Secreted PCSK9 promotes LDL receptor degradation independently of proteolytic activity (original) (raw)
Abstract
PCSK9 (proprotein convertase subtilisin/kexin 9) is a secreted serine protease that regulates cholesterol homoeostasis by inducing post-translational degradation of hepatic LDL-R [LDL (low-density lipoprotein) receptor]. Intramolecular autocatalytic processing of the PCSK9 zymogen in the endoplasmic reticulum results in a tightly associated complex between the prodomain and the catalytic domain. Although the autocatalytic processing event is required for proper secretion of PCSK9, the requirement of proteolytic activity in the regulation of LDL-R is currently unknown. Co-expression of the prodomain and the catalytic domain in trans allowed for production of a catalytically inactive secreted form of PCSK9. This catalytically inactive PCSK9 was characterized and shown to be functionally equivalent to the wild-type protein in lowering cellular LDL uptake and LDL-R levels. These findings suggest that, apart from autocatalytic processing, the protease activity of PCSK9 is not necessary for LDL-R regulation.
Keywords: atherosclerosis, coronary heart disease, hypercholesterolaemia, low-density lipoprotein receptor (LDL-R), proprotein convertase subtilisin/kexin 9 (PCSK9), _trans_-assembly
Abbreviations: ADH, autosomal dominant hypercholesterolaemia; CHD, coronary heart disease; DiI, 1,1′-dioctadecyl-3,3,3′,3′-tetramethyl-indocarbocyanine perchlorate; ER, endoplasmic reticulum; LDL, low-density lipoprotein; LDL-C, low-density lipoprotein cholesterol; LDL-R, low-density lipoprotein receptor; NARC-1, neural apoptosis-regulated convertase 1; PCSK9, proprotein convertase subtilisin/kexin 9; ta, _trans_-assembled; wt, wild-type
INTRODUCTION
PCSK9 (proprotein convertase subtilisin/kexin 9) also known as NARC-1 (neural apoptosis-regulated convertase 1) was first described in 2003 as the ninth member of the proprotein convertase subtilisin/kexin family [1]. Various genetic studies subsequently defined PCSK9 as the third gene [in addition to LDLR (low-density lipoprotein receptor) and APOB (apolipoprotein B)] to cause ADH (autosomal dominant hypercholesterolemia) and a number of PCSK9 gain-of-function mutations were identified from families affiliated with ADH [2–5]. Consistent with these findings, subjects carrying loss-of-function mutations (Y142X and C679X) exhibited a 28% reduction of plasma LDL-C (low-density lipoprotein cholesterol) levels and an 88% decrease in the risk of CHD (coronary heart disease) in a 15-year follow-up survey [6]. The human genetic data are in agreement with the observations in mice. Plasma cholesterol levels are approx. 50% lower in Pcsk9 knockout mice owing to increased clearance of lipoproteins from plasma [7]. No apparent physiological or behavioural abnormality was observed from knockout mice or from a human subject carrying compound heterozygous loss-of-function mutations [7,8].
Several studies have shown that PCSK9 exerts its role on cholesterol metabolism through post-translational down-regulation of the LDL-R, the receptor responsible for clearing the majority of LDL-C from the plasma. Maxwell and Breslow [9] discovered that adenoviral expression of PCSK9 efficiently increased plasma LDL-C levels in normal mice, but not in LDL-R-deficient mice. They also found that transfection of PCSK9 in McA-RH777 cells caused a reduction in LDL-R protein and LDL uptake with no effect on LDLR mRNA levels. Consistent with these observations, genetic deletion of PCSK9 in mice resulted in increased LDL-R protein levels, but not mRNA levels [7]. PCSK9 mediated reduction of LDL-R protein has also been reported in a recent mouse parabiosis study by Lagace et al. [10] where a loss of liver LDL-R protein was observed in recipients of parabiosed PCSK9 protein.
