ATRIP OLIGOMERIZATION IS REQUIRED FOR ATR-DEPENDENT CHECKPOINT SIGNALING (original) (raw)
. Author manuscript; available in PMC: 2006 Feb 2.
Published in final edited form as: J Biol Chem. 2005 Jul 15;280(36):31390–31396. doi: 10.1074/jbc.M504961200
Summary
The ATM and ATR kinases signal cell cycle checkpoint responses to DNA damage. Inactive ATM is an oligomer that is disrupted to form active monomers in response to ionizing radiation. We examined whether ATR is activated by a similar mechanism. We found that the ATRIP subunit of the ATR kinase and ATR itself exist as homo-oligomers in cells. We did not detect regulation of ATR or ATRIP oligomerization after DNA damage. The predicted coiled-coil domain of ATRIP is essential for ATRIP oligomerization, stable ATR binding, and accumulation of ATRIP at DNA lesions. Additionally, the ATRIP coiled-coil is also required for ATRIP to support ATR-dependent checkpoint signaling to Chk1. Replacing the ATRIP coiled-coil domain with a heterologous dimerization domain restored stable binding to ATR and localization to damage-induced intranuclear foci. Thus, the ATR-ATRIP complex exists in higher order oligomeric states within cells and ATRIP oligomerization is essential for its function.
DNA damage causes the activation of signaling pathways that promote cell cycle arrest, DNA repair, and apoptosis. Ataxia-Telangiectasia Mutated (ATM)1 and ATM and Rad3 related (ATR), the two kinases at the apex of the checkpoint response, are members of a family of atypical kinases that preferentially phosphorylate serine or threonine residues followed by glutamine (1-3). ATM initiates the immediate cell cycle checkpoint response to DNA double strand breaks while ATR is the predominant initiator of the checkpoint in response to lesions that stall replication forks (2). ATM deficiency causes the human disease ataxia telangiectasia. Cells and animals lacking ATR are not viable (4,5), but a hypomorphic allele of ATR was recently associated with rare cases of seckel syndrome (6,7).
ATR and ATM share significant sequence homology and many substrates but are activated by different stimuli. ATM is held inactive in undamaged cells as an oligomer, with the kinase domain of one molecule bound intermolecularly to the FAT domain of another molecule (8). Cellular irradiation induces rapid intermolecular autophosphorylation of serine 1981 and dimer dissociation, leading to the activation of ATM kinase signaling. Association with the Mre11/Rad50/Nbs1 complex can also facilitate monomerization and activation of ATM (9). The ATR activation mechanism is less well understood and may involve recruitment to sites of DNA lesions and interactions with specific DNA structures (10,11).
ATR exists in a stable complex with an associated protein ATRIP (ATR interacting protein)(4). Similarly, in S. pombe and S. cerevisiae, the ATR homologs Rad3 and Mec1 are found closely associated with ATRIP homologs Rad26 and Ddc2 respectively (12-15). Mutations in either Rad3 or Rad26 yield almost identical phenotypes, as do mutations in either Mec1 or Ddc2. Depletion of ATRIP by RNA inhibition also causes similar phenotypes to loss of ATR in mammalian cells (4). Furthermore, depletion of either ATRIP or ATR from cells causes a decrease in the intracellular levels of the other protein. ATR and ATRIP colocalize to distinct nuclear foci following exposure to DNA damage (4). These nuclear foci are accumulations of checkpoint and DNA repair proteins at DNA lesions although it is unclear whether the large accumulation of checkpoint proteins is required for their proper function (16,17). ATRIP functions as a recruitment protein for ATR through a direct RPA-single-stranded DNA-ATRIP interaction at stalled replication forks (18). However, there may be multiple activities that assist in recruiting ATR to DNA lesions (17,19-22). Additionally, ATRIP-RPA binding is dispensible for the role of ATRIP in ATR signaling to its primary substrate Chk1 (17). Conflicting data also exists regarding the size of the protein complex in which ATR and ATRIP are normally associated in vivo. Wright et al. demonstrated that ATR eluted from a size exclusion column in a large complex of approximately 1,000 kDa in size (23) whereas, Unsal-Kacmaz et al. found that ATR and ATRIP exist as a loosely associated heterodimer that elutes in fractions consistent with its predicted size of less than 400kDa (24).
We recently isolated ATRIP in a yeast two-hybrid screen for ATRIP-interacting proteins. Therefore, we investigated ATR-ATRIP oligomerization in cells. We found that both ATR and ATRIP are associated in oligomeric complexes. Our results demonstrate that the predicted coiled-coil domain of ATRIP is essential for ATRIP oligomerization and for the formation of a stable ATR-ATRIP complex. Furthermore, ATRIP oligomerization through the coiled-coil domain is required for proper ATRIP localization to damage-induced nuclear foci and to promote ATR-dependent Chk1 phosphorylation after DNA damage.
Materials and Methods
Cell culture, siRNA, and transfections
All cell lines were maintained in DMEM+10% fetal bovine serum. Retroviruses were packaged using calcium phosphate transfections of the Phoenix-amphotropic packaging cell line. Plasmid DNA transfections in other cell types were accomplished using lipofectamine (Invitrogen). siRNA transfections were performed with oligofectamine (Invitrogen). The ATRIP siRNA target sequence is AAGGUCCACAGAUUAUUAGAU. The ATR siRNA target sequence is AACCUCCGUGAUGUUGCUUGA. All RNA oligonucleotides were purchased from Dharmacon.
DNA constructs
Myc, Flag, and HA-tagged ATRIP expression vectors for transient expression were generated using the univector plasmid fusion system with pUNI50 ATRIP as the host vector (25). A retroviral vector containing HA-tagged ATRIP was described previously (17). The HA-ATRIPΔ112-225 expression vector was generated by digesting pLPCX HA-ATRIP with BstB1 and AvrII, blunting with T4 DNA polymerase, and religation. The GCN4 dimerization domain was used to replace the ATRIP coiled-coil domain by PCR with the following primers: GAGAGTTCGAACCAAGCACCCCTGGAT CCTCAAGAATGAAACAACTTGAAGACAAG and GAGAGCCTAGGGCTACTACCTGGAGTA CAAGGCGGTTTAGGTCCGCGTTCGCCA ACTAATTTCTTTAATC. The PCR product was digested with BstB1 and AvrII and subloned into the corresponding sites of pLPCX HA-ATRIP. All constructs generated using PCR were confirmed by sequencing.
