min at 16,000 × g at 4°C. The membrane/matrix fraction was transferred to a clean tube containing 4 ml acetone and precipitated at -20°C for 1 h before spinning at 17,000 × g for 10 min (Sorvall, Newton, CT; SS-34 rotor). A matrix fraction was generated by freeze/thaw treatment as previously described (Cole et al., 1998). In short, flagella suspended in HMDEK-A were frozen in liquid nitrogen, thawed at room temperature, and sedimented in a microfuge as above. Each flagella, axoneme, matrix, or membrane/matrix fraction was resuspended in 50 μl HMDEK-A and mixed with an equal volume of 2× SDS-PAGE buffer.
Antibodies, SDS-PAGE, and Western Blots
To generate antibodies against ODA16p, a bacterial fusion protein was produced as follows. Using primers AV64Bam-f (5′-GGCGCGAGCAGATTCGGATCCATGGCTGC) and AV64Aat-r (5′-CCTCCACATCGACGTCCGGCGTCAGG), a _Bam_HI site (underlined) was added just before the start codon (bold) in pOda16-cDNA. The PCR product was digested with _Bam_HI and _Aat_I, and the 141-base pair PCR product was cloned into pOda16-cDNA to make pOda16-cDNA-B/A. A 2-kB pOda16-cDNA-B/A fragment resulting from a _Bam_HI and _Xma_I double digestion was cloned into pGEX-4T-2 (Amersham Biosciences, Piscataway, NJ). The resulting plasmid was transformed into E. coli strain BL21 and a GST fusion protein was expressed and purified as described (Fowkes and Mitchell, 1998). Gel strips containing the fusion protein were sent for immunization in rabbits (Covance Research Products, Madison, WI). Anti-oda16 antibody was affinity-purified by incubating preblocked membrane strips of fusion protein with whole sera. The membrane was washed with 10 mM Tris, pH 7.5, and antibodies were released with sequential 5-min incubations in 500 μl 100 mM glycine, pH 2.5, and 500 μl 100 mM triethylamine, pH 11.5. The tubes were pooled, neutralized, and dialyzed against tris-buffered saline overnight.
The anti-DC2 antibody (Wakabayashi et al., 2001) was a kind donation from Dr. Ritsu Kamiya (University of Tokyo, Japan) and used at a 1:500 dilution. The anti-IC2 outer dynein arm IC antibody C11.4 (Mitchell and Rosenbaum, 1986) was used at a 1:1000 dilution. A rat anti-α-tubulin antibody, YOL1/34 (Sera-lab), was used at a 1:50 dilution.
SDS-PAGE gels contained 5% acrylamide in the stacking gel and 10% in the running gel prepared from a stock of 30% acrylamide [wt/vol], 0.8% bisacrylamide [wt/vol]. The Benchmark Protein Ladder (Invitrogen) containing 220-, 160-, 120-, 100-, 90-, 80-, 70-, 60-, 50-, 40-, 30-, 25-, 20-, 15-, and 10-kDa proteins was run alongside samples to estimate molecular weight. For immunoblots, proteins were transferred onto a PVDF membrane (Immobillon-P, Millipore, Bedford, MA) with a Bio-Rad minigel transfer apparatus (Richmond, CA), and antibodies were detected on Biomax Light film using ECL-Plus Western Blotting Detection Reagents (Amersham Biosciences) or Super Signal West Dura Extend Duration Substrate (Pierce Chemical, Rockford, IL). Images were scanned into Adobe Photoshop 6.0 (San Jose, CA) and cropped. For quantification of antibody binding, chemiluminescence was detected with a Storm 480 phosphorimager (Molecular Devices, Menlo Park, CA) and analyzed with ImageQuant software (Amersham Biosciences).
Electron Microscopy
Specimens for thin section electron microscopy were prepared as previously described (Mitchell and Sale, 1999). Images were taken using a JEOL 100CXII microscope (Peabody, MA) operated at 80 kV. Negatives were scanned and imported in Adobe Photoshop 6.0, the images were then inverted and adjusted for contrast and median density. Axoneme cross sections were reoriented so the dyneins projected clockwise and the central pair was horizontal.
RESULTS
Mutant Selection
To identify new proteins needed for axonemal dynein assembly, Chlamydomonas insertional mutants were screened for strains with the reduced motility phenotype characteristic of outer row dynein assembly-defective strains. Two new slow swimming mutants, 14E2 and 19F11, displayed a typical outer row dynein assembly (oda) mutant phenotype, including reduced beat frequencies compared with wild type (Figure 1A). Reduced swimming rates were not linked to reductions in flagellar length (unpublished data). The waveforms for both mutants appeared normal during forward swimming (asymmetric waveform), but neither mutant switched to a robust symmetric waveform after exposure to a photoshock stimulus, another characteristic of oda strains (Kamiya and Okamoto, 1985; Mitchell and Rosenbaum, 1985). Recombination analysis indicated that neither mutation was allelic to previously characterized oda mutants oda1-oda10, therefore these two mutations were considered as potential alleles of new oda loci.
