Reduced expression of C1q-mRNA in monocytes from patients with systemic lupus erythematosus (original) (raw)
Abstract
Inherited C1q deficiency is associated strongly with the development of systemic lupus erythematosus (SLE). The aim of our study was to evaluate the ability of monocytes from SLE patients without inherited C1q deficiency to up-regulate C1q-mRNA upon stimulation. Furthermore, we wanted to elucidate the physiological stimulus for up-regulation of C1q-mRNA. Peripheral blood mononuclear cell (PBMC)-derived monocytes from 10 SLE patients, 10 patients with rheumatoid arthritis (RA) and 10 healthy controls (HC) were stimulated with dexamethasone (DXM), interferon-gamma or both. Additionally, purified monocytes from HC were stimulated with interleukin (IL)-10. C1q-mRNA expression was measured by quantitative reverse transcription–polymerase chain reaction (RT–PCR). C1q protein was detected using the standard alkaline phosphatase/anti-alkaline phosphatase (APAAP) technique. SLE monocytes were significantly less able to up-regulate C1q-mRNA when compared to RA or HC. IL-10 was identified as an important stimulus for C1q synthesis. In SLE patients there is a significant functional impairment of monocytes to synthesize C1q upon stimulation. As C1q is linked to the process of recognition and removal of apoptotic cells, this relative C1q deficiency is likely to contribute to the reduced phagocytosis of apoptotic material observed in SLE and thereby might be a central pathogenetic factor.
Keywords: apoptosis, C1q, IL-10, lupus, monocytes
Introduction
C1q deficiency is closely linked to the development of systemic lupus erythematosus (SLE) and is the most powerful known susceptibility factor [1]. Genetically determined C1q deficiency affects less than 1% of SLE patients. The mechanism by which missing C1q contributes to the pathogenesis of SLE is not known. However, recent works link C1q to the so-called ‘defective waste disposal hypothesis’ [2].
It is widely accepted that defective or decreased phagocytosis of apoptotic cells is an important mechanism in the development of SLE (for review see Munoz et al. [3]) During the early phase of apoptosis intracellular proteins are translocated to the cell surface, as shown by landmark works by Rosen and coworkers [4]. Under physiological conditions early apoptotic cells are recognized immediately and removed by phagocytes leading to the release of interleukin (IL)-10 and transforming growth factor (TGF)-β [5,6]. If this process is altered, intracellular proteins become exposed to the adaptive immune system and autoimmunity can be triggered. Herrmann and coworkers [7,8] have shown an impaired phagocytosis of apoptotic cells by macrophages and an accumulation of apoptotic cells in the germinal centres of lymph nodes in SLE patients. Furthermore, the number of macrophages containing apoptotic nuclei were reduced in such lymph nodes and apoptotic cells were in close proximity to follicular dendritic cells.
C1q plays an essential role in the interaction of apoptotic cells and phagocytes. As one important soluble bridging molecule (besides many others) in a redundant system of receptors and apoptotic cell surface molecules, C1q targets apoptotic cells to macrophages as well as to dendritic cells [9]. This process leads to non-immunogenic and non-phlogistic removal of apoptotic cells [10]. The impaired clearance of apoptotic cells observed in C1q-deficient mice can be rectified by bone marrow transplantation leading to restoration of C1q levels [11].
C1q is important not only in the removal of early apoptotic material but also in the handling of cell debris. Cellular necrosis, secondary to apoptosis or as primary event, can lead to the release of chromatin. C1q is essential for the effective removal of degraded chromatin by phagocytes and co-operates with DNAse-I in the degradation of chromatin [12].
Finally, complement and especially C1q is essential for the clearance of immune complexes from circulation and from tissue. Failure in this clearing process leads to inflammation and tissue damage [13].
Apoptosis and removal of apoptotic cells occur in different body compartments, e.g. in the dermis and in lymph nodes. C1q from plasma might not be available in microcompartments such as the dermis or the central nervous system (CNS), or its concentration might not be sufficient for opsonization of apoptotic material. In lymph fluid C1q concentration is only about 5% of the plasma concentration [14]. It can therefore be assumed that C1q is provided by local cells ‘on demand’, as is the case in the CNS in response to ischaemia. The source of C1q in the CNS is microglia; in general, the main sources are monocytes, macrophages and immature dendritic cells [15,16].
Our hypothesis was that monocytes from SLE patients are less able to produce C1q upon stimulation when compared to monocytes from healthy people or from patients with rheumatoid arthritis.
To identify possible physiological stimuli for C1q-mRNA up-regulation, additional in vitro experiments were performed with pure monocytes from healthy donors.