Secretory subtilisin-like serine proteases are typically translated into the ER (endoplasmic reticulum) as zymogen precursor proteins that undergo autocatalytic cleavage of the N-terminal prodomain from the C-terminal catalytic domain [11]. The prodomain remains non-covalently bound to the catalytic domain inhibiting proteolytic activity until a second cleavage event in the prodomain occurs that disrupts the interaction allowing for full catalytic activity [11]. Like the other subtilisin-like proteases, PCSK9 also requires intramolecular processing for proper folding and trafficking as active-site mutations result in retention of the unprocessed PCSK9 zymogen in the ER [1,12]. However, unlike other subtilisin-like proteases, there is no evidence that PCSK9 prodomain ever dissociates from the catalytic domain. Even upon secretion the prodomain is associated with the catalytic domain, as evidenced by several studies that have characterized the secreted PCSK9 complex [10,13]. Furthermore, two newly published crystal structures of secreted PCSK9 verify that the prodomain remains non-covalently associated with the mature protein and sterically blocks the active-site cleft [14,15].
Despite the apparent inhibition of the catalytic activity by the prodomain, the secreted PCSK9 complex is functional, as the purified protein or conditioned medium from PCSK9-producing cells decreases cellular LDL-R levels and LDL-C uptake [10,16]. The precise mechanism underlying PCSK9-induced degradation of the LDL-R is unknown, largely due to a lack of tools with which to characterize the dependence of PCSK9 proteolytic activity in this process. In the present paper, we report the production of the secreted PCSK9 complex through co-expression in trans of the PCSK9 prodomain and catalytic domain. The PCSK9 protein complex produced in this manner allows for the mutation of residues in the catalytic domain without the disruption of the autocatalytic processing and trafficking. Using this method, an active-site mutant of PCSK9 was produced and compared with wt (wild-type) PCSK9 in functional cellular assays of LDL-R and LDL-C regulation.
METHODS
Plasmid construction
Plasmid (pRS5a/PCSK9) was constructed to express wild-type PCSK9 (sequence identical with that of Q8NBP7). To produce secreted S386A mutant, two plasmids [SigPro and SigMature(S386A)] were constructed to direct the co-translation of the prodomain and the mature S386A PCSK9 in the ER. (Details of each construction can be found in the Supplementary material at http://www.BiochemJ.org/bj/406/bj4060203add.htm).
Protein expression and purification
All PCSK9 and control proteins were expressed in HEK-293 (human embryonic kidney 293) Freestyle cells and were affinity-purified from culture media (details are available in the Supplementary material at http://www.BiochemJ.org/bj/406/bj4060203add.htm).
LDL-uptake assay
HepG2 cells were seeded in a collagen-coated 96-well plate (BD Biosciences) and incubated overnight at 37°C (under 5% CO2) to allow cell attachment. The following day, PCSK9 proteins were diluted into each well to the desired concentrations and incubated at 37°C for 3 h. DiI-LDL (LDL labelled with 1,1′-dioctadecyl-3,3,3′,3′-tetramethyl-indocarbocyanine perchlorate; Biomedical Technologies) was then added to a final concentration of 5 μg/ml and incubated for an additional 1 h. Cells were trypsinized and transferred to a 96-well V-bottom plate (Nunc) containing equal volume of 2 μg/ml DAPI (4′,6-diamidino-2-phenylindole). Finally, cells were pelleted by centrifugation and resuspended in ice-cold FACS buffer [5% (v/v) foetal bovine serum, 0.2% sodium azide and 2 mM EDTA in PBS) for flow-cytometric analysis on a BD LSR-II cytometer. Geometric means were analysed using FlowJo 5.7.2 software, normalized to buffer control, and non-linearly fitted to the Sigmoidal dose–response equation using Prism 4 software.
Surface LDL-R assay
HepG2 cells were trypsinized, seeded on a collagen-coated plate and incubated overnight at 37°C under 5% CO2 to allow the recovery of LDL-R expression. The following day, proteins samples were added, and cells were incubated for 4 h before harvesting with Versene treatment (Invitrogen). Harvested cells were blocked with donkey serum (Jackson ImmunoResearch Laboratories) prior to staining with a rabbit anti-(human LDL-R) polyclonal antibody (Fitzgerald), followed by APC (allophycocyanin)-conjugated donkey anti-rabbit IgG antibody (Jackson ImmunoResearch Laboratories). After washing, cells were fixed with 2% (w/v) paraformaldehyde and subjected to flow-cytometric analysis on a BD LSR-II cytometer. The averages of geometric means were calculated using FlowJo 5.7.2 software and reported in (Figure 2B) and Supplementary Figure 1 (http://www.BiochemJ.org/bj/406/bj4060203add.htm).
