Disparate effects of acute and chronic infection with SIVmac239 or SHIV-89.6P on macaque plasmacytoid dendritic cells (original) (raw)
Abstract
Blood plasmacytoid dendritic cells (pDCs) contribute to both innate and adaptive immune responses by secreting high levels of IFN-α following acute bacterial and viral infections and indirectly by augmenting cell-mediated immunity. Cross-sectional studies have shown that the number of circulating pDCs in HIV patients, compared to that in uninfected individuals, is reduced. However, since the time of infection is usually unknown in HIV-infected patients, pDC–virus interactions that occur immediately after virus exposure are poorly understood. The current study investigated pDC dynamics during acute and chronic infections of macaques with either SIVmac239 or the pathogenic SIV–HIV chimera, SHIV-89.6P, as models for HIV infection. In three rhesus and three pig-tailed macaques infected intravenously with SIVmac239, the percentages of pDCs in blood declined 2- to 6-fold during the first 6 weeks after infection and remained depressed throughout the disease course. Surprisingly, no consistent, comparable decline in peripheral blood pDCs was observed in six macaques infected with SHIV-89.6P. In this latter group, percentages of pDCs did not correlate with CD4+ T cells, but there was an inverse relationship with viral load. In addition, when compared to naïve controls, the percentages of pDCs were reduced in spleens and peripheral lymph nodes of SIVmac239- but not SHIV-89.6P-infected animals that had progressed to AIDS. Proviral DNA was detected during the acute phase in pDCs isolated from macaques infected with either virus. These results imply that, even though macaque pDCs can be infected by both SIVmac239 and SHIV-89.6P, the subsequent effects on in vivo pathogenesis differ. The underlying mechanism(s) for these differences is unclear, but the selection of SIV or SHIV as a challenge virus might influence the outcome of some studies, such as those evaluating vaccines or the therapeutic efficacy of drugs.
Keywords: Plasmacytoid dendritic cells, SIVmac239, SHIV-89.6P, Macaque, Pathogenesis
Introduction
Plasmacytoid dendritic cells (pDCs) are key effectors in innate immunity, are among the first responders to bacterial or viral infections, and produce more interferon (IFN)-α than any other cell type (Colonna et al., 2004, Siegal et al., 1999). Phenotypically, human pDCs are characterized as CD123+HLA-DR+ lineage-negative (Lin−, CD3−CD14−CD16−CD20−CD56−) cells and are distinct from the other major blood DC type, myeloid DCs (mDCs), which express CD11c, but not CD123. In response to Toll-like receptor 9 (TLR9) recognition of unmethylated CpG motifs found in the genomes of bacteria and some DNA viruses, pDCs become activated, undergo maturation, and secrete cytokines (Bauer et al., 2001, Del Corno et al., 2005, Krug et al., 2001, Lund et al., 2003, Teleshova et al., 2004, Teleshova et al., 2006). Based on their ability to activate pDCs, short oligodeoxynucleotides containing CpG motifs have been used as adjuvants in studies evaluating various vaccine candidates for immunogenicity (Cafaro et al., 2001, Jones et al., 1999). Since pDCs also express TLR7 and TLR8, that recognize single-stranded RNA, these cells are activated following infection by RNA viruses, including the human immunodeficiency virus (HIV) (Beignon et al., 2005, Diebold et al., 2004, Lund et al., 2004).
Innate and adaptive immune systems are linked indirectly by pDCs, which produce cytokines that augment NK-cell and virus-specific cytotoxic T-cell responses and induce the generation of T-helper 1 (Th1) and T-regulatory cells (Fonteneau et al., 2003, Gerosa et al., 2005, Megjugorac et al., 2004, Moseman et al., 2004, Teleshova et al., 2004). During viral and bacterial infections, pDCs migrate to inflamed lymph nodes through high endothelial venules (Cella et al., 1999, Yoneyama et al., 2004). Furthermore, pDCs are known to accumulate in nasal and vaginal mucosae following respiratory syncytial and herpes simplex type 2 virus infections, respectively, and can potentiate inflammatory and autoimmune diseases, such as allergic rhinitis and systemic lupus erythematosus, by producing large amounts of IFN-α and other inflammatory cytokines and by aggregating in lesion sites (Farkas et al., 2001, Gill et al., 2005, Jahnsen et al., 2000, Lund et al., 2006).
In vitro exposure of pDCs either to infectious or inactivated HIV or to purified gp120 results in production of high levels of IFN-α and upregulation of activation markers, such as CD40, CD83, and CD86 (Del Corno et al., 2005, Ferbas et al., 1994, Fong et al., 2002, Fonteneau et al., 2004, Herbeuval et al., 2005, Schmidt et al., 2005, Yonezawa et al., 2003). Circulating pDCs in HIV-infected patients also express higher levels of CD86 than do these cells in uninfected individuals (Barron et al., 2003). However, in the blood of HIV-infected patients, percentages and absolute numbers of pDCs and pDC-dependent IFN-α production by peripheral blood mononuclear cells (PBMCs) were reduced (Chehimi et al., 2002, Feldman et al., 2001, Pacanowski et al., 2001, Soumelis et al., 2001). In general, these decreases correlated positively with CD4+ T cell numbers and inversely with viral loads (Barron et al., 2003, Donaghy et al., 2001, Finke et al., 2004, Pacanowski et al., 2001, Schmidt et al., 2006, Soumelis et al., 2001). Of interest, pDCs isolated from patients not only were infected with HIV, as shown by virus production during co-culture of pDCs with CD4+ cells or detection by immunohistochemistry or PCR amplification of proviral DNA, but also were impaired in their ability to stimulate allogeneic T cells, suggesting that HIV infection of pDCs might contribute to the observed loss of these cells (Donaghy et al., 2003, Fong et al., 2002, Schmidt et al., 2004). That HIV can infect human pDCs is supported by in vitro studies demonstrating that human pDCs can be productively infected by both CCR5 (R5)- and CXCR4 (X4)-using HIV isolates and, subsequently, can form syncytium (Lore et al., 2005, Patterson et al., 2001, Schmidt et al., 2004, Schmitt et al., 2006, Smed-Sorensen et al., 2005). However, when patients were placed on highly active antiretroviral therapy (HAART), which can restore CD4+ T cell numbers in blood and lead to undetectable plasma viremia, there was only a partial restoration of pDC numbers and IFN-α production (Chehimi et al., 2002, Finke et al., 2004, Schmidt et al., 2006, Zhang et al., 2006). Consistent with HIV infections, pDCs are reduced in other viral infections, including hepatitis B virus (Duan et al., 2004), hepatitis C virus (Goutagny et al., 2004), SARS coronavirus (Zhang et al., 2005), and human T-lymphotropic virus (Hishizawa et al., 2004). Furthermore, in severe hemorrhagic fevers resulting from dengue virus infection of children, decreased percentages and numbers of pDCs are associated with high levels of viremia (Pichyangkul et al., 2003). The exact mechanisms responsible for the loss of this cell type in association with these viruses are unknown.