Patients
Ten patients with SLE gave informed written consent to donate 65 ml blood. All patients fulfilled the American College of Rheumatology (ACR) criteria for the diagnosis of SLE. The median age was 38·6 years. Only female patients with a positive anti-nuclear antibody (ANA) test and a current prednisolone dose of maximum 10 mg per day were admitted. Ten patients with rheumatoid arthritis (median age 47·9 years) served as the disease control group. They fulfilled ACR criteria for the diagnosis of rheumatoid arthritis and gave informed written consent. Only female patients with positive rheumatoid factor (RF) and a prednisolone dose of maximum 10 mg per day were allowed. Ten healthy female volunteers (median age 38·2 years) served as additional controls. Characteristics of patients and controls are given in Table 1.
Table 1.
Characteristics of patients and controls.
| Pat. no. | Age (years) | Disease duration (years) | ANA titre 1 | Anti-ds-DNA IU/ml | Prednisolone dose/day | Other medication |
|---|---|---|---|---|---|---|
| SLE1 | 30·7 | 0·9 | 5 120 | 58 | 8 | HQ, MFM |
| SLE2 | 65·4 | 11 | 640 | 0 | 10 | MTX |
| SLE3 | 33·3 | 2·5 | 20 480 | 92 | 0 | HQ |
| SLE4 | 22·6 | 4·6 | 5 120 | 125 | 5 | MFM |
| SLE5 | 28·8 | 1·6 | 1 280 | 0 | 5 | AZA |
| SLE6 | 44·7 | 3·9 | 1 280 | 92 | 0 | HQ, AZA |
| SLE7 | 40·4 | 11·5 | 20 480 | 0 | 4 | MTX |
| SLE8 | 54·7 | 9·6 | 20 480 | 23 | 7 | None |
| SLE9 | 36·8 | 14·6 | 5 120 | 73 | 4 | CSA |
| SLE10 | 46·3 | 4·5 | 20 480 | 150 | 0 | HQ, MTX |
| RF IU/ml | Anti-ccp-AK U/ml | |||||
|---|---|---|---|---|---|---|
| RA1 | 22·7 | 2 | 9 | 1 | 5 | MTX |
| RA2 | 44·7 | 1·4 | 0 | 55 | 5 | None |
| RA3 | 52·2 | 20·8 | 45 | 15 | 2·5 | MTX |
| RA4 | 68·1 | 8·7 | 703 | 0 | 0 | LEF |
| RA5 | 26·9 | 16·5 | 48 | 7 | 6 | MTX |
| RA6 | 50·5 | 2 | 159 | 100 | 4 | HQ |
| RA7 | 36·5 | 4·9 | 72 | 100 | 5 | None |
| RA8 | 67·1 | 7·1 | 180 | 7 | 4 | MTX |
| RA9 | 45·3 | 0·7 | 123 | 100 | 10 | MTX |
| RA10 | 60·9 | 0·9 | 73 | 20 | 7·5 | None |
| HC1 | 39·4 | |||||
| HC2 | 24·5 | |||||
| HC3 | 45·4 | |||||
| HC4 | 26·9 | |||||
| HC5 | 32·3 | |||||
| HC6 | 45·4 | |||||
| HC7 | 36·9 | |||||
| HC8 | 34·8 | |||||
| HC9 | 54·4 | |||||
| HC10 | 65 |
Methods
C1q mRNA quantification in SLE, rheumatoid arthritis (RA) and healthy controls (HC)
Peripheral blood mononuclear cells (PBMCs) were isolated from whole blood by density gradient centrifugation (Ficoll-Paque, Amersham Bioscience, Uppsala, Sweden). Vitality was checked using trypan blue staining. Isolated cells were resuspended in RPMI-1640 plus 10% fetal calf serum (FCS) and 1% l-glutamine at a concentration of 1 × 106 cells per ml with and without the addition of dexamethasone (DXM) (10–5 M), interferon (IFN)-γ (100 IU/ml, R&D Systems, Wienbaden, Germany) or both. For several reasons a 24-h cell culture duration was chosen: according to our own experiments C1q-mRNA was sufficiently detectable after 24 h and longer cell culture would have led to differentiation into macrophages which was not wanted. Furthermore, this culture duration is in accordance with published data. Loos et al. were able to detect C1q-mRNA in freshly isolated monocytes using a comparable reverse transcription–polymerase chain reaction (RT–PCR) technology, whereas Northern blotting was found to be less sensitive [17]. After culture at 37°C, 96% humidity and 5% CO2 cells were incubated on ice for 10 min and adhering cells, i.e. monocytes, were detached using a cellscraper (Sarstedt, Nümbrecht, Germany) and washed twice in phosphate-buffered saline (PBS). Monocyte purity was confirmed by fluorescein isothiocyanate (FACS) analysis using a polyclonal antibody to CD14 (M0825, Dako Cytomation, Glostrup, Denmark) and was always above 90%.
Total RNA from the adhering cells was isolated using the QIAamp RNA Blood Mini kit (Qiagen, Hilden, Germany), according to the manufacturer’s protocol. Whole RNA underwent a DNAse digestion for 15 min at 25°C. RNA concentration was measured photometrically. One µg of fresh or −70°C cryoconserved RNA was retrotranscribed to cDNA modified according to Suttorp et al. [18] and cDNA was stored at −20°C. A ‘no template’ and a ‘no RT’ reaction served as additional controls.
