Unique morphological features of the proliferative zones and postmitotic compartments of the neural epithelium giving rise to striate and extrastriate cortex in the monkey (original) (raw)
. Author manuscript; available in PMC: 2007 Jul 26.
Published in final edited form as: Cereb Cortex. 2002 Jan;12(1):37–53. doi: 10.1093/cercor/12.1.37
Abstract
We examined the development of the occipital lobe in fetal monkeys between embryonic day 37 (E37) and E108 in Nissl-stained and acetylcholine esterase (AChE)-reacted sections. We paid particular attention to features that distinguish the development of presumptive area 17. At E46 the neuroepithelium consists of a ventricular zone and a monolayer cortical plate sandwiched between a thin marginal zone and a minimal presubplate. Between E55 and E65 an augmented subplate emerges and continues to expand up to E94 to become a major compartment of the developing cortex. A mitotic subventricular zone is established by E55. Peaking in depth at E72, it constitutes the principal germinal zone. By E78 an invading fibre tract divides it into an outer radially organized zone and a more conventional inner zone. AChE staining reveals the future area 17/18 border from E86 onwards. Proceeding from presumptive area 17 to area 18 there is a progressive thinning of the radially structured subventricular zone. Comparison of these results with corticogenesis in rodents suggests a number of potentially unique primate features: (i) a minimal preplate stage; (ii) a radially augmented germinal zone not previously described in non-primates; (iii) a fibre tract dividing the subventricular zone into two laminae; (iv) late generation and expansion of the subplate.
Keywords: Acetylcholinesterase, metabolism, Animals, Epithelial Cells, enzymology, physiology, Epithelium, embryology, enzymology, Female, Immunohistochemistry, Macaca fascicularis, Mice, Mitosis, physiology, Neurons, physiology, Neuropeptide Y, metabolism, Occipital Lobe, anatomy & histology, embryology, enzymology, Pregnancy, Telencephalon, cytology, embryology, Visual Cortex, anatomy & histology, embryology, enzymology
Introduction
Much of our understanding of the processes of proliferation, migration and differentiation underlying cortical development has been obtained in rodents (Angevine and Sidman, 1961; Berry and Rogers, 1965; Smart and Smart, 1982; Bayer and Altman, 1991; Caviness and Takahashi, 1995). Theories of the peripheral control of the development of functional areas have largely emerged from work on the rodent barrel field (Van der Loos and Woolsey, 1973; Killackey et al., 1976, 1994; O’Leary, 1989; O’Leary and Stanfield, 1989). More recently, work on rodent transgenics has furthered our understanding on the intrinsic constraints of cortical development (Barbe and Levitt, 1991; Arimatsu et al., 1992; Cohen-Tannoudji et al., 1994; Rakic and Caviness, 1995; Miyashita-Lin et al., 1999; Bishop et al., 2000).
In contrast to rodent, primate cortical development has been relatively little studied, although the histogenesis and specification of cortical areas in this order have been shown to share key features with development in the rodent, including peripheral control of arealization, molecular evidence of early specification of functional domains and regionalization of the proliferative activity of the germinal zone (Rakic, 1988; Dehay et al., 1989, 1993; Rakic et al., 1991; Donoghue and Rakic, 1999). However, the developing primate cortex appears to be more than a scaled-up version of the developing rodent cortex. For example, a recent study on early human development has shown that a complex succession of transient neuronal populations appear during the earliest stages of the cortical plate that have not been described during rodent development (Meyer et al., 2000). A second example is provided by the subplate, which in primates is considerably deeper and more conspicuous than in rodents, but more importantly appears at much later stages of corticogenesis (Kostovic and Rakic, 1990; Meinecke and Rakic, 1992).
There are several advantages in studying cortical development in monkey as opposed to rodents. Firstly, the 60 day period of neuron production in monkey is 10 times longer than in mouse or rat (Rakic, 1972, 1974). The long period of primate development provides a high temporal resolution, making it possible to obtain a fine-grained picture of the events occurring during neuron production and migration. Secondly, the large surface area and the morphologically well-defined areal boundaries allow a high spatial resolution. Because the areal borders of area 17 are sharply defined at early stages, the occipital lobe in the monkey provides an excellent model for the study of the formation of areal boundaries and the developmental control of areal dimensions (Rakic, 1988; Dehay et al., 1989, 1996a, b; Rakic et al., 1991; Kennedy and Dehay, 1993) as well as investigating the role of proliferation in the specification of cortical areas (Dehay et al., 1993). Thirdly, post-mortem examination of the embryonic human brain shows that there are developmental features which are quantitatively and qualitatively different from those reported in non-primates (Sidman and Rakic, 1973; Kostovic and Rakic, 1984, 1990; Meyer et al., 2000). Studying the development of the monkey cortex introduces the possibility of implementing an experimental investigation of these unique primate features.
The cell-cycle kinetics of cortical precursors of areas 17 and 18 of the monkey have been shown to differ significantly (Dehay et al., 1993). A primary aim of the present study was to investigate whether these neurogenic differences are underpinned by morphological differences in the germinal zone. Because there is a spatial gradient in the rate of neuron production going from high values in area 17 to lower values in area 18 (Kennedy and Dehay, 1993), it is important to examine the morphology of the germinal zone in the rostral caudal axis going from presumptive area 17 to presumptive area 18. To address this issue we have focused on the in utero histogenesis of the monkey cortex and have given a special focus to the occipital pole. By using sagittal sections of the brain we are able to directly observe the 17/18 transition and its relation to the rostrocaudal gradient of histogenesis. In coronal sections this would not be possible because the radial structure of the transition zone is obscured due to a plane of section which cuts obliquely through the curving surface of the occipital pole of the hemisphere.
Although we have tried to retain the basic terminology of the Boulder Committee (Boulder Committee, 1970), this terminology is too limited to describe the sequence of changes that emerge during primate neocortical histogenesis, and so for the early stages of this process we have keyed our descriptions to the terminology used for the very early sequences of the human by Meyer et al. (Meyer et al., 2000), and for the later stages that of Kostovic and Rakic (Kostovic and Rakic, 1990), developed for the human and monkey. Throughout the text we provide explanations on how our terminology correlates with that of these authors.
Materials and Methods
Anesthesia and Surgery
Experiments were carried out on cynomolgus monkeys (Macaca fascicularis). Following premedication with atropine (1.25 mg, i.m.) and dexamethasone (4 mg, i.m.), pregnant monkeys were prepared for surgery under ketamine hydrochloride (20 mg/kg, i.m.), chlorpromazine (2 mg/kg, i.m.) and isoxsuprine (2.5 mg i.m). After intubation, anaesthesia was continued with halothane in a N2O/O2 (70/30) mixture. The heart rate was monitored and artificial respiration was adjusted to maintain the end-tidal CO2 at 4.5–6%. The rectal temperature was monitored and maintained at 37°C. A midline abdominal incision allowed uterotomy to be performed. The pregnant monkey received post-operative medication consisting of a muscular relaxant (isoxsuprine chlorydrate) and an analgesic (tiemonium methylsulfate). All the procedures used follow the national and European regulations concerning animal experiments and have been approved by the authorized national and veterinary agencies.
Histological Processing
Fetuses were deeply anaesthetized before being perfused transcardially with 200 ml of 2.7% saline, 1–3 I of 8% paraformaldehyde/0.5% glutaraldehyde mixture in phosphate buffer (0.1 M, pH 7.4), 0.5 I of 10% sucrose, 0.5 I of 20% sucrose, and 1 I of 30% sucrose in phosphate buffer (0.1 M, pH 7.4). After removal of the brain, some of the later fetuses (post E70) were cut on a freezing microtome (section thickness 40 mm). One section in three was mounted in saline onto gelatinized slides. Sections at regular intervals were reacted for acetylcholinesterase activity (Hardy et al., 1976; Mesulam and Geula, 1994).
NPY immunoreactivity was revealed according to the following three-step immunostaining procedure: (i) paraformaldehyde-fixed sections were incubated in primary antibody (NPY from Peninsula Laboratories IHC 7172), 1/40 at 4°C overnight; (ii) biotinylated goat anti-rabbit (DAKO; E432) (1:400 in TBS) for 1 h at RT; (iii) peroxidase-conjugated streptavidine (DAKO, PO 397) 1:500 in TBS, 1 h at RT. Peroxidase activity was revealed by incubating the sections in DAB (Zymed kit) for 5 min and then adding 3% H2O2 for 10 min.
For the younger fetuses and in brains where we wanted to optimize the quality of the histological material, fixation was carried out in bouin, the brains embedded in paraffin wax and 10 μm thick sections cut on a microtome. Sections were counterstained for Nissl substance.
Results
We begin by describing the low-power view of the gross histological changes occurring at the occipital pole of the cerebral hemisphere (Fig. 1). We then proceed to describe in more detail the histological changes observed in transects from the extreme pole of the hemisphere through the region corresponding to the location of future area 17 (Fig. 2). Finally we provide medium-power microphotographs and interpretative drawings which serve to identify major features of the transition from parietal to occipital lobes (Figs 3 and 4).
Figure 1.
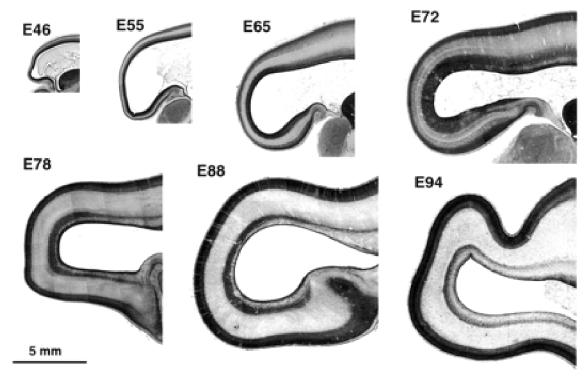
Parasagittal sections of the occipital lobe at different stages of development between E46 and E94. Note the increase in depth of the germinal zone at the occipital pole of the hemisphere between E65 and E72. Note also the subdivision of the germinal zone between E72 and E78 into an inner and outer part by an intruding fibre tract.
Figure 2.