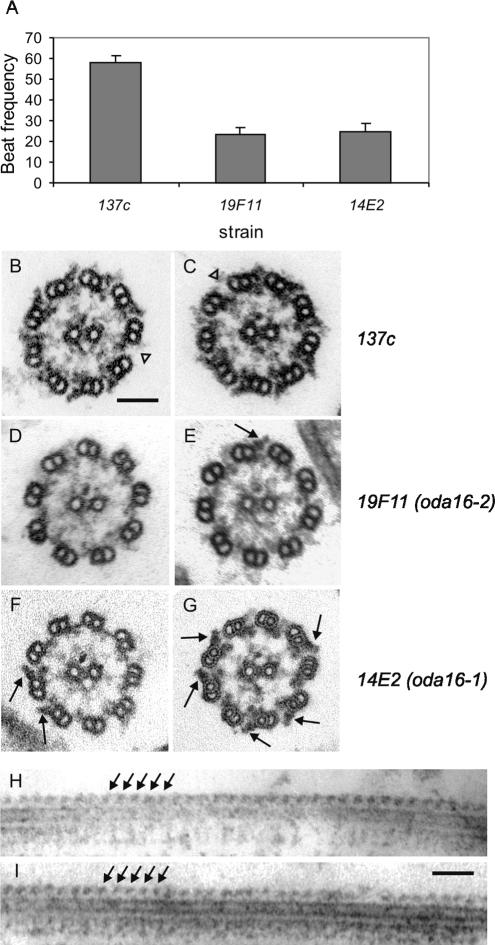
Figure 1.
(A) Beat frequency analysis of flagella on wild-type (137c), 19F11, and 14E2 cells show that these two new mutants both swim at half the wild-type beat frequency. Wild type (B and C), 19F11 (D and E), and 14E2 (F and G) axonemal cross sections show that both mutants have a reduction but not an absence of outer arm dyneins on the axoneme (black arrows). Outer arm dynein distribution appears to be random around the circumference of the axoneme in both 14E2 and 19F11. (H and I), electron micrographs of longitudinal-sections of mutant axonemes show that outer arm dyneins (arrows), when present, extend in rows along each doublet microtubule. Values in A are mean ± SD (n = 20). Scale bars, (B-I) 100 nm.
Electron micrographs of axonemal cross sections show that both 14E2 and 19F11 display a reduction but not a complete absence of outer arm dyneins, with no other apparent structural abnormality (Figure 1, B-G). Thin sections of wild-type axonemes had 8.0 ± 0.0 (n = 23) outer arms per axoneme compared with 1.3 ± 1.2 (n = 53) or 1.1 ± 1.4 (n = 32) outer arms per axoneme for 14E2 or 19F11, respectively. In comparison, axonemes from a typical oda mutant, pf28 (oda2), had 0.0 ± 0.2 (n = 24) outer arm images per axoneme cross section. The outer arms appeared to be randomly distributed with respect to doublet position in both new mutants. To determine if outer arm dyneins were randomly distributed along the length of the axonemes as well, longitudinal sections were analyzed (Figures 1, H and I). Although outer arms were not visible along most doublets, when present they were seen in a continuous row extending up to 1 μm or more along a doublet microtubule. This result suggests that outer arm dyneins are mutually stabilizing or cooperative in their attachment and that the initial attachment or nucleation phase of assembly may be defective in these mutants.
Cloning the ODA16 Gene
The similarity between 14E2 and 19F11 suggested that these mutations might result from disruptions in the same gene. When 14E2 and 19F11 were crossed with each other, all products from 240 zygotes retained the mutant phenotype, indicating tight linkage with a recombination frequency of <0.2 cM. Lack of complementation in 14E2/19F11 diploids confirmed that these mutations disrupt the same locus, and therefore we consider 14E2 and 19F11 to be allelic. A 2.5-kb genomic fragment flanking the insertion site in 19F11 was cloned by plasmid rescue following _Sal_I digestion of 19F11 genomic DNA, to generate plasmid p19F11-Sal (Figure 2A; see Materials and Methods for details). A 0.5-kb _Sal_I + _Xma_I fragment of this plasmid was determined to contain single-copy sequences and used as a probe for RFLP mapping studies (Kathir et al., 2003). The published data placed ODA16 on linkage group V close to Dhc6, an inner arm dynein heavy chain. Additional mapping data indicates that the ODA16 locus is telomeric to Dhc6. This genomic region does not include any previously characterized motility loci and the locus has therefore been designated ODA16 and the two mutant strains renamed oda16-1 (14E2) and oda16-2 (19F11).
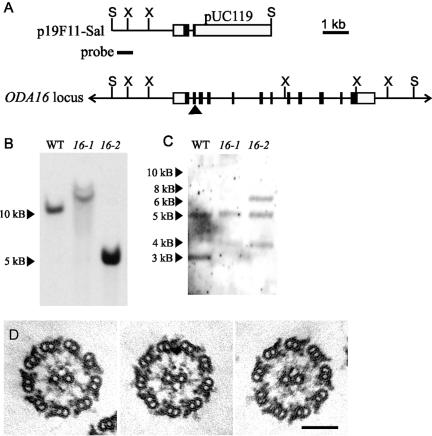
Figure 2.
Molecular analysis of the ODA16 locus. (A) Restriction maps of plasmid p19F11-Sal (top), which was rescued from insertional mutant oda16-2 and includes pUC119 vector sequences and genomic sequences flanking the insertion site, and the wild-type genomic ODA16 locus (bottom) showing coding exons (▪), noncoding exons (□), and the insertion break point in oda16-2 (arrowhead). Bar below the p19F11-Sal map indicates the _Sal_I-_Xma_I fragment used as a hybridization probe for Southern blots and for selection of BAC clones. (B and C) Southern blots of wild type, oda16-1, and oda16-2 genomic DNA digested with _Sal_I and probed with the 19F11-Sal-Xma fragment (B), or digested with _Xma_I and probed with the coding region from pOda16-cDNA (C). (D) Electron micrographs of axonemes from oda16-1 transformed with pGenD-oda16 to generate strain oda16-1R, in which outer row dynein assembly has been restored. S, _Sal_I; X, _Xma_I. Scale bar, (D) 100 nm.
