Human Dcp2: a catalytically active mRNA decapping enzyme located in specific cytoplasmic structures (original) (raw)
Abstract
We have cloned cDNAs for the human homologues of the yeast Dcp1 and Dcp2 factors involved in the major (5′–3′) and NMD mRNA decay pathways. While yeast Dcp1 has been reported to be the decapping enzyme, we show that recombinant human Dcp2 (hDcp2) is enzymatically active. Dcp2 activity appears evolutionarily conserved. Mutational and biochemical analyses indicate that the hDcp2 MutT/Nudix domain mediates this activity. hDcp2 generates m7GDP and 5′-phosphorylated mRNAs that are 5′–3′ exonuclease substrates. Corresponding decay intermediates are present in human cells showing the relevance of this activity. hDcp1 and hDcp2 co-localize in cell cytoplasm, consistent with a role in mRNA decay. Interestingly, these two proteins show a non-uniform distribution, accumulating in specific foci.
Keywords: mRNA cap/mRNA decay/MutT/Nudix/nuclease/turnover
Introduction
mRNA degradation plays a key role in the regulation of gene expression. Several mRNA decay pathways exist in eukaryotic cells (Caponigro and Parker, 1996). In the 5′–3′ mRNA decay pathway, poly(A) shortening is followed by cleavage of the mRNA cap, exposing the mRNA body to 5′–3′ exonucleases. The nonsense-mediated decay (NMD) pathway involves, likewise, decapping followed by 5′–3′ digestion of the mRNA. However, during NMD these steps are preceded by the recognition of a premature stop codon present in the (pre-)mRNA rather than by deadenylation (Czaplinski et al., 1999). The two other known eukaryotic mRNA turnover pathways rely on the activity of the exosome, a multisubunit complex endowed with 3′–5′ exonuclease activity (Butler, 2002). These are the 3′–5′ decay and the non-stop decay (NSD) pathways. The former process involves deadenylation followed by 3′–5′ decay of the mRNA body. In contrast, during NSD, the exosome-mediated degradation of the mRNA is triggered by the absence of a stop codon. In yeast and human cells, NMD and NSD allow the removal of aberrant mRNAs in the cytoplasm and unspliced pre-mRNAs that could be deleterious to the cell (He et al., 1993; Frischmeyer et al., 2002; van Hoof et al., 2002). Various proposals have been made for the location of NMD in metazoan cells: some models suggest that NMD occurs in the cytoplasm while others suggest that it happens exclusively, or in addition, in the nucleus where translation and ribosomes have been detected (Iborra et al., 2001; Ishigaki et al., 2001; Brogna et al., 2002). The 3′–5′ mRNA turnover pathway has also been shown to be involved in the decay of pre-mRNAs in the yeast nucleus (Bousquet-Antonelli et al., 2000).
As in yeast, both the 5′–3′ and 3′–5′ pathways probably contribute to mRNA decay in mammals. Indeed, deadenylated and decapped mRNAs, which are characteristic decay intermediates of the 5′–3′ pathway, have been detected (Couttet et al., 1997). In contrast, in vitro experiments indicated that substrate mRNAs are degraded mostly by the 3′–5′ pathway (Chen et al., 2001; Mukherjee et al., 2002). This pathway is activated in vitro by the presence of AU-rich elements (AREs) in the mRNA substrate, consistent with observations demonstrating that these sequences increase mRNA instability. These latter observations led to the suggestion that the 3′–5′ pathway is the major pathway for mRNA degradation in human cells. However, the proportion of (specific) mRNAs degraded by the 3′–5′ or the 5′–3′ decay pathway in vivo remains to be established.
Several cellular factors implicated in mRNA decay have been identified. These include deadenylases (Daugeron et al., 2001; Tucker et al., 2001) and exonucleases (Larimer et al., 1992; Mitchell et al., 1997). Two different decapping activities have been described. In yeast, Dcp1 was reported to be the major decapping enzyme cleaving capped RNA to release m7GDP and a 5′-phosphorylated downstream product that is a substrate for the Xrn1 5′–3′ exonuclease (Beelman et al., 1996). dcp1 mutants are unable to perform this process, leading to the stabilization of numerous mRNAs. dcp1 mutants are also defective in NMD. Dcp2 was identified as a multicopy suppressor of dcp1 mutants (Dunckley and Parker, 1999). dcp2 mutants also abolish a large fraction of mRNA turnover in yeast cells. Dcp2 was proposed to be an activator of Dcp1 since no intrinsic decapping activity was observed for Dcp2 (Dunckley and Parker, 1999). Interestingly, the Dcp2 protein contains a highly conserved MutT/Nudix motif. This motif has been found in a wide variety of proteins that possess the capability to hydrolyse a nucleoside diphos phate linked to some other moiety, X (nudiX) and has been shown to be critical for catalytic activity (Bessman et al., 1996). The Dcp2 protein is also required for NMD and was shown to interact with Upf1 (He and Jacobson, 1995). It is noteworthy that the decapping activity mediated by Dcp1 and Dcp2 is required, and tightly controlled both for the 5′–3′ and NMD mRNA decay pathways. Pat1 and a group of seven Lsm proteins are required for mRNA decapping in the 5′–3′ mRNA decay pathway but not in the NMD pathway (Bouveret et al., 2000; Tharun et al., 2000). Conversely, the Upf1, Upf2 and Upf3 factors specifically required to initiate NMD do not affect decapping in the 5′–3′ pathway (Czaplinski et al., 1999).
Recently, a different decapping activity was identified in human cell extracts. This activity, cleaving caps that have been released from mRNA through the activity of 3′–5′ exonucleases, has been named DcpS denoting the scavenger decapping activity of the corresponding factor (Wang and Kiledjian, 2001). The products of the reaction mediated by DcpS and using m7GpppG as a substrate are m7GMP and GDP and differ thus from the products of the Dcp1-mediated reaction. Because DcpS activity requires prior degradation of the mRNA body it is likely to act downstream of the exosome in the 3′–5′ and NSD mRNA decay pathways.
To gain further insight into the process of mRNA decapping and turnover, we set out to identify and characterize human homologues of the yeast Dcp1 and Dcp2 proteins. Surprisingly, analysis of recombinant human Dcp2 (hDcp2) revealed that it has decapping activity, identifying it as new decapping enzyme. Interestingly, hDcp2 co-localizes with hDcp1 in specific sub-structures in the cytoplasm of human cells suggesting that mRNA decay may occur in a dedicated compartment.
Results
Identification and cloning of cDNAs encoding hDcp1 and hDcp2
Because decapped mRNAs inconsistent with the DcpS properties (Wang and Kiledjian, 2001) have been identified in human cells (Couttet et al., 1997), we sought to identify the factor responsible for this activity. As yeast Dcp1 was reported several years ago to mediate decapping (Beelman et al., 1996) but related human factors have not been reported, we initiated low stringency database searches. Searches for yeast Dcp2 homologues conducted simultaneously were facilitated by the presence of a conserved Nudix/MutT domain (Dunckley and Parker, 1999). Expressed sequence tags (ESTs) encoding partial proteins that show weak similarity to yeast Dcp1 and strong homology to yeast Dcp2 were identified. Sequencing of the corresponding clones confirmed the similarity. A sequence lacking at the 5′ end of the cDNA clone encoding human Dcp2 (hDcp2) was recovered by RACE. This allowed the reconstruction of a clone encoding a full-length hDcp2 protein while the first five nucleotides of the cDNA encoding hDcp1 were similarly reintroduced by PCR (Materials and methods).
