Biomaterial adherent macrophage apoptosis is increased by hydrophilic and anionic substrates in vivo (original) (raw)
Abstract
An in vivo rat cage implant system was used to identify potential surface chemistries that prevent failure of implanted biomedical devices and prostheses by limiting monocyte adhesion and macrophage fusion into foreign-body giant cells while inducing adherent-macrophage apoptosis. Hydrophobic, hydrophilic, anionic, and cationic surfaces were used for implantation. Analysis of the exudate surrounding the materials revealed no differences between surfaces in the types or levels of cells present. Conversely, the proportion of adherent cells undergoing apoptosis was increased significantly on anionic and hydrophilic surfaces (46 ± 3.7 and 57 ± 5.0%, respectively) when compared with the polyethylene terephthalate base surface. Additionally, hydrophilic and anionic substrates provided decreased rates of monocyte/macrophage adhesion and fusion. These studies demonstrate that biomaterial-adherent cells undergo material-dependent apoptosis in vivo, rendering potentially harmful macrophages nonfunctional while the surrounding environment of the implant remains unaffected.
The host foreign-body response ensues immediately after implantation of biomedical devices and prostheses. This response progresses through stages of inflammation and wound healing with different cell types as hallmark indicators of the particular stage of the reaction. Neutrophils and mononuclear cells immediately infiltrate the tissue–material interface and are associated with acute inflammation. Chronic inflammation is evident when the number of infiltrating neutrophils decrease and the local monocyte population adheres to the surface of the implant and differentiates into macrophages. Lymphocytes also become more predominant during the chronic phase of inflammation. The total number of infiltrating cells normally decreases as the inflammatory response resolves and the wound-healing process progresses to the formation of granulation tissue and subsequent fibrous encapsulation of the implanted material. Although formation of this foreign-body capsule is thought to be beneficial to the implant, cells already adherent to the surface eventually may cause failure of the device. These adherent cells consist of monocyte-derived macrophages, which may fuse into foreign-body giant cells (FBGCs) that concentrate degradative and phagocytic properties leading to structural and chemical damage of the implant (1, 2). Therefore, methods of effectively modulating the presence and activity of adherent monocytes/macrophages/FBGCs would enhance the in vivo lifetime of implanted devices.
The rat cage implant system is an in vivo model that focuses on the types and levels of infiltrating and adherent inflammatory cells on or surrounding the biomaterial after s.c. implantation. With such studies, although the presence and levels of acute or chronic cell types may indicate the overall response to an implanted device, very little information is available regarding the functional capabilities of the exudate or adherent-cell populations. This information is paramount for engineering surfaces that modify the presence and function of adherent monocytes, macrophages, and FBGCs. One such mechanism can be provided by the induction of apoptosis of biomaterial-adherent cells.
We have reported that biomaterial-adherent cell apoptosis is influenced by the chemistry of the surface of adhesion in vitro (3). Particular surface chemistries were chosen to represent a broad spectrum of biomaterial applications. In other studies, we have identified the detrimental apoptotic effects of fluid shear stress on biomaterial-adherent neutrophils, which may lead to increased susceptibility of cardiovascular prostheses to bacterial infection (4). Although the literature pertaining to biomaterial-related apoptosis is expanding rapidly, only limited numbers of studies have explored this topic in vivo. Honma and Hamasaki examined apoptotic FBGCs adherent to collagen sponges (5), but these studies lacked information with respect to the area surrounding the implant, i.e., the overall response to the material. Others have identified material- and time-dependent apoptosis of neutrophils contained within exudate (6); however, these studies focused on short-term analyses (2 days) and provided no information regarding the biomaterial-adherent cell population. Our in vivo studies were performed by using the rat cage implant system with examination of both adherent and exudate cells. We correlated the induction of adherent-cell apoptosis to possible alterations, if any, in the types and levels of cells present surrounding the implant, indicating the progression and resolution of the inflammatory response. Adherent-cell densities, rates of fusion, and levels of apoptosis as well as exudate cell types, levels, and rates of apoptosis were determined. Importantly, these studies demonstrate that, in vivo, hydrophilic and anionic surfaces promote decreased monocyte adhesion and increased proportions of adherent apoptotic monocytes/macrophages, potentially reducing the risk of implant damage and failure caused by these cells.