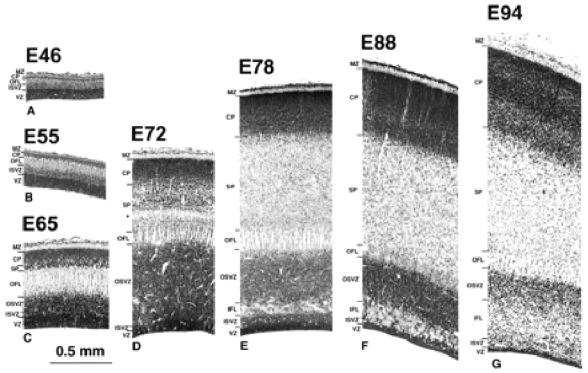
Transects of presumptive area 17 taken from the brains shown in Figure 1. An early appearing outer fibre layer (OFL) with a distinctly pallisaded appearance forms a major landmark throughout the period of development. The ventricular zone (VZ) declines progressively after E65. The subventricular zone, by contrast, increases progressively in depth and by E72 is divided into an inner subventricular zone (ISVZ) and outer subventricular zone (OSVZ) by an intruding inner fibre layer (IFL). The increase in the OSVZ is particularly important between E65 and E72 and occurs as the VZ declines. The cortical plate (CP), or its ‘pioneer plate precursor’ (Meyer et al., 2000), appears as early as E46. It increases progressively in depth and shows little change in packing density of its cells until after E88. Little in the way of the ‘presubplate’ of Kostovic and Rakic is evident in this type of preparation (Kostovic and Rakic, 1990). The subplate proper (SP), however, is evident after E55. Subsequently it increases in depth in two stages: between E65 and E72 the increase is densely cellular in character, and between E72 and E78 the increase appears to be derived mainly from a decrease in the packing density of the component cells. The marginal zone (MZ) is minimal before E65. A curiously conspicuous ‘clear layer’, marked by an asterisk, located in the deep SP, is transiently present at E72. It may be a new feature or an elaboration of the original ‘presubplate’. At later stages it appears to merge into the SP.
Figure 3.
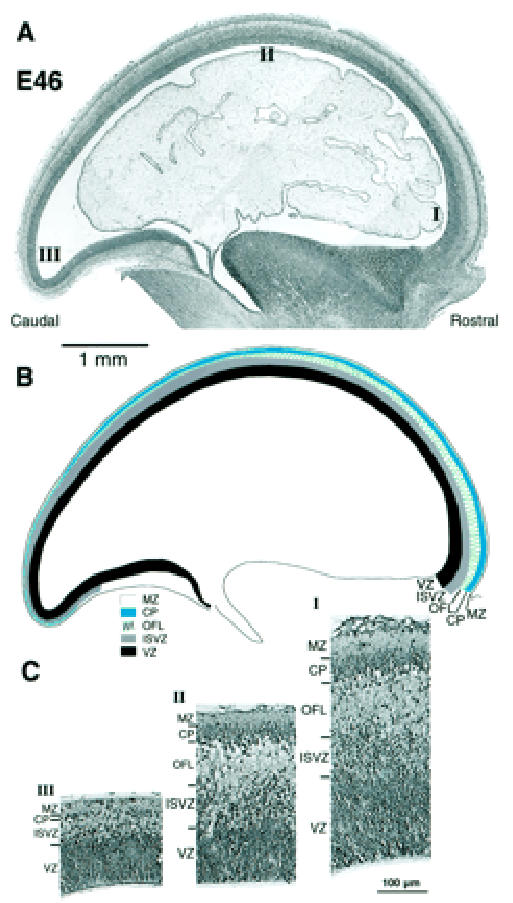
Rostrocaudal gradient of cortical development at E46. (A) Photomicrograph of a parasagittal section of an E46 Nissl-stained section. (B) Drawing of above identifying various layers. (C) High-power views of transects taken through levels I (rostral), II (dorsal) and III (caudal) in (A). Note the rostrocaudal gradient of decreasing depth of all layers except the VZ. At the caudal pole they are of minimal depth and, compared to more rostral areas, ill-defined. Abbreviations as in Figure 2.
Figure 4.
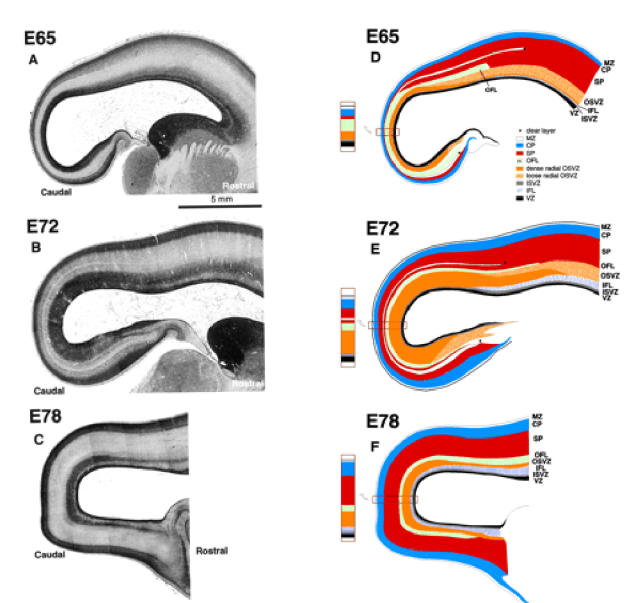
High-power photomicrographs of parasagittal sections. On the left are photomicrographs through the caudal pole of cerebral hemispheres at stated ages. On the right are matching drawings outlining various layers identified by reference to colour coding set out in the inset diagram. Abbreviations as in Figure 2.
Gross Histological Changes of the Telencephalic Wall
Inspection of parasagittal sections from E46, E55, E65, E72, E78, E88 and E94 brains (Fig. 1) shows that the total depth of the wall of the cerebral hemisphere diminishes progressively when traced rostrocaudally. Initially (E46–E55) the rostrocaudal decrease in thickness affects all the layers of the wall, including germinal and supragerminal compartments. That this is not due to the plane of section can be seen by examining the stacked sections throughout the full medial–lateral extent of the brain at E65 and E72 (not shown).
After E65 further differential changes in the various layers of the neural wall are evident. The most conspicuous of these is the substantial local increase in depth and density of the germinal zone at the caudal pole of the hemisphere. This is particularly evident at E72 and E78, where the expanded segment of the germinal zone forms a darkly staining cap over the pole of the hemisphere. The expanded germinal zone continues rostrally but the packing density of its cells is considerably reduced. The boundaries of the polar cap of densely staining germinal zone at E72 and E78 correspond approximately to a region of rapid decrease in depth of the neural wall. Between E65 and E78 there is a progressive increase in depth of both the cortical plate and the subplate at the caudal pole (Fig. 2C,D). Both compartments, however, remain thinner in caudal compared to more rostral regions.
The germinal layers begin to decline after E72, while the total depth of the cerebral wall is gradually increasing to accommodate released post-mitotic cells and their attendant fibre systems. The decrease of the germinal zones is, however, least at the pole of the hemisphere. At the E72 stage an inner fibre layer (IFL) appears rostrally on the dorsal and ventral aspect of the ventricle within the germinal compartment. By E78, the IFL has advanced caudally to extend over the occipital pole dividing the germinal compartment into a narrow inner subventricular zone (ISVZ) and a broader outer subventricular zone (OSVZ) (Fig. 2E). After E78 the germinal compartments continue to decline, while the postmitotic compartments increase in depth. The general picture suggests a progressive rostrocaudal gradient of histogenesis and differentiation with a major local variation to the sequence at the caudal extremity of the gradient in the putative region of area 17.
Detailed Histological Changes in the Caudal Pole of the Hemisphere
We shall now review the histological changes evident in a transect through the neural wall at the extreme caudal pole of the hemisphere in putative area 17.
Histological Status at E37
At E37 the cerebral wall consists of ventricular zone and a thin, cell-free marginal zone (not shown). The total depth of the epithelium is ~0.1mm. The ventricular zone is characterized by crowded, elongated, radially orientated nuclei. All mitotic figures lie at the ventricular surface, indicating that nuclei are migrating interkinetically (Sauer, 1935, 1936). Within the ventricular zone, a few rounded nuclei are present at its outer surface. These doubtless belong to the earliest differentiating cells which have not yet separated from the ventricular zone to form a supragerminal compartment.
Histological Status at E46
Developmental changes at E46 in the dimensions of the different compartments along the rostrocaudal axis of the neuroepithelium are shown in Figure 3. This reveals a rostrocaudal gradient of decreasing depth and differentiation of the neural wall. At the rostral pole of the hemisphere (Fig. 3C1), the neuroepithelium is composed of a typical ventricular zone, deeper than at E37. External to the ventricular zone is a narrow layer of cells with rounded or irregularly shaped nuclei among which are scattered mitotic figures. We refer to this as the ISVZ (so as to distinguish it from the later forming OSVZ to be described later). The ISVZ is applied to the deep surface of an overlying outer fibre layer (OFL) of similar depth. This fibre layer tends to be divided into flattened bundles by strands of radially orientated cells. This is the first indication of the pallisaded appearance of this layer, a feature which is more conspicuous at later stages in the development of the caudal hemisphere. Our OFL occupies the same position as the intermediate layer of Kostovic and Rakic (Kostovic and Rakic, 1990). The cortical plate lies superficial to the OFL and is composed of closely apposed cells with rounded nuclei of greater diameter than elsewhere in the epithelium. The cortical plate at this rostral location is five or six cells deep. There is no frank evidence of a subplate at this age at any rostrocaudal level. The boundary between the deep surface of the cortical plate and the OFL is, however, disrupted and corresponds to the site of the presubplate of Kostovic and Rakic (Kostovic and Rakic, 1990) and Meyer et al. (Meyer et al., 2000). A sparsely cellular marginal zone intervenes between the cortical plate and the pial surface.
Traced caudally towards the dorsum of the hemisphere, all layers decrease in depth (Fig. 3C, II) but otherwise retain the same characteristics as at the rostral pole. Traced to the extreme caudal pole of the hemisphere, the site of putative area 17, the neural epithelium is less than half as deep as at the rostral pole. The decrease in depth affects all layers (Fig. 3C, III). At the caudal pole a thin layer of scattered cells with round or irregularly shaped nuclei lie between a thin ventricular zone and a much reduced cortical plate, the latter now about one cell deep. The intervening layer of scattered cells probably consists of early neurons, the initial cells of the subventricular zone and a minimal caudal extension of the OFL. The E46 stage illustrated in Figure 3 corresponds to Meyer et al.’s ‘pioneer plate’ stage (Meyer et al., 2000) in which the cells of the plate lack frank radiality.
The initial presubplate stage is thus an inconspicuous event in the monkey involving relatively few cells (Fig. 3C). The monolayer of early-arriving cortical plate cells presumably divides the minimal preplate population into the inner and outer plexiform layer as described in rodent and carnivore (Rickmann et al., 1977; Marin-Padilla, 1978; Raedler and Raedler, 1978; Wolff, 1978; Konig and Marty, 1981; Caviness, 1982; Derer and Nakanishi, 1983; Luskin and Shatz, 1985; Chun et al., 1987; Bayer and Altman, 1990).