Antibodies and localization
The ATRIP antibody has been previously described (4). Antibodies to ATR were purchased from Santa Cruz; FITC and RRX conjugated secondary antibodies were obtained from Jackson Immunoresearch; Flag antibodies were purchased from Sigma. HA and Myc antibodies were purchased from Covance. Immunofluorescence was performed by plating cells directly on glass cover slips. Cells were fixed with paraformaldehyde and permeabalized with triton X-100 as previously described (26). Images were obtained with a Zeiss Axioplan microscope equipped with a Zeiss camera and software.
Cell lysis and immunoprecipitation
Cells were lysed in CHAPS lysis buffer (50mM Tris pH=7.5, 150mM NaCl, 0.75% CHAPS, 5μg/ml aprotinin, 5μg/ml leupeptin, 1mM NaF, 20mM μ-glycerolphosphate, 1mM sodium vanadate, 1mM dithiothreitol, and 1mM phenylmethylsulfonate). In selected experiments 0.5% Igepal was substituted for the CHAPS detergent. Immunoprecipitations were generally performed with 2ug of antibody and 15ul of protein-A/G agarose for 3 hours at 4°C.
ATR-ATRIP Crosslinking
HeLa cells transiently expressing ATRIP protein (mutant or wild-type) were trypsinzed, washed with PBS, and resuspended in PBS to approximately 5% cell density. A stock of 25 mM DSP (dithiobis(succinimidylpropionate)) in 100% DMSO was diluted to 1 mM and cells were rotated at room temperature for 3 minutes to crosslink. Cells were pelleted for 3 minutes (1,000 × g) and the crosslinking reaction was quenched by resuspending cells in PBS + 20 mM glycine. Cells were pelleted, frozen and then lysed as described in the immunoprecipitation protocol above using 1% triton X-100 detergent in place of CHAPS.
RPA-ssDNA binding
The 14 kDa and 70kDa subunits were tagged with a His6 epitope tag (27). RPA was purified from E. coli using nickel affinity chromatography followed by Superdex fractionation. 20 pmol of biotin-labeled 69 base pair single-stranded oligonucleotide was bound to streptavidin beads and incubated either with binding buffer (10mM Tris pH=7.5, 100mM NaCl, 10% glycerol, 0.02% Igepal CA-630, 10μg/ml BSA) alone or a 4 molar excess of RPA in binding buffer. The RPA-ssDNA-streptavidin beads were washed three times with binding buffer prior to use. 293T cells transiently transfected with HA-ATRIP encoding vectors were lysed in NETN buffer (50mM Tris pH=7.5, 150mM NaCl, 0.5% Igepal CA-630, 5μg/ml aprotinin, 5μg/ml leupeptin, 1mM NaF, 20 mM μ-glycerolphosphate, 1mM sodium vanadate, 1mM dithiothreitol, and 1mM phenylmethylsulfonate). Lysates were cleared by centrifugation. Beads containing the ssDNA with or without RPA were added to the cleared lysates, incubated for 1.5 hours at 4°C, and washed three times with NETN buffer. Proteins bound to beads were eluted and separated by SDS-PAGE prior to blotting.
RESULTS
ATRIP forms oligomeric complexes in mammalian cells
To identify ATRIP-interacting proteins we screened a B-cell cDNA two-hybrid library with full-length ATRIP fused to the Gal4 DNA binding domain. Two of the interacting clones contained ATRIP cDNAs. To validate ATRIP oligomerization in mammalian cells, we co-expressed Myc-ATRIP and Flag-ATRIP in 293T cells. Lysates were subjected to reciprocal co-immunoprecipitation using antibodies specific to the epitope tags and assessed by western blotting. Myc-ATRIP isolated using a Myc antibody was able to co-immunoprecipitate Flag-ATRIP (Fig. 1_A_). The reverse immunoprecipitation using Flag antibody to immunoprecipitate Flag-ATRIP also isolated co-associated Myc-ATRIP. Flag immunoprecipitation from cells expressing only Myc-ATRIP showed no non-specific association of Myc-ATRIP in the Flag immunoprecipitation (Fig. 1_A_, center), nor did Myc immunoprecipitations isolate any Flag-ATRIP from cells not expressing Myc-ATRIP (Fig. 1_A_, right). Reciprocal co-IP of Myc- and Flag-ATRIP is consistent with the two-hybrid data and confirms that ATRIP can form multimers in mammalian cells.
Figure 1.
ATRIP forms oligomeric complexes. A, Myc- and Flag epitope-tagged ATRIP proteins were co-expressed in 293T cells. Proteins were immunoprecipitated using antibodies to the Myc or Flag epitopes. Isolated proteins were separated by SDS-PAGE and detected by immunoblotting with the indicated antibodies. Flag immunoprecipitations from cells expressing only Myc ATRIP was blotted with Myc antibody (middle) and Myc immunoprecipitations from cells expressing only Flag-ATRIP were blotted with Flag antibody (right) as controls for antibody specificity. B, Cells expressing near endogenous levels of HA-ATRIP were generated by retroviral infection and selection. HA immunoprecipitates from lysates of these cells or parental cells (−) were performed, separated by SDS-PAGE and blotted with ATRIP antibodies. Total cell lysates were also blotted to show the relative expression levels of the HA-ATRIP and endogenous protein.
To demonstrate that ATRIP also oligomerizes when expressed at endogenous levels we generated a stable HCT116 cell line expressing HA3-ATRIP at levels equivalent to endogenous protein using retroviral infection and antibiotic selection. Western blotting with an ATRIP antibody that recognizes both endogenous and HA-tagged ATRIP demonstrated that endogenous ATRIP and HA-ATRIP proteins are expressed at approximately equal levels and the 3XHA tag allows separation of tagged and untagged proteins (Fig. 1_B_). Therefore, we were able to assess whether immunoprecipitates of the HA-ATRIP protein contained endogenous ATRIP. Indeed, HA antibodies immunoprecipitated both the tagged and untagged proteins from lysates of cells containing both proteins (Fig. 1_B_). These results confirm that ATRIP forms oligomeric complexes in mammalian cells.