To characterize the ODA16 gene, we mapped the regions disrupted by the insertions in oda16-1 and oda16-2. The 0.5-kb _Sal_I + _Xma_I probe used for RFLP analysis hybridized to different sized fragments on genomic Southern blots of _Sal_I-digested wild type, oda16-1 and oda16-2 genomic DNA (Figure 2B), confirming that this probe sequence is close to the insertion site for both oda16-1 and oda16-2. To isolate a wild-type copy of the ODA16 gene, the 0.5-kb _Sal_I + _Xma_I probe was used to screen a genomic Chlamydomonas BAC library. The smallest of the four BAC clones selected by the library screen, 26i15, was completely contained within the other three BAC clones (27a20, 27c18, 39j20). 26i15 was able to rescue the oda16-1Arg2 mutant to wild-type motility (visual assessment); however, the 26i15 insert is ∼50 kB and therefore likely to contain several genes. Southern blot analysis of _Sal_I-digested 26i15 showed the p19F11-_Sal_I/_Xma_I probe hybridized to the same sized fragment (∼12 kb) as the _Sal_I-digested wild-type genomic DNA, but attempts to subclone this _Sal_I fragment out of 26i15 were unsuccessful.
When the p19F11-Sal insert sequence was used to BLAST the Chlamydomonas genome database V1.0 (http://genome.jgi-psf.org/chlre1/chlre1.home.html), the insertion site matched sequence within the 5′-end of a partial predicted gene (genie.1022.2) at the end of scaffold 1022 (base position 24259-26189). A cDNA clone that generated an EST sequence matching this predicted gene, AV6424932, was obtained (Kazusa DNA Research Institute, Japan) and completely sequenced. The 3′ end of this cDNA matched a partial gene on scaffold 1246 (genie.1246.3) of the V1.0 database. The middle of the cDNA was not represented in V1.0 of the genome sequence. Both ends of this cDNA were identified on scaffold 132 (gene model C_1320001) of the V2.0 database (http://genome.jgi-psf.org/chlre2/chlre2.home.html) but the center of C_1320001 contains large sequence gaps. Scaffold 132 also contains noncontiguous sequence from another linkage group. Information from the database and the sequence of fragments amplified from BAC DNA permitted assembly of the complete gene (Figure 2A). Genomic Southern blots of wild type, oda16-1, and oda16-2 DNA probed with the coding region of the cDNA (Figure 2C) show that both mutants have altered hybridization patterns compared with wild type, and confirm that oda16-1 and oda16-2 both disrupt the coding region of this gene, which we tentatively designated ODA16.
To determine if the oda16 motility phenotype was the result of a disruption in this putative ODA16 gene, the oda16-1arg2 strain was transformed with pGenD-oda16, which contains the ODA16 cDNA coding region driven by flanking sequences from the Chlamydomonas PsaD gene. This construct was able to rescue oda16-1arg2 to wild-type beat frequency (58.1 ± 2.9 Hz; n = 39). Thin-section electron microscopy of a rescued strain, oda16-1R, confirmed that a full compliment of outer arm dyneins was restored (Figure 2D); similar results were obtained by transformation of the oda16-2 allele. Therefore, both the motility and assembly phenotypes of these strains are due to a disruption of this gene, confirming its identity as the ODA16 gene.
The ODA16 Gene Product Is a Novel WD Repeat Protein
The open reading frame in the ODA16 cDNA encodes a novel 446 amino acid protein (ODA16p) with a relative molecular mass of 49.2 × 103 (Figure 3). The N-terminus (residues 1-80) contains 6 prolines and is predicted to alternate between α-helical and β-sheet secondary structures. The central domain consists of 8 WD repeats spanning amino acids 81-416, each of which follows closely the characteristic pattern {X6-94[GHX23-41WD]}N4-8 common among proteins in this structural class (Neer et al., 1994). Seven of these repeats are identical in length (42 residues), but the second WD repeat contains one additional amino acid, an Asn inserted after residue 23 of this repeat. The C-terminal 29 amino acids constitute a glycine-, alanine-, and proline-rich flexible tail that immediately follows the last WD repeat.
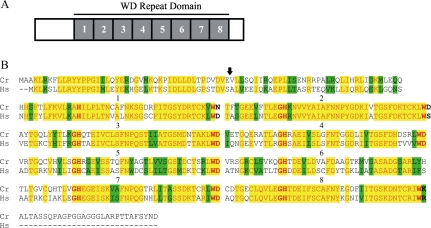
Figure 3.
ODA16 encodes a WD repeat domain protein. (A) ODA16p is a 449 amino acid protein that contains a central domain with 8 WD repeats (gray boxes). (B) The Chlamydomonas (Cr) ODA16p shares 58% identity (yellow shading), and 70% similarity (green shading) with an uncharacterized human protein (Hs). Amino acid pairs that define each repeat (GH...WD) are shown in bold face, and each repeat is set off by a space and numbered (1-8). The insertion of pMN24 in oda16-2 disrupts regions 3′ of codon 44 (arrow). Accession numbers are FLJ25955 for Hs and DQ151642 for Cr.