Histological Status at E55
At the caudal pole of the hemisphere at E55, the same basic layers are present as at E46 but each has increased substantially in depth and the borders between them are better defined (Fig. 2B). The depth of the ISVZ has considerably increased and is now only a little thinner than the ventricular zone. The cells of the ISVZ are interdigitated with a now more evidently pallisaded OFL, which in turn has deepened and is also closely applied to the irregular deep surface of the overlying cortical plate. The pallisaded bundles contain fibres cut in cross section. The pallisaded appearance of the OFL is imposed by radially running strands of migrating cells dividing up the tract into flattened fibre bundles containing very few nuclei. The radially orientated strands are presumably the processes of radial glia, partioning the migration lanes for the cells leaving the germinal layers and entering the more superficial neuronal compartments
The overlying cortical plate is composed of five or six layers of nuclei. These radially orientated nuclei presumably represent neurons of the cortical plate proper settling within the pioneer plate of Meyer et al. (Meyer et al., 2000). The interface between the OFL and the cortical plate appears to be pale and loculated. This appearance is probably the result of artefactual tearing during sectioning or differential shrinkage between layers of different texture, justifying the use of the term ‘disrupted zone’ (Kostovic and Rakic, 1990). The disrupted interface zone is regarded as a ‘presubplate’ by these authors. Using Golgi preparations, they found it to be a plexiform layer that contained a ‘population of large neurons that are for the most part not radially organized’. In addition, it contained ‘numerous dendritic processes descending from the deeply situated neurons of the cortical plate’. The deep layer of the later pioneer plate of Meyer et al. (Meyer et al., 2000) has a similar structure. Meyer et al. observed in the human that in the more mature lateral part of the plate ‘the radial disposition of the deep pioneer plate component was progressively more accentuated’ and where the cells ‘assumed a pyramidal shape with a prominent apical dendrite’. The latter neurons seem to be equivalent to Kostovic and Rakic’s ‘deeply situated neurons of the cortical plate’ (Kostovic and Rakic 1990) and to the layer VII neurons of Marin-Padilla (Marin-Padilla, 1978).
Histological Status at E65
By this stage the first major feature distinguishing the neuroepithelium of the caudal pole from more rostral areas has became evident. This is a marked increase in the depth and cell packing density of the subventricular zone, which at this stage is twice the depth of an undiminished ventricular zone (Figs 2C and 4). A major change in the subventricular zone is that the cells in its outer part have acquired a frank radial orientation (this distinctive histology is shown in Fig. 5). From E65 onwards there are two clearly defined subcompartments of the subventricular layer — an outer, radially structured compartment (OSVZ) and an inner compartment (ISVZ) with randomly organized cells showing a histology that is typical of the subventricular zone described in rodents (Fig. 8) (Boulder Committee, 1970). Mitotic figures are present at all levels in the ISVZ and OSVZ. This contrasts with the underlying ventricular zone where mitotic figures are only evident at the ventricular surface, indicating that ventricular zone cells continue to undergo interkinetic migration. The OFL has also increased in depth and its bundles interdigitate closely with the underlying OSVZ (Fig. 2C). The compartment external to the OFL consists of an outer layer of very crowded cells with radially arranged nuclei and an inner layer of similar depth of less crowded cells with more rounded nuclei. We regard the former as the cortical plate and the latter as the subplate proper making its first appearance.
Figure 5.
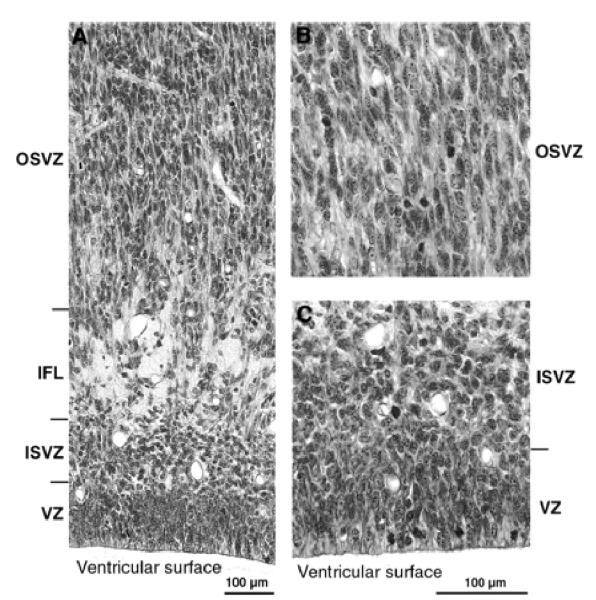
Photomicrographs at higher-power of the germinal zones at E78. (A) Note the radial texture of the dominant OSVZ and of the lesser VZ. Note also the IFL separating the radial OSVZ from the more irregularly orientated nuclei of the ISVZ. (B) Higher-power view of the OSVZ. Note radial texture of tissue and the presence of mitotic figures. (C) View of the ISVZ and VZ. Note the presence of mitotic figures among the irregularly orientated nuclei of the ISVZ. In the VZ, nuclei are radially arranged and mitotic figures lie towards the ventricular surface, indicating the continuation of interkinetic nuclear migration.
Figure 8.
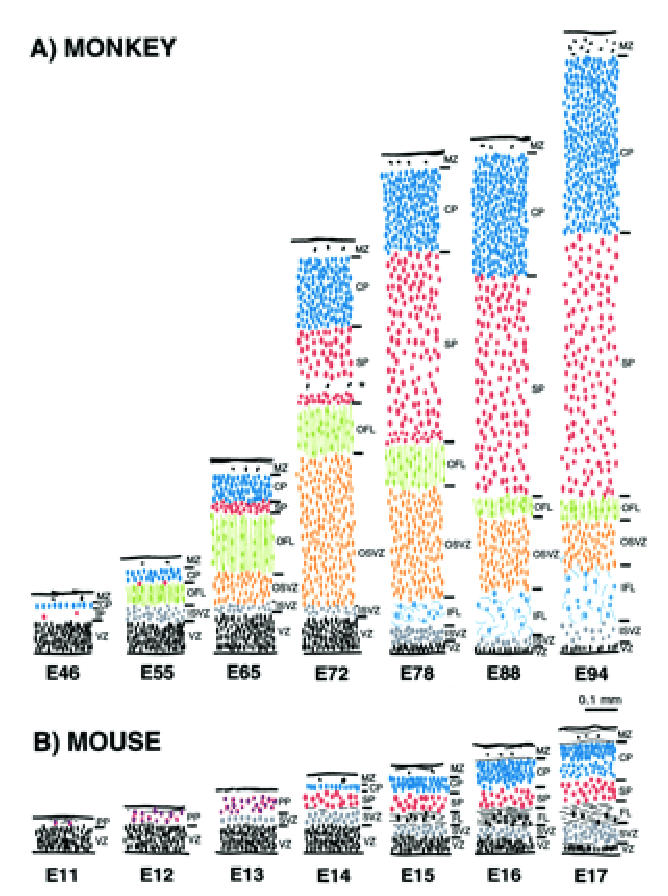
Comparison of histological sequences in mouse and monkey telencephalic wall. These drawings are of transects through putative Area 17 in (A) monkey and (B) mouse at comparable developmental stages. The depth of each layer is drawn to a common scale. The internal detail of each layer is not to scale but depicts the orientation, shape and relative packing density of nuclei in each layer. The drawings of monkey neuroepithelium were taken from the present material. The drawings from the mouse cortex were of material from the library of serially sectioned, Bouin-fixed mouse embryonic brains prepared for previous studies by I.H.M.S. The vertically aligned pairs have been chosen with reference to birthdating experiments so as to illustrate corticogenesis at equivalent developmental stages (Angevine and Sidman, 1961; Rakic, 1974; Smart and Smart, 1982; Caviness et al., 1995). Abbreviations and color coding as in Figures 2–4.
The more global picture at E65 (Fig. 4) shows that the OSVZ has a much higher cell packing density towards the pole of the occipital lobe. Traced rostrally, the OSVZ separates into a narrow inner zone of the same cell packing density as the rostral cap and an outer zone of lesser density which, however, retains a radial component. Between the OFL and overlying neuronal compartment there is a narrow clear zone, a minimal feature marked by an asterisk in Figure 4D. At this stage it corresponds in position to the presubplate of Kostovic and Rakic (Kostovic and Rakic, 1990). The clear zone diminishes when traced from rostral to caudal. As a discrete entity it does not extend very far beyond the boundary with the parietal region (indicated by an asterisk in Fig. 4). The OFL, when traced rostrally, also merges with the deeper subplate of the parietal lobe, and agrees with the earlier observation that the border between the subplate and the deep fibre pathway was sharper in visual compared to somatosensory cortex (Kostovic and Rakic, 1990). A feature, minimally evident at this stage, but later to prove important, is an inner fibre layer (IFL) which intrudes into the rostral subventricular zone separating the ISVZ from the OSVZ (Fig. 4A,D).
Histological Status at E72
Three major events take place at the caudal pole of the hemisphere between E65 and E72: (i) the ventricular zone diminishes in depth by ~50%; (ii) the OSVZ increases four times in depth to become the major germinal zone; and (iii) the subplate increases by a similar extent (Fig. 2C,D). During the preceding seven days there has evidently been a decline in the population of ventricular cells and a surge in both the number of non-interkinetically migrating precursor cells and in the neuron output of the germinal zones. The cells of the thinned ventricular zone continue to migrate to the ventricular surface to divide (as shown in Fig. 5B at E78). Within the radially augmented OSVZ, mitotic figures are frequent at all levels.
The OFL at E72 is thinner than at E65 and remains frankly pallisaded; the intervening migratory lanes appeared to be more crowded than at E65. External to the OFL is a layer of crowded nuclei of mixed shape: some are rounded, others elongated and radially orientated. This population, which was not present at E65, is bounded externally by the clear layer (marked by an asterisk in Fig. 2D), which is a conspicuous feature at this stage. Within the clear layer, the scarcity of cell nuclei suggests that it is composed of a plexus of fine fibres. It is, nevertheless, transient as a conspicuous feature, being barely evident at E78 and not detectable by E88 (Fig. 2E). At E72, the overlying subplate has increased three or four times in depth since E65 and its cells appear less densely packed. The cortical plate has also increased in depth by a factor of two and its nuclei have become progressively more crowded when traced towards the marginal layer.
The global picture at E72 is shown in Figure 4. As at earlier stages, all layers except the OSVZ decrease in depth when traced from rostral to caudal. The OSVZ, which extends to the subicular/hippocampal region ventrally and dorsally into the parietal region, is densest at the pole of the hemisphere. The wedged-shaped IFL is thicker than at E65 but does not yet extend to the pole of the hemisphere. The clear layer marked by an asterisk in Figure 4 is a conspicuous feature extending unequivocally round the pole of the hemisphere from the hippocampal boundary ventrally to the putative boundary of the parietal lobe dorsally where it blends into the subplate. If it corresponds to the original ‘preplate’, its late manifestation into a more conspicuous clear layer lying within the subplate is an interesting development in its history. The clear expanded layer may represent a barrier to later generations of radially migrating neurons. The layer of nuclei beneath it may possibly owe their crowding to being delayed by the clear layer in their migration.