ATR Forms Oligomeric Complexes in Mammalian Cells
We next examined whether ATR is also an oligomer in cells. ATR oligomerization was analyzed by co-expressing Flag- and Myc-tagged ATR proteins in 293T cells and assessing ATR oligomerization by co-immunoprecipitation. Immunoprecipitation of Flag-ATR using Flag antibodies isolated Myc-ATR (Fig. 2_A_, lane 6), and Myc antibodies co-immunoprecipitated Flag-ATR (Fig. 2_A_, lane 11), demonstrating that ATR also forms oligomeric complexes in cells. Flag antibodies did not immunoprecipitate any Myc-ATR when Flag-ATR was not expressed, nor did Myc antibodies immunoprecipitate Flag-ATR without Myc-ATR (Fig. 2_A_, lanes 10 and 14).
Figure 2.
ATR forms oligomeric complexes. A, HeLa cells were transfected with vectors encoding Flag-ATR, Myc-ATR, HA-ATRIP and/or a siRNA duplex targeted against ATRIP as indicated. Protein expression was assessed by blotting total cell lysates after SDS-PAGE separation with antibodies to the Flag, Myc or HA epitope tags. Efficiency of ATRIP siRNA knockdown was assessed by blotting with anti-ATRIP antibody. Flag or Myc immunoprecipitates were separated by SDS-PAGE and blotted with the indicated antibodies. B, Clonal cell lines stably expressing low levels of either Myc-ATR or both Myc-ATR and Flag-ATR were created by transfection and selection. Lysates from these cells or parental cells (−) were separated by SDS-PAGE and blotted with the Flag, Myc or ATR antibodies to show expression levels. Flag immunoprecipitations from the indicated cell lysates were performed, separated by SDS-PAGE and blotted with the indicated antibodies to assess the co-precipitation of Myc-ATR and ATRIP. C, A vector encoding HA-ATRIP and/or an siRNA duplex targeting ATR were transfected into HeLa cells as indicated. After two days, cells were lysed and immunoprecipitations performed with anti-HA antibodies. Immunoprecipitated proteins were separated by SDS-PAGE and blotted with the indicated antibodies. Cell lysates were also blotted to examine the levels of the proteins in the cell lysates prior to immunoprecipitation.
To confirm that ATR oligomerization is not an artifact of overexpression, we repeated these experiments in clonal cell lines that contained stable Myc- and Flag-ATR proteins expressed at levels no greater than the endogenous protein. The cell lines were first created by sequential transfection of linearized expression plasmids followed by selection of individual clones that express low amounts of the tagged ATR proteins. Western blotting with Myc or Flag antibodies revealed expression of the tagged proteins (Fig. 2_B_). Although the Myc and Flag ATR proteins cannot be separated from endogenous ATR on SDS-PAGE gels, blotting with ATR antibodies indicates that expression of Myc and Flag ATR increase total ATR levels less than two-fold. Thus, they must be expressed at levels less than the endogenous proteins (Fig. 2_B_). We then performed the co-immunoprecipitation analysis using a Flag antibody and again confirmed that the Myc and Flag-tagged ATR proteins co-immunoprecipitate (Fig. 2_B_).
ATRIP Oligomerization is Independent of ATR
To assess the role of ATR in mediating ATRIP oligomerization we compared ATRIP oligomerization in the presence and absence of ATR. ATR protein levels were reduced by transfecting HeLa cells with siRNA specific to ATR. HA-ATRIP was co-expressed with ATR siRNA and ATRIP oligomerization was assayed as the amount of endogenous ATRIP co-immunoprecipitated with HA-ATRIP from cell lysates using an HA antibody. ATR siRNA depleted ATR protein levels by approximately 85% (Fig. 2_C_). The amount of endogenous ATRIP associated with HA-ATRIP was not altered in cells depleted of ATR (Fig. 2_C_), suggesting that ATRIP oligomerization is independent of ATR. Thus, ATRIP exists as an oligomer in cells and ATRIP oligomerization is not mediated by ATR.
ATR Oligomerization is Independent of ATRIP
To assess the role of ATRIP in mediating ATR oligomerization, we co-expressed Myc-ATR and Flag-ATR in the presence of endogenous ATRIP levels, overexpressed ATRIP by co-expressing HA-ATRIP or in the presence of ATRIP-specific siRNA which reduced ATRIP protein levels by approximately 90% (Fig. 2_A_). The amount of co-association of Myc and Flag-ATR was not changed by the ATRIP status of the cell (Fig. 2_A_, lanes 6-8, 11-13). These results suggest that ATR oligomerization is independent of ATRIP. The lack of dependency of ATR oligomerization upon ATRIP or ATRIP oligomerization upon ATR suggests that both ATR and ATRIP contribute to the oligomeric status of the ATR-ATRIP complex.
ATRIP oligomers are not dissociated by DNA damage.
ATM is held as an inactive dimer that autophosphorylates and dissociates into active monomers in response to ionizing radiation (8). To investigate the possibility that a change in ATR or ATRIP oligomerization could regulate ATR activity we co-expressed Myc- and Flag-tagged ATRIP proteins and analyzed oligomerization by co-immunoprecipitating Flag- and Myc-ATRIP from lysates of cells harvested before and after treatment with ultraviolet light (UV), hydroxyurea (HU), or IR (Fig. 3_A_). We consistently found no change in the amount of ATRIP oligomerization after exposure to DNA damaging agents as the intensity of the co-associated Flag- or Myc-ATRIP band was not altered after damage (Fig. 3_A_).
Figure 3.