Most studies have characterized HIV infection of human pDCs ex vivo; however, a cell type phenotypically and functionally equivalent to human pDCs has been identified in rhesus macaques (Macaca mulatta), thus making the simian immunodeficiency virus (SIV)-macaque model available for use when asking questions about pDCs and lentivirus infections (Chung et al., 2005, Coates et al., 2003). Although considerable research has focused on understanding the dynamics of pDCs during HIV infection, much is still unclear, particularly specific interactions between HIV and pDCs during acute disease and whether any alterations in phenotype or numbers of pDCs that are detected in blood also occur in other tissues. Therefore, in this study we phenotypically characterized cellular counterparts of human pDCs in both rhesus and pig-tailed (Macaca nemestrina) macaques and assessed the effects on pDCs of acute and chronic lentivirus infection with an R5 virus, SIVmac239, and an X4 SIV/HIV chimera, SHIV-89.6P, both of which cause an AIDS-like disease in these macaque species.
Results
Enumeration and phenotypic characterization of macaque pDCs
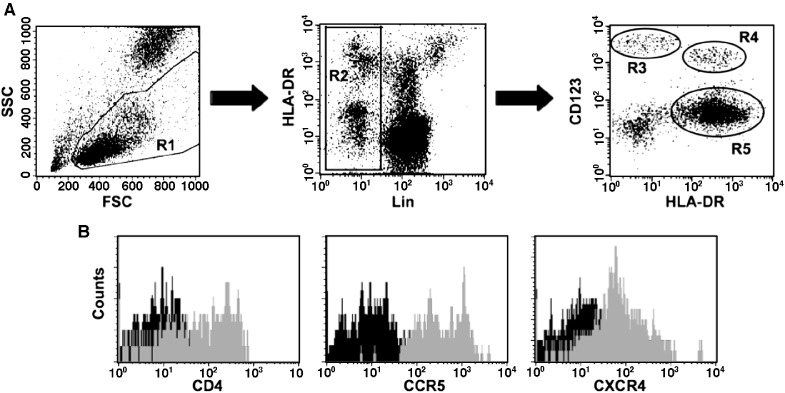
Human pDCs in blood are phenotypically characterized as CD123+HLA-DR+Lin− and represent less than 1% of all mononuclear cells (Barron et al., 2003, Pacanowski et al., 2001). To identify cells of the same phenotype in pig-tailed and rhesus macaques, human antibodies cross-reactive for antigens of both species were used. Macaque pDCs in blood (Fig. 1A, gate R4) were identified by flow cytometry and were distinguishable from CD123++HLA-DR− basophils (Fig. 1A, gate R3). Macaque pDCs were also distinct from the other major blood dendritic cell subset, mDCs, which are CD123lo/−HLA-DR+Lin− (Fig. 1A, gate R5) and express high levels of the cell-surface integrin, CD11c (data not shown). Thus, the defining phenotypes of human and macaque pDCs appear to be identical. Because human pDCs also express high levels of CD4 (Patterson et al., 2001), we analyzed macaque pDCs for expression of CD4 and the HIV/SIV/SHIV major coreceptors, CCR5 and CXCR4 (Fig. 1B). Although one pig-tailed macaque was an outlier with fewer (11.5%) pDCs that expressed CD4 than all other animals, in general, pig-tailed and rhesus macaque pDCs had comparable levels of CD4 on the cell surface (Table 1). In both species expression of CCR5 suggested a high cell surface density, as indicated by the MFI, with CCR5 present on 90% to 100% of pDCs. In contrast, the ranges of CXCR4+pDCs were broad, and the median MFI for CXCR4 expression was approximately tenfold lower than that for CCR5 (p < 0.0001).
Fig. 1.
Phenotypic analysis of macaque pDCs. (A) Using flow cytometry, pDCs (R4 gate) were identified among blood mononuclear cells as those cells expressing no lineage (Lin) markers (CD3, CD14, CD16, and CD20), but staining positive for HLA-DR and CD123. Differential expression of HLA-DR and Lin markers also identified CD123++ basophils (R3 gate) and CD123lo/−HLA-DR+Lin− mDCs (R5 gate). (B) Expressions of CD4, CCR5, and CXCR4 on macaque pDCs in blood are shown in representative histograms.
Table 1.
Expression of SIV/SHIV receptors on macaque pDCs
| Receptor | No.a | MFIb | Percent positivec |
|---|---|---|---|
| Rhesus | |||
| CD4 | 7 | 112 (70.5–236) | 97.7 (79.1–100) |
| CCR5 | 8 | 427 (103–629) | 98.5 (89.1–100) |
| CXCR4 | 8 | 53.7 (35.6–99.4) | 53.6 (25.5–73.9) |
| Pig-tailed | |||
| CD4 | 7 | 134 (25.0–289) | 82.8 (11.5–98.8) |
| CCR5 | 11 | 416 (141–1290) | 97.6 (91.9–100) |
| CXCR4 | 10 | 72.7 (14.1–91.6) | 48.9 (19.6–65.0) |
A comparison of the proportions of pDCs in whole blood and PBMC samples from pig-tailed (n = 22) and rhesus (n = 11) macaques revealed that the median percentage of pDCs in pig-tailed macaques (0.198%) was significantly higher (p < 0.001, Mann–Whitney U test) than that in rhesus macaques (0.118%) (Fig. 2). However, the absolute numbers of pDCs/ml of peripheral blood in pig-tailed (median, 5794; range, 3772 to 16,799) and rhesus (median, 6697; range, 5124 to 15,924) macaques were comparable. This discrepancy might be related, in part, to the observation that normal, uninfected rhesus macaques have significantly higher (p = 0.03) numbers of peripheral blood lymphocytes than pig-tailed macaques; median values, as determined from complete blood cell counts and differentials, were 4207 and 2982 for rhesus (n = 7) and pig-tailed (n = 15) macaques, respectively.