The Housekeeping Gene Selection Set (Roche Diagnostics, Mannheim, Germany) was applied and porphobilinogen deaminase (PBGD) was identified using the optimal housekeeping gene as internal standard because it was not regulated under various culture conditions and stimulations.
Amplification was performed as described by the manufacturer.
C1q mRNA expression was analysed using light cycler technology (Roche Diagnostics). Specific primers and light-cycler probes for the C1q a-chain were designed located at exon 1 and exon 2 to amplify C1q mRNA and not contaminating DNA. Primer and probe sequences and locations were as follows: forward primer exon 1: 5′-CCCCAgggATAAAAggAg; reverse primer exon 2: 5′-ggCAgAgAAggCgATT; probe 1: 3FL; exon 2: 5′-AgggCTggCTggAgACC-fluorescein; probe 2: LC red 640; and exon 2: 5′-LC Red640- ggTgAgTTCggAgAgAAggg-phosphate (all TIB Molbiol Laboratory, Berlin, Germany). PCR was performed using 2 µl Mastermix (light-cycler DNA hybridization probes; Roche Diagnostics); 0·5 µM of each primer, 0·2 µM probe 1 and 0·4 µM probe 2 and 4 µM MgCl2 in a final volume of 20 µl. Two µl cDNA was amplified using a 45-cycle three-step amplification protocol after an initial denaturation of 95°C for 10 min (denaturation: 95°C 10 s, annealing: 60°C for 10 s with fluorescence detection, amplification: 72°C for 10 s). The fluorescence intensity of the ratio of the emission of LC Red640 and fluorescein was plotted against the cycle number. Normalized baseline adjustments and the second derivative calculation were used to identify the crossing-point (CP), which is correlated with the initial copy number. The amount of C1q expression was calculated by using light cycler relative quantification software (Roche Diagnostics) using these gene-specific standard curves following the manufacturer’s guidelines. Changes in the expression of C1q in stimulated cells were calculated as a ratio of PBGD normalized to the C1q to a PBGD ratio of the same cell population without stimulation.
C1q staining on cytospins
Cytospins were prepared from several experiments as an additional control using a monoclonal antibody to human C1q (DPC Biermann, Bad Nauheim, Germany). In brief, purified monocytes for indirect immunofluorescence (IIF) staining or whole PBMCs using the standard alkaline phosphatase/anti-alkaline phosphatase (APAAP) method were centrifuged for 10 min at 500 g on cytoslides (Shandon cytoslides, Shandon cytospin) at a concentration of 2 × 105 cells/ml. After 24 h drying cells were fixed and stained.
For APAAP staining, a kit was used according to the manufacturer’s protocol (Dako Cytomation, Glostrup, Denmark) with a monoclonal anti-C1q antibody (DPC Biermann).
Staining for IIF was as follows: fixed cytospins were incubated with an anti-CD14 mouse polyclonal antibody (Dako) and a secondary fluorescence-labelled anti-mouse immunoglobulin (Alexa fluor 486; Invitrogen, Karlsruhe, Germany). For C1q staining a rabbit polyclonal antibody (Dako) and a fluorescence-labelled anti-rabbit immunoglobulin (Alexa fluor 594) was used. Finally the nuclei were counterstained using a commercial kit (Cytological Counterstain kit; Invitrogen) according to the manufacturer’s protocol. Washing steps with PBS were interposed as appropriate. Analysis was performed using a fluorescence microscope.
IL-10 stimulation
Pure monocytes were isolated from PBMCs using counterflow centrifugation (Beckman Instruments Inc., Fullerton, CA, USA). Cells were cultured as described above under various conditions, including stimulation with DXM (10–5 M; Sigma, Munich, Germany) added to different concentrations of IL-10 (0·1–100 ng/ml, R&D Systems). After 24 h cells were harvested and processed as described above. After mRNA isolation C1q Real-time PCR was performed.
Monocytes from three SLE patients were isolated as described above and stimulated with DXM and IL-10 (50 ng/ml) for 24 h. mRNA isolation and RT–PCR were performed as described.
Statistics
Statistical analysis was performed using the GraphPad Prism 3·02 software. The non-parametric Mann–Whitney, Wilcoxon’s matched pairs and Spearman’s rank order tests were used as appropriate. Significance was defined as P < 0·05 in a two-sided analysis.
Results
C1q mRNA expression in monocytes from SLE, RA and HC
The baseline C1q-mRNA expression in non-stimulated cells was not significantly different between RA, HC and SLE. The mean CPs ± s.d. were 31 ± 1·54 in HC, 30·1 ± 1·39 in RA and 29·8 ± 1·02 in SLE.
In all three populations studied, stimulation with DXM and IFN-γ led to a significant up-regulation of C1q-mRNA expression when compared to non-stimulated cells. An over-additive effect was seen with both stimuli in combination.