ATR and ATRIP oligomeric complexes are not dissociated by DNA damage. A, Vectors encoding Myc- and Flag-ATRIP proteins were transfected into HeLa cells. Cells were mock treated or treated with ionizing radiation (IR), hydroxyurea (HU), or ultraviolet light (UV) then incubated for the indicated amount of time prior to harvesting. Flag or Myc immunoprecipitations were performed from cell lysates, separated by SDS-PAGE, and blotted with Flag or Myc antibodies. B, HCT116 cells stably expressing HA-ATRIP and endogenous ATRIP at near equal levels were damaged with 50 J/m2 UV and lysed at 2 or 5 hours after damage. HA immunoprecipitates were separated by SDS-PAGE then blotted with ATRIP antibody to visualize both the HA-ATRIP and endogenous co-immunoprecipitated ATRIP proteins. Cell lysates were also blotted to ensure equal amounts of protein were analyzed. C, HEK293 cells were transfected with vectors encoding Flag-ATR, Myc-ATR, and HA-ATRIP then mock treated or exposed to 50 J/m2 of UV light. After 2 hours the cells were lysed and immunoprecipitations were performed with either anti-Myc or anti-Flag antibodies. Imunoprecipitates were separated by SDS-PAGE and blotted with Myc, Flag, or HA antibodies. D, Parental HeLa cells (−) or the HeLa cell line stably expressing low levels of Myc and Flag ATR were treated with 50 J/m2 of UV, 2mM HU or left untreated. Flag-ATR was immunoprecipitated from cell lysates and separated by SDS-PAGE. Flag-ATR and co-associated Myc-ATR and ATRIP were identified using specific antibodies as indicated.
To confirm that regulation of endogenous ATRIP is similar to that of overexpressed protein we repeated this experiment in HCT116 cells stably expressing HA-ATRIP at near endogenous protein levels. ATRIP oligomerization was indicated by the amount of endogenous ATRIP co-immunoprecipitated with HA-ATRIP in the presence and absence of DNA damage. ATRIP oligomerization is unchanged at 2 hr and 5 hr after UV exposure as compared to the amount of oligomerization seen in undamaged cells (Fig. 3_B_). HA antibodies do not immunoprecipitate endogenous ATRIP from HCT116 cells not expressing HA-ATRIP (Fig. 1_B_). A similar experiment using hTERTimmortalized epithelial cells stably expressing HA-ATRIP yielded similar results (data not shown). These data suggest that the ATRIP oligomer is not dissociated after DNA damage.
ATR oligomers are not disrupted by DNA damage
To test whether ATR oligomerization is disrupted by DNA damage, we co-expressed Myc-ATR, Flag-ATR and HA-ATRIP in 293T cells, exposed cells to UV radiation, and assessed ATR oligomerization before and after damage. Immunoprecipitation of Flag-ATR from cell lysates isolated co-associated Myc-ATR and HA-ATRIP (Fig.3_C_). The amount of co-associated Myc-ATR protein was not changed by exposing the cells to UV (Fig. 3_C_). Reciprocally, isolation of co-associated Flag-ATR with Myc-ATR using Myc antibodies was not altered by UV damage (Fig. 3_C_). Additionally, the amount of HA-ATRIP associated with either Myc-ATR or Flag-ATR was not affected by UV treatment.
We repeated these experiments using the clonal cell lines expressing less than endogenous amounts of Myc- and Flag-tagged ATR proteins. Again we found that Flag immunoprecipitations contained both Myc- and Flag-ATR proteins, and the amount of the co-precipitation was not altered by exposing the cells to UV or HU damage (Fig. 3_D_).
We have found that ATR and ATRIP in cell lysates co-elute from gel filtration columns in complexes larger than 1 MDa that are not detectably altered by DNA damage (data not shown). We also have not detected a difference in oligomerization of chromatin bound ATR-ATRIP after DNA damage or in S-phase synchronized cells (data not shown). These results suggest that the ATR-ATRIP multimeric complex is not detectably dissociated by DNA damage.
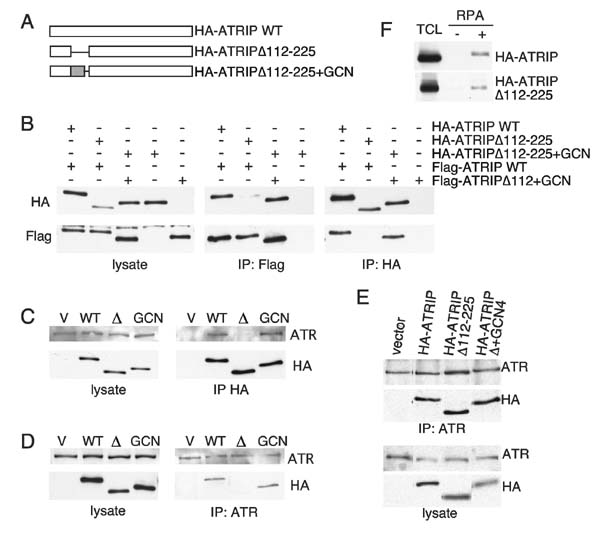
ATRIP coiled-coil domain is required for ATRIP oligomerization and stable ATR-ATRIP complex formation
ATRIP contains a predicted coiled-coil domain stretching from amino acids 108-217. Many coiled-coil domains function as oligomerization motifs (28). To determine if this region is necessary for ATRIP oligomerization, we generated a mutant of ATRIP lacking amino acids 112-225 (ATRIPΔ112-225) (Fig. 4_A_). Deletion of the ATRIP coiled coil domain disrupted ATRIP oligomerization. HA-ATRIP WT or HA-ATRIPΔ112-225 were co-transfected with Flag-ATRIP in HeLa cells. Full-length Flag-ATRIP isolated with Flag antibodies associated with full-length HA-ATRIP but not HA-ATRIPΔ112-225 (Fig. 4_B_, center panel). Reciprocally, immunoprecipitation of HA-ATRIP WT with HA antibodies co-precipitated full-length Flag-ATRIP, but immunoprecipitates of HA-ATRIPΔ112-225 did not contain Flag-ATRIP (Fig. 4_B_, right panel).
Figure 4.