Materials and Methods
Surface Preparation.
Biomaterial surfaces displaying distinct surface chemistries were prepared with a custom-designed semiautomatic apparatus for laboratory-scale mass production as described (7, 8). The surfaces consisted of polyethylene terephthalate film coated with poly(benzyl N,_N_-diethyldithiocarbamate-co-styrene) (BDEDTC) and either polyacrylamide, sodium salt of poly(acrylic acid), or methiodide of poly(dimethylaminopropyl-acrylamide) were photograft-copolymerized to the BDEDTC surface. These surfaces were characterized in other studies (3) and are considered slightly hydrophobic, hydrophobic, hydrophilic, anionic, and cationic, respectively (Table 1).
Table 1.
Surface properties
| Surface | Chemical property | Advancing water contact angle |
|---|---|---|
| PET base surface | Slightly hydrophobic | 47.6 ± 2.1 |
| BDEDTC-coated surface | Hydrophobic | 83.3 ± 1.7 |
| PAAm | Hydrophilic | 28.4 ± 3.2 |
| PAANa | Anionic | 25.9 ± 2.0 |
| DMAPAAmMeI | Cationic | 28.0 ± 1.1 |
Rat Cage Implant System.
Implants were performed as described (9, 10). Briefly, surfaces were placed in surgical-grade stainless steel wire mesh cages (1.5 cm in length, 0.5 cm in diameter, 0.25-mm wire diameter, and 0.8-mm opening width with 58% open area) and sterilized with ethylene oxide. Cages containing surfaces were implanted s.c. in the backs, at the level of the panniculus carnosus, of anesthetized 3-month-old 250–300 g female Sprague–Dawley rats according to National Institutes of Health guidelines.
Exudate Cell Analyses.
On days 7, 14, and 21, 300–1,000 μl of exudate surrounding the implant within the cages were drawn before explantation. One half of the exudate was processed for total leukocyte and differential cell counting (described in ref. 10), and the other half was stained for apoptosis markers for analysis by flow cytometry. Collected cells were pelleted and washed twice before staining with annexin V-FITC and propidium iodide (R & D Systems) according to manufacturer instructions. The double-stained cells then were washed again and analyzed immediately by flow cytometry. Leukocytes were gated to eliminate consideration of erythrocytes or platelets, and cells staining annexin V-positive/propidium iodide-negative were counted as apoptotic. The results are expressed as the number of apoptotic cells per 100,000 events counted.
Adherent-Cell Analyses.
The rats were killed and the implants retrieved on days 7, 14, and 21. Explanted surfaces were washed and adherent cells stained for apoptosis by using annexin V-FITC conjugate (R & D Systems) according to manufacturer instructions. As described elsewhere (3, 4, 11, 12), treated samples were viewed by confocal scanning laser microscopy, and adherent cells staining positive were counted as apoptotic. Apoptotic levels were confirmed for some samples by using the terminal deoxynucleotidyltransferase-mediated dUTP end-labeling method (R & D Systems).
Adherent-cell densities and macrophage fusion were determined after staining by May–Grünwald/Giemsa. Surfaces were rinsed with PBS twice, and adherent cells were fixed by the addition of methanol for 5 min. Cells then were washed with PBS, and May–Grünwald reagent was added for 1 min followed by another PBS wash. Giemsa reagent was added for 5 min followed by a final wash with distilled water. Cell densities were determined from ×5–20 objective fields for each sample and are expressed as cells per mm2. Nuclei contained within multinucleated FBGCs each were counted as individual cells for determination of cell densities. Percent fusion was calculated by dividing the number of nuclei contained within giant cells by the total number of nuclei in the field of view. This process was repeated for ×3–20 objective fields.
Statistics.
The data are expressed as the average of replicate experiments using cells from three different animals ± the standard error of the mean. Statistical comparisons were performed by using the Student's unpaired t tests by using STATVIEW 4.1 (Abacus Concepts, Berkeley, CA).
Results
Surface Chemistry-Dependent Induction of Adherent-Cell Apoptosis.