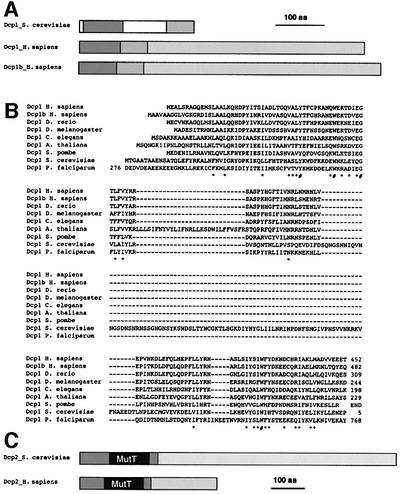
Analysis of the full-length cDNA thus obtained indicated that the hDcp1 gene is located on chromosome 3. While this work was in progress, a second gene encoding a protein related to yeast Dcp1 located on chromosome 12 was revealed by the Human Genome Project. The corresponding protein was named hDcp1b. A single locus on chromosome 5 encodes hDcp2. Comparison of the hDcp1 and hDcp1b proteins with their yeast counterparts revealed the presence of two conserved domains despite the overall low similarity level. These domains are located at the N- and C-terminus of the yeast protein, being separated by a short spacer domain (Figure 1A and B). Interestingly, in the human proteins, these domains are contiguous and correspond to the region of highest similarity between them. Both human factors contain long and poorly conserved C-terminal extensions that make them much larger than their yeast counterpart (63–68 versus 26 kDa) (Figure 1A). While the similarity between hDcp1, hDcp1b and yeast Dcp1 is relatively low (20% identity shared between the yeast protein and either hDcp1 or hDcp1b in the conserved N-terminal domain, Figure 1), the validity of the sequence alignment is confirmed using comparison with Dcp1 proteins identified in a wide variety of eukaryotic species extending from metazoa to unicellular fungi and parasites and including plants. These data indicate that the hDcp1 and hDcp1b proteins are functionally related to yeast Dcp1. For Dcp2 and hDcp2, a high level of amino acid similarity was detected at the N-terminus of the protein (33% identity over 249 amino acids), consistent with analysis of proteins from other species. This region contains a Nudix/MutT domain (Bessman et al., 1996) preceded by a short N-terminal region specific to the Dcp2 proteins (Figure 1C). Both proteins contain a C-terminal tail that presents a low level of sequence similarity. These C-terminal extensions are of very different length, making the yeast protein much larger than the human factor (109 versus 48 kDa).
Fig. 1. Sequence similarities and domain structures of yeast Dcp1 and Dcp2 and human homologues. (A) Schematic representation of the structure of yeast Dcp1 and the human homologues hDcp1 and hDcp1b. The two shaded areas in Dcp1 are conserved between yeast and human, yeast-specific sequences are indicated in white. The long C-terminal tails in hDcp1 and hDcp1b that are not present in yeast Dcp1 and show little sequence similarity between the two human proteins are shown as distinct stippled areas. (B) Sequence alignment of the conserved domain of Dcp1 from a variety of eukaryotic species. Identical residues are indicated by a ‘#’ while similar residues are indicated by an asterisk (*). (C) Schematic structures of yeast and human Dcp2. The highly conserved MutT(/Nudix) domain is indicated in grey. The regions marked with diagonal stripes are conserved between yeast and human, while the C-terminal tails are divergent (stippled).
Recombinant hDcp2 catalyses mRNA decapping
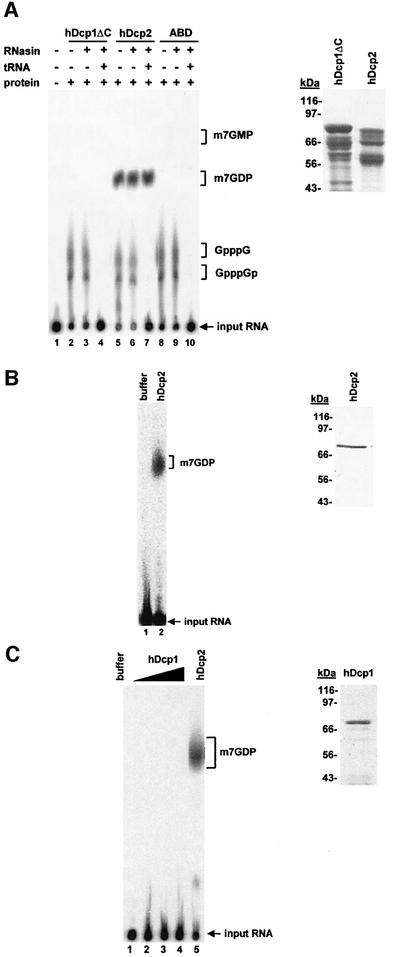
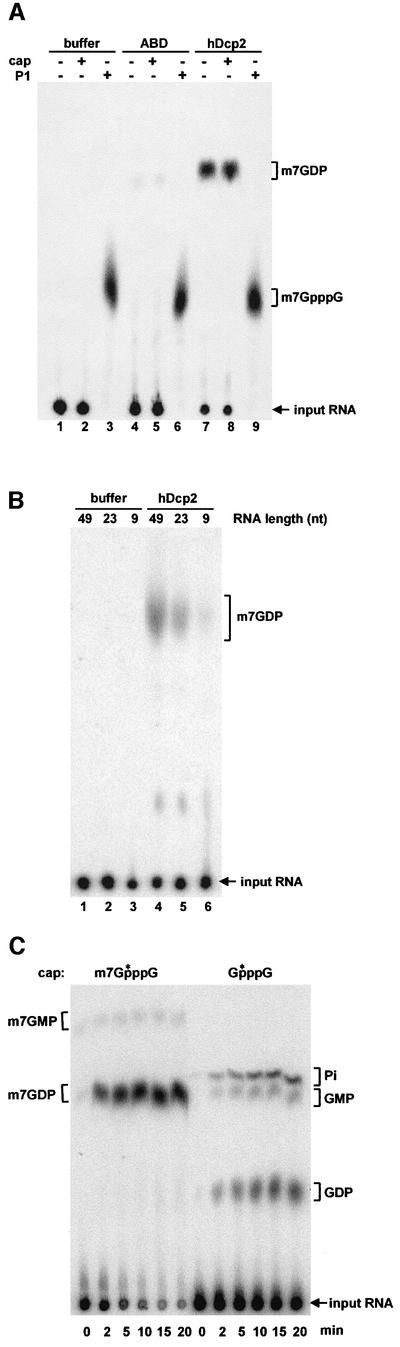
Because yeast Dcp1 was reported to cleave mRNA cap structures (Beelman et al., 1996; LaGrandeur and Parker, 1998), we expressed recombinant proteins to test whether the same is true for hDcp1. As yeast Dcp2 was proposed to activate yeast Dcp1 (Dunckley and Parker, 1999), we also prepared recombinant hDcp2 protein for these assays. Full-length hDcp2 and a truncated hDcp1 protein lacking 154 non-conserved amino acids at its C-terminus (hDcp1ΔC) were expressed as His6–GST fusions in Escherichia coli and purified by Ni-NTA chromatography. In both cases, the complete translation products were contaminated by shorter fragments resulting from premature termination and/or degradation. As a negative control, we used an unrelated His6–GST fusion to a domain of human α-actinin (ABD). To test for decapping activity, these proteins were incubated with an in vitro transcribed RNA 49 nucleotides in length carrying a labelled cap structure. The products of the reaction were fractionated by thin layer chromatography (TLC), revealed by autoradiography and identified by comparison with the mobility of known standards. Surprisingly, a decapping activity generating m7GDP was observed using hDcp2 but not when hDcp1ΔC or the ABD control were incubated with the substrate (Figure 2A). This activity was insensitive to the presence of RNasin or of an excess uncapped tRNA (Figure 2A). Interestingly, mixing hDcp1 and hDcp2 produced in E.coli failed to stimulate the reaction catalysed by hDcp2 alone (see supplementary data, available at The EMBO Journal Online). To ascertain that the full-length proteins were active and rule out the presence of a contaminating activity, we repeated the decapping assays using full-length proteins obtained from different vectors and/or different expression systems. A second version of recombinant hDcp2 was expressed from a vector containing an N-terminal GST and a C-terminal His6 tag. This protein was purified by chromatography on glutathion agarose and Ni-NTA resulting in the production of full-length protein devoid of contaminants (Figure 2B). This protein was also active in decapping (Figure 2B). Expression of full-length hDcp1 in E.coli turned out to be difficult. Therefore, we expressed full-length His6-tagged-hDcp1 by large-scale in vitro translation (see Materials and methods) and purified it. This protein was also not active in decapping (Figure 2C). In contrast, hDcp2 produced by in vitro translation was active. Overall, these data indicate that hDcp2 is a new decapping enzyme.