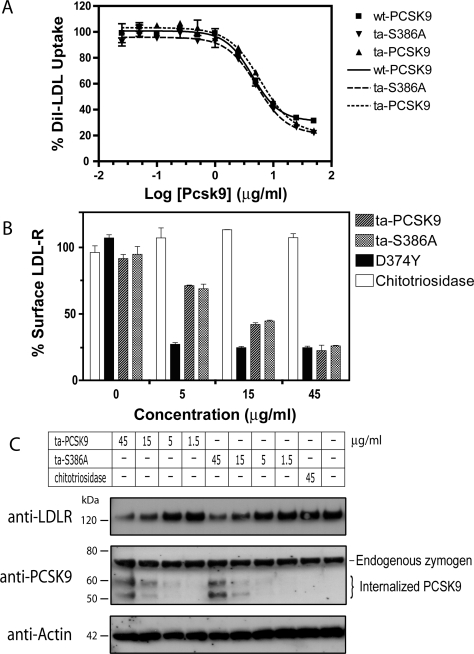
Figure 2. Ta-S386A is functionally equivalent to wt-PCSK9.
(A) Effect on LDL uptake by HepG2 cells. HepG2 cells were incubated with purified wt-PCSK9, ta-S386A or ta-PCSK9 at the indicated concentrations for 3 h and subsequently exposed to 5 μg/ml DiI-LDL for an additional 1 h prior to flow-cytometric analysis. Geometric means of duplicates were nonlinearly fitted to a Sigmoidal dose–response equation and representative fitting curves were shown as: solid line, wt-PCSK9; large dashes, ta-S386A; small dashes, ta-PCSK9. The calculated EC50 values (concentration of PCSK9 required to reach 50% of the maximal reduction) were 4.4±1.1 μg/ml (wt-PCSK9), 6.1±1.1 μg/ml (ta-S386A) and 5.2±1.1 μg/ml (ta-PCSK9) (_n_=2). (B) Effect on cell-surface LDL-R level. HepG2 cells were incubated with ta-PCSK9, ta-S386A, gain-of-function mutant D374Y or control protein chitotriosidase for 4 h, stained with anti-LDL-R polyclonal antibody and subjected to FACS analysis. The averages of the geometric means (_n_=3) are plotted as percentage of buffer control. (C) Effect on cellular concentration of LDL-R. HepG2 cells were incubated with the indicated concentrations of ta-PCSK9, ta-S386A or chitotriosidase control for 4 h before harvesting. Soluble cell lysates (50 μg) were subjected to reducing SDS/PAGE and transferred on to nitrocellulose membranes for immunoblotting with anti-LDLR (top) and anti-PCSK9 (middle) antibodies. Approximately equal sample loading was demonstrated using an anti-actin antibody (bottom).
Total LDL-R assay
HepG2 cells were treated with PCSK9 proteins for 4 h before lysis in RIPA (radioimmunoprecipitation) buffer supplemented with protease inhibitor cocktail (Roche). The lysates were cleared by centrifugation, resolved in a 4–12% Bis/Tris NuPage gel (50 μg per sample) and transferred on to nitrocellulose membranes. After blocking with 1% (w/v) casein, the membranes were incubated overnight with rabbit polyclonal anti-(human LDL-R), anti-PCSK9 (Cayman) or anti-actin (Sigma) antibodies. The membranes were subsequently incubated with an HRP (horseradish peroxidase)-conjugated goat anti-rabbit antibody (BioRad) followed by reaction with ECL (enhanced chemiluminesence) Western blotting substrate (Pierce) and visualization with Hyperfilm ECL® (Amersham Biosciences).