Fig. 2.
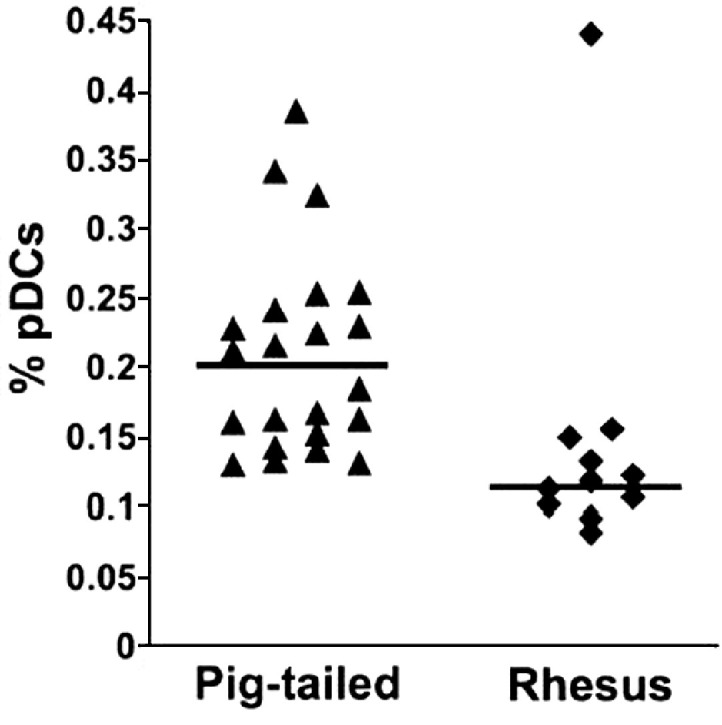
Frequencies of pDCs in macaque whole blood or isolated PMBC. Percentages of circulating CD123++HLA-DR+Lin− pDCs in normal pig-tailed (n = 26) and rhesus (n = 11) macaques were determined by multi-color flow cytometry. To enlarge the sample size, fresh whole blood and cryopreserved PBMC were analyzed and results were combined after determining that they were comparable. The horizontal lines indicate medians.
Dynamics of pDCs in blood of SIV/SHIV-infected macaques
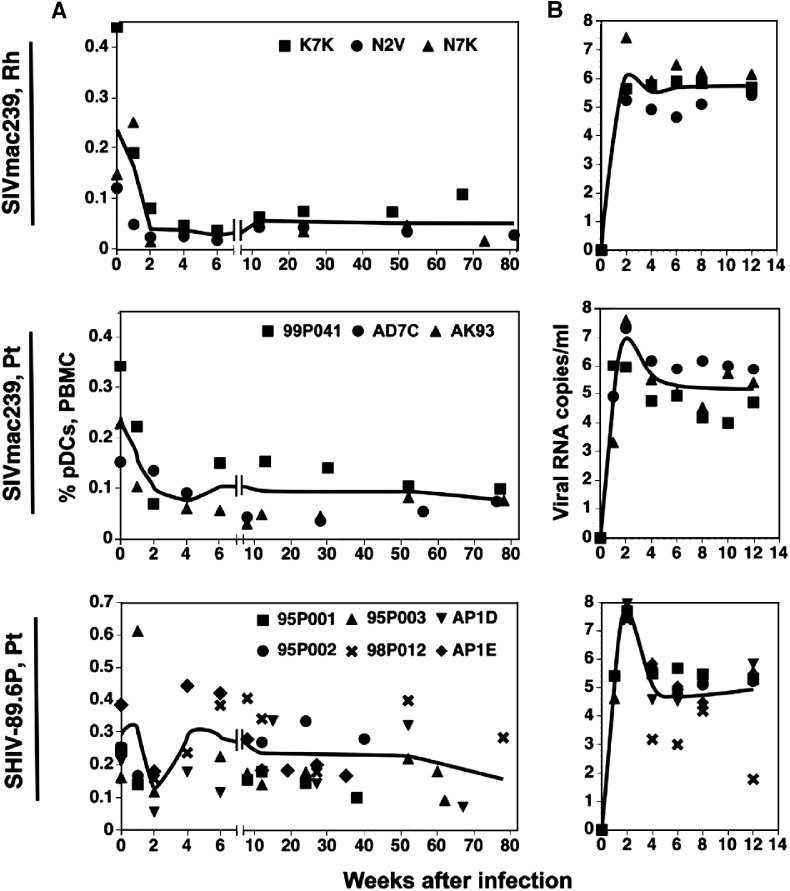
To evaluate changes in the percentages and numbers of circulating pDCs during acute and chronic lentivirus infections, we performed a retrospective longitudinal analysis using cryopreserved PBMC from three rhesus and three pig-tailed macaques infected with SIVmac239 and six pig-tailed macaques infected with SHIV-89.6P (Fig. 3A). In the three rhesus macaques the percentages of pDCs in PBMC declined rapidly during the first weeks post-infection, with the mean reaching a low of 0.028% at 6 weeks compared to 0.237% on day 0. Furthermore, no appreciable recovery of pDCs was observed during the chronic phase of infection. Similarly, the percentages of pDCs declined during acute SIVmac239 infection of the pig-tailed macaques, from a mean of 0.241% on day 0 to 0.076% by week 4, with minimal to no recovery of pDCs observed during chronic infection. A possible exception was noted for macaque 99P041: an increase in the percentages of pDCs, which persisted at least 24 weeks before declining, was noted initially at 6 weeks after infection; however, the increase never reached 50% of the day-0 value. In contrast to the SIVmac239-infected animals, the mean percentages of pDCs from the six SHIV-89.6P-infected macaques declined from 0.291% on day 0 to 0.128% at 2 weeks post-infection, a reduction of approximately 56%. The frequencies of pDCs then returned to normal levels by week 4 and showed only a slight downward trend during late-stage disease.