Fig. 2. hDcp2 has decapping activity. (A) Analysis of decapping activity from purified His6–GST-tagged recombinant proteins hDcp1ΔC (lanes 2–4), hDcp2 (lanes 5–7) and the control protein ABD (a fragment of human α-actinin) (lanes 8–10) (2 µg each). Cap-labelled RNA was incubated with the indicated proteins as described in Materials and methods. The presence or absence of protein, RNasin (lanes 3, 4, 6, 7, 9 and 10) or tRNA (lanes 4, 7 and 10) in the reactions is indicated by (+) and (–) symbols above the lanes, respectively. Lane 1 contains a control reaction with buffer alone. The reaction products were separated by PEI cellulose TLC along with unlabelled standards; their positions of migration are indicated on the right. The position of the input RNA, which remained at the origin of loading, is also indicated. A Coomassie Blue-stained gel containing His6–GST-tagged hDcp1ΔC and hDcp2 is shown on the right. (B) Decapping activity of purified GST–hDcp2-His6. Cap-labelled RNA was incubated with buffer alone (lane 1) or 100 ng of full-length GST–hDcp2-His6 purified by two successive affinity purification steps (lane 2). The purified GST–hDcp2-His6 protein detected by Coomassie Blue staining is shown on the right. (C) Full-length in vitro translated Dcp1 is not active for decapping. Various amounts of purified His6-tagged hDcp1 protein (30–90 ng) were assayed for decapping (lanes 2–4). hDcp2 was used as a positive control (lane 5) while enzyme storage buffer was used as a negative control (lane 1) for the decapping reaction. An aliquot of the in vitro translated, purified His6-Dcp1 was fractionated by gel electrophoresis and stained with Coomassie Blue. This protein contains only a His6 tag and thus migrates at a position lower than the truncated protein expressed in E.coli (A) that contains in addition a GST tag.
hDcp2 cleaves mRNA generating m7GDP and a 5′-phosphorylated RNA fragment
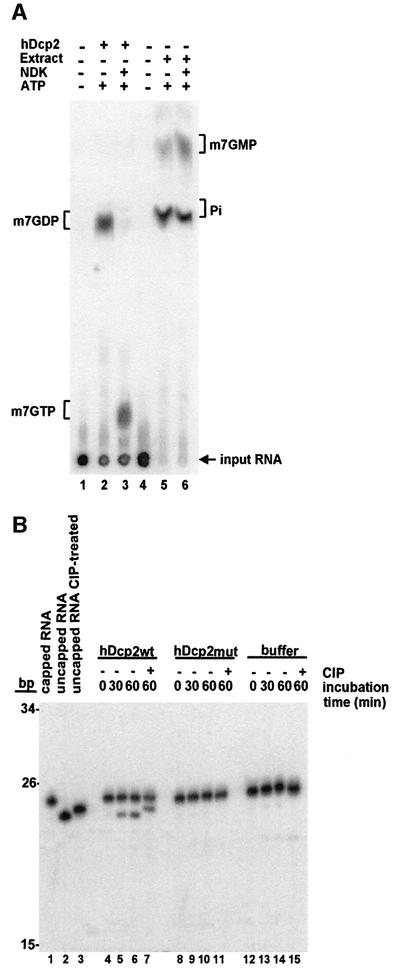
A scavenger decapping activity, DcpS, present in human cell extracts and generating m7GMP was identified recently (Wang and Kiledjian, 2001). To prove that the catalytic activity of hDcp2 is different from the previously identified DcpS activity, we characterized the requirements and products of the reaction. Recombinant hDcp2 releases a product co-migrating with m7GDP on TLC plates using a variety of developing solutions (Figure 2 and supplementary data). This product does not co-migrate with m7GMP (Figures 2 and 3 and supplementary data). Consistently, the products obtained upon incubation of the cap-labelled RNA substrate in human cell extracts, and thus resulting from DcpS activity, fail to co-migrate with the product generated by hDcp2 (Figure 3A) but migrate at the expected positions compared to unlabelled standards (m7GMP and inorganic phosphate, Pi). To prove definitively that the product of the reaction catalysed by hDcp2 contains two phosphates, we incubated the reaction products with nucleotide di-phosphate kinase (NDK) in the presence of ATP. NDK converted the product in a species co-migrating with m7GTP (Figure 3A). In contrast, the products generated in human cell extracts were not modified by NDK. We conclude that hDcp2 releases a nucleotide harbouring two phosphates. To characterize the other product generated by hDcp2, we performed in vitro decapping reactions using a 23 nucleotide long internally labelled RNA carrying a non-radioactive cap. Incubation of this internally labelled substrate with hDcp2 resulted in the release of a 23 nucleotide long uncapped RNA, demonstrating that the RNA body is not degraded during the reaction (Figure 3B). This product was specifically generated by hDcp2 but was not detected following mock incubation or in control reactions with a mutant inactive hDcp2 (Figure 3B, see also Figure 5). The mobility of the 23 nucleotide product generated by hDcp2 was retarded following incubation with calf intestinal phosphatase (CIP) indicating that it contains a phosphorylated extremity (Figure 3B). Because no phosphate source is present in the reaction, it can be deduced from these results that this product contains a 5′ monophosphate.
Fig. 3. Decapping by hDcp2 generates m7GDP and a 5′-phosphorylated downstream product. (A) Cap-labelled RNA (lanes 1 and 4) was incubated with recombinant hDcp2 (lanes 2 and 3) or HeLa cell extract as a source of DcpS (lanes 5 and 6) and the products were separated by TLC. Both the hDcp2 and the DcpS reactions were treated with nucleotide diphosphate kinase (NDK), which specifically converts diphosphates into triphosphates in the presence of ATP. The migration positions of unlabelled standards are indicated on the sides. Lanes 1 and 4 contain control reactions with buffer alone. (B) Analysis of the downstream product of decapping. Internally labelled capped RNA (23 nt) was incubated with recombinant hDcp2wt (lanes 4–7), hDcp2mut (lanes 8–11) or buffer alone (lanes 12–15). Aliquots were taken after 0, 30 and 60 min, and reaction products were separated on a 7 M urea–8% polyacrylamide denaturing gel. Part of the 60 min aliquots was treated with calf intestinal phosphatase (CIP) and run on the gel along with the other samples (lanes 7, 11 and 15). Lanes 1 and 2 contain untreated capped and uncapped RNA, respectively. Lane 3 contains uncapped RNA treated with CIP. Note that CIP treatment results in a slower migration because of the loss of a negative charge. The migration positions of fragments from a DNA size marker are indicated on the left.