Histological Status at E78
By E78 the ventricular zone has further diminished in depth (Fig. 2E). Its nuclei are still radially orientated and mitotic figures restricted to the ventricular surface, indicating continued interkinetic movement (Fig. 5B). The IFL now extends round the pole of the occipital lobe separating the randomly organized ISVZ and the OSVZ. The ISVZ is narrow, has an irregular boundary with the VZ, and superficially blends into the fibre bundles of the IFL. The ISVZ is composed of mostly rounded nuclei, some in mitosis (Fig. 5). The IFL is made up of fibres cut transversely or obliquely with many interspersed, irregularly shaped nuclei, possibly of neuroglia cells (Fig. 5). The OSVZ, by contrast, remains radially organized and contains numerous mitotic figures (Fig. 5A). Some of these are dividing horizontally, others vertically to give side-by-side daughter cells. The OFL has the same characteristics as at E72. Between E72 and E78 the subplate increases in depth threefold, and at this stage the clear layer is no longer visible. The increase stems both from the acquisition of more cells and a major decrease in their packing density, the latter indicating a correspondingly substantial increase in neuropil and fibre content. The cortical plate has likewise increased in depth during the same period. The cell packing density of its nuclei is greatest towards the marginal zone and less so in its inner half. The decline of the ventricular zone and simultaneous burgeoning of the OSVZ indicates that the latter may be the source of the major increase in the cells of the subplate and cortical plate.
The global picture at E78 (Fig. 4) shows that the IFL now circumscribes the caudal pole of the hemisphere. Compared to area 17, presumptive area 18 has a deeper IFL and a much decreased OSVZ. Both the subplate and cortical plate are thinner in presumptive area 17 than in more rostral regions.
Histological status at E88
In presumptive area 17 at E88, the ventricular zone has diminished (Fig. 2F). The ISVZ is also thinner and nuclei appear to stream from it to join the nuclei within the IFL which is deeper than at E78. The OSVZ is somewhat thinner than at E78 but still has a radial organisation. Both the subplate and cortical plate are deeper than at E78. The cortical plate has retained an outer zone of densely packed neurons and an inner zone of lesser packing density.
The global picture at E88 (Fig. 1) indicates that the cortical plate at the pole of the hemisphere is thinner than at more rostral levels. The cell packing density is, however, higher in the caudal region compared to rostral levels. The depth of the cortical plate is constant over the pole of the hemisphere.
Histological Status at E94
At this stage the ventricular zone in presumptive area 17 remains reduced, while the ISVZ is deeper but more diffuse than at E88, and its nuclei appear to grade into the dispersed nuclei lying among the fibres of an expanded IFL (Figs 1 and 2G). The OSVZ is thinner and its nuclei less crowded. The OFL remains distinct and pallisaded. The subplate is about the same depth as at E88 but its cells are more widely separated. The cortical plate, however, is deeper, and five layers can be distinguished according to their different degrees of nuclear crowding.
The global picture at E94 (Fig. 1) is similar to that at E88. The cortical plate, however, has started to fold. The underlying subplate, while thinner than in the more rostral areas, varies in depth inversely with the undulations of the incipient folding of the cortical plate. The OSVZ remains thicker and denser round the occipital pole than at more rostrally levels.
Appearance of Areal Boundaries
At early stages of corticogenesis (E46–E72) the various layers of the neural tissue forming the caudal pole of the hemisphere are thinnest at the extreme pole and increase gradually in depth as they are traced from putative area 17 to area 18. The first incontrovertible evidence of the area 17/18 boundary occurs at E86. The boundary is marked by a decrease in the amount of stainable AChE (Fig. 6). The OFL contains AchE-labelled fibres which emanate from the lateral geniculate nucleus and are composed of fibres of the optic radiation (Fig. 6A). Histologically the transition from area 17 to area 18 at E88 is characterized by a stepping up of the depth of the cortical plate and a gradual reduction in the OSVZ, which remains deeper and denser under area 17 (Figs 1 and 6). With hindsight these changes in the supragerminal layers can be traced back to E55.
Figure 6.
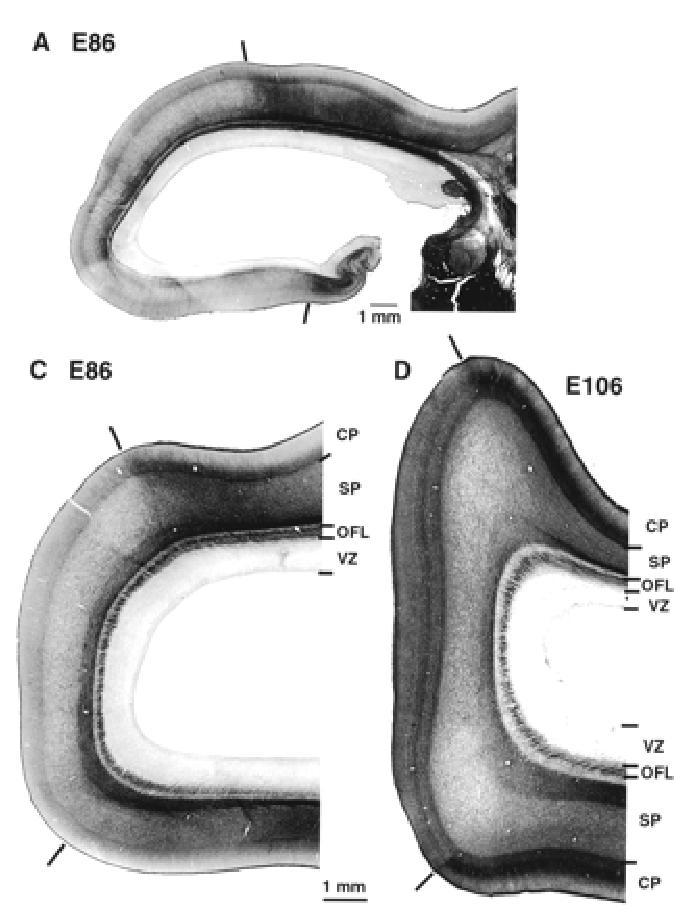
Acetylcholinesterase reactivity in the occipital lobe and thalamus. Dark bars show the 17/18 border at E86 and E106. At all ages there is a strong labelling of fibres in OFL. Note that AchE reactivity reveals lamination of the lateral geniculate nucleus at this early developmental stage. Abbreviations as in Figure 2.
Identification of Subplate
The present results suggest that the subplate at the occipital pole can first be detected at E65 which is some 20 days after the cortical plate appears. The subplate continues to expand upto E100 by which time it has become the largest post-mitotic cellular compartment before proceeding to disappear around birth (Kostovic and Rakic, 1990; Meinecke and Rakic, 1992).
At E95 we used NPY immunohistochemistry to identify the subplate (Fig. 7). Comparison of adjacent sections which have been reacted for AChE and NPY shows that at E95 the region between the OFL and the cortical plate is NPY reactive and corresponds to the location of the subplate. Hence at late gestation, the region of low cell density located between the cortical plate and the OFL is not homologous with the white matter of the mature cortex containing ascending, descending and cortico-cortical fibre tracts. Instead, these fibre tracts, including corticofugal and corticopetal fibres, are restricted at these developmental stages to the OFL.
Figure 7.
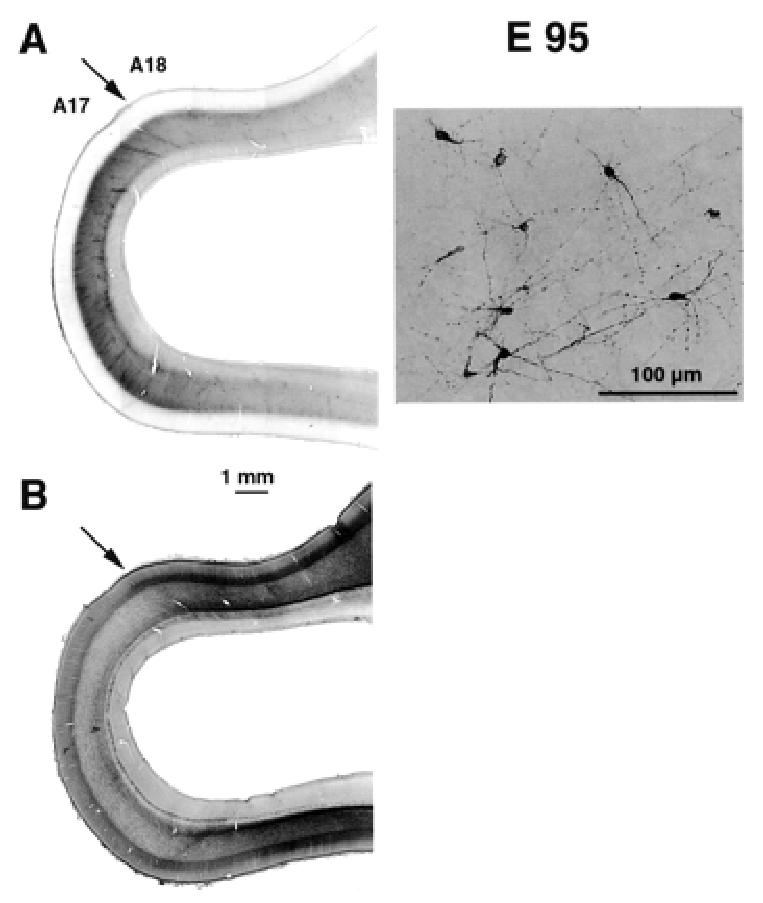
Identification of subplate and areal borders at E95. (A) Neuropeptide Y labelling identifies the subplate. Low magnification (left panel) shows that there is a difference in the intensity of labelling in the region of the putative area 17/18 border. This is due to changes in the density of labelled subplate neurons (right panel). (B) Adjacent section reacted for acetylcholinesterase activity. This confirms the location of the area 17/18 border (for further details, see Fig. 6).
Discussion
By first comparing morphological features of the developing cortex in the mouse and monkey it is possible to identify a number of morphological features in the monkey that are either unique to the developing primate cortex or at least more pronounced than in the non-primate. Secondly, by focusing on area 17/18 as a model system we review the relevance of the present morphological findings for understanding the development of areal boundaries and dimensions.
Histogenetic Sequence of the Mouse Cortex
The histogenetic events observed in the development of putative area 17 in the mouse are summarised in Figure 8. An initial ventricular zone gives rise by E12 to the early neuron populations of the marginal zone and preplate. By late E13 or early E14 a thin line of cortical plate cells is thought to split the preplate neurons into an outer plexiform layer and an inner subplate (Marin-Padilla, 1978; Caviness, 1982; Del Rio et al., 2000) [reviewed elsewhere (Bayer and Altman, 1991)].
A subventricular zone is apparent at E13, just before the appearance of the cortical plate. Initially the subventricular zone is evident as a line of cells with round nuclei at the border between the ventricular zone and the preplate (Smart, 1973). Mitotic figures, the majority of which cleave horizontally, can be observed among these nuclei, which therefore belong to cells that are no longer undergoing interkinetic nuclear migration (Smart, 1973). During E15 the ventricular layer starts to decline, signalling the end of the major period of neuron production (Smart, 1973; Smart and McSherry, 1982). Also at E15, the subventricular layer begins to diminish and to be invaded by fibres, and from this point on produces mostly or uniquely neuroglia (Smart and McSherry, 1982). During the transition into mature cortex the ventricular zone transforms into a single line of ependymal cells, the subventricular zone persists as a minimal subependymal layer, the fibre bundles coalesce into the subcortical white matter, the subplate largely disappears, and the cortical plate cells mature into the conventional inner five cortical layers, the marginal zone remaining as layer 1.