Fig. 3.
Changes in percentages of pDCs and viremia in macaques infected with SIVmac239 or SHIV-89.6P. (A) Percentages of pDCs in cryopreserved PBMC were determined by flow cytometry. Fewer data points are present at later times because some animals had been euthanized due to terminal disease. Trend lines were generated from the means of percentages of pDCs for each week from weeks 0 to 6, and then from the means of all values for periods from 7 to 12, 12 to 52, or greater than 52 weeks. Note differences in scales on the _y_-axes. (B) Plasma viral loads during the acute phase of infection were quantified by real-time PCR. Curves represent the mean RNA copy numbers for each time point. Rh, rhesus macaques; Pt, pig-tailed macaques.
The absolute number of pDCs also declined early after SIVmac239 infection and remained lower than day-0 values throughout the 9- to 81-week chronic infection period (Table 2). Five of the six SIVmac239-infected animals experienced decreases in the total number of pDCs at 1 week post-infection, the exception being macaque N7K, which had a transient increase before exhibiting a decline in numbers of pDCs. Because of limited availability of cryopreserved PBMC obtained during the first 8 weeks from the SHIV-89.6P-infected animals, definitive conclusions cannot be made; however, there appeared to be an overall loss in absolute numbers of pDCs, but these numbers fluctuated more than those in the SIVmac239-infected animals (Table 2). Overall, relative to day-0 values, the absolute numbers of circulating pDCs in the SIVmac239-infected animals during the chronic phase were lower than those in the SHIV-89.6P-infected animals. Most of the latter group had severe lymphopenia that developed during chronic infection (data not shown), which probably accounted for a portion of the observed decreases in pDC numbers.
Table 2.
Changes in absolute numbers of pDCs in macaque peripheral blood after SIV/SHIV infection
| Macaque | Weeks after infection | ||||
|---|---|---|---|---|---|
| 0 | 1 | 2 | 3–8 | 9–81 | |
| SIVmac239 – Rha | |||||
| K7K | 6789b | 5319 | 3586 | 943 ± 144c | 2247 ± 864 |
| N2V | 5124 | 1174 | 1263 | 885 ± 20 | 1811 ± 630 |
| N7K | 6605 | 10,312 | 555 | 1824 ± 241 | 1887 ± 1314 |
| SIVmac239 – Pt | |||||
| AD7C | 5179 | 1979 | NAd | 910 ± 228 | 675 ± 233 |
| AK93 | 9722 | 3480 | NA | 1296 ± 614 | 1626 ± 507 |
| 99P041 | 12,895 | 6158 | 1835 | 3990e | 4833 ± 747 |
| SHIV-89.6P – Pt | |||||
| 95P001 | 7270 | 2968 | NA | 2421e | 1904 ± 620 |
| 95P002 | 3772 | 3774 | NA | NA | 4990 ± 1649 |
| 95P003 | 5388 | 15,814 | 1042 | 3473 ± 2294 | 3060 ± 1412 |
| 98P012 | 10,228 | NA | 4006 | 4860 ± 3885 | 7832 ± 3316 |
| AP1D | 6799 | NA | 905 | 3252 ± 1073 | 2552 ± 2469 |
| AP1E | 13,933 | NA | 1834 | 5237 ± 1675 | 2901 ± 1205 |
Association of viral load with loss of pDCs
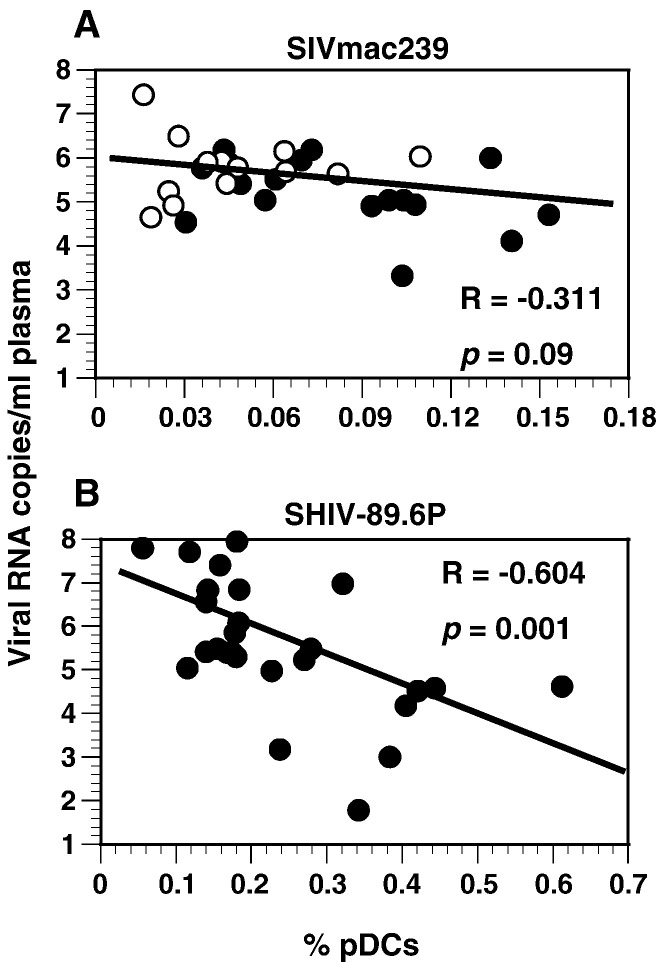
Viral loads, measured as virion RNA copies/ml of plasma, generally peaked at about 2 weeks for most animals; maximum titers for the SHIV-89.6P-infected macaques were at least fivefold higher than those for the SIVmac239-infected animals (geometric means: SIVmac239-infected rhesus macaques, 1.3 × 106 copies/ml; SIVmac239-infected pig-tailed macaques, 9.1 × 106 copies/ml; and SHIV-89.6P-infected pig-tailed macaques, 5.0 × 107 copies/ml) (Fig. 3B). Peak viremia also coincided with decreases in numbers of pDCs in SIVmac239-infected rhesus and pig-tailed macaques and the transient loss of pDCs observed at 2 weeks after infection in the SHIV-89.6-infected pig-tailed macaques. Although the relationship between viral loads and percentages of pDCs in SIVmac239-infected macaques was not statistically significant (Fig. 4A), it is possible that the persistently low percentages of pDCs in these animals compromised the accuracy of a correlative test. However, there was an inverse correlation between viral loads and percentages of pDCs in SHIV-89.6P-infected animals (Fig. 4B). When the relationships of absolute numbers of pDCs and viral loads were evaluated, the results were similar (data not shown).