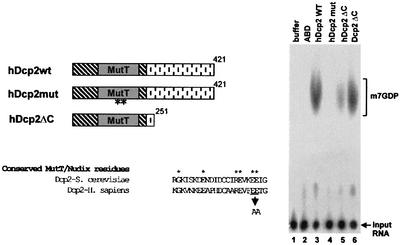
Fig. 5. The MutT/Nudix domain in hDcp2 is essential for decapping. On the left, the schematic structures of hDcp2wt and two derivatives, hDcp2mut and hDcp2ΔC, are shown. In hDcp2mut, two highly conserved glutamic acid residues in the MutT/Nudix domain (E147 and E148) were mutated to alanine. hDcp2ΔC contains a C-terminal truncation of 170 amino acids. An alignment of a fragment of the MutT domain of yeast Dcp2 and human Dcp2 is shown together with the amino acids mutated in hDcp2mut. hDcp2wt, hDcp2mut and hDcp2ΔC (2 µg) were incubated with cap-labelled RNA and the reaction products were separated by TLC (lanes 3, 4 and 5, respectively; high amounts of protein were used to ensure detection of weak activities). Mutating the MutT/Nudix domain reduced activity to background levels while deleting the C terminus caused only a moderate reduction in hDcp2 activity. A recombinant fragment of yeast Dcp2 (lacking the C-terminal 657 amino acids) was also active (lane 6) demonstrating that this property is evolutionarily conserved. Control reactions were performed with buffer alone and ABD (lanes 1 and 2).
We next assessed the size of the mRNA body required for the hDcp2 reaction. hDcp2 was unable to cleave a free cap structure generated by P1 nuclease treatment of a cap-labelled RNA (Figure 4A). Consistently, free cap (m7GpppG) was unable to inhibit competitively the hDcp2 reaction. In contrast, DcpS is able to cleave a free cap. Assaying the activity of hDcp2 with capped RNAs of various sizes revealed that a short RNA containing nine nucleotides was a very poor substrate for hDcp2, while a 23 nucleotide RNA was processed more efficiently (∼2-fold) and a 49 nucleotide species even more so (∼6-fold, Figure 4B). Interestingly, for DcpS in contrast, decreasing RNA length leads to higher activity (Wang and Kiledjian, 2001).
Fig. 4. Requirements for decapping by hDcp2. (A) Cap-labelled RNA was incubated with hDcp2 in the absence (–) or presence (+) of a 1000-fold molar excess unlabelled m7GpppG cap analog (lanes 7 and 8, respectively). Cap-labelled RNA digested with nuclease P1 as a source of radiolabelled free cap (m7GpppG) was also incubated with hDcp2 (lane 9). As controls, the same reactions were performed with buffer alone (lanes 1–3) or ABD (lanes 4–6). The positions of the input RNA and unlabelled standards are indicated on the right. (B) Inefficient decapping of short RNAs. 49, 23 and 9 nt long RNAs were incubated with hDcp2 (lanes 4–6) or buffer (lanes 1–3). Input RNAs and m7GDP locations are indicated. (C) In a time-course experiment, cap-labelled RNA with either a methylated or an unmethylated cap structure was incubated with hDcp2. Aliquots were taken from the reactions after 0, 2, 5, 10, 15 and 20 min and run on a TLC plate. The presence or absence of a methyl group on the cap is indicated above the lanes, the asterisk marks the radiolabelled phosphate. Positions of unlabelled standards and the input RNA are indicated on the sides. With both methylated and unmethylated substrates, some of the diphosphate products were converted to monophosphate and free phosphate due to the presence of contaminating phosphatase that was more active on the unmethylated substrate. This contaminating activity was not detected in other hDcp2 preparations.
To examine the role of the cap methyl group on hDcp2 decapping activity, we performed a time-course experiment using methylated and non-methylated substrates (Figure 4C). This revealed that an RNA with a natural methylated cap structure is a much better substrate for hDcp2 than an RNA harbouring a non-methylated cap. Furthermore, this time-couse experiment showed that the substrate was nearly entirely cleaved by hDcp2 in 10 min. This analysis thus reveals highly specific substrate recognition and efficient catalysis by hDcp2.
Taken together, our data demonstrate that hDcp2 cleaves the mRNA cap to generate m7GDP and an intact 5′-phosphorylated downstream product. hDcp2 requires the presence of an mRNA body attached to the cap to be active and preferentially cleaves methylated cap structure. The properties of hDcp2 are consistent with a role of this enzyme in generating an entry point for 5′–3′ exonucleases such as Xrn1.
The MutT/Nudix domain of hDcp2 catalyses decapping
hDcp2 contains near its N-terminus a MutT/Nudix domain similar to those found in a variety of proteins (Bessman et al., 1996). These domains contain conserved residues that participate to the formation of a catalytic centre. Enzymes from this family have a basic pH optimum and invariably cleave substrates to release a pyrophosphate-containing product. A wide variety of substrates have been identified for various members of this protein family, including nucleotides and dinucleotides. Because the MutT/Nudix domain of hDcp2 is the most obvious feature of this protein, we tested whether it is involved in decapping. First, we expressed and purified a truncated version of hDcp2 lacking the C-terminal extension, which is not conserved in the yeast protein (hDcp2ΔC). This protein was also active to cleave the cap structure of mRNA indicating that the divergent C-terminal tail is not required for this process (Figure 5). The truncated protein was somewhat less efficient than the full-length version indicating that the C-terminal extension may contribute to the activity either directly (e.g. through substrate binding) or indirectly (e.g. by stabilizing the protein in a folded state). We also used a mutated version of the protein harbouring substitutions of residues at the putative catalytic centre. The wild-type and mutant proteins were expressed and purified from E.coli giving very similar yields. Interestingly, mutation of two highly conserved glutamic acid residues (Bessman et al., 1996; Figure 5) completely abolished hDcp2 activity, while a lysine to glutamic acid substitution at position 154 (i.e. six residues downstream) dramatically reduced cap cleavage (data not shown). Although we cannot formally rule out the possibility that the mutations disrupt an interaction that leads to co-purification of a contaminating activity present both in bacteria and in the in vitro translation system, these results provide very strong evidence that decapping is mediated by hDcp2. Finally, we determined the pH optimum of the hDcp2 enzyme and found that it was in the basic range (pH ∼9, data not shown) like other MutT/Nudix enzymes (O’Handley et al., 2001).
We also expressed a fragment of the yeast Dcp2 protein in E.coli. This fragment contains the MutT/Nudix domain but lacks the non-conserved C-terminal tail. Interestingly, this fragment was also active in decapping (Figure 5, lane 6). This confirms that the Dcp2 activity is mediated by the MutT/Nudix domain. Importantly, this experiment indicates that the decapping activity of Dcp2 is evolutionarily conserved.
Taken together, these data reveal that the MutT/Nudix domain of hDcp2 and its yeast homologue catalyses the decapping reaction.