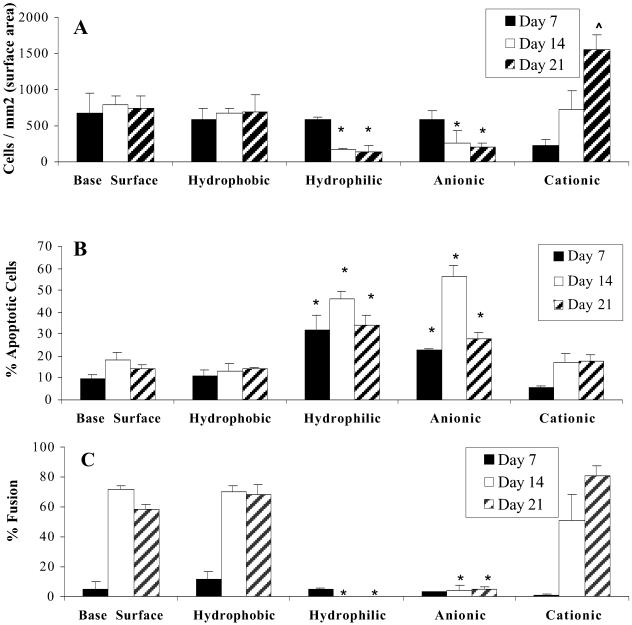
Surfaces displaying distinct chemical characteristics were placed in cages and implanted into the dorsum of rats. As shown in Fig. 1 A, hydrophilic and anionic surfaces promoted significantly (P ≤ 0.021) lower rates of adhesion (142 ± 84 and 214 ± 38 cells per mm2, respectively) when compared with the base surface (746 ± 174 cells per mm2). In contrast, monocyte adhesion increased on cationic surfaces, reaching significantly increased levels on day 21 (1,551 ± 210 cells per mm2) when compared with all other surfaces tested.
Fig 1.
Adherent-macrophage adhesion, apoptosis, and fusion. Cages were explanted on days 7 (black bars), 14 (white bars), and 21 (hatched bars), surfaces were washed with PBS, and adherent cells were stained with May–Grünwald/Giemsa (A and C) or annexin V-FITC in situ (B). (A) Cell densities were determined from ×5–20 objective fields for each sample and are expressed as cells per mm2. (B) Samples were viewed by confocal microscopy, and cells staining positive were counted as apoptotic. A total of 200 cells were counted, and the results are expressed as the percentage of the cells that were counted as apoptotic. (C) Percent fusion was calculated by dividing the number of nuclei contained within giant cells by the total number of nuclei in the field of view. This process was repeated for ×3–20 objective fields. The data represent mean ± SEM. *, statistical significance, where P < 0.05 when compared with the base surface by using the Student's t test.
Adherent cells were stained in situ with annexin V-FITC to determine the percentage of adherent cells undergoing apoptosis. As shown in Fig. 1B, hydrophilic and anionic surfaces promoted adherent-macrophage apoptosis at increased levels at all time points tested when compared with base surface (polyethylene terephthalate) controls (P ≤ 0.048 and P ≤ 0.038, respectively). The largest differences in apoptotic rates were observed on day 14 when hydrophilic surfaces promoted 46 ± 3.7% apoptosis and anionic surfaces 57 ± 5.0% apoptosis, whereas the proportion of apoptotic cells remained relatively low on the base surface (18 ± 1.5%).
Fusion of adherent macrophages was determined by May–Grünwald/Giemsa staining and light microscopy. As shown in Fig. 1C, hydrophilic and anionic surfaces provided the lowest levels of macrophage fusion into FBGCs on days 14 and 21 (no fusion observed on hydrophilic surfaces and 4.4–4.6% fusion observed on anionic surfaces), whereas cationic surfaces promoted the highest levels of fusion (80.5 ± 6.7%) on day 21 (P = 0.03).