In HC, DXM led to a 24·5-fold, IFN-γ to a 26·1-fold and the combination of both to a 159-fold up-regulation of C1q-mRNA. The difference between IFN-γ- and DXM-stimulated cells was not significant (P = 0·48), while the difference between cells stimulated with the combination and those stimulated with the single agents was highly significant (P = 0·002).
The results in RA were very similar to HC, with a 20·7-fold C1q-mRNA up-regulation with DXM stimulation, 22·5-fold with IFN-γ stimulation and 156·2-fold under stimulation with both. There was no difference between C1q-mRNA expression in cells stimulated with the single agents (P = 1), while both single agents were significantly less potent than the combination (P = 0·002) to stimulate C1q-mRNA expression.
In cells from SLE patients, the single agents as well as the combination resulted in a much weaker up-regulation of C1q-mRNA expression: 9·7-fold with DXM, 11·7-fold with IFN-γ and 45·3-fold with both. Again, the difference between DXM- and IFN-γ-stimulated cells was not significant (P = 0·65), whereas the difference between stimulation with a single agent versus the combination of both was highly significant (P = 0·004).
The increase in C1q mRNA expression was not significantly different between HC and RA with all three stimulation conditions. The lower C1q-mRNA expression in SLE was highly significant when compared to HC or RA for all three stimulations. The results are shown in Fig. 1.
Fig. 1.
C1q-mRNA expression in unstimulated cells and under various stimulations. The figure shows the C1q mRNA expression in monocytes from healthy controls (HC), systemic lupus erythematosus (SLE) patients and patients with rheumatoid arthritis (RA) as determined by reverse transcription–polymerase chain reaction (RT–PCR). (a) CP values obtained in unstimulated cells are depicted. There was no significant difference between the three groups. (b) Results from interferon (IFN)-γ-stimulated cells; (c) from dexamethasone (DXM)-stimulated cells; and (d) from cells stimulated with IFN-γ and DXM depicted as x-fold C1q-mRNA increase compared to non-stimulated monocytes. The error bars represent the standard deviation. With all stimulations, there was no significant difference between monocytes from HC and RA and there was a highly significantly reduced C1q-mRNA expression in monocytes from SLE patients with an over-additive effect using both stimuli.
C1q protein expression
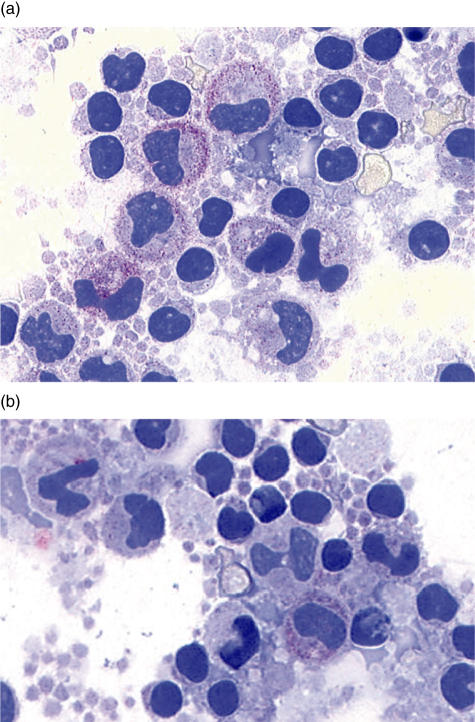
From several experiments cytospins were prepared and stained for C1q-protein expression using either a standard APAAP method on whole PBMCs or with IIF in purified monocytes. Although a quantitative analysis was not performed, a more intensive staining after stimulation was observed in monocytes from HC and RA patients than in those from SLE patients. A representative example is given in Fig. 2.
Fig. 2.
Alkaline phosphatase/anti-alkaline phosphatase (APAAP) staining for C1q protein. The figure shows cytospins from cultured whole peripheral blood mononuclear cells (PBMCs) after culture stained for C1q protein expression. The cells in (a) are from a healthy control; and (b) from a systemic lupus erythematosus (SLE) patient. A less intense staining was observed in cells from SLE patients.
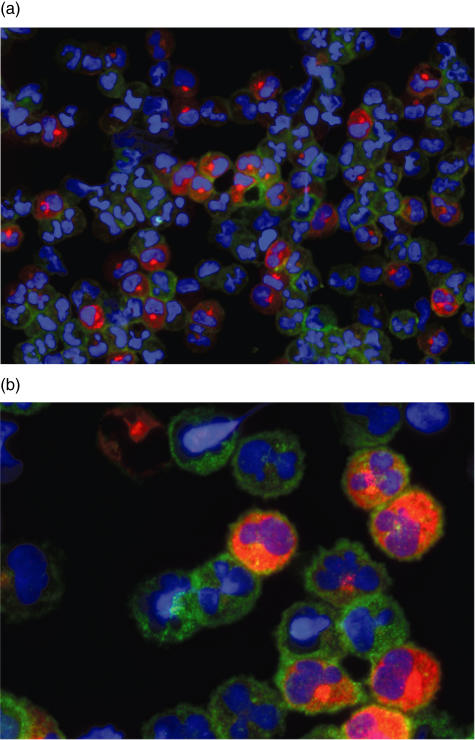
In particular, the IIF staining demonstrated an inhomogeneous C1q expression with only approximately one-third of the cells staining positive for C1q. An example is given in Fig. 3.