hDcp2 co-localizes with hDcp1 in specific cytoplasmic structures
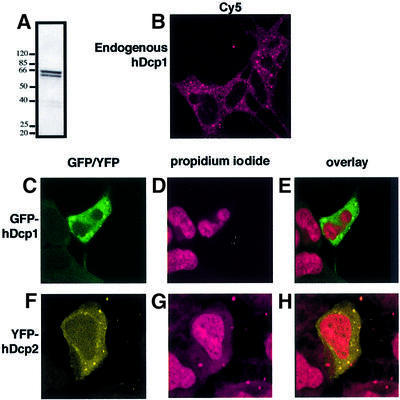
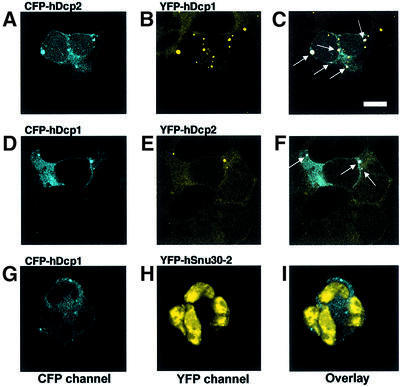
Having established that hDcp2 is a decapping enzyme, we determined its localization in human cells to test whether its distribution would be consistent with its potential role in mRNA decay. Constructs carrying GFP, YFP or CFP fused to hDcp2 and hDcp1 were transiently transfected into HEK293 human embryonic kidney cells and protein location was determined by confocal microscopy. hDcp2 was located mostly in the cytoplasm with, at most, very low amounts present in the nucleus (Figure 6). hDcp1 was found exclusively in the cytoplasm with no detectable nuclear signal (Figure 6). Interestingly, the distribution of both hDcp1 and hDcp2 was not uniform in the cytoplasm (Figure 6). For both proteins, a punctated staining pattern was observed. Several controls were performed to ascertain that these structures were not artefacts resulting from over-expression of tagged proteins. First, transient transfections were repeated using various amounts of DNA of the expression vectors. Secondly, stable cell lines expressing GFP fused to either hDcp1 or hDcp2 were established. Thirdly, the location of hDcp1 was also determined following transient transfection of the vectors in other human cell lines, namely HeLa and C33 cells (data not shown). Finally, the location of endogenous hDcp1 was determined by indirect immunofluorescence using a specific serum (Figure 6A and B). The punctate staining pattern characteristic of hDcp1 was always detected with the number of dots often being lower for hDcp2 (data not shown). These results suggested that hDcp1 and hDcp2 are located in specific structures in the cytoplasm and also raised the possibility that hDcp1 and hDcp2 would co-localize. To test this hypothesis, we simultaneously analysed the location of CFP and YFP variants of hDcp1 and hDcp2 by transiently transfecting one of the fusions into stable cell lines expressing the cognate partner. These experiments revealed that hDcp2 and hDcp1 do indeed co-localize in cytoplamic dots (Figure 7). Overlapping signals are not detected when the control protein hSnu30-2 is used, indicating that they do not result from aggregation mediated by the CFP and YFP fusions or from a confocal artefact resulting from non-specific detection of YFP and/or CFP proteins in the two channels. Co-localization of hDcp1 and hDcp2 was further confirmed following analysis of the sub-cellular location of endogenous hDcp1 using immunofluorescence in a stable cell line stably expressing a YFP-tagged hDcp2 (data not shown). Although not proving homology, the co-localization of hDcp1 and hDcp2 is in agreement with our sequence analysis indicating that yeast Dcp1 and hDcp1 are homologous despite a low level of sequence similarity. Indeed, co-immunoprecipitation and biochemical studies have revealed that the yeast Dcp1 and Dcp2 proteins interact inside a complex. In addition, our study reveals specific cytoplamic structures that are likely to be involved in mRNA decay because they contain both hDcp1 and hDcp2.
Fig. 6. hDcp1 and hDcp2 localize to dots in human cell cytoplasm. (A) Western blot demonstrating the specific recognition of hDcp1 in human cell extracts by the corresponding anti-serum. (B) Indirect immunoflorescence of HEK293 cells using the antiserum directed against hDcp1 and Cy5 labelled secondary antibodies. (C–H) Analysis of HEK293 cells transfected with a GFP-hDcp1 expression plasmid (C–E) or a YFP-hDcp2 expression plasmid (F–H). (C and F) Visualiza tion of GFP-hDcp1 and YFP-hDcp2. (D and G) Propidium iodide staining of the nuclei of transfected cells. (E and H) Overlay of the protein and the propidium iodide signals.
Fig. 7. hDcp1 and hDcp2 co-localize. HEK293 cells stably expressing YFP-hDcp1 (top row; A–C) or YFP-hDcp2 (middle row; D–F) were transiently transfected respectively with CFP-hDcp2 or CFP-hDcp1 expression contructs. (A) CFP-hDcp2 signal in a YFP-hDcp1-expressing cell. (B) YFP-hDcp1 signal in the stably transfected cell line. (C) Overlay of CFP-hDcp2 and YFP-hDcp1 signals. The dots containing both CFP-hDcp2 and YFP-hDcp1 appear as white and are indicated by arrows. (D) CFP-hDcp1 signal in a YFP-hDcp2-expressing cell. (E) YFP-hDcp2-signal in the stably transfected cell line. (F) Overlay of CFP-hDcp1 and YFP-hDcp2 signals. The dots that contain both CFP-hDcp1 and YFP-hDcp2 appear as white and are indicated by arrows. As a control for CFP/YFP aggregation and for the lack of cross-contamination of CFP and YFP signals we co-expressed CFP-hDcp1 with the YFP-hSnu30-2 splicing factor. (G) CFP-hDcp1 signal. (H) YFP-hSnu30-2-signal. (I) Overlay of CFP-hDcp1 and YFP-hSnu30-2 signals. The bar depicted in (C) indicates a size of 8 µm.
Discussion
Our results demonstrate that hDcp2 is a new mRNA decapping enzyme. Recombinant hDcp2 catalyses the decapping of capped RNA generating m7GDP and a 5′-phosphorylated RNA fragment that is a substrate for 5′–3′ exonucleases such as Xrn1. hDcp2 differs from human DcpS activity by the nature of the product and by its ability to cleave cap structures attached to long mRNA molecules. Recombinant yeast Dcp2 has been reported to be inactive for decapping in vitro (Dunckley and Parker, 1999). We were unable to test the activity of full-length yeast Dcp2 as expression of this large (108 kDa) protein in E.coli was not successful (F.Wyers, personal communication). Our work indicates, however, that a recombinant fragment of yeast Dcp2 containing the conserved N-terminal moiety was active for decapping (Figure 5). The region required for hDcp2 activity is also located at the N-terminus of the protein, which is the most conserved part. This region contains a MutT/Nudix domain and mutation of conserved residues in this domain ablates the decapping activity of hDcp2. Similar mutations completely abolish the activity of yeast Dcp2 in vivo, thus blocking decapping (Dunckley and Parker, 1999). The decapping activity of hDcp2 has an alkaline pH optimum, a property that is also consistent with characteristics of known enzymes harbouring an active MutT/Nudix catalytic domain. The various enzymes of this family have been shown to cleave substrates carrying a pyrophosphate group to release a phosphate containing product and the remaining part of the substrate molecule. Similarly, the reaction catalysed by hDcp2 produces m7GDP and a downstream 5′-phosphorylated RNA. Interestingly, hDcp2 requires the presence of a long RNA molecule attached to the cap and is stimulated by the presence of the methyl7 group. This substrate specificity differs from what is known for other MutT/Nudix proteins, demonstrating that capped mRNAs are natural substrates for Dcp2. Taken together, these data strongly suggest that hDcp2 is a bona fide decapping enzyme.