RESULTS AND DISCUSSION
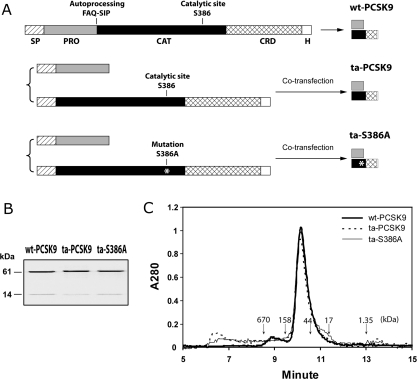
Prodomains of secretory subtilisin-like serine proteases serve as intramolecular chaperones that are required for proper folding of the zymogen. The zymogen subsequently undergoes autocatalytic cleavage between the N-terminal prodomain and the C-terminal catalytic domain. Intramolecular autocatalytic processing is a prerequisite for proper folding and secretion of the functionally mature enzyme [11,17]. The prodomains of bacterial serine proteases have been shown to fold and activate the protease domain not only in an intramolecular fashion, but also in an intermolecular fashion [18,19]. An in trans co-expression strategy was used in the present study to produce a secreted catalytically inactive PCSK9 mutant. Two expression plasmids were constructed to direct the co-expression of PCSK9 prodomain and the S386A catalytic domain mutant in the ER (Figure 1A). At 48 h after co-transfection of the plasmids, the prodomain and catalytic domain of PCSK9 were both detected in the culture medium by a polyclonal antibody that recognizes both the prodomain and the catalytic domain of PCSK9 (results not shown). The secreted PCSK9 mutant was purified via a hexahistidine tag tethered to the C-terminus of mature PCSK9 (Figure 1B). This ta (_trans_-assembled) catalytic-site mutant (ta-S386A) was found composed of the prodomain (14 kDa) and catalytic domain (61 kDa) that showed similar electrophoretic mobility to their counterparts from autoprocessed wt-PCSK9 or ta-PCSK9. Gel filtration analysis revealed that the apparent molecular mass of ta-S386A was 75.6 kDa, confirming that ta-S386A forms a similar complex as wt-PCSK9 with 1:1 stoichiometry between prodomain and mature PCSK9 (Figure 1C).
Figure 1. Production of the ta-S386A mutant.
(A) Schematic diagram of the expression strategy for wt-PCSK9, ta-PCSK9 and ta-S386A proteins. The wt-PCSK9 protein is expressed from a single construct as described in the Methods section. The single-chain precursor is biosynthesized in the ER, auto-cleaved between Gln152 and Ser153, and secreted as a prodomain–catalytic domain bound complex. The ta-PCSK9 and ta-S386A proteins are produced with a two-plasmid co-transfection strategy, as described in the Methods section. The prodomain and catalytic domain of PCSK9, either wt or bearing the S386A mutation, are translated in the ER via separate secretion signal peptides, then folded in trans, assembled and secreted in the same complex form as wt-PCSK9. Segments on each construct are indicated as follows: SP, signal peptide; PRO, prodomain; CAT, catalytic domain; CRD, cysteine-rich domain; H, hexahistidine tag. (B) Electrophoretic analysis of purified PCSK9 variants. All PCSK9 variants were affinity purified via the C-terminal hexahistidine tag and were subjected to reducing SDS/PAGE (0.5 μg of protein per lane). The prodomains (14 kDa) and mature PCSK9 subunits (61 kDa) were visualized by Coomassie Blue staining. (C) Analytic size-exclusion chromatography of PCSK9 variants wt-PCSK9, ta-PCSK9 and ta-S386A. Purified samples (30 μg) were subjected to gel filtration on a Shodex KW-803 column equilibrated with 20 mM Tris/HCl (pH 7.6), 200 mM NaCl, 0.5 mM TCEP [tris-(2-carboxyethyl)phosphine]. Molecular mass references are indicated by downward arrows: thyroglobulin (670 kDa), γ-globulin (158 kDa), ovalbumin (44 kDa), myoglobin (17 kDa) and vitamin B12 (1.35 kDa).