Fig. 3.
Indirect immunofluorescence (IIF) staining for C1q protein and CD14. The cytospin shown was prepared from isolated monocytes from a HC stimulated with interferon (IFN)-γ and dexamethasone (DXM) and stained for C1q protein, CD14 and the nuclei. (a) Overview; (b) higher magnification. Whereas nearly all monocytes stained positive for CD14, approximately only one-third stained for C1q.
Monocyte stimulation with IL-10
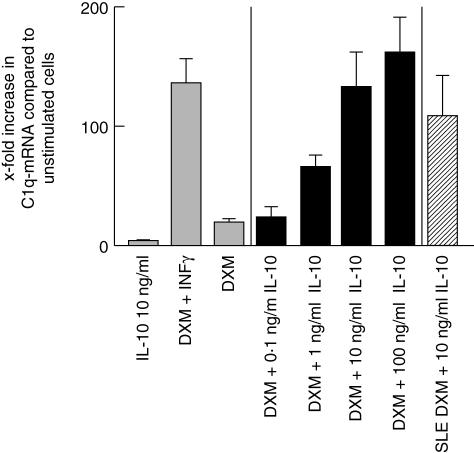
In monocytes from healthy donors, DXM stimulation (10–5 M) led to a 20-fold increase in C1q-mRNA expression. IL-10 (10 ng/ml) stimulation increased C1q-mRNA only fivefold. IL-10 added to a basal stimulation with DXM (10–5 M) led to over-additive and dose-dependent stimulation of C1q-mRNA expression, with a maximum 162-fold increase with 100 ng/ml IL-10. Under these experimental conditions the combination of DXM and IFN-γ increased C1q-mRNA 152 times, which is similar to the results obtained in monocytes from PBMCs.
Data obtained from experiments with monocytes from three SLE patients show that DXM plus IL-10 was the most potent C1q-mRNA-inducing stimulus, leading to a C1q-mRNA expression almost reaching that seen in monocytes from HC. However, the later results are preliminary and for methodological reasons statistics were not applied. The results are shown in Fig. 4.
Fig. 4.
C1q-mRNA expression upon interleukin (IL)-10 stimulation. The graph shows C1q-mRNA expression in monocytes from healthy controls (HC) under various stimuli including different concentrations of IL-10 and from three systemic lupus erythematosus (SLE) patients after stimulation with dexamethasone (DXM) and IL-10 depicted as x-fold increase compared to non-stimulated cells. The error bars represent the standard deviation. Whereas IL-10 alone did not induce C1q-mRNA expression, the combination with DXM led to an exponentiated increase in C1q-mRNA. With 100 ng/ml IL-10 a maximum stimulation was observed, which exceeded that seen with DXM and interferon (IFN)-γ stimulation. In monocytes from SLE patients, IL-10 addition to DXM led to a C1q-mRNA up-regulation, nearly reaching the level observed with cells from HC. It was approximately twice as strong as in SLE monocytes with DXM and IFN-γ.
Discussion
We hypothesized that monocytes from SLE patients might be less able to produce C1q ‘on demand’. IFN-γ and DXM are well-known stimuli for C1q expression with over-additive effects [19]. We used this stimulation to produce a maximal C1q induction in PBMC-derived monocytes in vitro. Monocytes from SLE patients were less able to up-regulateC1q-mRNA, probably resulting in a lower C1q protein expression.
According to the ‘defective waste disposal hypothesis’ a reduced ability to remove apoptotic cells in a physiological, i.e. non-inflammatory way is regarded a major contribution to the development of SLE. Such a defect has been demonstrated impressively by Herrman and colleges in lymph nodes from SLE patients [7,8]. There is an ongoing debate about the nature of the reduced phagocytic capacity: whereas some authors assume a general defect of phagocytosis (for a review see Pablos and Gomez-Reino [20]), other and more recent works did not show such a global deficiency but suggest reduced serum complement levels as causal [21,22]. In this context C1q plays a major role as an opsonizing and bridging molecule between apoptotic cell surfaces and phagocytes. In a knock-out mouse model, C1q deficiency leads to a severe phagocyte dysfunction which can be rectified by bone marrow transplantation, i.e. by restoration of C1q synthesis [11]. Furthermore, in several C1q knock-out mice the development of autoimmune phenomena resembling SLE and an increase in unremoved apoptotic bodies has been demonstrated [23]. In humans inherited C1q deficiency is associated strongly with the development of SLE [1].