The developmental sequence in the monkey occipital cortex, although generally similar, differs in a number of important respects from that described above in the mouse. Its generation is considerably prolonged compared to the mouse — months rather than weeks — so we are comparing a system deploying its sequences at a more leisurely pace with a telescoped system.
Comparison of Presubplate Stage in Mouse and Monkey
Very early in the monkey developmental sequence (by E46), a cortical plate of a single line of cells overlies a minimal subplate [or ‘presubplate’ in primate terminology (Kostovic and Rakic, 1990; Meyer et al., 2000)] and a small but distinguishable fibre layer (Fig. 3C). Thus, in the monkey the cortical plate neurons are born early and there does not appear to be much in the way of a preplate population to be split into two laminae by the arrival of the cortical plate neurons. In the mouse, by contrast, the cortical plate only appears after a substantial preplate population has been released (Smart and McSherry, 1982). The early presence of a discrete fibre layer (our OFL) is absent in the mouse. Hence a comparison of the E55 monkey and the E12/E13 mouse transects in Figure 8 shows that from very early on, the monkey histogenetic programme differs in important respects from that of the mouse.
Comparison of Germinal Zones in Mouse and Monkey
At later stages, the monkey occipital cortex displays other major features not found in the mouse. The first of these is a compartment of the subventricular zone which shows a highly radial organization and which we term OSVZ to distinguish it from a classically randomly organized subventricular zone which we refer to as ISVZ. At E72 there is a relatively early decline of the ventricular zone. This decline is preceded by a massive increase from E65 onwards in the size of the OSVZ. The decrease in the ventricular zone and the increase in the OSVZ occur prior to the major phase of neuron production, indicating that the OSVZ is the principal source of cortical neurons in the monkey. Note that the OSVZ may be unique to the primate; at least no counterpart has been described in non-primates.
The OSVZ and ISVZ are later separated by the IFL. An interesting property of the OSVZ is that the cell processes and their oval nuclei are radially arranged, resembling the structure of the ventricular zone rather than the looser and more variable organization classically associated with the subventricular zone in the mouse. The monkey OSVZ, in spite of its radial appearance, is distinguished from the ventricular zone in that species by its lesser cell density and the abundance of mitotic figures throughout the layer. Hence, while the ventricular zone of the monkey continues to show interkinetic migration, the OSVZ does not. At late stages (E16, E17) in the mouse, the cells of the subventricular zone resemble the monkey ISVZ in that they appear to merge into the fibre bundles that intrude relatively late in histogenesis (Smart and McSherry, 1982).
The strongly radial organization of the OSVZ gives a clue to its possible cellular composition. In mouse one possible constraint on the orientation of precursors in the ventricular zone is the presence of large numbers of proliferating radial glial cells (Misson et al., 1988, 1991; Hartfuss et al., 2001). The random organization of the subventricular zone is compatible with this compartment containing few nuclei of radial glial cells. In the monkey, radial glial cells have been shown to undergo proliferation during the peak of neurogenesis and nuclei of the radial glial cells have been observed in the region that we have identified as the OSVZ in the present study (Schmechel and Rakic, 1979; Levitt et al., 1981). In comparison with the mouse, we show that the ventricular zone in the monkey becomes very reduced in depth at early stages in corticogenesis. This might be incompatible with the increase in surface area during the development of the monkey occipital lobe and the need to maintain densities of radial glial. One could speculate that this is overcome by allocating radial glial cells to the OSVZ and these cells acquiring a proliferate mode that does not necessitate interkinetic migration, thereby avoiding nuclear congestion at the ventricular surface (Smart, 1973). This speculation is compatible with the OSVZ being the major site of neuron production given that radial glial cells are known to fulfil a role of neuronal precursors (Malatesta et al., 2000; Hartfuss et al., 2001; Noctor et al., 2001).
Rostrocaudal Gradients in Mouse and Monkey
The rostrocaudal gradient of histogenesis and maturation described in the mouse cortex (Smart, 1983) is also present in monkey (Figs 1 and 3). Similar neurogenic gradients have also been described in the rat (Hicks and D’Amato, 1968; Raedler and Raedler, 1978; Raedler et al., 1980), in carnivores (Luskin and Shatz, 1985; Jackson et al., 1989), in the rabbit (Fernandez and Bravo, 1974) and in human (Sidman and Rakic, 1973).
The rostrocaudal sequence of neuron release and plate formation depicted in Figure 3 spans the early stages of plate formation described in the human (Meyer et al., 2000). According to these authors, the initial neurons condensing to form a plate are themselves separated by the later arrival of neurons that form the radially organized cortical plate proper. In the early stages of plate formation in human, the following neuron populations appear in succession and can be distinguished by differences in antibody expression, cell orientation and branching pattern of their processes: marginal zone, preplate, ‘monolayer’ plate, pioneer plate, presubplate, subplate and cortical plate. While most of these subpopulations cannot be distinguished using the general histological preparations of the present study, the work of Meyer et al. (Meyer et al., 2000) indicates the underlying intricacy of the sequences we are describing at a more macro level.
Comparison of the Subplate Origin in Mouse and Monkey
In non-primates, numerous authors have described the early formation of the preplate, which is later split into a marginal zone and subplate populations by the arrival of cortical plate (Konig et al., 1975; Raedler and Sievers, 1975; Rickmann et al., 1977; Raedler and Raedler, 1978; Wolff, 1978; Konig and Marty, 1981; Caviness, 1982; Luskin and Shatz, 1985; Chun etal., 1987; Bayer and Altman, 1990). Hence in the mouse the postmigratory cells from E11 to E13 form the loosely packed preplate, which is split to form the marginal layer and the subplate at E14 by the dense cortical plate. It is implied that the entire population of mouse subplate neurons is formed prior to the birth of cortical plate neurons. In the monkey, on the other hand, we do not find evidence of a numerically important early population of loosely packed cells. The earliest plate population occurs in a numerically minimal, albeit complex, preplate population (Fig. 3). In the monkey the preplate cells deep to the cortical plate form a configuration termed ‘presubplate’ by Kostovic and Rakic (Kostovic and Rakic, 1990). Monkey ‘presubplate’ is thus equivalent to mouse ‘subplate’. The monkey presubplate may persist as the clear layer of our description.
Hence, the monkey subplate is not the residue of an early preplate but instead appears some time after the cortical plate has been formed. A similar developmental process may occur in human cortex where early transient populations of neurons form a dense accumulation at very early stages (Meyer et al., 2000).
Our results show that the earliest coalescence of postmigratory cells at E46 forms a dense cortical plate rather than subplate, and that subplate cells begin to accumulate below the cortical plate at E65 (Figs 1 and 2). By this stage the cortical plate is relatively well formed and birthdating experiments show that it is largely composed of cells destined to become neurons belonging to layers 5/6 (Rakic, 1974; Dehay et al., 1993). E65 marks the onset of the rapid expansion of the subplate and it is reported to reach its maximum depth between E100 and E120 (Kostovic and Rakic, 1990; Meinecke and Rakic, 1992). In the monkey, the subplate lies between the crowded nuclei of the cortical plate and the OFL, occupying the position of the presubplate zone of Kostovic and Rakic (Kostovic and Rakic, 1990), which has been equated with the inner layer of the divided pioneer plate by Meyer et al. (Meyer et al., 2000). The subplate is composed of fibres, neuropil and resident cells, as well as cells transiting to the cortical plate (Chun and Shatz, 1988; Kostovic and Rakic, 1990; Meinecke and Rakic, 1992). Within the subplate, mature neurons can be identified by immunohistochemical staining for neuropeptide Y (Chun et al., 1987; Wahle and Meyer, 1987; Chun and Shatz, 1989a, b). The subplate is a transient structure and is reported to have been largely eliminated by birth in the monkey (Kostovic and Rakic, 1990).
These findings suggest that major differences exist in the timing and relative dimensions of the maturing cortical plate and subplate in mouse and monkey. The results in the monkey indicate that the presubplate is subsumed into the subplate. The presubplate in the monkey, although histologically complex (Kostovic and Rakic, 1990), is morphologically minimal and is comprised of few neurons. The first indication of the subplate proper (by E65 in our material) is accompanied by a substantial increase in the cellularity in the region of the presubplate (Fig. 2C,D). While Kostovic and Rakic concluded that the expansion of the subplate at E65–E70 is due to the ingrowth of fibres and is accompanied by a decrease in its cell density, we find that the decrease in density occurs later between E72 and E78 (Fig. 2D,E). Given the size of the primate subplate and the fact that it does not begin to get assembled until sometime between E55 and E65, some 10–20 days after the initiation of the cortical plate, it would seem very unlikely that all subplate neurons are the earliest generated neurons as has been described in the rodent and carnivore and in earlier studies of the monkey (Marin-Padilla, 1978; Kostovic and Rakic, 1980; Rakic, 1982; Luskin and Shatz, 1985).
In rat it has been claimed that occasional late-generated neurons contribute to the subplate (Rickmann et al., 1977), and in our diagrams of the mouse sequence in Figure 8 there does indeed seem to be an increase in the subplate population after the cortical plate has formed. Clearly the origin of subplate neurons as opposed to presubplate neurons requires further investigation. Birthdating experiments in monkey have not examined the time of origin of subplate neurons, and have failed to detect late generation of interstitial neurons in the white matter below area 17 3–5 months postnatal (Kostovic and Rakic, 1980). The failure of Kostovic and Rakic’s 1980 study to detect late generation of subplate neurons in the monkey could be due to the fact that the brains were examined late, after many subplate neurons have presumably died (Kostovic and Rakic, 1990).
Subplate Expansion is Characteristic of the Primate
An expanded subplate is the hallmark of primate corticogenesis, including monkey and man, and it has been speculated that this feature could be related to the extensive development of cognitive functions in this order (Kostovic and Rakic, 1990). The present results suggest that the expansion of the subplate may entail a shift to cell production for this compartment to later stages of corticogenesis. This means that neurons destined for the cortical plate and subcortical plate may be generated simultaneously in the monkey, whereas they are generated sequentially in the rodent and cat (Luskin and Shatz, 1985; Bayer et al., 1991). The existence of additional germinal compartments in the primate might facilitate the simultaneous generation of two different sets of neurons destined for the cortical plate and the subplate.
The subplate continues to expand up to E100, by which time it has become the deepest postmitotic cellular compartment (Kostovic and Rakic, 1990) (see also present study). As the cortical plate starts to fold, the depth of the subplate varies with the folding, being deeper in the gyri and thinnest at the sulci (Fig. 1). Hence, the subplate appears to mould itself to fill the space between the OFL — whose curvature remains, initially at least, relatively unchanged — and the undulations of the cortical plate.