Purified PCSK9 protein and conditioned medium from PCSK9-producing cells have been reported to decrease cellular LDL uptake and LDL-R protein levels [10,16]. To test if ta-S386A is functional in regulating LDL-C uptake, HepG2 cells were incubated with a range of concentrations of wt-PCSK9, ta-S386A or ta-PCSK9 proteins prior to measuring LDL-C uptake. All three variants exhibited essentially identical dose-dependent reduction of LDL-C uptake (Figure 2A) with EC50 values of 4.4 μg/ml (wt-PCSK9), 5.2 μg/ml (ta-S386A) and 6.1 μg/ml (ta-PCSK9). To confirm that LDL-R levels are decreased as suggested by the LDL-C uptake data, cell-surface LDL-R levels were measured by flow cytometry after HepG2 cells were treated with PCSK9 proteins or control. PCSK9 [D374Y], a known gain-of-function mutant, reduced the surface LDL-R level to the maximal extent at concentrations as low as 5 μg/ml. Both ta-S386A and ta-PCSK9 decreased cell-surface LDL-R levels in a similar concentration-dependent fashion. In contrast, chitotriosidase, a non-relevant protein, which was expressed and purified in the same manner as the PCSK9 variants, showed no effects at any of the concentrations tested (Figure 2B). In a separate LDL-R flow-cytometric experiment, wt-PCSK9, ta-S386A and ta-PCSK9 were tested side-by-side, and the activities were found to be similar among the three variants (see Supplementary Figure 1). Individual LDL-R molecules can cycle between the cell surface and endosome more than 100 times to import LDL-C before their eventual degradation [20]. To ensure that ta-S386A indeed promotes LDL-R degradation instead of simply sequestering it into subcellular compartments, lysates from PCSK9-treated HepG2 cells were subject to immunoblotting with a polyclonal antibody against mature LDL-R. It was clearly observed that incubation with ta-S386A (15 and 45 μg/ml) caused the same extent of decrease in intracellular LDL-R content as ta-PCSK9 treated cells (Figure 2C). At lower concentrations (1.5 and 5 μg/ml), the intracellular LDL-R levels of ta-S386A or ta-PCSK9 treated cells remain the same as that of untreated or chitotriosidase controls. Interestingly, the levels of PCSK9 zymogen are constant across all samples, whereas exogenous PCSK9 mature domain and a breakdown product were seen in cells exposed to higher concentrations of PCSK9 proteins that resulted in LDL-R decrease (15 and 45 μg/ml) (Figure 2C).
Purified PCSK9 binds directly to extracellular domain of LDL-R with over 100-fold lower affinity at neutral pH compared with acidic endosomal pH [14,21]. When added to cultured cells, PCSK9 is internalized in a LDL-R-dependent manner and relocates the LDL-R from the plasma membrane to the lysosomes for degradation [22]. PCSK9-induced degradation of the LDLR relies on acidic pH, but is insensitive to inhibitors of the proteasome, lysosomal cysteine proteases, aspartic acid proteases or metalloproteases [23]. Given the proteolytic nature of PCSK9, it is tempting to suggest that the prodomain-inhibited PCSK9 sequesters the membrane bound LDL-R into lysosomes and, upon pH change, becomes activated for a programmed LDL-R clearance. However, there is currently no evidence for catalytically active PCSK9 beyond autoprocessing or observation of recombinant LDL-R cleavage by PCSK9. The inactive mutant produced in the present study displayed activity equivalent to that of wt PCSK9 in reducing LDL-C uptake and surface/cellular LDL-R levels (Figure 2 and Supplementary Figure 1). Our finding provides direct evidence that the proteolytic activity is not required for secreted PCSK9 to down-regulate the LDL-R protein levels, and strongly implies that PCSK9-mediated LDL-R degradation is through a binding or chaperone activity of PCSK9. The protease(s) responsible for lysosomal degradation of accumulated LDL-R merits further investigation.
Caution should be taken in evaluating the overall role of PCSK9 protease activity in LDL-R regulation. First, intramolecular autocatalytic activity is required for processing the PCSK9 zymogen into the functional prodomain–catalytic domain complex, and entry into secretory pathway [1,12]. Secondly, for other members of proprotein convertase family such as furin, a secondary cleavage occurs post-autoprocessing to disrupt the prodomain and thereby removes the inhibition of the catalytic domain [11]. PCSK9 differs from the other members of the proprotein convertase family in that the PCSK9 prodomain remains tightly associated with the catalytic domain and blocks the active site. However, we cannot exclude the possibility that the prodomain dissociates from the catalytic domain resulting in a proteolytically competent enzyme. On the basis of the data presented in the present paper, we can only conclude that proteolytic activity of the secreted PCSK9 complex is not necessary to regulate LDL-R degradation and LDL-C uptake. Further understanding of the nature of the interaction and regulation of PCSK9 and LDL-R will provide additional guidance on targeting PCSK9 for the therapeutic intervention.
While the current manuscript was under review, another manuscript by McNutt et al. [24] was published that supports our claim that catalytic activity is not required for secreted PCSK9 to reduce LDL-R in HepG2 cells.