Our data show that in SLE patients without genetically determined C1q deficiency monocytes are invariably not sufficiently able to increase C1q production upon stimulation. Therefore it is reasonable to assume a relative C1q deficiency at sites of increased need, especially in lymph nodes. This might contribute to the lack of phagocytic efficacy and the redirection of apoptotic material to dendritic cells.
Whereas there is little doubt that a lack of C1q production plays a major role in the pathogenesis of SLE, the reason for the impairment observed here is not known.
Possible explanations are a reduced general synthetic capacity of SLE monocytes, a specific defect in C1q-mRNA synthesis or a decreased cell number in a C1q-producing monocyte subset.
Several works demonstrated impaired monocyte functions in SLE, possibly arguing in favour of a reduced overall synthetic capacity. However, while the synthesis of some cytokines, mediators and surface molecules such as IL-1 [24], prostaglandins [25] and CD14 [26] is decreased, the production of others factors such as IL-10 [27] and complement C3 [28] is increased. For further monocyte functions such as phagocytosis or TNF-α production, the cumulative data from the literature are poor and partially contradictive [29,30]. Therefore, a general defect or weakness of synthesis in SLE monocytes cannot be concluded.
Our IIF results suggest that C1q is produced mainly by a specialized subset of monocytes rather than by monocytes in general. It is conceivable that the C1q-mRNA quantity is not a result of reduced synthesis in single monocytes but of the reduction of a specialized cell subset. To date, however, this is still speculative and needs further investigation.
Finally, we wanted to address the question of what the physiological stimulus of C1q-mRNA production might be. DXM as used in this work is not a physiological agent, although it is known that glucocorticoids are able to increase non-inflammatory phagocytosis of apoptotic cells by an as yet unknown mechanism [31]. Increased C1q synthesis might offer an explanation for this observation.
IFN-γ could be one of the physiological stimuli. While the role of IFN-γ in lupus pathogenesis is not totally clear, knock-out mice models demonstrated a significant contribution of IFN-γ to the development of lupus nephritis [32]. In vitro experiments demonstrated that IFN-γ might promote polyclonal B cell activation [33]. However, there is evidence that its production is reduced in PBMCs from SLE patients [34]. As IFN-γ promotes C1q synthesis [34], it may be supposed that the decreased IFN-γ synthesis in SLE contributes directly to insufficient C1q production.
Because C1q is needed to remove apoptotic material, we were interested to determine whether or not apoptotic cells might themselves be a signal or might release a signal to up-regulate C1q production. In cell culture experiments, apoptotic neutrophils were not able to induce monocyte C1q-mRNA and the same expression levels as in non-stimulated control monocytes were observed. Furthermore, phagocyte ‘attraction signals’ known to be released from dying cells as lysophosphatidylcholine [35] and uric acid [36] did not stimulate C1q-mRNA synthesis; again, the same expression levels as in non-stimulated control monocytes were seen.
IL-10, together with TGF-β, is released from phagocytes after ingestion of complement opsonized apoptotic cells and is known to have a role in SLE pathogenesis. Furthermore, IL-10 production by monocytes is induced by apoptotic neutrophils in the presence of lipopolysaccharide (LPS) [5] and monocytes undergoing spontaneous apoptosis up-regulate IL-10 production upon stimulation [37]. We therefore tested the ability of IL-10 to induce C1q-synthesis and found an over-additive effect with DXM. Thus, arguably, the release of IL-10 by phagocytes can be regarded as a positive feedback mechanism to provide further C1q and thereby to enhance the capacity for non-inflammatory phagocytosis of apoptotic material.
In summary, our interpretation of the presented results is that in SLE there is a specific defect in C1q production upon stimulation, which can explain the reduced phagocytosis of apoptotic cells. As IL-10 up-regulates C1q-mRNA, the known increase in IL-10 production by monocytes and elevated IL-10 serum levels in SLE can be interpreted as a (insufficient) counter regulatory measure to overcome decreased C1q synthesis. Preliminary data suggest that the IL-10 induced C1q-mRNA expression might not be altered in SLE. These results, however, have to be interpreted very cautiously and further experiments are needed.
Besides the well-known anti-inflammatory actions of corticosteroids, the over-additive stimulation of C1q production is a possible specific, yet unrecognized, mechanism explaining the clinical efficacy of continuous low-dose steroid therapy in preventing exacerbations of the disease.
Future work should address the precise cause of the decreased C1q synthesis in SLE and seek to find more specific means than corticosteroids to rectify this defect.