The decline of the subplate occurs during a period of cortical expansion. In both carnivores and rodents the interstitial cells of the adult white matter are known to be the remnants of the subplate neurons (Valverde and Facal-Valverde, 1988; Chun and Shatz, 1989a, b; Woo et al., 1991). As gyrification proceeds, the area of the cortex increases tenfold between E95 and birth (H. Kennedy, unpublished). If the total volume of subplate does not change but merely follows the increase in cortical surface, it would become very attenuated as it follows the expansion of the cortex and might be no more than a very thin layer of cells and fibres. This would mean that part of the decrease in the subplate is by a dilution of its cells in to the white matter of the adult, as has been shown in other orders (Luskin and Shatz, 1985; Chun and Shatz, 1989a, b; Woo, et al., 1991).
Tangential Expansion of the Cortex
A striking contrast between the mouse and monkey development shown in Figure 7 is the greater depth of the neuroepithelium of the monkey compared to the mouse. It is not expected that the fivefold increase in depth in the monkey could be accounted for by the 50% increase in the numbers of neurons in the thickness of area 17 in the monkey compared to area 17 in the mouse (Rockel et al., 1980). Likewise, the increase in neuron size between the two orders is expected to make only a small contribution to differences in the thickness of the neuroepithelium, and this in any case would not be a factor before late stages of differentiation. One possibility, which is hinted at in our results, is that there is a large degree of tangential expansion in the occipital lobe of the monkey.
Up to and including E88, the cortical plate at the occipital pole where presumptive area 17 is located is actually thinner than at more rostral levels (Fig. 1). This is unexpected given that the germinal zone underlying the occipital pole is highly active and, compared to area 18, is the site of increased neuron production (Dehay et al., 1993). One possible explanation of the thinner cortical plate at the pole is that it is the site of increased tangential expansion.
Our observation in monkey that the cortical plate is thinner at the occipital lobe needs to be reconciled with the fact that (i) there are increased rates of neuron production in this region; and (ii) in the mature cortex area 17 is thicker than area 18 in terms of cell numbers (Rockel et al., 1980). One possibility is that some of the increased rates of proliferation serve to increase numbers of radial glia and founder neurons of area 17 relative to more anterior regions. This would lead to a tangential expansion of the occipital lobe which would allow the enlargement of visual system that is characteristic of the primates (Van Essen et al., 1984). The notion that there is an important tangential expansion of the occipital lobe is further supported by the progressive decrease in depth of the OFL after E65, while other layers are increasing in depth (Fig. 7). In so far as the tangential expansion underlines an enlarged visual cortex, this could be viewed as a primate feature.
Area 17/18 Border is Set on a Proliferative Gradient
One major aim of the present study was to determine if the area 17/18 boundary emerges from a specialization of the germinal zone. Although the 17/18 border did not emerge from a sharp discontinuity in the germinal zones there were gradual changes in the underlying germinal zone. Up to E78 the presumptive boundary between areas 17 and 18 coincides on the area 17 side of the boundary with a just perceptible reduction in depth of the cortical plate and an increase in depth of the OSVZ (Fig. 1). Hence, the present findings show that the 17/18 border emerges from the caudal end of a morphological gradient. This contrasts with birthdating experiments, which show that the 17/18 border emerges from the rostral end of a proliferative gradient (Kennedy and Dehay, 1993). These two sets of findings may have direct implications for the dynamics of boundary formation in the cortex.
Despite the fact that the area 17/18 border emerges on a gradient of morphological and proliferative features, it can be clearly distinguished as early as E86 by AchE histochemistry. These findings need to be considered along with the reduction in size of area 17 following early bilateral enucleation before E70 (Rakic, 1988; Dehay et al., 1989). Similar dependence of the dimensions of cortical areas on cortical afferents have been demonstrated in the somatosensory cortex of the rodent (Killackey et al., 1994). The consequence of bilateral removal of the retina during a critical developmental window is to reduce the number of thalamic afferents, which in turn leads to a reduction of the dimensions of area 17 (Rakic, 1988; Dehay et al., 1989, 1991). Despite the enormous reduction of the dimensions of area 17, the overall dimensions of the neocortex remain unaltered (Dehay et al., 1996a). This last result means that a primary consequence of the decrease in the numbers of thalamic afferents is a shift in the location of the area 17 border, so that cortex that was originally destined to become area 17 takes on an alternative phenotype which corresponds more closely to extrastriate cortex (Rakic, 1988; Rakic et al., 1991; Dehay et al., 1996b). These findings suggest that in normal development the final setting of the 17/18 border is in response to multiple factors which determine the position of the border along a caudal–rostral gradient of decreasing proliferation. The fact that the abrupt differences in the numbers of neurons on either side of the area 17/18 border following early enucleation does not differ from that found in the normal adult suggests that the periphery directly influences the gradient itself by modulating rates of proliferation of cortical precursors (Dehay et al., 2001).
Early Fibre Tracts in the Monkey Neuroepithelium
We have mentioned above that the IFL does not have a clear non-primate counterpart. It is also worth noting that in the low-power photograph at E65 the IFL is seen to extend from the ganglionic eminence in the front part of the brain back to the occipital lobe, reaching the pole at E78 (Fig. 4). This pathway could be in a highly strategic position given the recent evidence that the ganglionic eminence is the source of tangentially migrating neuroblasts that account for many, if not all, of the cortical interneurons in the adult (De Carlos et al., 1996; Anderson et al., 1997, 2001; Tamamaki et al., 1997; Lavdas et al., 1999; Wichterle et al., 1999). Neuroblasts from the lateral ganglionic eminence are known to migrate in the subventricular layer and hence could use the IFL as a migratory pathway if a similar origin of cortical interneurons is maintained in the monkey (Anderson et al., 2001). However, at this stage the ISVZ is much diminished and the IFL sparely nucleated, so that tangential migratory pathways might also be located in the OSVZ and subplate.
The present study shows AchE-labelled fibres emanating from the lateral geniculate nucleus and running into the OFL, suggesting that optic tract fibres run in the OFL (Kostovic and Rakic, 1990). Our results suggest that immature geniculostriate fibres in the monkey are transiently positive for AchE, as has been shown for thalamocortical fibres in rodents (Robertson et al., 1988, 1991; Schlaggar et al., 1993; Sendemir et al., 1996) and for pulvinar fibres in monkey (Kostovic and Rakic, 1984). It has been shown that corticothalamic and thalamocortical fibres fasciculate during their growth to their cortical and thalamic targets (Molnar et al., 1998; Molnar and Blakemore, 1999). If close proximity between these two sets of fibres is maintained in the monkey during development it would seem that the corticogeniculate fibres run dorsal to the geniculostriate fibres in the space immediately dorsal to the AchE labelling in the OFL (Shatz and Rakic, 1981). This is surprising given that in nonprimates the relationship of corticofugal and corticopetal fibres is inverted (Woodward and Coull, 1984; Miller et al., 1993; Bicknese et al., 1994).
In the present study we have strong evidence showing that geniculostriate fibres are located in the lower part of the OFL at E86. We can only speculate on the origin of the fibre pathways which reside in the OFL at earlier stages. While the OFL is present as early as E55 it would be surprising if geniculate fibres had reached the cortex by this age (Kennedy and Dehay, 1993). However, it should be noted that the earliest fibres reaching the cortex in rodent originate from the thalamus (Miller et al., 1993). Alternatively, the early OFL may contain fibres originating from the mesencephalon, as has been demonstrated in human (Marin-Padilla, 1983).
Conclusion
The sequence of developmental events, as well as qualitative and quantitative aspects of corticogenesis, differ in primates and non-primates as summarized in Figure 8. (i) In primates the preplate population is minimal and the cortical plate appears relatively earlier than in non-primates (compare mouse E11–E12 with monkey E46–E65). (ii) In non-primates there is no equivalent of an early arriving optic radiation in the form of an ‘outer fibre layer’ (compare E55–E72 monkey with E12–E14 mouse), (iii) The ventricular zone in primates declines before the commencement of the major phase of neuron production, and at E72 is largely replaced by an expanded, radially organized, mitotically active extension of the subventricular zone from which later generations of neurons arise, (iv) The subplate in non-primates reaches a depth equivalent to that of the cortical plate, whereas in primates the subplate at its maximum is twice the depth of a much deeper cortical plate.
In the monkey the early arrival of cortical plate cells and of a morphologically conspicuous fibre input in the form of the OFL are in striking contrast to the early development of the mouse neuroepithelium. The subsequent production in monkey of the OSVZ, the greater complexities of the subplate and deeper cortical plate must represent steps in the neurohistogenetic programme acquired since the evolutionary stems of rodent and primate diverged.
In all probability, the monkey represents a version of the basic programme evolved by higher primates. As such, it may be a useful model of the investigation of normal and abnormal human development. The differences between rodent and monkey also invite caution when applying experimental results gained from laboratory rodents to cortical histogenesis in general. The differences between monkey and human are probably less, but nevertheless these differences must be identified in order to use the monkey model to greatest advantage.
Acknowledgments
We thank Ghislaine Clain for animal care and Christelle Merrouche for excellent technical assistance. Financial support was provided by the EC, Quality of Life and Management of Living Resources (QLG3-2000–00158), the Region Rhône Alpes and the European Economic Community grant BIOMED BMH4 CT961604.