Online data
Supplemental online data
Acknowledgments
We thank Eric Peters, Scott Brittain and Daniel Mason (Genomics Institute) for MS analysis, Chris Trussell (Genomics Institute) for assistance with flow-cytometric studies, Edward Nigoghossian (Genomics Institute) for analytic chromatography, Julian Levell (Novartis Institute) and Andrew Schumacher (Genomics Institute) for helpful discussion and Peter Schultz (Genomics Institute) for continued support.
References
- 1.Seidah N. G., Benjannet S., Wickham L., Marcinkiewicz J., Jasmin S. B., Stifani S., Basak A., Prat A., Chretien M. The secretory proprotein convertase neural apoptosis-regulated convertase 1 (NARC-1): liver regeneration and neuronal differentiation. Proc. Natl. Acad. Sci. U.S.A. 2003;100:928–933. doi: 10.1073/pnas.0335507100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abifadel M., Varret M., Rabes J. P., Allard D., Ouguerram K., Devillers M., Cruaud C., Benjannet S., Wickham L., Erlich D., et al. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat. Genet. 2003;34:154–156. doi: 10.1038/ng1161. [DOI] [PubMed] [Google Scholar]
- 3.Shioji K., Mannami T., Kokubo Y., Inamoto N., Takagi S., Goto Y., Nonogi H., Iwai N. Genetic variants in PCSK9 affect the cholesterol level in Japanese. J. Hum. Genet. 2004;49:109–114. doi: 10.1007/s10038-003-0114-3. [DOI] [PubMed] [Google Scholar]
- 4.Timms K. M., Wagner S., Samuels M. E., Forbey K., Goldfine H., Jammulapati S., Skolnick M. H., Hopkins P. N., Hunt S. C., Shattuck D. M. A mutation in PCSK9 causing autosomal-dominant hypercholesterolemia in a Utah pedigree. Hum. Genet. 2004;114:349–353. doi: 10.1007/s00439-003-1071-9. [DOI] [PubMed] [Google Scholar]
- 5.Leren T. P. Mutations in the PCSK9 gene in Norwegian subjects with autosomal dominant hypercholesterolemia. Clin. Genet. 2004;65:419–422. doi: 10.1111/j.0009-9163.2004.0238.x. [DOI] [PubMed] [Google Scholar]
- 6.Cohen J. C., Boerwinkle E., Mosley T. H., Jr, Hobbs H. H. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N. Engl. J. Med. 2006;354:1264–1272. doi: 10.1056/NEJMoa054013. [DOI] [PubMed] [Google Scholar]
- 7.Rashid S., Curtis D. E., Garuti R., Anderson N. N., Bashmakov Y., Ho Y. K., Hammer R. E., Moon Y. A., Horton J. D. Decreased plasma cholesterol and hypersensitivity to statins in mice lacking Pcsk9. Proc. Natl. Acad. Sci. U.S.A. 2005;102:5374–5379. doi: 10.1073/pnas.0501652102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao Z., Tuakli-Wosornu Y., Lagace T. A., Kinch L., Grishin N. V., Horton J. D., Cohen J. C., Hobbs H. H. Molecular characterization of loss-of-function mutations in PCSK9 and identification of a compound heterozygote. Am. J. Hum. Genet. 2006;79:514–523. doi: 10.1086/507488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maxwell K. N., Breslow J. L. Adenoviral-mediated expression of Pcsk9 in mice results in a low-density lipoprotein receptor knockout phenotype. Proc. Natl. Acad. Sci. U.S.A. 2004;101:7100–7105. doi: 10.1073/pnas.0402133101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lagace T. A., Curtis D. E., Garuti R., McNutt M. C., Park S. W., Prather H. B., Anderson N. N., Ho Y. K., Hammer R. E., Horton J. D. Secreted PCSK9 decreases the number of LDL receptors in hepatocytes and in livers of parabiotic mice. J. Clin. Invest. 2006;116:2995–3005. doi: 10.1172/JCI29383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seidah N. G., Prat A. The proprotein convertases are potential targets in the treatment of dyslipidemia. J. Mol. Med. 2007 doi: 10.1007/s00109-007-0172-7. 10.1007/s00109-007-0172-7. [DOI] [PubMed]