References
- 1.Manderson AP, Botto M, Walport MJ. The role of complement in the development of systemic lupus erythematosus. Ann Rev Immunol. 2004;22:431–56. doi: 10.1146/annurev.immunol.22.012703.104549. [DOI] [PubMed] [Google Scholar]
- 2.Walport MJ. Complement. Second of two parts. N Engl J Med. 2001;344:1140–4. doi: 10.1056/NEJM200104123441506. [DOI] [PubMed] [Google Scholar]
- 3.Munoz LE, Gaipl US, Franz S, et al. SLE − a disease of clearance deficiency? Rheumatology (Oxford) 2005;44:1101–7. doi: 10.1093/rheumatology/keh693. [DOI] [PubMed] [Google Scholar]
- 4.Casciola-Rosen LA, Anhalt G, Rosen A. Autoantigens targeted in systemic lupus erythematosus are clustered in two populations of surface structures on apoptotic keratinocytes. J Exp Med. 1994;179:1317–30. doi: 10.1084/jem.179.4.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Byrne A, Reen DJ. Lipopolysaccharide induces rapid production of IL-10 by monocytes in the presence of apoptotic neutrophils. J Immunol. 2002;168:1968–77. doi: 10.4049/jimmunol.168.4.1968. [DOI] [PubMed] [Google Scholar]
- 6.Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM. Macrophages that have ingested apoptotic cells in vitro inhibit pro-inflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J Clin Invest. 1998;101:890–8. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baumann I, Kolowos W, Voll RE, et al. Impaired uptake of apoptotic cells into tingible body macrophages in germinal centers of patients with systemic lupus erythematosus. Arthritis Rheum. 2002;46:191–201. doi: 10.1002/1529-0131(200201)46:1<191::AID-ART10027>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 8.Herrmann M, Voll RE, Zoller OM, Hagenhofer M, Ponner BB, Kalden JR. Impaired phagocytosis of apoptotic cell material by monocyte-derived macrophages from patients with systemic lupus erythematosus. Arthritis Rheum. 1998;41:1241–50. doi: 10.1002/1529-0131(199807)41:7<1241::AID-ART15>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 9.Nauta AJ, Castellano G, Xu W, et al. Opsonization with C1q and mannose-binding lectin targets apoptotic cells to dendritic cells. J Immunol. 2004;173:3044–50. doi: 10.4049/jimmunol.173.5.3044. [DOI] [PubMed] [Google Scholar]
- 10.Fadok VA, Bratton DL, Henson PM. Phagocyte receptors for apoptotic cells. recognition, uptake, and consequences. J Clin Invest. 2001;108:957–62. doi: 10.1172/JCI14122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cortes-Hernandez J, Fossati-Jimack L, Petry F, et al. Restoration of C1q levels by bone marrow transplantation attenuates autoimmune disease associated with C1q deficiency in mice. Eur J Immunol. 2004;34:3713–22. doi: 10.1002/eji.200425616. [DOI] [PubMed] [Google Scholar]
- 12.Gaipl US, Beyer TD, Heyder P, et al. Cooperation between C1q and DNase I in the clearance of necrotic cell-derived chromatin. Arthritis Rheum. 2004;50:640–9. doi: 10.1002/art.20034. [DOI] [PubMed] [Google Scholar]
- 13.Stokol T, O’Donnell P, Xiao L, et al. C1q governs deposition of circulating immune complexes and leukocyte Fc-gamma receptors mediate subsequent neutrophil recruitment. J Exp Med. 2004;200:835–46. doi: 10.1084/jem.20040501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Olszewski WL, Engeset A. Haemolytic complement in peripheral lymph of normal men. Clin Exp Immunol. 1978;32:392–8. [PMC free article] [PubMed] [Google Scholar]
- 15.Schafer MK, Schwaeble WJ, Post C, et al. Complement C1q is dramatically up-regulated in brain microglia in response to transient global cerebral ischemia. J Immunol. 2000;164:5446–52. doi: 10.4049/jimmunol.164.10.5446. [DOI] [PubMed] [Google Scholar]
- 16.Castellano G, Woltman AM, Nauta AJ, et al. Maturation of dendritic cells abrogates C1q production in vivo and in vitro. Blood. 2004;103:3813–20. doi: 10.1182/blood-2003-09-3046. [DOI] [PubMed] [Google Scholar]
- 17.Kaul M, Loos M. Expression of membrane C1q in human monocyte-derived macrophages is developmentally regulated and enhanced by interferon-gamma. FEBS Lett. 2001;500:91–8. doi: 10.1016/s0014-5793(01)02592-3. [DOI] [PubMed] [Google Scholar]
- 18.Suttorp M, Ritgen M, von Neuhoff N, Schoch R, Schmitz N. Blood on filter paper as a readily available source of bcr-abl rearranged mRNA. Blood. 1997;90:1713–5. [PubMed] [Google Scholar]
- 19.Walker DG. Expression and regulation of complement C1q by human THP-1-derived macrophages. Mol Chem Neuropathol. 1998;34:197–218. doi: 10.1007/BF02815080. [DOI] [PubMed] [Google Scholar]