Fig. 4.
Relationship of percentages of pDCs and viral loads during infection of macaques with SIVmac239 or SHIV-89.6P. (A) Values were obtained from three rhesus and three pig-tailed macaques infected with SIVmac239 for 1 to 77 weeks and include four to seven time points for individual animals. Open circles are values for rhesus macaques and closed circles are those for pig-tailed macaques (Spearman's correlation). (B) Values were obtained from six pig-tailed macaques infected with SHIV-89.6P for 1 to 52 weeks and include two to six time points for individual animals.
To determine whether macaque pDCs were infected in vivo, these cells were purified to 98.2% homogeneity using adherence, Dynabead, and cell sorting procedures, and then DNA was isolated and tested by PCR for amplification of a region of the gag gene. During the first 12 weeks after infection, all DNA samples isolated from pDCs from SIVmac239-infected macaques were positive for provirus or cDNA (Table 3). For example, provirus was amplified from genomic DNA equivalent to 50 pDCS isolated from macaque N7K at 4 weeks after infection, indicating that at least one in 50 cells was infected with SIVmac239. However, provirus was not detected in genomic DNA equivalent to 4300 pDCs that were isolated from this same animal at 73 weeks. In the SHIV-89.6P-infected macaques, two of three different pDC DNA samples obtained during the first 12 weeks of infection were positive for provirus. Although DNA from pDCs from only two animals (one each from a SIVmac239- and a SHIV-89.6P-infected macaque) at late times of infection was analyzed, the results suggest not only that more pDCs were infected during the acute rather than the chronic phase but also that during the acute phase, the number of infected pDCs was lower in SHIV-89.6P-compared to SIVmac239-infected animals. There appeared to be no association, however, between the apparent number of infected pDCs and plasma viral loads.
Table 3.
Amplification of proviral DNA from purified macaque pDCs
| Animal | Weeksa | No. pDCsb | PCRc | G. eq.d | Copies/mle |
|---|---|---|---|---|---|
| SIVmac239 | |||||
| N2V | 2 | 7.0 × 104 | 3/3 | < 120 | 1.7 × 105 |
| 4 | 5.5 × 103 | 2/3 | ≤ 230 | 8.4 × 104 | |
| 12 | 1.6 × 104 | 2/3 | ≤ 670 | 2.6 × 105 | |
| N7K | 4 | 3.3 × 103 | 3/3 | < 50 | 8.4 × 105 |
| 73 | 1.3 × 104 | 0/2 | > 4300 | 1.3 × 105 | |
| SHIV-89.6P | |||||
| 95P003 | 6 | 1.8 × 103 | 0/2 | > 580 | 9.6 × 104 |
| 8 | 2.8 × 104 | 2/3 | ≤ 1200 | 2.4 × 105 | |
| 62 | 1.7 × 103 | 0/2 | > 570 | NDf | |
| 98P012 | 6 | 8.0 × 103 | 2/3 | ≤ 330 | 1.0 × 103 |
| Uninfected | |||||
| 98P058 | NAg | 8.7 × 103 | 0/2 | > 730 | NAg |
Relationship of CD4+ T cells to pDCs
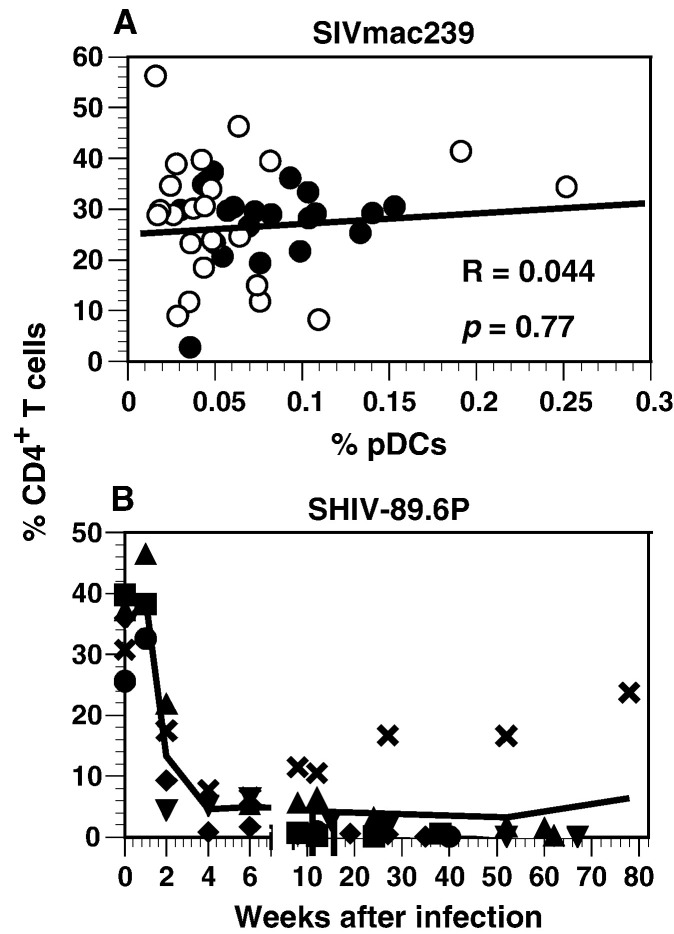
Both pDCs and CD4+ T cells in blood decline during SIVmac239 infection of macaques, suggesting that the decreases in both cell types might be related. However, no correlation was found between the percentages of CD4+ cells and pDCs in the SIVmac239-infected animals (Fig. 5A). As reported previously by others (Karlsson et al., 1998, Reimann et al., 1996), we found that peripheral blood CD4+ T cells rapidly declined during acute SHIV-89.6P infection and, in most animals, remained low thereafter (Fig. 5B). Although the onset of loss of circulating CD4+ T cells coincided with the transient loss of pDCs during acute SHIV-89.6P infections, numbers of CD4+ T cells were persistently low and rarely increased after the acute stage; therefore, it was impossible to determine whether there was a relationship between changes in percentages of CD4+ T cells and pDCs.