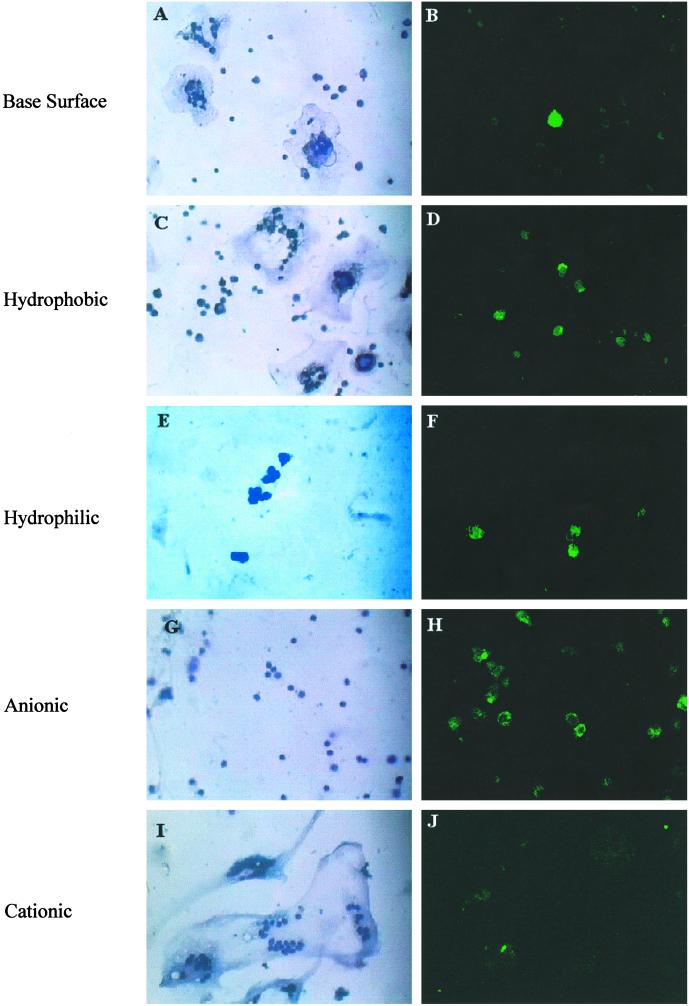
These differences were readily observed qualitatively at day 14 (Fig. 2). The polyethylene terephthalate base surface provides a moderate level of adhesion and fusion (Fig. 2A) and a very low percentage of cells staining positive for annexin V-FITC (Fig. 2B, note only one brightly stained cell compared with the background fluorescence of the surrounding cells). Hydrophobic surfaces provided a slightly increased level of adhesion and fusion (Fig. 3C) when compared with the base surface. Although it appears that the levels of apoptotic cells were increased on these surfaces by the given field of view (Fig. 2D), quantitative analyses revealed no statistical significance between the hydrophobic and base surface adherent-cell apoptotic levels (Fig. 1B). Adhesion and fusion levels were decreased dramatically on hydrophilic and anionic surfaces (Fig. 2 E and G), and a large proportion of the adherent cells stained positive for annexin V-FITC (Fig. 2 F and H, note the relative number of adherent cells on the left to the number of brightly stained cells on the right). Cationic surfaces promoted the highest rate of macrophage fusion (Fig. 2I), because the surface was covered almost entirely with FBGCs by this time point (day 14 after implantation).
Fig 2.
Morphological properties of macrophages adherent to various surface chemistries. Cages were explanted on day 14, surfaces were washed with PBS, and adherent cells were stained with May–Grünwald/Giemsa and observed by light microscopy (A, C, E, G, and I) or stained with annexin V-FITC in situ and observed by confocal microscopy (B, D, F, H, and J).
Fig 3.
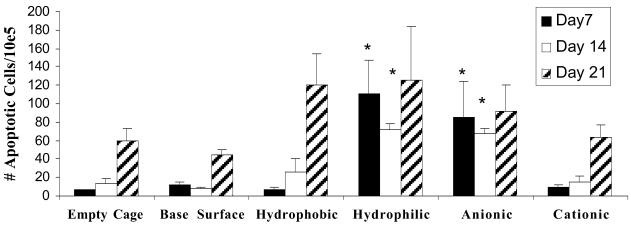
Exudate cell apoptosis. Exudate surrounding the implant was collected on days 7 (black bars), 14 (white bars), and 21 (hatched bars). One half of the exudate was centrifuged, and cells were stained with annexin V-FITC and propidium iodide. Cells were analyzed immediately by flow cytometry. Leukocytes were gated, and a total of 100,000 events were counted. The data are expressed as the number of cells staining positive out of the total number counted. The data represent mean ± SEM. *, statistical significance, where P < 0.05 when compared with the empty cage control using Student's t test.
Effects of Biomaterial Surface Chemistry on Infiltrating Cells.