References
- Anderson SA, Eisenstat DD, Shi L, Rubenstein LR. Interneuron migration from basal forebrain to neocortex: dependence on Dlx genes. Science. 1997;278:474–476. doi: 10.1126/science.278.5337.474. [DOI] [PubMed] [Google Scholar]
- Anderson SA, Marin O, Horn C, Jennings K, Rubenstein JL. Distinct cortical migrations from the medial and lateral ganglionic eminences. Development. 2001;128:353–363. doi: 10.1242/dev.128.3.353. [DOI] [PubMed] [Google Scholar]
- Angevine JB, Sidman RL. Autoradiographic study of cell migration during histogenesis of cerebral cortex in the mouse. Nature. 1961;192:766–768. doi: 10.1038/192766b0. [DOI] [PubMed] [Google Scholar]
- Arimatsu Y, Miyamoto M, Nihonmatsu I, Hirata K, Uratani Y, Hatanaka Y, Takiguchi-Hayashi K. Early regional specification for a molecular neuronal phenotype in the rat neocortex. Proc Natl Acad Sci USA. 1992;89:8879–8883. doi: 10.1073/pnas.89.19.8879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbe MF, Levitt P. The early commitment of fetal neurons to the limbic cortex. J Neurosci. 1991;11:519–533. doi: 10.1523/JNEUROSCI.11-02-00519.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer SA, Altman J. Development of layer I and the subplate in the rat neocortex. Exp Neurol. 1990;107:48–62. doi: 10.1016/0014-4886(90)90062-w. [DOI] [PubMed] [Google Scholar]
- Bayer SA, Altman J. Neocortical development. New York: Raven Press; 1991. [Google Scholar]
- Bayer SA, Altman J, Russo RJ, Dai XF, Simmons JA. Cell migration in the rat embryonic neocortex. J Comp Neurol. 1991;307:499–516. doi: 10.1002/cne.903070312. [DOI] [PubMed] [Google Scholar]
- Berry M, Rogers AW. The migration of neuroblasts in the developing cerebral cortex. J Anat. 1965;99:691–709. [PMC free article] [PubMed] [Google Scholar]
- Bicknese AR, Sheppard AM, O’Leary DDM, Pearlman AL. Thalamocortical axons extend along a chondroitin sulfate proteoglycan-enriched pathway coincident with the neocortical subplate and distinct from the efferent path. J Neurosci. 1994;14:3500–3510. doi: 10.1523/JNEUROSCI.14-06-03500.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop KM, Goudreau G, O’Leary DD. Regulation of area identity in the mammalian neocortex by Emx2 and Pax6. Science. 2000;288:344–349. doi: 10.1126/science.288.5464.344. [DOI] [PubMed] [Google Scholar]
- Boulder Committee. Embryonic vertebrate central nervous system: revised terminology. Anat Rec. 1970;166:257–261. doi: 10.1002/ar.1091660214. [DOI] [PubMed] [Google Scholar]
- Caviness VS. Neocortical histogenesis in the normal and reeler mice: a developmental study based upon [3H]-thymidine autoradiography. Dev Brain Res. 1982;4:293–302. doi: 10.1016/0165-3806(82)90141-9. [DOI] [PubMed] [Google Scholar]
- Caviness VS, Takahashi T. Proliferative events in the cerebral ventricular zone. Brain Dev. 1995;17:159–163. doi: 10.1016/0387-7604(95)00029-b. [DOI] [PubMed] [Google Scholar]
- Caviness VS, Takahashi T, Nowakowski RS. Numbers, time and neocortical neuronogenesis: a general developmental and evolutionary model. Trends Neurosci. 1995;18:379–383. doi: 10.1016/0166-2236(95)93933-o. [DOI] [PubMed] [Google Scholar]
- Chun JJM, Shatz CJ. Redistribution of synaptic vesicle antigens is correlated with the disappearance of a transient synaptic zone in the developing cerebral cortex. Neuron. 1988;1:297–310. doi: 10.1016/0896-6273(88)90078-5. [DOI] [PubMed] [Google Scholar]
- Chun JJM, Shatz CJ. The earliest-generated neurons of the cat cerebral cortex: characterization by MAP2 and neurotransmitter Immunohistochemistry during fetal life. J Neurosci. 1989a;9:1648–1667. doi: 10.1523/JNEUROSCI.09-05-01648.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun JJM, Shatz CJ. Interstitial cells of the adult neocortical white matter are the remnant of the early generated subplate neuron population. J Comp Neurol. 1989b;282:555–569. doi: 10.1002/cne.902820407. [DOI] [PubMed] [Google Scholar]
- Chun JJM, Nakamura MJ, Shatz CJ. Transient cells of the developing mammalian telencephalon are peptide-immunoreactive neurons. Nature. 1987;325:617. doi: 10.1038/325617a0. [DOI] [PubMed] [Google Scholar]
- Cohen-Tannoudji M, Babinet C, Wassef M. Early determination of a mouse somatosensory cortex marker. Nature. 1994;368:460–463. doi: 10.1038/368460a0. [DOI] [PubMed] [Google Scholar]
- De Carlos JA, Lopez-Mascaraque L, Valverde F. Dynamics of cell migration from the lateral ganglionic eminence in the rat. J Neurosci. 1996;16:6146–6156. doi: 10.1523/JNEUROSCI.16-19-06146.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehay C, Horsburgh G, Berland M, Killackey H, Kennedy H. Maturation and connectivity of the visual cortex in monkey is altered by prenatal removal of retinal input. Nature. 1989;337:265–267. doi: 10.1038/337265a0. [DOI] [PubMed] [Google Scholar]
- Dehay C, Horsburgh G, Berland M, Killackey H, Kennedy H. The effects of bilateral enucleation in the primate fetus on the parcellation of visual cortex. Dev Brain Res. 1991;62:137–141. doi: 10.1016/0165-3806(91)90199-s. [DOI] [PubMed] [Google Scholar]
- Dehay C, Giroud P, Berland M, Smart I, Kennedy H. Modulation of the cell cycle contributes to the parcellation of the primate visual cortex. Nature. 1993;366:464–466. doi: 10.1038/366464a0. [DOI] [PubMed] [Google Scholar]
- Dehay C, Giroud P, Berland M, Killackey HP, Kennedy H. The contribution of thalamic input to the specification of cytoarchitectonic cortical fields in the primate: effects of bilateral enucleation in the fetal monkey on the boundaries and dimensions of striate and extrastriate cortex. J Comp Neurol. 1996a;367:70–89. doi: 10.1002/(SICI)1096-9861(19960325)367:1<70::AID-CNE6>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Dehay C, Giroud P, Berland M, Killackey H, Kennedy H. Phenotypic characterisation of respecified visual cortex subsequent to prenatal enucleation in the monkey: development of acetylcholinesterase and cytochrome oxydase patterns. J Comp Neurol. 1996b;376:386–402. doi: 10.1002/(SICI)1096-9861(19961216)376:3<386::AID-CNE3>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Dehay C, Savatier P, Cortay V, Kennedy H. Cell-cycle kinetics of neocortical precursors are influenced by embryonic thalamic axons. J Neurosci. 2001;21:201–214. doi: 10.1523/JNEUROSCI.21-01-00201.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Rio JA, Martinez A, Auladell C, Soriano E. Developmental history of the subplate and developing white matter in the murine neocortex. Neuronal organization and relationship with the main afferent systems at embryonic and perinatal stages. Cereb Cortex. 2000;10:784–801. doi: 10.1093/cercor/10.8.784. [DOI] [PubMed] [Google Scholar]
- Derer P, Nakanishi S. Extracellular matrix distribution during neocortical wall ontogenesis in ‘normal’ and ‘Reeler’ mice. J Hirnforsch. 1983;24:209–224. [PubMed] [Google Scholar]
- Donoghue MJ, Rakic P. Molecular gradients and compartments in the embryonic primate cerebral cortex. Cereb Cortex. 1999;9:586–600. doi: 10.1093/cercor/9.6.586. [DOI] [PubMed] [Google Scholar]
- Fernandez V, Bravo H. Autoradiographic study of development of the cerebral cortex in the rabbit. Brain Behav Evol. 1974;9:317–332. doi: 10.1159/000123674. [DOI] [PubMed] [Google Scholar]
- Hardy H, Heimer L, Switzer R, Watkins D. Simultaneous demonstration of horseradish peroxidase and acetylcholinesterase. Neurosci Lett. 1976;3:1–5. doi: 10.1016/0304-3940(76)90090-2. [DOI] [PubMed] [Google Scholar]
- Hartfuss E, Galli R, Heins N, Gotz M. Characterization of CNS precursor subtypes and radial glia. Dev Biol. 2001;229:15–30. doi: 10.1006/dbio.2000.9962. [DOI] [PubMed] [Google Scholar]
- Hicks SP, D’Amato CJ. Cell migrations to the isocortex in the rat. Anat Rec. 1968;160:619–634. doi: 10.1002/ar.1091600311. [DOI] [PubMed] [Google Scholar]
- Jackson CA, Peduzzi JD, Hickey TL. Visual cortex development in the ferret. I. Genesis and migration of visual cortical neurons. J Neurosci. 1989;9:1242–1253. doi: 10.1523/JNEUROSCI.09-04-01242.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy H, Dehay C. Cortical specification of mice and men. Cereb Cortex. 1993;3:27–35. doi: 10.1093/cercor/3.3.171. [DOI] [PubMed] [Google Scholar]
- Killackey HP, Belford G, Ryugo R, Ryugo DK. Anomalous organization of thalamocortical projections consequent to vibrissae removal in the newborn rat and mouse. Brain Res. 1976;104:309–315. doi: 10.1016/0006-8993(76)90623-5. [DOI] [PubMed] [Google Scholar]
- Killackey HP, Chiaia NL, Bennett-Clarke CA, Eck M, Rhoades RW. Peripheral influences on the size and organization of somatotopic representations in the fetal rat cortex. J Neurosci. 1994;14:1496–1506. doi: 10.1523/JNEUROSCI.14-03-01496.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konig N, Marty R. Early neurogenesis and synaptogenesis in cerebral cortex. Bibl Anat. 1981;119:152–160. [PubMed] [Google Scholar]
- Konig N, Roch G, Marty R. The onset of synaptogenesis in rat temporal cortex. Anat Embryol. 1975;148:73–87. doi: 10.1007/BF00315564. [DOI] [PubMed] [Google Scholar]
- Kostovic I, Rakic P. Cytology and time of origin of interstitial neurons in the white matter in infant and adult human and monkey telencephalon. J Neurocytol. 1980;9:219–242. doi: 10.1007/BF01205159. [DOI] [PubMed] [Google Scholar]
- Kostovic I, Rakic P. Development of prestriate visual projections in the monkey and human fetal cerebrum revealed by transient cholinesterase staining. J Neurosci. 1984;4:25–42. doi: 10.1523/JNEUROSCI.04-01-00025.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostovic I, Rakic P. Develomental history of the transient subplate zone in the visual cortex of the macaque monkey and human brain. J Comp Neurol. 1990;297:441–470. doi: 10.1002/cne.902970309. [DOI] [PubMed] [Google Scholar]
- Lavdas AA, Grigoriou M, Pachnis V, Parnavelas JG. The medial ganglionic eminence gives rise to a population of early neurons in the developing cerebral cortex. J Neurosci. 1999;19:7881–7888. doi: 10.1523/JNEUROSCI.19-18-07881.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt P, Cooper ML, Rakic P. Coexistence of neuronal and glial precursor cells in the cerebral ventricular zone of the fetal monkey: an ultrastructural immunoperoxidase analysis. J Neurosci. 1981;1:27–39. doi: 10.1523/JNEUROSCI.01-01-00027.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luskin MB, Shatz CJ. Neurogenesis of the cat’s primary visual cortex. J Comp Neurol. 1985;242:611–631. doi: 10.1002/cne.902420409. [DOI] [PubMed] [Google Scholar]