Fig. 5.
Relationship of pDCs to CD4+ T cells. (A) Percentages of CD4+ T cells and pDCs in PBMC were obtained from weeks 1 to 81 during SIVmac239 infection. Values for rhesus and pig-tailed macaques are as in Fig. 4A above. (B) Percentages of CD4+ T cells during acute SHIV-89.6P infection of pig-tailed macaques were determined using cryopreserved PBMC. The trend line is based on the means of the percentages of CD4+ T cells for each week from weeks 0 to 6, and then on all means for percentages obtained during the periods from 7 to 12, 13 to 52, and 52 to 81 weeks. Each symbol represents the same animal as that indicated in Fig. 3A, bottom panel.
Distribution of pDCs in lymphoid tissues
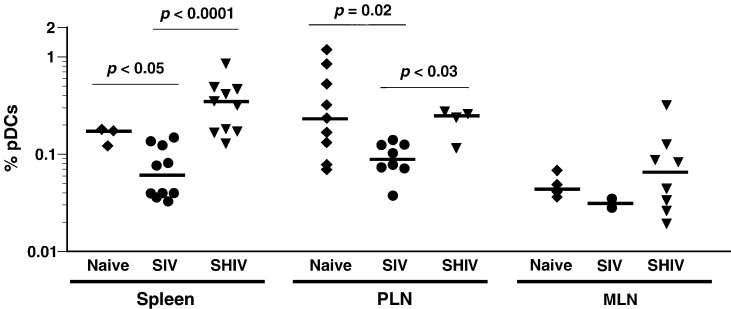
Since pDCs in humans, mice, and, recently, in rhesus macaques (Cella et al., 1999, Coates et al., 2003, Teleshova et al., 2006, Yoneyama et al., 2004) were shown to migrate to secondary lymphoid tissues, we determined the proportion of pDCs in different lymphoid tissues of naïve pig-tailed macaques. Flow cytometric analysis was performed on single-cell suspensions prepared from PLN and MLN as well as spleen tissues taken from animals at necropsy. Percentages of pDCs in tissues from naïve pig-tailed macaques were compared to those of pDCs in peripheral blood (discussed above); similar percentages of pDCs in the total mononuclear cell population were found in spleen tissue, but values were significantly higher in PLN (p = 0.04) and lower in MLN (p < 0.0001) (data not shown). Additionally, the proportions of pDCs in tissues from naïve macaques were compared to those in tissues from pig-tailed macaques that were infected with either SIVmac239 or SHIV-89.6P and were at end-stage disease (Fig. 6). Even though the median percentage of pDCs in spleens of SHIV-89.6P-infected animals was higher than that of naïve animals (0.354% and 0.158%, respectively), percentages of pDCs in spleens and PLN from naïve and SHIV-89.6P-infected macaques were not statistically different. In contrast, spleens and PLN from SIVmac239-infected animals contained significantly lower percentages of pDCs than those in both naïve and SHIV-89.6P-infected animals. There were no differences in the percentages of pDCs in MLN from any group.
Fig. 6.
Variability of percentages of pDCs in tissues from naïve and SIV- or SHIV-infected macaques. Percentages of pDCs in single-cell suspensions of spleens, peripheral lymph nodes (PLN), and mesenteric lymph nodes (MLN) from naïve and SIVmac239- and SHIV-89.6P-infected pig-tailed macaques at necropsy were determined by flow cytometry. Times after infection with SIVmac239 and SHIV-89.6P when the animals were euthanized ranged from 67 to 150 weeks and 28 to 211 weeks, respectively. Within a given tissue, whether there were significant differences between macaque groups in median percentages (horizontal lines within data sets) of pDCs was assessed using the Mann–Whitney U test; p values are not shown for medians that were not significantly different (p > 0.05). Cell samples for all tissues were not available from all animals.
Activation of pDCs
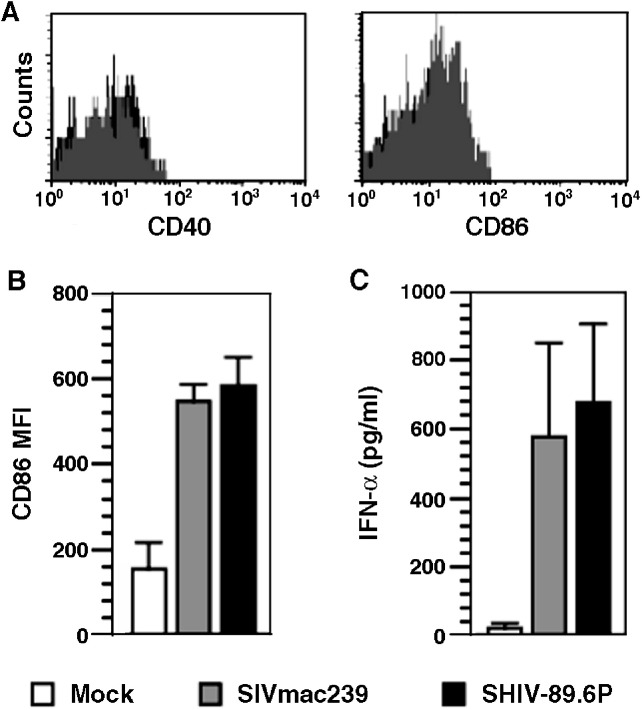
In general, pDCs in human blood are phenotypically immature cells. To determine whether this is true in macaques, multi-parametric flow cytometry was used to examine surface expression of the activation/maturation markers CD40 and CD86 on circulating pDCs in six pig-tailed and eight rhesus macaques. The mean percentages of pDCs that were positive for both markers was less than 1%, with MFIs comparable to those of background controls (Fig. 7A), strongly indicating that circulating pDCs in macaques also have an immature phenotype.