The yeast Dcp1 protein has been reported to be a decapping enzyme generating m7GDP (Beelman et al., 1996; LaGrandeur and Parker, 1998; Fischer and Weis, 2002). The involvement of the yeast Dcp1 protein in decapping in vivo is well established as deletion or mutation of the corresponding gene blocks decapping. Recombinant yeast Dcp1 expressed in E.coli was originally reported to be inactive in decapping (Beelman et al., 1996; LaGrandeur and Parker, 1998). Yeast Dcp1 overproduced and purified from yeast was, however, active. The difference in activity of the two protein preparations was attributed to the lack of phosphorylation of the recombinant protein. More recently, the preparation of active recombinant yeast Dcp1 was reported (Vilela et al., 2000). Thus, while there is no doubt that in yeast, Dcp1 is required for decapping in vivo, two models can be proposed to explain the function of Dcp1 in the decapping reaction. There is first the possibility that Dcp1, like Dcp2, is an active decapping enzyme. Our inability to detect activity associated using recombinant hDcp1 produced in E.coli or by in vitro translation under a variety of conditions (Figure 1 and data not shown) would thus reflect a lack of modification or aberrant folding of the protein. Alternatively, it remains possible that the decapping activity originally attributed to Dcp1 was not mediated by Dcp1 itself. Purification of yeast Dcp2 reveals that it copurifies with yeast Dcp1 but is quite unstable leading to the generation of a smear of truncated products probably through the action of non-specific proteases during the purification (F.Caspary and B.Séraphin, unpublished results). It remains therefore possible that Dcp1 fractions obtained from yeast contained a significant amount of truncated yeast Dcp2 responsible for the observed activity (even if the corresponding polypeptide was not detected by staining after gel electrophoresis). However, this possibility is somewhat difficult to reconcile with the fact that denaturing gel electrophoresis followed by renaturation of Dcp1 yields a protein capable of decapping (Beelman et al., 1996; LaGrandeur and Parker, 1998). The detection of cap-cleavage in the presence of recombinant yeast Dcp1 also suggests that this protein might be catalytically active. It is noteworthy, however, that the exact nature of the products generated by the recombinant protein purified from E.coli was not assessed. We have observed a background cap-cleaving activity from E.coli cleaving free cap but only weakly affected by cap methylation (data not shown). In this vein, it is important to note that recombinant Dcp1 proteins produced in E.coli appeared to have low activities per weight unit of protein. In summary, while we cannot exclude that hDcp1 is a second decapping enzyme, our results clearly demonstrate that both yeast and human Dcp2 are active. The exact function of hDcp1 remains therefore to be established. The phenotype of yeast Dcp1 mutants, the co-purification of yeast Dcp1 and Dcp2, sequence conservation throughout evolution and the co-localization of hDcp1 and hDcp2 in the cytoplasm leave however little doubt that hDcp1 is required for some aspect of the decapping reaction or more generally for mRNA decay. While this work was in progress, hDcp1 was also identified as a factor interacting with Smad4 and implicated in transcriptional regulation (Bai et al., 2002). Further studies will reveal whether Dcp1 affects mRNA degradation and transcriptional regulation independently.
In yeast, the 5′–3′ mRNA decay pathway appears to be the major mode of mRNA degradation in vivo (van Hoof and Parker, 2002). The situation is less clear for human cells. mRNA decay intermediates lacking a cap structure have been identified, indicating that the 5′–3′ mRNA decay pathway is also active in these cells (Couttet et al., 1997). In addition, a decapping activity potentially generating m7GDP has been reported (Gao et al., 2001). The finding that Dcp2 is active in decapping supports this conclusion and suggests further that it might have been responsible for the production of the intermediates detected. Previous studies have revealed that only a small proportion (3%) of poly(A)– RNA population lacks a cap (Couttet et al., 1997). However, given that the steady state level of intermediates will be governed by the relative kinetics of their formation and disappearance, further work will be required to determine the quantitative contribution of the 5′–3′ pathway to overall mRNA decay in human cells. Incubation of synthetic mRNAs in human cell extracts indicated that those are essentially degraded in a 3′–5′ manner in vitro and suggested that this pathway is specifically regulated by ARE elements (Chen et al., 2001; Mukherjee et al., 2002). It remains to be demonstrated, however, whether the natural mRNA degradation pathway with intricate regulation (e.g. deadenylation by bona fide enzymes preceding decapping or 3′–5′ exonucleolytic degradation by the exosome) is faithfully reproduced in vitro in the absence of processes such as translation. Indeed, while 5′–3′ degradation is the main route for mRNA degradation in yeast, RNA introduced into cells appear to be degraded by the 3′–5′ mRNA decay pathway, suggesting that degradation directionality may be highly sensitive to small perturbations of the system (Brown and Johnson, 2001). Overall, the existence of mRNA decay intermediates lacking a cap together with the conservation of factors implicated in the 5′–3′ pathway (Lsm proteins, Pat1, Xrn1) in human cells (Bouveret et al., 2000) clearly demonstrate that an mRNA decapping activity is functional in these cells. However, the contribution of hDcp2 to the overall mRNA decay process as well as its involvement in specific pathways remains to be established.
Our investigation of the intracellular localization of hDcp1 and hDcp2 led to the surprising observation that these proteins co-localize in specific substructures in the cytoplasm. While a cytoplasmic localization supports a role for both of these proteins in mRNA decay, their presence in specific substructures was unexpected. Two major suggestions can be made for the role of these structures. First, these structures could represent a storage compartment for mRNA decay factors. Alternatively, these structures may correspond to the location of an active site of mRNA degradation. We favour the latter hypothesis because there is no evidence for storage of mRNA decay factors that would have to be activated under specific conditions. It is worth mentioning that related structures containing RNA and RNA-binding proteins have been described recently in human cells (Eystathioy et al., 2002). If these two structures are indeed identical, substrate molecules would be present together with mRNA decay enzymes, supporting an active rather than a storage function. Interestingly, the Xrn1 protein has also been shown to be enriched in specific dots in the cytoplasm of mouse cells (Bashkirov et al., 1997). Beside the identification of a new pattern of cytoplasmic staining, the observation that mRNA decay factors are unevenly distributed in the cytoplasm suggests that mRNA decay may be precisely localized. Such a situation would enable cells to remove specific mRNAs from particular locations in the cytoplasm. Defining the exact function and composition of these structures in the near future should provide new insights into the mechanism and control of mRNA decay.
Material and methods
Plasmid construction
cDNA clones for hDcp1 (IMAGp998B07136Q2, pBS2154) and hDcp2 (IMAGp998B233419Q2, pBS2155) were obtained from the IMAGE EST project (Lennon et al., 1996).
To construct an expression clone of hDcp1, the original cDNA was PCR amplified with a 5′ oligo containing a _Nco_I site and the first five nucleotides (nt) of the hDCP1 coding sequence lacking in the cDNA; the 3′ oligo contained a _Xho_I site. The PCR product digested with _Nco_I and _Xho_I was cloned in the corresponding sites of an expression vector carrying a His6 and a GST tag. The coding sequence of the resulting plasmid (pBS2175) was free of mutations. Digestion of pBS2175 with _Nhe_I and _Xho_I, blunting and religation generated a C-terminal truncation of 154 amino acids (aa). The resulting plasmid (pBS2176) encodes the first 428 aa of hDcp1 followed by eight amino acids derived from the vector.
Approximately 200 nt lacking at the 5′ end of the original Dcp2 cDNA were recovered by RACE from HeLa cell poly(A)+ mRNAs using specific oligonucleotides (pBS2183). This fragment was combined with a cDNA containing the downstream Dcp2 ORF and inserted in an expression vector yielding pBS2204.
Point mutations were introduced in the hDcp2 coding sequence using the Quikchange kit (Stratagene). Two A/C nucleotide substitutions were introduced changed residues E147 and E148 to alanine (pBS2270). The region coding for the first 250 aa of hDcp2 was PCR amplified using appropriate primers containing a stop codon and _Xho_I site. The product was digested with _Bsr_GI and _Xho_I and inserted into the same sites of plasmid pBS2204, giving pBS2271.
Modification of pBS2204 allowed the insertion of the hDcp2 coding sequence between a N-terminal GST tag and a C-terminal His6 tag. The His6 tag of pBS2204 was deleted by replacing the _Xba_I–_Swa_I fragment by an _Xba_I–_Swa_I fragment from pGSTevCBP (L.Minvielle-Sebastia) yielding pBS2311. An _Nco_I–_Ava_II fragment of the hDcp2 coding sequence was fused with an adapter containing an _Ava_II site, a His6 coding sequence, a stop codon and an _Xho_I site. The fusion product was cloned in the _Nco_I–_Xho_I sites of pBS2311 yielding pBS2312.
For in vitro translation, the hDcp1 and hDcp2 ORF were cloned in the _Nco_I–_Sma_I sites of plasmid pIVEX2.4a (Roche) yielding plasmid pBS2229 and pBS2230, respectively.