ODA16p homologues were found in the databases for mammals (_Homo sapiens, Mus musculu_s), insects (Anopheles gambiae, Drosophila melanogaster) and chordates (Ciona intestinalis) No ODA16p homologues were found in the Saccharomyces cerevisiae or Caenorhabditis elegans databases, consistent with the apparent role of this protein in motile cilia and flagella. The human ODA16p homologue (hypothetical protein FLJ25955), whose sequence was obtained from a testis-derived cDNA library, shares 58% identity and 70% similarity with ODA16p from Chlamydomonas (Figure 3B). Chlamydomonas ODA16p was the only homologue from among the databases examined that had a C-terminal tail after the last WD repeat, suggesting that this region is not essential for ODA16p function. Identity between the human and Chlamydomonas homologues increases to 62% when this tail sequence is ignored. Within the WD domain there is 66% identity between these homologues, and this similarity between mammalian and algal homologues extends to the precise length of each WD repeat. Although dynein intermediate chains also contain WD repeats (Wilkerson et al., 1995), ODA16p appears unique and shows no greater similarity to cytoplasmic or axonemal dynein intermediate chains than to any other previously characterized WD repeat protein.
The Role of ODA16p in Motility and Dynein Assembly
Outer arm dynein assembly mutants oda1, oda3, and oda14 have disruptions that affect a docking complex and, despite assembling an apparently complete outer arm dynein motor in the cytoplasm (Fowkes and Mitchell, 1998), all three fail to assemble outer arm dyneins on the axoneme (Koutoulis et al., 1997; Takada et al., 2002; Casey et al., 2003). To determine if the partial loss of outer arm dynein in oda16 was due to failed assembly of this docking complex, Western blot analysis was used to compare the reduction in a docking complex subunit (DC2) with the loss of an outer arm motor complex IC (IC2) in oda16 and wild-type axonemes (Figure 4). Overall, there was an 82% decrease (range 75-90%) in outer arm protein in mutant axonemes compared with wild type, but only a 25% decrease in the docking complex. The slight decrease in DC2 is consistent with the previous observation that oda6, an IC2 mutant that fails to assemble outer arm dyneins (Mitchell and Kang, 1991), also shows a slight reduction in docking complex proteins (Wakabayashi et al., 2001). The slight decrease in docking complex abundance, coupled with a much greater decrease in dynein motor subunits, is consistent with a role for ODA16p in assembly of the motor but not the docking complex. Apparent differences between oda16-1 and oda16-2 were not consistent but instead showed variability among preparations. We were unable to identify growth conditions that might be responsible for this variability in dynein assembly or stability.
Figure 4.
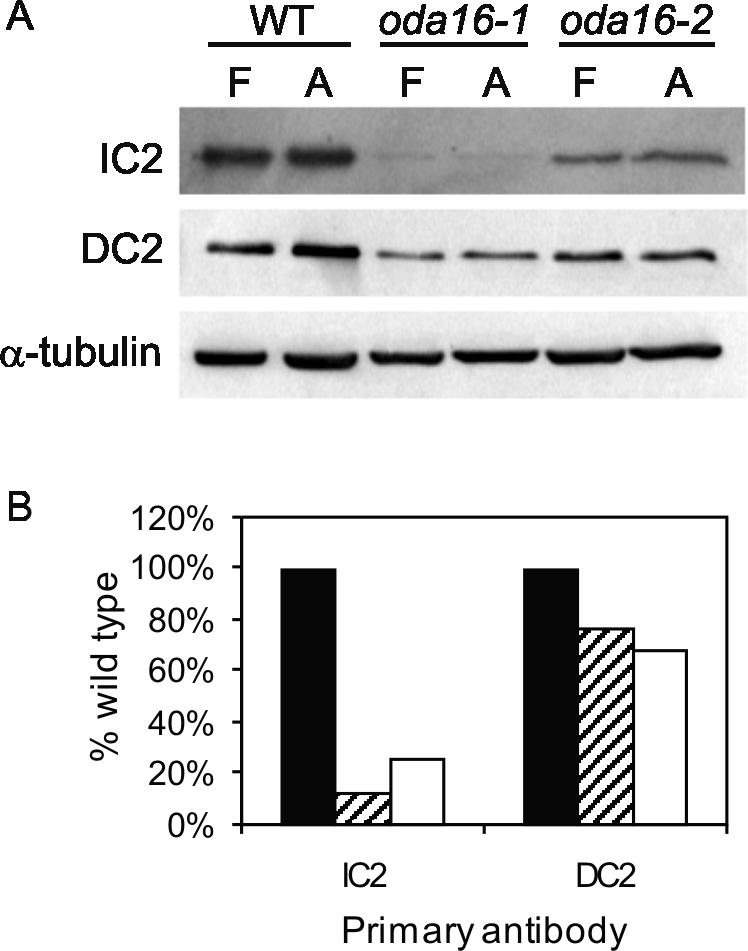
(A) Western blots compare the reduction in dynein and docking complex proteins in oda16 flagellar fractions. Blots of stoichiometrically equivalent amounts of wild-type and oda16 flagella (F) or detergent extracted flagellar axonemes (A) show a reduction in IC2 outer arm dynein IC in both oda16 mutants. There is also a slight reduction in DC2 (62-kDa docking complex protein). An anti-α-tubulin antibody was included as a loading control. (B) Signal intensities of wild-type (filled bar), oda16-1 (striped bar), and oda16-2 (empty bar) axonemal lanes from blots similar to those in A were quantified on a phosphorimager and normalized to tubulin. Each value is an average of four measurements based on two blots from each of two independent preparations of axonemes.