- 12.Park S. W., Moon Y. A., Horton J. D. Post-transcriptional regulation of low density lipoprotein receptor protein by proprotein convertase subtilisin/kexin type 9a in mouse liver. J. Biol. Chem. 2004;279:50630–50638. doi: 10.1074/jbc.M410077200. [DOI] [PubMed] [Google Scholar]
- 13.Benjannet S., Rhainds D., Essalmani R., Mayne J., Wickham L., Jin W., Asselin M. C., Hamelin J., Varret M., Allard D., et al. NARC-1/PCSK9 and its natural mutants: zymogen cleavage and effects on the low density lipoprotein (LDL) receptor and LDL cholesterol. J. Biol. Chem. 2004;279:48865–48875. doi: 10.1074/jbc.M409699200. [DOI] [PubMed] [Google Scholar]
- 14.Cunningham D., Danley D. E., Geoghegan K. F., Griffor M. C., Hawkins J. L., Subashi T. A., Varghese A. H., Ammirati M. J., Culp J. S., Hoth L. R., et al. Structural and biophysical studies of PCSK9 and its mutants linked to familial hypercholesterolemia. Nat. Struct. Mol. Biol. 2007;14:413–419. doi: 10.1038/nsmb1235. [DOI] [PubMed] [Google Scholar]
- 15.Piper D. E., Jackson S., Liu Q., Romanow W. G., Shetterly S., Thibault S. T., Shan B., Walker N. P. The crystal structure of PCSK9: a regulator of plasma LDL-cholesterol. Structure. 2007;15:545–552. doi: 10.1016/j.str.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 16.Cameron J., Holla O. L., Ranheim T., Kulseth M. A., Berge K. E., Leren T. P. Effect of mutations in the PCSK9 gene on the cell surface LDL receptors. Hum. Mol. Genet. 2006;15:1551–1558. doi: 10.1093/hmg/ddl077. [DOI] [PubMed] [Google Scholar]
- 17.Baker D., Shiau A. K., Agard D. A. The role of pro regions in protein folding. Curr. Opin. Cell Biol. 1993;5:966–970. doi: 10.1016/0955-0674(93)90078-5. [DOI] [PubMed] [Google Scholar]
- 18.Silen J. L., Agard D. A. The α-lytic protease pro-region does not require a physical linkage to activate the protease domain in vivo. Nature. 1989;341:462–464. doi: 10.1038/341462a0. [DOI] [PubMed] [Google Scholar]
- 19.Zhu X. L., Ohta Y., Jordan F., Inouye M. Pro-sequence of subtilisin can guide the refolding of denatured subtilisin in an intermolecular process. Nature. 1989;339:483–484. doi: 10.1038/339483a0. [DOI] [PubMed] [Google Scholar]
- 20.Brown M. S., Herz J., Goldstein J. L. LDL-receptor structure: calcium cages, acid baths and recycling receptors. Nature. 1997;388:629–630. doi: 10.1038/41672. [DOI] [PubMed] [Google Scholar]
- 21.Fisher T. S., Lo Surdo P., Pandit S., Mattu M., Santoro J. C., Wisniewski D., Cummings R. T., Calzetta A., Cubbon R. M., Fischer P. A., et al. PCSK9-dependent LDL receptor regulation: effects of pH and LDL. J. Biol. Chem. 2007;282:20502–20512. doi: 10.1074/jbc.M701634200. [DOI] [PubMed] [Google Scholar]
- 22.Zhang D. W., Lagace T. A., Garuti R., Zhao Z., McDonald M., Horton J. D., Cohen J. C., Hobbs H. H. Binding of PCSK9 to EGF-A repeat of LDL receptor decreases receptor recycling and increases degradation. J. Biol. Chem. 2007;282:18602–18612. doi: 10.1074/jbc.M702027200. [DOI] [PubMed] [Google Scholar]
- 23.Maxwell K. N., Fisher E. A., Breslow J. L. Overexpression of PCSK9 accelerates the degradation of the LDLR in a post-endoplasmic reticulum compartment. Proc. Natl. Acad. Sci. U.S.A. 2005;102:2069–2074. doi: 10.1073/pnas.0409736102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McNutt M. C., Lagace T. A., Horton J. D. Catalytic activity is not required for secreted PCSK9 to reduce LDL receptors in HepG2 cells. J. Biol. Chem. 2007 doi: 10.1074/jbc.C700095200. 10.1074/jbc.C700095200. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental online data