ATRIP oligomerization is essential for stable ATRIP-ATR binding. A, Schematic diagram of wild-type ATRIP, ATRIP lacking the coiled-coil domain (ATRIPΔ112-225) and ATRIP containing a heterologous dimerization domain from the GCN4 protein (ATRIPΔ112-225+GCN4). B, ATRIP oligomerization was assessed by co-transfection of vectors encoding HA-ATRIP, HA-ATRIPΔ112-225, HA-ATRIPΔ112-225+GCN4, Flag-ATRIP, and Flag-ATRIPΔ112-225+GCN4 in HeLa cells as indicated. Flag or HA immunoprecipitates as well as cell lysate were separated by SDS-PAGE and blotted with HA or Flag antibodies. (C and D) Retroviral infection was used to generate U20S cells stably expressing full-length HA-ATRIP, HA-ATRIPΔ112-225 or HA-ATRIPΔ112-225+GCN4. Protein expression levels were examined by blotting cell lysates with either HA or ATR antibodies after SDS-PAGE separation. C, HA or D, ATR immunoprecipitates from the indicated cell lines were separated by SDS-PAGE and blotted with HA or ATR antibodies to assess protein interactions. E, U20S cells expressing HA-ATRIP, HA-ATRIPΔ112-225 or HA-ATRIPΔ112-225+GCN4 were treated with crosslinking agent before lysing cells for immunoprecipitation as in C and D above. F, HEK293 cells were transfected with vectors expressing HA-ATRIP or HA-ATRIPΔ112-225. Cells were lysed and incubated with beads bound to single stranded DNA that had either been coated with recombinant RPA (+) or mock coated (−). After extensive washing, proteins bound to the beads were denatured and separated by SDS-PAGE followed by blotting with the HA antibody. TCL is 5% of the lysate used in the pull down.
We next tested whether ATRIPΔ112-225 could bind to ATR. ATRIP binds ATR through a C-terminal ATR binding domain (17,29). Therefore, we expected ATRIPΔ112-225 to retain the ability to bind ATR. HeLa cells were transiently transfected with HA-ATRIP WT or HA-ATRIPΔ112-225 and approximately equal amounts of the proteins were expressed (Fig. 4_C_, left panel). HA-ATRIP or HA-ATRIPΔ112-225 was immunoprecipitated from cell lysates with an HA antibody and co-associated ATR protein was detected using an ATR-specific antibody. HA-ATRIP WT bound to ATR efficiently based on co-immunoprecipitation. Surprisingly, binding between ATR and the HA-ATRIPΔ112-225 oligomerization mutant was severely compromised despite the retention of the ATR-binding domain in this mutant (Fig. 4_C_). Reciprocally, immunoprecipitating ATR from cell lysates and detecting associated HA-ATRIP proteins confirmed that HA-ATRIP WT but not HA-ATRIPΔ112-225 is associated with ATR (Fig. 4_D_). Longer exposures of these blots sometimes revealed small amounts of ATRIPΔ112-225 binding to ATR; suggesting that oligomerization increases the affinity of ATRIP for ATR but does not necessarily provide residues in contact with ATR. To further clarify the role of ATRIP oligomerization in the formation and stability of the ATR-ATRIP, we repeated the co-immunoprecipitation experiments described above in the presence of a crosslinking agent to trap loosely associated ATR-ATRIP complexes. In contrast to the results generated in the absence of crosslinking, when cells were treated with a crosslinking agent both HA-ATRIP WT and HA-ATRIPΔ112-225 were associated with ATR, as judged by the amount of HA protein co-immunopreciptated using ATR antibodies (Fig. 4_E_). These results indicate that the oligomerization mutant ATRIP (ATRIPΔ112-225) is able to associate with ATR in cells but that the stability of the ATR-ATRIP complex is compromised in the absence of ATRIP oligomerization and becomes too weak to be detected by co-immunoprecipitation in the absence of a crosslinking agent. Therefore, ATRIP oligomerization is essential for the formation of a stable ATR-ATRIP complex. Importantly the ATRIPΔ112-225 protein still retained the ability to interact with RPA-ssDNA similar to wild type ATRIP (Fig. 4_F_) indicating that the mutant protein was not completely misfolded.
To confirm that disruption of stable ATR binding in the ATRIPΔ112-225 mutant was due to the loss of oligomerization we sought to restore ATRIP oligomerization by adding a heterologous dimerization domain. We replaced the coiled-coil domain of ATRIP with the dimerization domain of the yeast transcription factor GCN4 to generate HA-ATRIPΔ112-225+GCN (Fig. 4_A_). Adding the GCN4 dimerization domain to the ATRIPΔ112-225 protein restored ATRIP oligomerization (Fig. 4_B_). HA-ATRIPΔ112-225 expressed in HeLa cells was unable to bind Flag-ATRIP, but HA-ATRIPΔ112-225+GCN4 was able to co-precipitate Flag-ATRIPΔ112-225+GCN4 when isolated with antibodies to the Flag or HA tags (Fig. 4_B_). We next tested whether restoring ATRIP oligomerization with the heterologous dimerization domain restored stable ATR binding in the absence of crosslinking agents as would be expected if the ATRIP oligomerization domain itself did not make any specific contacts with ATR. HA-ATRIPΔ112-225+GCN4 bound ATR with approximately equal efficiency as HA-ATRIP WT, as assessed by co-immunoprecipitation (Fig. 4_C_ & 4_D_). These findings demonstrate that ATRIP oligomerization through the coiled-coil domain is required for formation of the stable ATR-ATRIP complex.