If the dyneins in oda16 fail to bind as tightly to the axoneme as dyneins in wild-type flagella, one might expect a larger pool of unassembled dynein motor proteins in the flagellar matrix compartment. However, Western blots show that oda16 outer arm dynein IC levels are the same when stoichiometrically equivalent amounts of flagella and axoneme protein are compared (Figure 4A). Therefore, outer arm dyneins present in the mutant flagella are firmly attached to the axoneme.
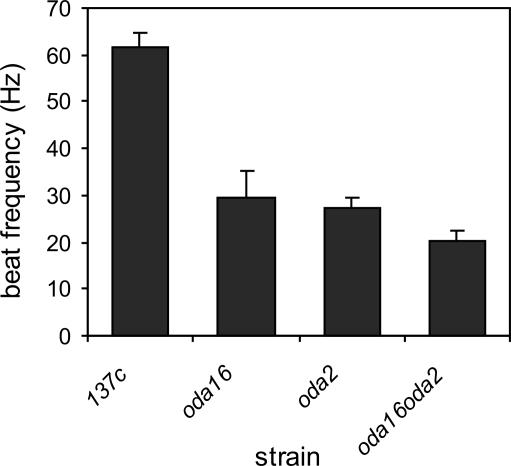
Even though oda16 mutant flagella retain ∼18% of the normal complement of outer arm dyneins, they display the same beat frequency as oda2, a typical outer armless mutant (Figure 5). This could result if the oda16 outer arm dyneins are not functional and therefore do not contribute to beat frequency. To test this, residual outer row dyneins in oda16 were prevented from assembling by combining the oda16 mutation with the oda2 mutation. The oda16oda2 double mutant cells had a lower beat frequency than oda16 alone, indicating that outer arm dyneins on oda16 axonemes are functional and contribute to beat frequency. The double mutant also had a lower beat frequency than oda2 alone, suggesting that the ODA16 gene product may be needed for assembly or regulation of one or more inner arm dynein.
Figure 5.
Oda16 and oda2 display a similar reduction in beat frequency when compared with wild type (137c). The beat frequency is further reduced in oda16oda2 double mutants below the level seen with either mutation alone (significant at p < 0.05; Duncan's multiple range test). Each measurement is the mean ± SD (n = 20).
ODA16p Localizes to the Flagellar Matrix
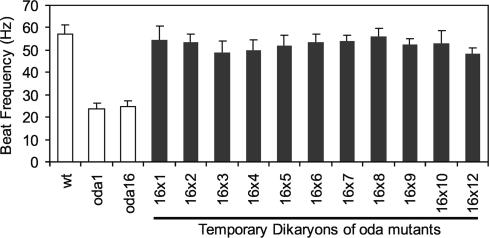
The reduction in outer arm dyneins on oda16 axonemes is not due to a complete absence of the docking complex (Figure 4) or due to a failure of outer arm dyneins to assemble into a functional motor (Figure 5), but could still result from the absence of a previously uncharacterized dynein subunit. To identify potential interactions between ODA16p and previously identified outer arm dynein complexes, we screened for noncomplementation in temporary diploids between oda16 and other oda strains (dikaryon rescue analysis). In these experiments, opposite mating type gametes of two different strains fuse to become quadriflagellate cells, which allows mixing of cytoplasmic pools of preassembled complexes. If two oda mutations do not disrupt the same preassembled complex, then cytoplasmic mixing allows assembly of a complete dynein arm and restoration of beat frequency within 1-2 h (Kamiya, 1988). Such noncomplementation studies have defined three complexes that form separately in the cytoplasm before their assembly onto axonemes, the docking complex, the catalytic complex, and the oda5 complex (Kamiya, 1988; Fowkes and Mitchell, 1998). All of the oda mutants tested were rescued to wild-type beat frequency as temporary dikaryons with oda16 (Figure 6). Therefore, the ODA16 gene product is not likely to be a component of any of the three previously defined preassembled complexes needed for outer arm assembly.
Figure 6.
Dikaryon complementation analysis. Beat frequencies were measured in wild-type, oda1, and oda16 gametes (□) and in temporary diploids (dikaryons) between the oda16 mutants and other previously characterized outer arm dynein assembly mutants (▪). All dikaryons between other oda mutants and oda16 show complementation (beat frequencies restored to near wild type). Error bars indicate std dev (n = 15).
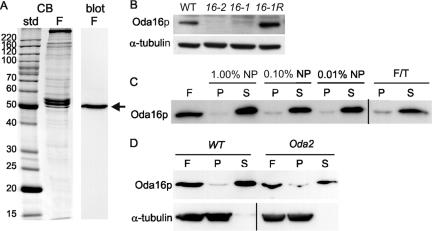
To localize ODA16p, the cDNA coding region was expressed as a bacterial fusion protein and used to raise antibodies in rabbits. Affinity-purified anti-ODA16p antibodies recognized a single 50-kDa protein band in Western blots of whole flagella (Figure 7A) that is missing in oda16 mutant flagella and recovered in the rescued oda16-1R strain (Figure 7B). After fractionation of flagella into membrane/matrix and axoneme by treatment with a nonionic detergent (NP40), ODA16p is predominantly in the membrane/matrix fraction rather than the microtubule-associated axoneme fraction (Figure 7C). The amount of ODA16p retained on axonemes was not affected by changes in detergent concentration between 1.00 and 0.01%. To distinguish between matrix and membrane fractions, freeze/thaw extraction was used to release soluble matrix components and leave membranes in the pelletable fraction (Cole et al., 1998). Because ODA16p was found predominately in the supernatant after freeze/thaw exposure (Figure 7C), we conclude that ODA16p is a matrix protein.