- Malatesta P, Hartfuss E, Gotz M. Isolation of radial glial cells by fluorescent-activated cell sorting reveals a neuronal lineage. Development. 2000;127:5253–5263. doi: 10.1242/dev.127.24.5253. [DOI] [PubMed] [Google Scholar]
- Marin-Padilla M. Dual origin of the mammalian neocortex and evolution of the subplate. Anat Embryol. 1978;152:109–126. doi: 10.1007/BF00315920. [DOI] [PubMed] [Google Scholar]
- Marin-Padilla M. Structural organization of the human cerebral cortex prior to the appearance of the cortical plate. Anat Embryol. 1983;168:21–40. doi: 10.1007/BF00305396. [DOI] [PubMed] [Google Scholar]
- Meinecke DL, Rakic P. Expression of GABA and GABAA receptors by reurons of the subplate zone in developing primate occipital cortex: evidence for transient local circuits. J Comp Neurol. 1992;317:91–101. doi: 10.1002/cne.903170107. [DOI] [PubMed] [Google Scholar]
- Mesulam M-M, Geula C. Chemoarchitectonics of axonal and perikaryal acetylcholinesterase along information processing systems of the human cerebral cortex. Brain Res Bull. 1994;33:137–153. doi: 10.1016/0361-9230(94)90244-5. [DOI] [PubMed] [Google Scholar]
- Meyer G, Schaaps JP, Moreau L, Goffinet AM. Embryonic and early fetal development of the human neocortex. J Neurosci. 2000;20:1858–1868. doi: 10.1523/JNEUROSCI.20-05-01858.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller B, Chou L, Finlay BL. The early development of thalamocortical and corticothalamic projections. J Comp Neurol. 1993;335:16–41. doi: 10.1002/cne.903350103. [DOI] [PubMed] [Google Scholar]
- Misson JP, Edwards MA, Yamamoto M, Caviness VSJ. Identification of radial glial cells within the developing murine central nervous system: studies based upon a new immunohistochemical marker. Dev Brain Res. 1988;44:95–108. doi: 10.1016/0165-3806(88)90121-6. [DOI] [PubMed] [Google Scholar]
- Misson JP, Austin CP, Takahashi T, Cepko CL, Caviness VSJ. The alignment of migrating neural cells in relation to the murine neopallial radial glial fiber system. Cereb Cortex. 1991;1:221–229. doi: 10.1093/cercor/1.3.221. [DOI] [PubMed] [Google Scholar]
- Miyashita-Lin EM, Hevner R, Wassarman KM, Martinez S, Rubenstein JL. Early neocortical regionalization in the absence of thalamic innervation. Science. 1999;285:906–909. doi: 10.1126/science.285.5429.906. [DOI] [PubMed] [Google Scholar]
- Molnar Z, Blakemore C. Development of signals influencing the growth and termination of thalamocortical axons in organotypic culture. Exp Neurol. 1999;156:363–393. doi: 10.1006/exnr.1999.7032. [DOI] [PubMed] [Google Scholar]
- Molnar Z, Adams R, Blakemore C. Mechanisms underlying the early establishment of thalamocortical connections in the rat. J Neurosci. 1998;18:5723–5745. doi: 10.1523/JNEUROSCI.18-15-05723.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noctor SC, Flint AC, Weissman TA, Dammerman RS, Kriegstein AR. Neurons derived from radial glial cells establish radial units in neocortex. Nature. 2001;409:714–20. doi: 10.1038/35055553. [DOI] [PubMed] [Google Scholar]
- O’Leary DDM. Do cortical areas emerge from a protocortex? Trends Neurosci. 1989;12:400–406. doi: 10.1016/0166-2236(89)90080-5. [DOI] [PubMed] [Google Scholar]
- O’Leary DDM, Stanfield BB. Selective elimination of axons extended by developing cortical neurons is dependent on regional locale: experiments utilizing fetal cortical transplants. J Neurosci. 1989;9:2230–2246. doi: 10.1523/JNEUROSCI.09-07-02230.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raedler A, Sievers J. The development of the visual system of the albino rat. Adv Anat Embryol Cell Biol. 1975;50:3–88. doi: 10.1007/978-3-642-45461-5. [DOI] [PubMed] [Google Scholar]
- Raedler E, Raedler A. Autoradiographic study of early neurogenesis in rat neocortex. Anat Embryol. 1978;154:267–284. doi: 10.1007/BF00345657. [DOI] [PubMed] [Google Scholar]
- Raedler E, Raedler A, Feldhaus S. Dynamical aspects of neocortical histogenesis in the rat. Anat Embryol. 1980;158:253–269. doi: 10.1007/BF00301816. [DOI] [PubMed] [Google Scholar]
- Rakic P. Mode of cell migration to the superficial layers of fetal monkey neocortex. J Comp Neurol. 1972;145:61–84. doi: 10.1002/cne.901450105. [DOI] [PubMed] [Google Scholar]
- Rakic P. Neurons in rhesus monkey visual cortex: systematic relation between time of origin and eventual disposition. Science. 1974;183:425–427. doi: 10.1126/science.183.4123.425. [DOI] [PubMed] [Google Scholar]
- Rakic P. Early developmental events: cell lineages, acquisition of neuronal positions and areal and laminar development. Neurosci Res Prog Bull. 1982;20:439–451. [PubMed] [Google Scholar]
- Rakic P. Specification of cerebral cortical areas. Science. 1988;241:170–176. doi: 10.1126/science.3291116. [DOI] [PubMed] [Google Scholar]
- Rakic P, Caviness VS., Jr Cortical development: view from neurological mutants two decades later. Neuron. 1995;14:1101–1104. doi: 10.1016/0896-6273(95)90258-9. [DOI] [PubMed] [Google Scholar]
- Rakic P, Suner I, Williams RW. A novel cytoarchitectonic area induced experimentally within the primate visual cortex. Proc Natl Acad Sci USA. 1991;88:2083–2087. doi: 10.1073/pnas.88.6.2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickmann M, Chronwall BM, Wolff JR. On the development of non-pyramidal neurons and axons outside the cortical plate: the early marginal zone as a pallial anlage. Anat Embryol. 1977;151:285–307. doi: 10.1007/BF00318931. [DOI] [PubMed] [Google Scholar]
- Robertson RT, Höhmann CF, Bruce JL, Coyle JT. Neonatal enucleations reduce specific activity of acetylcholinesterase but not choline acetyltransferase in developing rat visual cortex. Dev Brain Res. 1988;39:298–302. doi: 10.1016/0165-3806(88)90034-x. [DOI] [PubMed] [Google Scholar]
- Robertson RT, Mostamand F, Kageyama GH, Gallardo KA, Yu J. Primary auditory cortex in the rat: transient expression of acetylcholinesterase activity in developing geniculocortical projections. Dev Brain Res. 1991;58:81–95. doi: 10.1016/0165-3806(91)90240-j. [DOI] [PubMed] [Google Scholar]
- Rockel AJ, Hiorns RW, Powell TPS. The basic uniformity in structure of the neocortex. Brain. 1980;103:221–244. doi: 10.1093/brain/103.2.221. [DOI] [PubMed] [Google Scholar]
- Sauer FC. Mitosis in the neural tube. J Comp Neurol. 1935;62:377–405. [Google Scholar]
- Sauer F. The interkinetic migration of embryonic nuclei. J Morphol. 1936;60:1–11. [Google Scholar]
- Schlaggar BL, De Carlos JA, O’Leary DDM. Acetylcholinesterase as an early marker of the differentiation of dorsal thalamus in embryonic rats. Dev Brain Res. 1993;75:19–30. doi: 10.1016/0165-3806(93)90061-e. [DOI] [PubMed] [Google Scholar]
- Schmechel DE, Rakic P. Arrested proliferation of radial glial cells during midgestation in rhesus monkey. Nature. 1979;277:303–305. doi: 10.1038/277303a0. [DOI] [PubMed] [Google Scholar]
- Sendemir E, Erzurumlu RS, Jhaveri S. Differential expression of acetylcholinesterase in the developing barrel cortex of three rodent species. Cereb Cortex. 1996;6:377–387. doi: 10.1093/cercor/6.3.377. [DOI] [PubMed] [Google Scholar]
- Shatz CJ, Rakic P. The genesis of efferent connections from the visual cortex of the fetal rhesus monkey. J Comp Neurol. 1981;196:287–307. doi: 10.1002/cne.901960208. [DOI] [PubMed] [Google Scholar]
- Sidman RL, Rakic P. Neuronal migration, with special reference to developing human brain: a review. Brain Res. 1973;62:1–35. doi: 10.1016/0006-8993(73)90617-3. [DOI] [PubMed] [Google Scholar]
- Smart IHM. Proliferative characteristics of the ependymal layer during the early development of the mouse neocortex: a pilot study based on recording the number, location and plane of cleavage of mitotic figures. J Anat. 1973;116:67–91. [PMC free article] [PubMed] [Google Scholar]
- Smart IHM. Three dimensional growth of the mouse isocortex. J Anat. 1983;137:683–694. [PMC free article] [PubMed] [Google Scholar]
- Smart IHM, McSherry GM. Growth patterns in the lateral wall of the mouse telencephalon: II. Histological changes during and subsequent to the period of isocortical neuron production. J Anat. 1982;134:415–442. [PMC free article] [PubMed] [Google Scholar]
- Smart IHM, Smart M. Growth patterns in the lateral wall of the mouse telencephalon: I. Autoradiographic studies of the histogenesis of the isocortex and adjacent areas. J Anat. 1982;134:273–298. [PMC free article] [PubMed] [Google Scholar]
- Tamamaki N, Fujimori KE, Takauji R. Origin and route of tangentially migrating neurons in the developing neocortical intermediate zone. J Neurosci. 1997;17:8313–8323. doi: 10.1523/JNEUROSCI.17-21-08313.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valverde F, Facal-Valverde MV. Postnatal development of interstitial (subplate) cells in the white matter of the temporal cortex of kittens: a correlated golgi and electron microscopic study. J Comp Neurol. 1988;269:168–192. doi: 10.1002/cne.902690203. [DOI] [PubMed] [Google Scholar]
- Van der Loos H, Woolsey TA. Somatosensory cortex: structural alteration following early injury to sense organs. Science. 1973;179:395–398. doi: 10.1126/science.179.4071.395. [DOI] [PubMed] [Google Scholar]
- Van Essen DC, Newsome WT, Maunsell JHR. The visual field representation in striate cortex of the macaque monkey: asymetries, anisotropies, and individual variability. Neuroscience. 1984;24:429–448. doi: 10.1016/0042-6989(84)90041-5. [DOI] [PubMed] [Google Scholar]
- Wahle P, Meyer G. Morphology and quantitative changes of transient NPY-ir neuronal populations during early postnatal development of the cat visual cortex. J Comp Neurol. 1987;261:165–192. doi: 10.1002/cne.902610202. [ISI][Medline] [DOI] [PubMed] [Google Scholar]
- Wichterle H, Garcia-Verdugo JM, Herrera DG, Alvarez-Buylla A. Young neurons from medial ganglionic eminence disperse in adult and embryonic brain. Nat Neurosci. 1999;2:461–466.0. doi: 10.1038/8131. [DOI] [PubMed] [Google Scholar]
- Wolff JR. Ontogenetic aspects of cortical architecture: lamination. In: Bravier HP, editor. Architectonics of the cerebral cortex. New York: Raven Press; 1978. pp. 159–173. [Google Scholar]
- Woo TU, Beale JM, Finlay BL. Dual fate of subplate neurons in a rodent. Cereb Cortex. 1991;1:433–443. doi: 10.1093/cercor/1.5.433. [DOI] [PubMed] [Google Scholar]
- Woodward WR, Coull BM. Localization and organization of geniculocortical and corticofugal fiber tracts within the subcortical white matter. Neuroscience. 1984;12:1089–1099. doi: 10.1016/0306-4522(84)90005-8. [DOI] [PubMed] [Google Scholar]