Fig. 7.
Activation of macaque pDCs by infection with SIVmac239 and SHIV-89.6P. (A) Representative histograms showing no expression of CD40 and CD86 on pDCs in peripheral blood from a naïve macaque. (B) Upregulation of CD86 on enriched macaque pDCs is reflected as an increase in MFI. (C) Concentrations of IFN-α in culture supernatants of enriched macaque pDCs were determined by ELISA. CD86 and IFN-α were measured 48 h after addition of the indicated virus (moi = 0.2). Values are means and standard deviations of three independent experiments.
Since retroviruses appear to have an effect on pDCs, we determined whether infectious viruses activated these cells in vitro. Macaque CD123+HLA-DR+pDCs were enriched and cultured without or with SIVmac239 or SHIV-89.6P and, as indicators of activation, expression of cell surface CD86 and secretion of IFN-α were assessed. In mock-infected cultures, CD86 detection was characterized by a mean MFI of 154, while cells in the two virus-infected cultures had MFIs approximately threefold higher (547 and 583) (Fig. 7B). For comparison, pDCs in blood that were analyzed ex vivo had a mean MFI of 8.76. Like upregulation of CD86, supernatants of the same enriched and infected pDC cultures contained similar IFN-α concentrations, which were 30-fold greater than that in supernatants from the mock-infected cultures (Fig. 7C). Compared to pDCs in blood, the modest increase in CD86 expression in the mock-infected cultures (MFI increased from approximately 9 to 154) could be due, in part, to ligation of IL-3Rα (CD123) by antibodies during the sorting process and/or the addition of IL-3 to the culture media as a growth supplement for pDCs. Cross-linking of CD123 in the presence of both a primary mAb and secondary anti-IgG antibodies can induce maturation of pDCs and downregulate IFN-α expression (Fanning et al., 2006); however, we used only a primary anti-CD123 antibody for pDC enrichment. Furthermore, IL-3 in the medium would compete with any antibodies for binding to CD123; therefore, it is unlikely that antibody-mediated downregulation of IFN-α was a major factor in modulating IFN-α production after virus infection. The above results clearly show that SIV/SHIV infection of Lin− pDC-enriched cultures induced activation of these cells.
Discussion
When the adaptive immune system is impaired or fails, as in HIV-1 infections, host innate immune responses must play a larger role in defending against invading pathogens and controlling opportunistic infections and neoplasms than in normal individuals. Much of what is known regarding HIV pathogenesis and the development of efficacious vaccines is derived from analyses of SIV infections of nonhuman primates. Although both pig-tailed and rhesus macaques are considered comparable for a variety of studies, minor differences, such as in frequencies of some cell types in blood, are not uncommon. For example, Ibegbu et al. (2001) found significantly higher percentages and absolute numbers of NK cells in pig-tailed macaques than in either rhesus macaques or sooty mangabeys (Cercocebus atys). Limited information about pDCs in macaques is available, and it is restricted to rhesus macaques (Coates et al., 2003, Teleshova et al., 2004, Teleshova et al., 2006). We report here that (i) cells phenotypically similar to human pDCs are present in both pig-tailed and rhesus macaques, and (ii) the frequency of circulating pDCs in pig-tailed macaques is similar to, while the frequency in rhesus macaques is lower than, that in humans (Chehimi et al., 2002, Duan et al., 2004).
The disease courses and clinical outcomes of infection of rhesus and pig-tailed macaques with the same SIV or SHIV strains are equivalent (Batten et al., 2006, Fultz et al., 2001, Fultz et al., 2003, Reimann et al., 2005). In the present study, while the effect of SIVmac239 on pDCs appeared to be comparable in both macaque species, the effects of SIVmac239 and SHIV-89.6P on CD4+ T cells were disparate and distinct. SIVmac239 caused a transient decline in the number of circulating CD4+ T cells during acute infection, followed by a gradual decline during the chronic phase. In contrast, SHIV-89.6P caused a loss of > 95% of all circulating CD4+ T cells within the first 2 to 4 weeks after infection, as reported by others (Reimann et al., 1996). This difference is now known to be related to coreceptor usage: most SIV strains use CCR5, while SHIV-89.6P uses CXCR4 exclusively (Moore et al., 2004, Nishimura et al., 2004). Although memory and naïve CD4+ T cells can express CXCR4, the levels on naïve cells tend to be higher and, therefore, these cells may be more susceptible targets for SHIV-89.6P infection (Nishimura et al., 2005). In addition, the majority of SIV strains infect memory CD4+ T cells, which are almost completely depleted in gut and other lymphoid tissues during the first weeks of SIV infections (Mattapallil et al., 2005), a result consistent with events described in the gastrointestinal tracts of HIV-infected humans (Brenchley et al., 2004). However, our data demonstrated that, while the frequency of circulating pDCs correlated with viral load, it was unrelated to CD4+ T cell numbers. Similar studies of HIV patients have yielded conflicting results: some reported a strong relationship between pDC frequencies in blood and both viral load and CD4+ T cell counts, while others found essentially no relationship (Chehimi et al., 2002, Donaghy et al., 2001, Feldman et al., 2001, Schmidt et al., 2006). One possible explanation for the discrepancy is that these studies were a cross-sectional analysis of HIV patients at various stages of disease; they did not include many subjects, if any, who were acutely infected.
Since different primate lentiviruses target specific subsets of CD4+ T cells, based on their use of coreceptors, it is tempting to speculate that the effects of lentivirus pathogenesis on pDCs are also, at least in part, coreceptor-dependent. Almost 100% of macaque pDCs express levels of CCR5 that are logarithmically higher than what is required for infection of memory CD4+ T cells (Mattapallil et al., 2005), suggesting that pDCs might be especially susceptible to infection by CCR5-using (R5) viruses (Moore et al., 2004), which could explain the extensive decrease in numbers of these cells during acute infection with SIVmac239. This notion is supported by a recent report that suggests infection of pDCs in vitro with R5 HIV isolates produced higher proviral loads than comparably infected CD4+ T cells (Cameron et al., 2006). That the percentages of macaque pDCs that express CXCR4 are highly variable and CXCR4 has a lower surface density than CCR5 might explain the minor impact of SHIV-89.6P infection on pDC frequencies in blood. Independent of the route of transmission, R5 viruses tend to predominate early in HIV-1 infection, and the emergence of CXCR4-using (X4) viruses is associated with disease progression; however, at any given time viral quasispecies are likely to be heterogeneous, comprised of both R5 and X4 variants (Moore et al., 2004). Thus, the effect of HIV on pDCs early in infection might mimic what we observed during acute SIVmac239 infection. However, while several groups have shown that human pDCs express both CCR5 and CXCR4, little data exist on the relationship of coreceptor expression levels and virus infectivity (Lore et al., 2005, Schmitt et al., 2006, Smed-Sorensen et al., 2005).