Figure 7.
Characterization of anti-ODA16p antibodies and analysis of ODA16p distribution in flagellar fractions. (A) A Coomassie Blue-stained SDS-PAGE gel (CB) and corresponding Western blot of wild-type flagella shows that the anti-Oda16p antibody recognizes a single 50-kDa band (arrow). (B) A Western blot of wild-type, oda16, and oda16-1R (rescued strain) axonemal protein using the anti-ODA16p antibody confirms that the oda16 mutants are knockouts. An anti-α-tubulin antibody was used as a loading control. (C) A Western blot comparing stoichiometrically equivalent amounts of whole wild-type flagella (F) or flagella separated by centrifugation into pellet (P) and supernatant (S) fractions after treatment with the indicated concentration of NP40 detergent (NP) or after freeze-thaw treatment in the absence of detergent (F/T). After NP40 treatment, the pellet fraction contains axonemes and the supernatant fraction contains membranes and the matrix. After freeze/thaw (F/T) treatment, the pellet contains both axonemes and membranes and the supernatant contains the matrix. ODA16p fractionates predominately with the matrix. (D) A Western blot of stoichiometrically equivalent loads of whole flagella (F), axoneme (P), and membrane/matrix (S) fractions of NP40-treated wild-type and oda2 flagella shows that ODA16p localization is unchanged in the absence of outer arm dyneins. All lanes used for C and D are from the same blot but additional lanes in the center were removed for clarity.
If ODA16p is in fact a dynein subunit but only retains a weak association with other outer row dynein proteins, then its flagellar abundance should be reduced when outer row dyneins are missing. To determine if ODA16p localization changes in the absence of outer row dynein, we compared ODA16p levels in both whole flagella and detergent-extracted fractions from wild-type cells and dynein assembly mutant oda2. Western blots show that ODA16p localization was unaltered by the oda2 mutation (Figure 7D).
DISCUSSION
Axonemal dyneins are essential for movement of cilia and flagella, and their absence has been linked to immotile cilia syndrome and its associated symptoms of male infertility, chronic respiratory infections, and situs inversus (Milisav, 1998). Dynein assembly has been most extensively studied using C. reinhardtii flagella as a model system, where 16 motility mutants have been categorized as outer arm dynein assembly (oda) mutants (Mitchell, 2000). These mutants include pf13, pf22, and oda1-oda15, and the loci disrupted in these mutants can be assigned to one of three categories, based on the known properties of the protein encoded by the locus or on their dikaryon rescue profiles. Here we have characterized a new dynein assembly locus, ODA16, which does not fit any of these three previously defined categories and has several unusual properties.
The largest dikaryon noncomplementation group includes mutants that disrupt the core of the outer arm dynein motor complex (three heavy chains, two intermediate chains) and therefore fail to assemble a functional motor. This group includes oda2, oda4, oda6, oda7, oda9, oda12, and oda15, along with pf13 and pf22. Most have been characterized as loci encoding subunits of the motor. Although products of the oda7, pf13, and pf22 genes have not been identified, these mutations fail to rescue as dikaryons with any other member of this group, suggesting that they also encode subunits of the motor that preassemble in the cytoplasm. The second group has defects that disrupt the docking complex, a trimeric protein complex needed for attachment of outer arms to the axoneme (Casey et al., 2003). This group consists of oda1, oda3, and probably oda14, which encode the three subunits of this complex (oda14 encodes a docking complex subunit, but dikaryon complementation between mutations at this locus and other docking complex loci has not been tested). The last complementation group contains oda5, oda10, and possibly oda8, which may encode subunits of an accessory complex. The only gene in this group whose product is known, oda5, encodes an axonemal protein essential for association of a flagellar adenylate kinase (Wirschell et al., 2004). Because oda16 complemented members of all three groups during dikaryon analysis (Figure 6), ODA16p is unlikely to preassemble with other subunits of the motor, docking or oda5/accessory complexes. This conclusion is supported by beat frequency comparisons of oda16 and oda16oda2 (Figure 5), which suggest that a functional motor can assemble correctly (at low efficiency) on oda16 axonemes, and Western blot analysis (Figure 4A), which shows that docking complex proteins can assemble in oda16 mutants. Additional work is needed to confirm that assembly of the ODA5 gene product is unaffected by oda16.
A second unique characteristic of ODA16 is that the gene product fractionates predominately with the flagellar matrix and not the axoneme (Figure 7C), whereas the products of all other characterized dynein assembly loci are axonemal proteins. The requirement of a matrix protein for outer arm assembly suggests that ODA16p is needed during intraflagellar transport of precursor complexes, or for final assembly of such complexes onto the axoneme. Because homologues were only found in organisms with motile cilia and/or flagella, ODA16p may only be needed for assembly of select components essential for motility. Our knockout alleles of ODA16 show no defects in overall flagellar length, indicating no general disruptions of flagellar assembly. In contrast, IFT motor and particle subunits are required for the assembly of both motile and nonmotile cilia (Scholey, 2003). Detergent-extracted wild-type Chlamydomonas axonemes can be reactivated to beat in vitro with motility properties similar to those seen in vivo (Omoto and Brokaw, 1985). Because these axonemes are depleted of most of their ODA16p (Figure 7), ODA16p may not be needed directly for motility but only for dynein assembly.