In vitro decapping assays
Unlabelled and uncapped RNA was produced by in vitro transcription with T7 polymerase (Promega). Plasmid pBS2266 (a pGEM-3Zf+ derivative containing a cloned synthetic insert) digested with _Fok_I was used as template, yielding a transcript of 49 nt. Digestion of pBS2266 with _Sma_I or _Eco_RI generated transcripts of 23 and nine nt. RNAs were purified from a 7 M urea–8% polyacrylamide gel, eluted overnight in elution buffer (0.3 M NaAc, 50% phenol, 1 mM EDTA pH 5.2), chloroform-extracted and ethanol-precipitated. Between 20 and 50 pmol of these RNAs were cap-labelled using the vaccinia virus capping enzyme (Higman et al., 1994) in capping buffer (50 mM Tris–HCl pH 7.9, 1.2 mM MgCl2, 6.0 mM KCl, 2.5 mM DTT) in the presence of 1 mM SAM, 2.5 µM unlabelled GTP, 0.132 µM [α-32P]GTP, 40 units RNasin and capping enzyme. Specific activity, determined after gel-filtration and ethanol precipitation, was measured by counting; 5000–9000 c.p.m. used per reaction corresponded to 0.01–0.02 pmol RNA.
Recombinant proteins were expressed and purified on Ni-NTA (Qiagen). For the GST–hDcp2-His6 protein, the Ni-agarose purification was followed by GST purification using glutathione–Sepharose 4B beads (Amersham). Purified proteins were dialysed against 20 mM HEPES–KOH pH 7.6, 0.01 % NP-40, 20 mM KCl, 1 mM MgCl2, 10% glycerol, 0.1 mM EDTA, 1 mM DTT. In vitro translated full-length hDcp1 and hDcp2 were obtained using the Rapid Translation System RTS500 E.coli HY kit (Roche) and the RTS500 Instrument (Roche).
The decapping reactions (Zhang et al., 1999) were performed at 37°C for 30 min in decapping buffer (45 mM Tris–HCl pH 8, 27 mM (NH4)2SO4, 9 mM MgAc) in the presence of 0.25 µg/µl tRNA and 26 ng to 2 µg of recombinant protein. While an absolute specific activity was not determined, we observed that, at non-saturating substrate concentrations, <26 ng of recombinant Dcp2 were sufficient for the decapping of 0.1 pmol of substrate in 10 min. Aliquots of the reactions were run on PEI-cellulose TLC plates (Merck) in 0.3–0.5 M LiCl, 1 M formic acid. TLC plates were dried and exposed to PhosphorImager screens (Molecular Dynamics). Nuclease P1 treatment of cap-labelled RNA was carried out with 1 U enzyme in 10 mM Tris pH 7.5.
Downstream product analysis
Uniformly labelled RNAs were transcribed in vitro with T7 polymerase (Promega) in the presence of [α-32P]UTP with or without m7GpppG for capped or uncapped RNA, respectively, using pBS2266 linearized with _Sma_I as template. RNAs were purified as described above. 3000 c.p.s. were used for in vitro decapping. Reactions were stopped with formamide loading dye and run on 7 M urea–8% polyacrylamide denaturing gels. Dried gels were exposed to PhosphorImager screens. CIP (Biolabs) treatment was carried out with 10 U of enzyme for 30 min at 37°C.
Cell culture and transfection for GFP localization
The cDNAs encoding hDcp1 or hDcp2 were cloned in peGFP-C2, peCFP-C1 and peYFP-C1 plasmids (Clontech). This yielded the following constructs: hDcp1-GFP (pBS2201), hDcp1-CFP (pBS2278), hDcp1-YFP (pBS2265), hDcp2-GFP (pBS2315), hDcp2-CFP (pBS2269) and hDcp2-YFP (pBS2277). As a control, the hSnu30-2 coding sequence from pBS2108 was inserted in the peYFP-C1 vector giving plasmid pBS2293.
For stable transfection, 3 × 106 HEK293 cells were transfected with 1 µg plasmid DNA using the Effectene Transfection Reagent (Qiagen) and were cultured for 2 days without selection agent. Cells were then treated with 400 µg/ml G418 (Invitrogen) and selected for 2–3 months on selective media. Cells were cloned and selected for expression of the fluorescent protein. Transient transfections were performed using the same protocol but using 10 µg of plasmid DNA. For the co-localization experiments, stably transfected cell lines were transiently transfected, either with CFP-hDcp1, CFP-hDcp2 and/or YFP-hSnu30-2, as described above. Next, the preparations were analysed using a confocal microscope (Leica RCS SP2) on an inverted stand using objectives HC Plan APO OS 100X oil NA 1.4.
Immunofluorescence
HEK293 cells were grown in DMEM Glutamax (Invitrogen) and 10% FCS on clean glass slides to 70% confluence. The cells were washed in PBS and fixed for 20 min in 4% _p_-formaldehyde. After several washes, cells were permeabilized in 0.1% Triton X-100 for 10 min. Then, cells were saturated for 30 min with 0.2% BSA/PBS and after several washes cells were immunostained for 1 h at 37°C with a rabbit anti-hDcp1 serum (1:1000). After subsequent washes, cells were incubated 1 h at 37°C with secondary antibody [Fluorolink Cy 5-labelled goat anti-rabbit IgG (Amersham) or goat anti-rabbit IgG FITC-conjugated (Sigma) (1:1000)]. The anti-hDcp1 serum used for immunofluorescence revealed specifically hDcp1 in western blotting analyses (Figure 6A). Nuclei were stained with propidium iodide (5 µg/ml) for 30 min in a dark chamber. Finally preparations were analysed by confocal microscopy as described above.
Supplementary data
Supplementary data are available at The EMBO Journal Online.
Acknowledgments
Acknowledgements
We thank members of the Séraphin laboratory for comments and critical reading of the manuscript. We are indebted to F.Wyers for the yeast Dcp2 expression plasmids and for sharing unpublished information. We acknowledge L.Minvielle-Sebastia, E.Niles, B.Schwer and S.Shuman for gifts of material. The microscopy was performed in the core facility of the CNRS campus supported by the Institut Fédératif de Recherche 87 ‘La plante et son environnement’ with the expert assistance of S.Brown. This work was funded by La Ligue, le Ministère de la Recherche Scientifique (programme PRFMMIP) and an ATIPE from the CNRS (to B.S.) and by the Fonds der Chemischen Industrie (to E.W.).
Note added in proof
While this work was in progress, mRNA decapping by hDcp2 was independently reported by Wang et al. (Proc. Natl Acad. Sci. USA, 2002, 99, 12663–12668) and Lykke-Andersen (Mol. Cell. Biol., 2002, in press).