- 20.Pablos JL, Gomez-Reino JJ. Defective phagocytosis in systemic lupus erythematosus. Eur J Clin Invest. 1998;28:864–5. doi: 10.1046/j.1365-2362.1998.00261.x. [DOI] [PubMed] [Google Scholar]
- 21.Bijl M, Reefman E, Horst G, Limburg PC, Kallenberg CG. Reduced uptake of apoptotic cells by macrophages in systemic lupus erythematosus (SLE): correlates with decreased serum levels of complement. Ann Rheum Dis. 2006;65:57–63. doi: 10.1136/ard.2005.035733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moosig F, Graf D, Knorr-Spahr A, Zeuner RA, Böttcher S, Schröder JO. Monocyte and granulocyte phagocytosis of Escherichia coli particles in systemic lupus erythematosus is not reduced when compared to healthy controls. Lupus. 2003;12:490–2. doi: 10.1191/0961203303lu415xx. [DOI] [PubMed] [Google Scholar]
- 23.Botto M, Dell’Agnola C, Bygrave AE, et al. Homozygous C1q deficiency causes glomerulonephritis associated with multiple apoptotic bodies. Nat Genet. 1998;19:56–9. doi: 10.1038/ng0598-56. [DOI] [PubMed] [Google Scholar]
- 24.Sierakowski S, Kucharz EJ, Lightfoot RW, Jr, Goodwin JS. Interleukin-1-production by monocytes from patients with systemic lupus erythematosus. Clin Rheumatol. 1987;6:403–7. doi: 10.1007/BF02206840. [DOI] [PubMed] [Google Scholar]
- 25.Cruchaud A, Despont JP, Roth A, Dayer JM. Phagocytosis, bactericidal capacity, and PGE2 production of monocytes in systemic lupus erythematosus and rheumatoid arthritis. Diagn Immunol. 1984;2:203–12. [PubMed] [Google Scholar]
- 26.Steinbach F, Henke F, Krause B, Thiele B, Burmester GR, Hiepe F. Monocytes from systemic lupus erythematous patients are severely altered in phenotype and lineage flexibility. Ann Rheum Dis. 2000;59:283–8. doi: 10.1136/ard.59.4.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu TF, Jones BM. Impaired production of IL-12 in systemic lupus erythematosus. I. Excessive production of IL-10 suppresses production of IL-12 by monocytes. Cytokine. 1998;10:140–7. doi: 10.1006/cyto.1997.0268. [DOI] [PubMed] [Google Scholar]
- 28.Tsukamoto H, Ueda A, Nagasawa K, Tada Y, Niho Y. Increased production of the third component of complement (C3) by monocytes from patients with systemic lupus erythematosus. Clin Exp Immunol. 1990;82:257–61. doi: 10.1111/j.1365-2249.1990.tb05436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Horwitz DA, Gray JD, Behrendsen SC, et al. Decreased production of interleukin-12 and other Th1-type cytokines in patients with recent-onset systemic lupus erythematosus. Arthritis Rheum. 1998;41:838–44. doi: 10.1002/1529-0131(199805)41:5<838::AID-ART10>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 30.Kyttaris VC, Juang YT, Tsokos GC. Immune cells and cytokines in systemic lupus erythematosus: an update. Curr Opin Rheumatol. 2005;17:518–22. doi: 10.1097/01.bor.0000170479.01451.ab. [DOI] [PubMed] [Google Scholar]
- 31.Liu Y, Cousin JM, Hughes J, et al. Glucocorticoids promote nonphlogistic phagocytosis of apoptotic leukocytes. J Immunol. 1999;162:3639–46. [PubMed] [Google Scholar]
- 32.Haas C, Ryffel B, Le Hir M. IFN-gamma receptor deletion prevents autoantibody production and glomerulonephritis in lupus-prone (NZB × NZW) F1 mice. J Immunol. 1998;160:3713–8. [PubMed] [Google Scholar]
- 33.Funauchi M, Sugishima H, Minoda M, Horiuchi A. Effect of IFN-gamma on B lymphocytes of patients with systemic lupus erythematosus. J Rheumatol. 1991;18:368–72. [PubMed] [Google Scholar]
- 34.Hagiwara E, Gourley MF, Lee S, Klinman DK. Disease severity in patients with systemic lupus erythematosus correlates with an increased ratio of interleukin-10: IFN-gamma-secreting cells in the peripheral blood. Arthritis Rheum. 1996;39:379–85. doi: 10.1002/art.1780390305. [DOI] [PubMed] [Google Scholar]
- 35.Lauber K, Bohn E, Krober SM, et al. Apoptotic cells induce migration of phagocytes via caspase-3-mediated release of a lipid attraction signal. Cell. 2003;113:717–30. doi: 10.1016/s0092-8674(03)00422-7. [DOI] [PubMed] [Google Scholar]
- 36.Shi Y, Evans JE, Rock KL. Molecular identification of a danger signal that alerts the immune system to dying cells. Nature. 2003;425:516–21. doi: 10.1038/nature01991. [DOI] [PubMed] [Google Scholar]
- 37.Bzowska M, Guzik K, Barczyk K, Ernst M, Flad HD, Pryjma J. Increased IL-10 production during spontaneous apoptosis of monocytes. Eur J Immunol. 2002;32:2011–20. doi: 10.1002/1521-4141(200207)32:7<2011::AID-IMMU2011>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]