A third unique characteristic of oda16 is that it may affect other structures in addition to outer row dyneins. The reduced beat frequency of oda16oda2 double mutants compared with oda2 alone (Figure 5) implies that oda16 affects the assembly or function of another component of the axoneme needed for motility in addition to outer row dyneins. This additional component could be one or more inner arm dynein or a component that regulates inner arm dyneins. Two other oda mutations whose gene products have not been characterized, pf13 and pf22, generate more severe motility phenotypes than oda16 and may also affect both inner and outer row dyneins.
Interestingly, Oda16p does not appear in any of the recent proteomic (Pazour et al., 2005), genomic (Li et al., 2004), and transcriptional (Stolc et al., 2005) identifications of Chlamydomonas flagellar-associated gene products. In the first study, the failure to identify Oda16p likely reflects the difficulty of gel-based proteomics to identify proteins near the size of more abundant proteins, such as tubulin. The Stolc et al. (2005) analysis depended on apparently small changes in deflagellation-induced message abundance typical of proteins important for flagellar assembly and failed to identify all known flagellar proteins. Finally, proteins with common structural motifs, like WD repeat domains, were excluded from the Li et al. (2004) study.
We conclude that ODA16p is necessary for efficient outer arm dynein assembly onto axonemal microtubules through an as yet unknown mechanism. As a WD repeat protein, it likely interacts with other subunits to fulfill its role, which may include selection of dynein complexes for transport from the cytoplasm into flagella, release of dynein from IFT particles for assembly, or modification of dynein or axonemal attachment sites into a form that is competent for assembly.
Acknowledgments
We thank Judy Freshour for her help in making constructs and raising antibodies, Masako Nakatsugawa for her help with electron microscopy, Ritsu Kamiya for providing an antibody to DC2, and Carolyn D. Silflow for gene mapping. This work was supported by National Institutes of Health Grant GM044228 to D.R.M.
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E05-07-0627) on August 10, 2005.
Abbreviations used: IFT, intraflagellar transport.
References
- Casey, D. M., Inaba, K., Pazour, G. J., Takada, S., Wakabayashi, K., Wilkerson, C. G., Kamiya, R., and Witman, G. B. (2003). DC3, the 21-kDa subunit of the outer dynein arm-docking complex (ODA-DC), is a novel EF-hand protein important for assembly of both the outer arm and the ODA-DC. Mol. Biol. Cell 14, 3650-3663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole, D. G., Diener, D. R., Himelblau, A. L., Beech, P. L., Fuster, J. C., and Rosenbaum, J. L. (1998). Chlamydomonas kinesin-II-dependent intraflagellar transport (IFT): IFT particles contain proteins required for ciliary assembly in Caenorhabditis elegans sensory neurons. J. Cell Biol. 141, 993-1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutcher, S. K. (1995). Flagellar assembly in two hundred and fifty easy-to-follow steps. Trends Genet. 11, 398-404. [DOI] [PubMed] [Google Scholar]
- Fischer, N., and Rochaix, J.-D. (2001). The flanking regions of PsaD drive efficient gene expression in the nucleus of the green alga Chlamydomonas reinhardtii. Mol. Genet. Genomics 265, 888-894. [DOI] [PubMed] [Google Scholar]
- Fowkes, M. E., and Mitchell, D. R. (1998). The role of preassembled cytoplasmic complexes in assembly of flagellar dynein subunits. Mol. Biol. Cell 9, 2337-2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris, E. H. (1989). The Chlamydomonas Sourcebook, San Diego: Academic Press.