Exudates surrounding the implanted surfaces were collected, inflammatory cells were identified and quantified, and the percentage of apoptotic cells was determined by flow cytometry. As shown in Fig. 3, the percentage of exudate cells surrounding the implants staining positive for apoptosis also was significantly higher for hydrophilic and anionic surfaces at days 7 and 14 (P ≤ 0.003 and P ≤ 0.01, respectively). However, these differences in the levels of apoptotic exudate cells around these surfaces were absent by day 21. The percentages of apoptotic cells present in the exudate surrounding all surfaces tested were extremely low, rarely reaching 0.1%.
Total leukocyte counts taken from the exudates surrounding each of the implants revealed that no significant differences were observed in the number of exudate cells between the different chemistries (data not shown). Differential cell counts also displayed no variations in the types or levels of exudate neutrophils, monocytes, or lymphocytes as resolution of the inflammatory response was observed by day 14. Taken together, these data indicate that increased apoptosis is associated with hydrophilic and anionic surfaces, but there is no apparent effect on the surrounding environment, resulting in no prolonged acute or chronic inflammatory reactions.
Discussion
Achieving methods to limit the presence and/or activity of biomaterial-adherent macrophages is essential to promote the functional longevity of implanted medical devices by reducing the cell–material and cell–cell interactions. Adherent-macrophage products such as reactive oxygen species, lysosomal enzymes, and low pH act locally to oxidize and damage the implant surface through mechanisms such as oxidative chain cleavage, hydrolysis, or environmental stress cracking (13, 14). We have shown previously that the fusion of biomaterial-adherent macrophages into FBGCs concentrates these degradative activities to facilitate an increase of surface damage (1, 2). Additionally, adherent monocytes/macrophages/FBGCs play key roles in guiding the response to implanted materials by orchestrating the foreign-body reaction through the production and release of cytokines. The cytokines released by biomaterial-adherent macrophages guide cell–cell interactions by either autocrine or paracrine mechanisms. Chemoattractants such as IL-8 released from adherent macrophages may recruit other leukocytes, namely neutrophils and lymphocytes, to the tissue–material interface. Neutrophils can increase surface damage by generating degradative products through the respiratory burst, whereas lymphocytes interact with adherent macrophages to amplify local cytokine concentrations. The cytokines released by either the lymphocytes (IL-2, IL-4, IL-13, and IL-1β) or adherent macrophages (IL-1β, IL-1RA, TNF-α, and IL-10) further influence the activity of surrounding cells. Additionally, IL-4 or IL-13 produced by these cells induce the fusion of macrophages into FBGCs (15, 16), leading to increased damage of the material as described above. Therefore, methods of reducing these damaging activities by limiting the presence and activity of adherent macrophages are needed to increase the in vivo lifetime of implanted biomaterials, prostheses, and medical devices. Our results from the in vivo studies identify apoptosis of biomaterial-adherent monocytes/macrophages as a mechanism for the removal of these cells without generating a prolonged inflammatory response. To this effect, we show that hydrophilic and anionic surfaces promote low levels of adhesion, increased percentages of apoptosis, and decreased levels of macrophage fusion.
It is likely that differences in adhesion rates result from differences in the adsorbed protein layer that coats the surface of implants immediately after introduction into the body. Previous studies have demonstrated that the types and levels of proteins adsorbed to the implant surface vary among surface chemistries (17, 18). The adsorbed protein layer may include fibrinogen (Fg), fibronectin (Fn), von Willebrand factor (vWF), IgG, and vitronectin (Vn), which act as ligands for protein-specific receptors on the surface of monocytes (19). Therefore, surface chemistry-dependent protein adsorption ultimately may determine the degree of monocyte adhesion. To this extent, decreased rates of monocyte adhesion onto hydrophilic and anionic surfaces may be caused by insufficient numbers or altered conformations of ligands present in the adsorbed protein layer.