References
- Bai R.Y., Koester,C., Ouyang,T., Hahn,S.A., Hammerschmidt,M., Peschel,C. and Duyster,J. (2002) SMIF, a Smad4-interacting protein that functions as a co-activator in TGFβ signalling. Nat. Cell Biol., 4, 181–190. [DOI] [PubMed] [Google Scholar]
- Bashkirov V.I., Scherthan,H., Solinger,J.A., Buerstedde,J.M. and Heyer,W.D. (1997) A mouse cytoplasmic exoribonuclease (mXRN1p) with preference for G4 tetraplex substrates. J. Cell Biol., 136, 761–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beelman C.A., Stevens,A., Caponigro,G., LaGrandeur,T.E., Hatfield,L., Fortner,D.M. and Parker,R. (1996) An essential component of the decapping enzyme required for normal rates of mRNA turnover. Nature, 382, 642–646. [DOI] [PubMed] [Google Scholar]
- Bessman M.J., Frick,D.N. and O’Handley,S.F. (1996) The MutT proteins or ‘Nudix’ hydrolases, a family of versatile, widely distributed, ‘housecleaning’ enzymes. J. Biol. Chem., 271, 25059–25062. [DOI] [PubMed] [Google Scholar]
- Bousquet-Antonelli C., Presutti,C. and Tollervey,D. (2000) Identification of a regulated pathway for nuclear pre-mRNA turnover. Cell, 102, 765–775. [DOI] [PubMed] [Google Scholar]
- Bouveret E., Rigaut,G., Shevchenko,A., Wilm,M. and Séraphin,B. (2000) A Sm-like protein complex that participates in mRNA degradation. EMBO J., 19, 1661–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brogna S., Sato,T.A. and Rosbash,M. (2002) Ribosome components are associated with sites of transcription. Mol. Cell, 10, 93–104. [PubMed] [Google Scholar]
- Brown J.T. and Johnson,A.W. (2001) A _cis_-acting element known to block 3′ mRNA degradation enhances expression of poly(A)– mRNA in wild-type yeast cells and phenocopies a ski mutant. RNA, 7, 1566–1577. [PMC free article] [PubMed] [Google Scholar]
- Butler J.S. (2002) The yin and yang of the exosome. Trends Cell Biol., 12, 90–96. [DOI] [PubMed] [Google Scholar]
- Caponigro G. and Parker,R. (1996) Mechanisms and control of mRNA turnover in Saccharomyces cerevisiae. Microbiol. Rev., 60, 233–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C.Y. et al. (2001) AU binding proteins recruit the exosome to degrade ARE-containing mRNAs. Cell, 107, 451–464. [DOI] [PubMed] [Google Scholar]
- Couttet P., Fromont-Racine,M., Steel,D., Pictet,R. and Grange,T. (1997) Messenger RNA deadenylylation precedes decapping in mammalian cells. Proc. Natl Acad. Sci. USA, 94, 5628–5633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czaplinski K., Ruiz-Echevarria,M.J., Gonzalez,C.I. and Peltz,S.W. (1999) Should we kill the messenger? The role of the surveillance complex in translation termination and mRNA turnover. BioEssays, 21, 685–696. [DOI] [PubMed] [Google Scholar]
- Daugeron M.C., Mauxion,F. and Séraphin,B. (2001) The yeast POP2 gene encodes a nuclease involved in mRNA deadenylation. Nucleic Acids Res., 29, 2448–2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunckley T. and Parker,R. (1999) The DCP2 protein is required for mRNA decapping in Saccharomyces cerevisiae and contains a functional MutT motif. EMBO J., 18, 5411–5422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eystathioy T., Chan,E.K., Tenenbaum,S.A., Keene,J.D., Griffith,K. and Fritzler,M.J. (2002) A phosphorylated cytoplasmic autoantigen, GW182, Associates with a unique population of human mRNAs within novel cytoplasmic speckles. Mol. Biol. Cell, 13, 1338–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer N. and Weis,K. (2002) The DEAD box protein Dhh1 stimulates the decapping enzyme Dcp1. EMBO J., 21, 2788–2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frischmeyer P.A., van Hoof,A., O’Donnell,K., Guerrerio,A.L., Parker,R. and Dietz,H.C. (2002) An mRNA surveillance mechanism that eliminates transcripts lacking termination codons. Science, 295, 2258–2261. [DOI] [PubMed] [Google Scholar]
- Gao M., Wilusz,C.J., Peltz,S.W. and Wilusz,J. (2001) A novel mRNA-decapping activity in HeLa cytoplasmic extracts is regulated by AU-rich elements. EMBO J., 20, 1134–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He F. and Jacobson,A. (1995) Identification of a novel component of the nonsense-mediated mRNA decay pathway by use of an interacting protein screen. Genes Dev., 9, 437–454. [DOI] [PubMed] [Google Scholar]
- He F., Peltz,S.W., Donahue,J.L., Rosbash,M. and Jacobson,A. (1993) Stabilization and ribosome association of unspliced pre-mRNAs in a yeast upf1-mutant. Proc. Natl Acad. Sci. USA, 90, 7034–7038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higman M.A., Christen,L.A. and Niles,E.G. (1994) The mRNA (guanine-7-)methyltransferase domain of the vaccinia virus mRNA capping enzyme. Expression in Escherichia coli and structural and kinetic comparison to the intact capping enzyme. J. Biol. Chem., 269, 14974–14981. [PubMed] [Google Scholar]
- Iborra F.J., Jackson,D.A. and Cook,P.R. (2001) Coupled transcription and translation within nuclei of mammalian cells. Science, 293, 1139–1142. [DOI] [PubMed] [Google Scholar]
- Ishigaki Y., Li,X., Serin,G. and Maquat,L.E. (2001) Evidence for a pioneer round of mRNA translation: mRNAs subject to nonsense-mediated decay in mammalian cells are bound by CBP80 and CBP20. Cell, 106, 607–617. [DOI] [PubMed] [Google Scholar]
- LaGrandeur T.E. and Parker,R. (1998) Isolation and characterization of Dcp1p, the yeast mRNA decapping enzyme. EMBO J., 17, 1487–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larimer F.W., Hsu,C.L., Maupin,M.K. and Stevens,A. (1992) Characterization of the XRN1 gene encoding a 5′→3′ exoribonuclease: sequence data and analysis of disparate protein and mRNA levels of gene-disrupted yeast cells. Gene, 120, 51–57. [DOI] [PubMed] [Google Scholar]
- Lennon G., Auffray,C., Polymeropoulos,M. and Soares,M.B. (1996) The I.M.A.G.E. Consortium: an integrated molecular analysis of genomes and their expression. Genomics, 33, 151–152. [DOI] [PubMed] [Google Scholar]
- Mitchell P., Petfalski,E., Shevchenko,A., Mann,M. and Tollervey,D. (1997) The exosome: a conserved eukaryotic RNA processing complex containing multiple 3′→5′ exoribonucleases. Cell, 91, 457–466. [DOI] [PubMed] [Google Scholar]
- Mukherjee D., Gao,M., O’Connor,J.P., Raijmakers,R., Pruijn,G., Lutz,C.S. and Wilusz,J. (2002) The mammalian exosome mediates the efficient degradation of mRNAs that contain AU-rich elements. EMBO J., 21, 165–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Handley S.F., Dunn,C.A. and Bessman,M.J. (2001) Orf135 from Escherichia coli is a Nudix hydrolase specific for CTP, dCTP, and 5-methyl-dCTP. J. Biol. Chem., 276, 5421–5426. [DOI] [PubMed] [Google Scholar]
- Tharun S., He,W., Mayes,A.E., Lennertz,P., Beggs,J.D. and Parker,R. (2000) Yeast Sm-like proteins function in mRNA decapping and decay. Nature, 404, 515–518. [DOI] [PubMed] [Google Scholar]
- Tucker M., Valencia-Sanchez,M.A., Staples,R.R., Chen,J., Denis,C.L. and Parker,R. (2001) The transcription factor associated Ccr4 and Caf1 proteins are components of the major cytoplasmic mRNA deadenylase in Saccharomyces cerevisiae. Cell, 104, 377–386. [DOI] [PubMed] [Google Scholar]
- van Hoof A. and Parker,R. (2002) Messenger RNA degradation: beginning at the end. Curr. Biol., 12, R285–R287. [DOI] [PubMed] [Google Scholar]
- van Hoof A., Frischmeyer,P.A., Dietz,H.C. and Parker,R. (2002) Exosome-mediated recognition and degradation of mRNAs lacking a termination codon. Science, 295, 2262–2264. [DOI] [PubMed] [Google Scholar]
- Vilela C., Velasco,C., Ptushkina,M. and McCarthy,J.E. (2000) The eukaryotic mRNA decapping protein Dcp1 interacts physically and functionally with the eIF4F translation initiation complex. EMBO J., 19, 4372–4382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z. and Kiledjian,M. (2001) Functional link between the mammalian exosome and mRNA decapping. Cell, 107, 751–762. [DOI] [PubMed] [Google Scholar]
- Zhang S., Williams,C.J., Wormington,M., Stevens,A. and Peltz,S.W. (1999) Monitoring mRNA decapping activity. Methods, 17, 46–51. [DOI] [PubMed] [Google Scholar]