Oligomerization is required for ATRIP damage-induced foci formation
ATRIP and ATR are localized to distinct nuclear foci after exposure to DNA damaging agents such as HU, UV and IR (4). The role of ATRIP oligomerization in the localization of ATRIP to DNA-damage induced nuclear foci was assessed using indirect immunoflourescence. U20S cells expressing either HA-ATRIP WT, HA-ATRIPΔ112-225 or HA-ATRIPΔ112-225+GCN4 were generated by retroviral infection and selection. The cells were exposed to 8 Gy of IR and harvested 3.5 hours post-damage, or exposed to 2mM HU for 5 hours. Localization of the ATRIP proteins was assessed using antibodies to the HA tag. HA-ATRIP, HA-ATRIPΔ112-225 and HA-ATRIPΔ112-225+GCN4 were located diffusely throughout the nucleus in undamaged cells. After exposure to HU, HA-ATRIP WT formed foci in 48% of cells, the oligomerization mutant HA-ATRIPΔ112-225 failed to form foci (0%) and restoring oligomerization with the GCN4 domain (HA-ATRIPΔ112-225+GCN4) restored foci formation (30%) (Fig. 5_A_, 5_B_). Similarly, after exposure to IR, HA-ATRIP WT formed foci in 47% of cells, HA-ATRIPΔ112-225 oligomerization mutant formed foci in only 9% of cells and restoring oligomerization in the HA-ATRIPΔ112-225+GCN4 mutant restored foci in 29% of cells (Fig. 5_A_, B). The ATRIPΔ112-225 mutant is completely defective in forming foci after HU treatment; however, we did observe ATRIPΔ112-225 foci that were generally smaller and fainter than those formed by wild-type ATRIP in 9% of IR-treated cells. In contrast, ATRIPΔ112-225+GCN4 formed large, bright foci in cells after IR (Fig. 5_A_, B). The slightly greater ability of ATRIPΔ112-225 to form foci after IR compared with an almost complete defect after HU may reflect a difference in the mechanism of ATR-ATRIP recruitment to these different DNA lesions. Since ATRIPΔ112-225 binds RPA-ssDNA in vitro (Fig. 4_F_), its inability to accumulate into foci suggests that RPA-ssDNA binding is not sufficient for ATRIP accumulation at DNA lesions.
Figure 5.
ATRIP oligomerization is required for proper ATRIP localization to damage-induced foci and ATR-dependent checkpoint signaling. A, Indirect immunoflouresence with HA antibodies was performed on U20S cells stably expressing HA-ATRIP, HA-ATRIPΔ112-225 or HA-ATRIPΔ112-225+GCN4 that had been left undamaged (−), treated with 8 Gy of IR and incubated for 3.5 hours post-damage, or treated with 2mM of HU for 5 hours. Representative micrographs of the cells are shown. B, Quantitation of HA-ATRIP, HA-ATRIPΔ112-225 and HA-ATRIPΔ112-225+GCN4 foci formation. Cells containing 5 or more easily visualized foci were counted as positive. Error bars represent standard deviations from three separate counts of 100 cells each. C, U2OS cells expressing no cDNA (vector), HA-ATRIP (WT), HA-ATRIPΔ112-225 or HA-ATRIPΔ112-225+GCN4 were transfected with ATRIP siRNA to remove endogenous ATRIP. The cDNAs were made immune to the ATRIP siRNA by mutations of wobble base pairs in the target sequence. Cells were either mock treated (−) or treated with 50 J/m2of UV light then incubated for 2 hours. Cell lysates were separate by SDS-PAGE and blotted with antibodies to HA-ATRIP, Chk1 or phospho-S345-specific antibodies to Chk1. The bar graphs are quantitations of the Chk1 phosphorylation data in which the Chk1 P-S345 signal was divided by the total Chk1 signal. All values were compared to the value obtained in the damaged HA-ATRIP (WT) expressing cells which was set at 100%.
ATRIP coiled-coil domain is essential for ATR-ATRIP checkpoint signaling
To assess the role of ATRIP oligomerization on ATR-dependent checkpoint signaling, we examined the ability of wild-type ATRIP or ATRIP mutants to complement the loss of endogenous ATRIP in U20S cells. Stable cell lines were generated expressing HA-ATRIP WT, HA-ATRIPΔ112-225, or HA-ATRIPΔ112-225+GCN4 containing wobble base pair mutations to provide immunity to siRNA treatment with ATRIP oligos. Cells were treated with ATRIP-specific oligos to reduce endogenous ATRIP protein levels, damaged with UV and harvested 2 hours after damage. The ability of the expressed proteins to compensate for the loss of endogenous ATRIP was assessed by western blotting for ATR-ATRIP-dependent phosphorylation of Chk1 on Ser345. Cells expressing empty vector had greatly diminished levels of phosphorylated Chk1 after UV, whereas cells expressing HA-ATRIP WT were able to complement the loss of endogenous ATRIP as seen by the robust induction of Chk1 phosphorylation after damage (Fig. 5_C_). Cells expressing the oligomerization mutant HA-ATRIPΔ112-225 were severely compromised in the induction of Chk1 phosphorylation after UV damage (Fig. 5_C_). Restoration of ATRIP oligomerization in the HA-ATRIPΔ112-225+GCN4 expressing cells was unable to significantly restore the UV-induced Chk1 phoshorylation. These data indicate that oligomerization through the ATRIP coiled-coil domain is essential for ATRIP to support ATR signaling and also suggests there may be a function of the coiled-coil domain beyond dimerization that is important to allow Chk1 phosphorylation.
DISCUSSION
We have demonstrated that ATR and ATRIP form oligomeric complexes in cells, and ATRIP oligomerization is essential for stable binding to ATR. Additionally, ATRIP oligomerization is essential to localize ATRIP to intranuclear foci following DNA damage and to support ATR-dependent checkpoint signaling. Our data on ATRIP oligomerization is consistent with the observation that the yeast ATRIP homolog Rad26 can form homodimers when made in rabbit reticulocyte lysates (30). Our data is also consistent with Bentley et al. who demonstrated S.Pombe Rad3 forms homo-oligomeric complexes that are not disrupted by DNA damage (31).
ATR and ATRIP oligomerization are inconsistent with a recent report concluding that purified ATR and ATRIP exist as a loosely associated heterodimer with no higher order oligomerization (24). The cause of this discrepancy is not clear, but may relate to the specific methods of purification used in the in vitro study. We have found that ATR and ATRIP co-elute from gel filtration columns in a complex larger than 1,000 kDa (data not shown), consistent with other reports (23) but inconsistent with the elution profile of the purified ATR and ATRIP proteins that UnsalKacmaz et al. studied. While the exact stoichiometry of the complex in vivo remains to be determined, our data conclusively demonstrate that in cells both ATR and ATRIP exist as oligomers and that oligomerization of ATRIP is essential for its function.