The mechanism behind the described surface-dependent apoptosis is not clear; however, as mentioned above the surface-property influence of protein adsorption (17) further modulates the types and levels of cytokines produced (W.G.B., Y.N., T.M., E. Colton, N.P.Z., and J.M.A., unpublished data) because of selective receptor–ligand interactions between the adsorbed protein layer and adherent macrophages. In turn, the levels of cytokines produced by biomaterial-adherent cells locally influence the induction of (by TNF-α or IL-10) or resistance to (by IL-4) apoptosis (12). In accordance with this, we have demonstrated that hydrophilic and anionic surfaces promote increased production of IL-10 (W.G.B., Y.N., T.M., E. Colton, N.P.Z., and J.M.A., unreported observations), a cytokine known to induce apoptosis of adherent cells (20). Additionally, apoptosis is induced by stress caused by geometric constraints (21). Cells adherent to low ligand-containing surfaces (such as the hydrophilic and anionic surfaces) experience low ligand-mediated cell spreading, and maturation is absent, leading to apoptosis by complex signaling mechanisms passed from the nonengaged adhesion receptors to the apoptotic machinery. These two induction mechanisms (increase of IL-10 production and geometric constraints) explain the increased percentage of adherent-macrophage apoptosis observed on hydrophilic and anionic surfaces.
Fusion is a result also of cytokine signaling and protein adsorption. As mentioned above, the wound-healing cytokines IL-4 and IL-13 have been used in vitro to promote fusion of adherent macrophages (15, 16). Proximity of adherent cells and appropriate receptor engagements both are needed to promote fusion of adherent macrophages. Once again, hydrophilic and anionic surface chemistries lack the correct adsorbed proteins to promote these mechanisms, whereas cationic surfaces contain abundant ligands to promote adhesion, cell survival, and fusion, which lends support to our previous work linking the parameters of fusion and apoptosis (3), in addition to the fact that no apoptotic FBGCs were observed in any of these studies.
It is of interest to note that the greatest differences in apoptotic levels of cells adherent to the varying surface chemistries were observed on day 14 after implantation. In this respect, it is essential to limit the presence of adherent monocytes/macrophages at early time points to prevent FBGC formation and cytokine release, which would limit long-term structural and chemical damage of the implant by FBGCs and prevent the cytokine-orchestrated formation of an avascular collagenous capsule, providing prolonged implant function.
Although the proportion of adherent cells undergoing apoptosis on hydrophilic and anionic surfaces was increased, relatively few apoptotic cells were observed in the exudate. As time progressed from day 14 to 21, the levels of apoptotic exudate cells increased for all surfaces to the extent where no significant differences were seen among the surfaces. The levels of apoptotic cells observed in the exudate could be accounted for by apoptotic cells released from the surface to the surrounding area, explaining the low levels of apoptotic cells in the exudates, the differences in the levels observed at the earlier time points, and the overall increase over time (because of the accumulation of released cells). These findings also indicate that the mechanism of apoptosis induction is caused by direct interactions between adherent cells and the biomaterial surface and not by released, cell-derived soluble mediators such as cytokines, thereby limiting apoptotic signals to the adherent-cell population. This observation implies a mechanism of induction mediated through the adsorbed protein layer as a function of the surface chemistry. Notably, the induction of apoptosis does not affect the duration or intensity of the inflammatory response as evidenced by the material-independent inflammatory-cell populations in the exudate.
The results presented here agree with previous data regarding implanted prostheses and devices and have implications in their future development. For example, device surfaces used in total hip and knee replacements are hydrophobic and result in eliciting the foreign-body reaction, which is in agreement with expected findings from the data presented here. According to our data, joint prostheses, which use tissue integration into porous surfaces, probably could benefit from surface modifications resulting in hydrophilic and/or anionic properties, because they would reduce the interfacial foreign-body response yet promote bony ingrowth and formation; however, these modifications are not warranted on articular surfaces or with bone cement because of the lack of mechanical properties. The benefits of hydrophilic surface properties are observed easily with synthetic tendons, where there is no foreign-body reaction to the hydrophilic surface, or cardiovascular and catheter materials, in which reduced blood and tissue interactions are observed with hydrophilic surfaces as opposed to hydrophobic surfaces. Although there is a strong correlation between clinical data and the data presented here regarding surface properties, the role of early surface chemistry-dependent induction of adherent-cell apoptosis in these clinical outcomes is yet to be defined.
Hydrophilic and anionic surface chemistries are the first to be identified to promote increased apoptotic levels of adherent macrophages while limiting adhesion and fusion. This phenomenon represents an ideal mechanism for eliminating the presence of potentially harmful cells from the surface of implanted medical devices and prostheses.
Acknowledgments
We thank the National Institutes of Health for funding through Grants HL-33849, HL-55714, and GM-20380 (fellowship awarded to W.G.B.).