- Iomini, C., Babaev-Khaimov, V., Sassaroli, M., and Piperno, G. (2001). Protein particles in Chlamydomonas flagella undergo a transport cycle consisting of four phases. J. Cell Biol. 153, 13-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya, R. (1988). Mutations at twelve independent loci result in absence of outer dynein arms in Chylamydomonas reinhardtii. J. Cell Biol. 107, 2253-2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya, R., and Okamoto, M. (1985). A mutant of Chlamydomonas reinhardtii that lacks the flagellar outer dynein arm but can swim. J. Cell Sci. 74, 181-191. [DOI] [PubMed] [Google Scholar]
- Kathir, P., LaVoie, M., Brazelton, W. J., Hass, N. A., Lefebvre, P. A., and Silflow, C. D. (2003). Molecular map of the Chlamydomonas reinhardtii nuclear genome. Eukaryot. Cell 2, 362-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King, S. M., Patel-King, R. S., Wilkerson, C. G., and Witman, G. B. (1995). The 78,000-Mr intermediate chain of Chlamydomonas outer arm dynein is a microtubule-binding protein. J. Cell Biol. 131, 399-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutoulis, A., Pazour, G. J., Wilkerson, C. G., Inaba, K., Sheng, H., Takada, S., and Witman, G. B. (1997). The Chlamydomonas reinhardtii ODA3 gene encodes a protein of the outer dynein arm docking complex. J. Cell Biol. 137, 1069-1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, J. B. et al. (2004). Comparative genomics identifies a flagellar and basal body proteome that includes the BBS5 human disease gene. Cell 117, 541-552. [DOI] [PubMed] [Google Scholar]
- Milisav, I. (1998). Dynein and dynein-related genes. Cell Motil. Cytoskelet. 39, 261-272. [DOI] [PubMed] [Google Scholar]
- Mitchell, D. R. (2000). Chlamydomonas flagella. J. Phycol. 36, 261-273. [Google Scholar]
- Mitchell, D. R. (2004). Speculations on the evolution of 9+2 organelles and the role of central pair microtubules. Biol. Cell 96, 691-696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell, D. R., and Kang, Y. (1991). Identification of _oda_6 as a Chlamydomonas dynein mutant by rescue with the wild-type gene. J. Cell Biol. 113, 835-842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell, D. R., and Rosenbaum, J. L. (1985). A motile Chlamydomonas flagellar mutant that lacks outer dynein arms. J. Cell Biol. 100, 1228-1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell, D. R., and Rosenbaum, J. L. (1986). Protein-protein interactions in the 18S ATPase of Chlamydomonas outer dynein arms. Cell Motil. Cytoskelet. 6, 510-520. [DOI] [PubMed] [Google Scholar]
- Mitchell, D. R., and Sale, W. S. (1999). Characterization of a Chlamydomonas insertional mutant that disrupts flagellar central pair microtubule-associated structures. J. Cell Biol. 144, 293-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neer, E. J., Schmidt, C. J., Nambudripad, R., and Smith, T. F. (1994). The ancient regulatory-protein family of WD-repeat proteins. Nature 371, 297-300. [DOI] [PubMed] [Google Scholar]
- Omoto, C. K., and Brokaw, C. J. (1985). Bending patterns of Chlamydomonas flagella: II. Calcium effects on reactivated Chlamydomonas flagella. Cell Motil. 5, 53-60. [DOI] [PubMed] [Google Scholar]
- Pazour, G. J., Agrin, N., Leszyk, J., and Witman, G. B. (2005). Proteomic analysis of a eukaryotic cilium. J. Cell Biol. 170, 103-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piperno, G., and Mead, K. (1997). Transport of a novel complex in the cytoplasmic matrix of Chlamydomonas flagella. Proc. Natl. Acad. Sci. USA 94, 4457-4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin, H., Diener, D. R., Geimer, S., Cole, D. G., and Rosenbaum, J. L. (2004). Intraflagellar transport (IFT) cargo: IFT transports flagellar precursors to the tip and turnover products to the cell body. J. Cell Biol. 164, 255-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum, J. L., and Witman, G. B. (2002). Intraflagellar transport. Nat. Rev. Mol. Cell. Biol. 3, 813-825. [DOI] [PubMed] [Google Scholar]
- Scholey, J. M. (2003). Intraflagellar transport. Annu. Rev. Cell Dev. Biol. 19, 423-443. [DOI] [PubMed] [Google Scholar]
- Stolc, V., Samanta, M. P., Tongprasit, W., and Marshall, W. F. (2005). Genome-wide transcriptional analysis of flagellar regeneration in Chlamydomonas reinhardtii identifies orthologs of ciliary disease genes. Proc. Natl. Acad. Sci. USA 102, 3703-3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada, S., Wilkerson, C. G., Wakabayashi, K., Kamiya, R., and Witman, G. B. (2002). The outer dynein arm-docking complex: composition and characterization of a subunit (Oda1) necessary for outer arm assembly. Mol. Biol. Cell 13, 1015-1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam, L.-W. and Lefebvre, P. A. (1993). Cloning of flagellar genes in Chlamydomonas reinhardtii by DNA insertional mutagenesis. Genetics 135, 375-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam, L.-W. and Lefebvre, P. A. (1995). Insertional mutagenesis and isolation of tagged genes in Chlamydomonas. In: Cilia and Flagella, ed. W. Dentler and G. Witman, San Diego: Academic Press, 519-523. [DOI] [PubMed]
- Wakabayashi, K., Takada, S., Witman, G. B., and Kamiya, R. (2001). Transport and arrangement of the outer-dynein-arm docking complex in the flagella of Chlamydomonas mutants that lack outer dynein arms. Cell Motil. Cytoskelet. 48, 277-286. [DOI] [PubMed] [Google Scholar]
- Walther, Z., Vashishtha, M., and Hall, J. L. (1994). The Chlamydomonas FLA10 gene encodes a novel kinesin-homologous protein. J. Cell Biol. 126, 175-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkerson, C. G., King, S. M., Koutoulis, A., Pazour, G. J., and Witman, G. B. (1995). The 78,000 _M_r intermediate chain of Chlamydomonas outer arm dynein is a WD-repeat protein required for arm assembly. J. Cell Biol. 129, 169-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirschell, M., Pazour, G., Yoda, A., Hirono, M., Kamiya, R., and Witman, G. B. (2004). Oda5p, a novel axonemal protein required for assembly of the outer dynein arm and an associated adenylate kinase. Mol. Biol. Cell 15, 2729-2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witman, G. B., Plummer, J., and Sander, G. (1978). Chlamydomonas flagellar mutants lacking radial spokes and central tubules. J. Cell Biol. 76, 729-747. [DOI] [PMC free article] [PubMed] [Google Scholar]