ATM is an inactive dimer in cells that dissociates into active monomers in response to DNA damage (8). We did not observe any regulation of ATRIP or ATR oligomerization by DNA damage. It is possible that a population of ATR-ATRIP molecules that are not detected using our methodology is regulated by DNA damage. A portion of ATRIP and ATR are associated with chromatin, and this specific population may not be completely solubilized by our cell lysis conditions. However, co-immunoprecipitation assays were performed from soluble nuclear or chromatin bound ATRIP populations and no differences in regulation of ATRIP oligomerization in these different protein populations was revealed (data not shown). In addition, our methodologies would not have detected a change in the complex if at least two molecules of ATRIP and ATR each remained associated or if only a small proportion of ATR-ATRIP oligomers were dissociated. Therefore, although we were unable to detect regulation of ATR or ATRIP oligomerization by DNA damage, we can not completely rule out the possibility that it is occuring.
The role of the ATRIP coiled-coil domain
Deletion of the ATRIP coiled-coil domain demonstrated that the coiled-coil is essential for ATRIP oligomerization and that ATRIP oligomerization is required for the formation of a stable ATR-ATRIP complex. This data clarifies previous data on the region of ATRIP which is required for ATR binding. We previously reported that upon deletion of the first 380 amino acids of ATRIP, ATR could no longer be co-immunoprecipitated with ATRIP (4). However, we later mapped the ATR-binding domain of ATRIP to the C-terminus (17), which was independently confirmed (29). The requirement for ATRIP oligomerization mediated by the coiled-coil domain for stable ATR-ATRIP interaction explains these seemingly discrepant data because while the C-terminus of ATRIP does contain the ATR interaction domain, stable association with ATR requires N-terminal coiled-coil domain-mediated oligomerization.
The coiled-coil domain clearly functions in mediating ATRIP oligomerization. Failure of the heterologous GCN4 coiled-coil domain to restore the ability of ATRIPΔ112-225+GCN to promote Chk1 phosphorylation suggests that the ATRIP coiled-coil domain could have additional roles other than oligomerization. One possible explanation is that the ATRIP coiled-coil domain promotes the binding of other uncharacterized ATRIP interacting proteins. Alternatively, the GCN4 coiled-coil domain could be restoring an incorrect form of ATRIP oligomerization. The GCN4 coiled-coil is a well characterized dimerization domain but we have not determined the exact stoichiometry of the ATRIP oligomer. Therefore, it is possible that the GCN4 coiled-coil may not restore the correct ATR-ATRIP quaternary structure. Further experiments using alternate heterologous dimerization or oligomerization domains could help to clarify this possibilty.
ATRIP localization to sites of DNA damage
ATR and ATRIP are know to localize to DNA damage-induced nuclear foci (4). This ATR-ATRIP accumulation requires RPA, and ATRIP can bind to RPA-coated single stranded DNA in vitro (18). ATRIP lacking the RPA-binding domain fails to accumulate at sites of DNA damage (17). Thus, an interaction between RPA and ATRIP may be one method by which ATR is recruited to DNA lesions. However, the ATRIPΔ112-225 oligomerization mutant can still bind to RPA-coated ssDNA, but fails to accumulate into damage-induced nuclear foci, suggesting that RPA-ATRIP binding alone is not sufficient to localize ATR-ATRIP. Indeed, we have recently shown that ATR-ATRIP binding is also required for ATRIP to properly localize to nuclear foci after DNA damage (17) and ATR kinase activity is required to localize both ATR and RPA to sites of damage (19). Thus, it is likely that the inability of the ATRIPΔ112-225 mutant to accumulate in foci is due, at least in part, to its inability to stably bind ATR. Interestingly, there was a greater defect in ATRIPΔ112-225 foci formation after hydroxyurea treatment than after ionizing radiation suggesting that these two types of genotoxic stress may require different molecular interactions to be sensed by ATR-ATRIP. Additionally, the significance of ATR-ATRIP relocalization on ATR checkpoint signaling is unclear. Formation of bright ATRIP nuclear foci is not required for ATR-ATRIP checkpoint signaling after UV damage (17). Data presented here demonstrates that the formation of nuclear foci is not sufficient for checkpoint signaling since ATRIPΔ112-225+GCN4 forms damage-inducible nuclear foci but fails to support ATR-dependent Chk1 phosphorylation. Further experiments are required to clarify how the ATR-ATRIP complex is recruited to and retained at DNA lesions and how recruitment and retention influence ATR function.
In conclusion, the ATR protein kinase and its regulatory subunit ATRIP exist as multimers in cells similar to the related ATM kinase. There is no evidence that ATR-ATRIP oligomerization is regulated in response to DNA damage; however, it remains possible that while the majority of ATR-ATRIP oligomers do not dissociate, there is a subset of activated molecules that are indeed disrupted. Both ATR and ATRIP appear to contain homo-oligomerization domains and ATRIP oligomerization requires an intact coiled-coil domain. Disruption of ATRIP oligomerization prevents stable binding to ATR but does not disrupt ATRIP-RPAssDNA association. However, the ATRIP-RPA-ssDNA association is insufficient to target ATRIP to DNA lesions since the oligomerization mutant is defective in DNA damage-induced foci formation. Replacing the ATRIP coiled-coil domain with a heterologous dimerization domain restored ATR binding and proper localization after DNA damage. However, despite restoring proper ATRIP localization, restoring ATRIP oligomerization did not restore ATR-ATRIP checkpoint signaling, suggesting that ATRIP localization to damage-inducible nuclear foci is not sufficient for ATR-dependent checkpoint signaling.
Footnotes
*
This work was supported by NCI grants K01CA93701 and RO1CA102729 to D.C. D.C. is also supported by the Pew Scholars Program in the Biological Sciences sponsored by the Pew Charitable Trusts. HB is supported by training grant T32 CA78136. We thank Walter Chazin and Melissa Stauffer for the His-RPA expression vector, Stephen Elledge for the two-hybrid cDNA library, and Jennifer Pietenpol for the GCN4 cDNA.