Abbreviations
- FBGC, foreign-body giant cell
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Zhao Q., Topham, N. S., Anderson, J. M., Hiltner, A., Lodoen, G. & Payet, C. R. (1991) J. Biomed. Mater. Res. 25**,** 177-183. [DOI] [PubMed] [Google Scholar]
- 2.Ziats N. P., Miller, K. M. & Anderson, J. M. (1988) Biomaterials 9**,** 5-13. [DOI] [PubMed] [Google Scholar]
- 3.Brodbeck W. G., Shive, M. S., Colton, E., Nakayama, Y., Matsuda, T. & Anderson, J. M. (2001) J. Biomed. Mater. Res. 55**,** 661-668. [DOI] [PubMed] [Google Scholar]
- 4.Shive M. S., Salloum, M. L. & Anderson, J. M. (2000) Proc. Natl. Acad. Sci. USA 97**,** 6710-6715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Honma T. & Hamasaki, T. (1996) Virchows Arch. 428**,** 165-176. [DOI] [PubMed] [Google Scholar]
- 6.Fabre T., Belloc, F., Dupuy, B., Schappacher, M., Soum, A., Bertrand-Barat, J., Baquey, C. & Durandeau, A. (1999) J. Biomed. Mater. Res. 44**,** 429-435. [DOI] [PubMed] [Google Scholar]
- 7.Nakayama Y. & Matsuda, T. (1996) Macromolecules 29**,** 8622-8630. [Google Scholar]
- 8.DeFife K. M., Colton, E., Nakayama, Y., Matsuda, T. & Anderson, J. M. (1999) J. Biomed. Mater. Res. 45**,** 148-154. [DOI] [PubMed] [Google Scholar]
- 9.Marchant R. E., Hiltner, A., Hamlin, C., Rabinovich, A., Slobodkin, R. & Anderson, J. M. (1983) J. Biomed. Mater. Res. 17**,** 301-325. [DOI] [PubMed] [Google Scholar]
- 10.Suggs L. J., Shive, M. S., Garcia, C. A., Anderson, J. M. & Mikos, A. G. (1999) J. Biomed. Mater. Res. 46**,** 22-32. [DOI] [PubMed] [Google Scholar]
- 11.Shive M. S., Brodbeck, W. G., Colton, E. & Anderson, J. M. (2001) J. Biomed. Mater. Res. 60**,** 148-158. [DOI] [PubMed] [Google Scholar]
- 12.Brodbeck W. G., Shive, M. S., Colton, E. & Anderson, J. M. (2001) J. Lab. Clin. Med. 139**,** 90-100. [DOI] [PubMed] [Google Scholar]
- 13.Pinchuk L. (1994) J. Biomater. Sci. Polym. Ed. 6**,** 225-267. [DOI] [PubMed] [Google Scholar]
- 14.Labow R. S., Santerre, J. P. & Waghray, G. (1997) J. Biomater. Sci. Polym. Ed. 8**,** 779-795. [DOI] [PubMed] [Google Scholar]
- 15.McInnis A. & Rennick, D. M. (1988) J. Exp. Med. 167**,** 598-611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DeFife K. M., Jenney, C. R., McNally, A. K. & Anderson, J. M. (1997) J. Immunol. 158**,** 3385-3390. [PubMed] [Google Scholar]
- 17.Jenney C. & Anderson, J. (2000) J. Biomed. Mater. Res. 49**,** 435-447. [DOI] [PubMed] [Google Scholar]
- 18.Horbett T. A. (1993) Cardiovasc. Pathol. 2**,** 137S-148S. [Google Scholar]
- 19.Anderson J. M., DeFife, K. M., McNally, A. K., Collier, T. O. & Jenney, C. R. (1999) J. Mater. Sci. Mater. Med. 10**,** 579-688. [DOI] [PubMed] [Google Scholar]
- 20.Wang Z. Q., Bapat, A. S., Rayanade, R. J., Dagtas, A. S. & Hoffmann, M. K. (2001) Immunology 102**,** 331-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen C. S., Mrksich, M., Huang, S., Whitesides, G. M. & Ingber, D. E. I. (1997) Science 276**,** 1425-1427. [DOI] [PubMed] [Google Scholar]