1
The abbreviations used are: ATM, ataxia-telangiectasia mutated; ATR, ATM and Rad3 related; HU, hydroxyurea; UV, ultraviolet; IR, ionizing radiation; WT, wild-type; TCL, total cell lysate; PCR, polymerase chain reaction, kDa, kilodalton.
REFERNCES
- 1.Bakkenist CJ, Kastan MB. Cell. 2004;118:9–17. doi: 10.1016/j.cell.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 2.Abraham RT. Genes Dev. 2001;15:2177–2196. doi: 10.1101/gad.914401. [DOI] [PubMed] [Google Scholar]
- 3.Shiloh Y. Curr Opin Genet Dev. 2001;11:71–77. doi: 10.1016/s0959-437x(00)00159-3. [DOI] [PubMed] [Google Scholar]
- 4.Cortez D, Guntuku S, Qin J, Elledge SJ. Science. 2001;294:1713–1716. doi: 10.1126/science.1065521. [DOI] [PubMed] [Google Scholar]
- 5.Brown EJ, Baltimore D. Genes Dev. 2000;14:397–402. [PMC free article] [PubMed] [Google Scholar]
- 6.O'Driscoll M, Ruiz-Perez VL, Woods CG, Jeggo PA, Goodship JA. Nat Genet. 2003;33:497–501. doi: 10.1038/ng1129. [DOI] [PubMed] [Google Scholar]
- 7.Alderton GK, Joenje H, Varon R, Borglum AD, Jeggo PA, O'Driscoll M. Hum Mol Genet. 2004;13:3127–3138. doi: 10.1093/hmg/ddh335. [DOI] [PubMed] [Google Scholar]
- 8.Bakkenist CJ, Kastan MB. Nature. 2003;421:499–506. doi: 10.1038/nature01368. [DOI] [PubMed] [Google Scholar]
- 9.Lee JH, Paull TT. Science. 2005;308:551–554. doi: 10.1126/science.1108297. [DOI] [PubMed] [Google Scholar]
- 10.Byun TS, Pacek M, Yee MC, Walter JC, Cimprich KA. Genes Dev. 2005;19 doi: 10.1101/gad.1301205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cortez D. Genes Dev. 2005;19 doi: 10.1101/gad.1316905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Edwards RJ, Bentley NJ, Carr AM. Nat Cell Biol. 1999;1:393–398. doi: 10.1038/15623. [DOI] [PubMed] [Google Scholar]
- 13.Paciotti V, Clerici M, Lucchini G, Longhese MP. Genes Dev. 2000;14:2046–2059. [PMC free article] [PubMed] [Google Scholar]
- 14.Wakayama T, Kondo T, Ando S, Matsumoto K, Sugimoto K. Mol Cell Biol. 2001;21:755–764. doi: 10.1128/MCB.21.3.755-764.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rouse J, Jackson SP. Embo J. 2000;19:5801–5812. doi: 10.1093/emboj/19.21.5801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lisby M, Rothstein R. Curr Opin Cell Biol. 2004;16:328–334. doi: 10.1016/j.ceb.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 17.Ball HL, Myers JS, Cortez D. Mol Biol Cell. 2005;16:2372–2381. doi: 10.1091/mbc.E04-11-1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zou L, Elledge SJ. Science. 2003;300:1542–1548. doi: 10.1126/science.1083430. [DOI] [PubMed] [Google Scholar]
- 19.Barr SM, Leung CG, Chang EE, Cimprich KA. Curr Biol. 2003;13:1047–1051. doi: 10.1016/s0960-9822(03)00376-2. [DOI] [PubMed] [Google Scholar]
- 20.Bomgarden RD, Yean D, Yee MC, Cimprich KA. J Biol Chem. 2004;279:13346–13353. doi: 10.1074/jbc.M311098200. [DOI] [PubMed] [Google Scholar]
- 21.Parrilla-Castellar ER, Karnitz LM. J Biol Chem. 2003;278:45507–45511. doi: 10.1074/jbc.C300418200. [DOI] [PubMed] [Google Scholar]
- 22.Unsal-Kacmaz K, Makhov AM, Griffith JD, Sancar A. Proc Natl Acad Sci U S A. 2002;99:6673–6678. doi: 10.1073/pnas.102167799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wright JA, Keegan KS, Herendeen DR, Bentley NJ, Carr AM, Hoekstra MF, Concannon P. Proc Natl Acad Sci U S A. 1998;95:7445–7450. doi: 10.1073/pnas.95.13.7445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Unsal-Kacmaz K, Sancar A. Mol Cell Biol. 2004;24:1292–1300. doi: 10.1128/MCB.24.3.1292-1300.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Q, Li MZ, Leibham D, Cortez D, Elledge SJ. Curr Biol. 1998;8:1300–1309. doi: 10.1016/s0960-9822(07)00560-x. [DOI] [PubMed] [Google Scholar]
- 26.Wang Y, Cortez D, Yazdi P, Neff N, Elledge SJ, Qin J. Genes Dev. 2000;14:927–939. [PMC free article] [PubMed] [Google Scholar]
- 27.Stauffer ME, Chazin WJ. J Biol Chem. 2004;279:25638–25645. doi: 10.1074/jbc.M400029200. [DOI] [PubMed] [Google Scholar]
- 28.Lupas A. Trends Biochem Sci. 1996;21:375–382. [PubMed] [Google Scholar]
- 29.Falck J, Coates J, Jackson SP. Nature. 2005;434:605–611. doi: 10.1038/nature03442. [DOI] [PubMed] [Google Scholar]
- 30.Lindsay HD, Griffiths DJ, Edwards RJ, Christensen PU, Murray JM, Osman F, Walworth N, Carr AM. Genes Dev. 1998;12:382–395. doi: 10.1101/gad.12.3.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bentley NJ, Holtzman DA, Flaggs G, Keegan KS, DeMaggio A, Ford JC, Hoekstra M, Carr AM. Embo J. 1996;15:6641–6651. [PMC free article] [PubMed] [Google Scholar]