Even though we analyzed a limited number of purified pDC populations, our results suggest that, after transmission of both SIVmac239 and SHIV-89.6P, macaque pDCs were infected early in the disease course and harbored proviral DNA. There are no reports that positively identify SIV proviruses in macaque pDCs, but several groups have shown not only that human pDCs are infected in vivo but also that these cells can be infected by HIV in vitro (Donaghy et al., 2003, Gurney et al., 2004, Lore et al., 2005, Patterson et al., 2001, Smed-Sorensen et al., 2005). Furthermore, human pDCs support more efficient replication of an R5 virus (HIVBaL) than an X4 virus (HIVIIIB). These differences between R5 and X4 HIV strains might partially explain the diverse effects on macaque pDCs that we observed during SIVmac239 and SHIV-89.6P infections. In agreement with our results, R5 and X4 HIV isolates induced production of similar amounts of IFN-α and upregulation of CD86 on pDCs in vitro (Lore et al., 2005, Smed-Sorensen et al., 2005, Yonezawa et al., 2003). Since both R5 and X4 viruses stimulate pDCs equally, cell-mediated responses to both types of SIV and SHIV strains most likely are similar and have little impact on the disparate disease pathogenesis we observed. Because virus stocks generally contain more noninfectious virus particles than infectious virions, these noninfectious particles also might activate pDCs and contribute to induction of IFN-α, thus enhancing antiviral activity. In vitro experiments have shown that infectious HIV is not required and that recombinant gp120 alone is sufficient to activate human pDCs (Del Corno et al., 2005, Fonteneau et al., 2004, Yonezawa et al., 2003). Furthermore, Teleshova et al. (2004) showed that after the addition of aldrithiol-2-inactivated SIV to cultures of enriched macaque dendritic cells, CD80 and CD86 were upregulated on CD123+ pDCs and IFN-α accumulated in culture supernatants in a dose-dependent manner. A current goal is to define more rigorously interactions between SIV and macaque pDCs, but this objective has been hindered because antibodies that are used for in vitro purification of pDCs from human blood, such as the pDC-specific antigens BDCA-2 and -4, crossreact poorly with the analogous nonhuman primate antigens (Reeves and Fultz, unpublished data; Dzionek et al., 2000). Thus, to facilitate characterization of macaque pDCs, similar antibodies specific for macaque BDCA-2 and -4, or other unidentified pDC-specific proteins, need to be generated.
Information regarding changes in pDC populations during SIV and SHIV infections is limited. Teleshova et al. (2004) reported that during chronic infections there were significantly lower frequencies of pDCs in the blood of rhesus macaques vaccinated with the attenuated SIVmac239Δnef strain and then challenged with SIVmac239 compared to those in SHIV-162P-infected macaques; however, neither of these frequencies were statistically different from those in naïve controls. One caveat is that both of these cohorts were examined after 2 to 6 years of infection, and only the percentages of pDCs among HLA-DR+Lin− cells in the periphery, and not absolute numbers, were determined. Prior vaccination of the SIVmac239-infected animals also may have provided some protection against substantial decreases in pDCs. After infection with SHIV-162P, also an R5 virus, some animals can control viremia such that either a viral set point that is lower (< 1 log) than that associated with SIVmac239 infection is established or virion RNA becomes undetectable (Batten et al., 2006, Harouse et al., 1999). Since our data suggest that pDC frequency is related to viral load, low or undetectable levels of viremia could lead to the re-emergence of pDCs in the peripheral blood of long-term infected animals exhibiting no signs of disease progression. Relative to this possibility, several studies have shown that in HIV-infected patients whose viremia was suppressed due to successful treatment with HAART, a partial restoration of peripheral blood pDCs was observed (Chehimi et al., 2002, Finke et al., 2004, Schmidt et al., 2006, Zhang et al., 2006).
In addition to HIV, and now SIVmac239, decreases in circulating pDCs in a variety of viral (Duan et al., 2004, Goutagny et al., 2004, Hishizawa et al., 2004, Zhang et al., 2005), bacterial (Lichtner et al., 2006), and parasitic (Pichyangkul et al., 2004) infections, as well as in some non-microbial pathologies (Baumgart et al., 2005, Blomberg et al., 2003), have been demonstrated. Such peripheral alterations might be explained by migration to and accumulation of pDCs at sites of inflammation or in lymphoid organs (Cella et al., 1999, Farkas et al., 2001, Gill et al., 2005, Jahnsen et al., 2000, Pashenkov et al., 2002, Yoneyama et al., 2004); however, the sustained decrease of pDCs in both blood and lymphoid tissues at end-stage SIVmac239 infection makes this explanation unlikely and suggests that infection and loss of pDCs occur early and systemically, as is the case for CCR5+ memory CD4+ T cells (Mattapallil et al., 2005). In contrast, our findings of elevated percentages of pDCs in spleens and mesenteric lymph nodes of some SHIV-89.6P-infected macaques in end-stage disease suggest there might be a continual influx and accumulation of pDCs in lymphoid tissues during acute and/or chronic infections with SHIV-89.6P that was not associated with extensive loss of circulating pDCs. Since mucosal infections represent the major route of HIV transmissions worldwide, it would be of interest to determine whether pDCs accumulate in the genital mucosa following rectal or vaginal infection, as was shown for herpes simplex virus type 2 infection of mice (Lund et al., 2006).
In conclusion, our results using the SIV/SHIV macaque models suggest that, in patients chronically infected with HIV, loss of pDCs occurs primarily during acute infection and may be linked to coreceptor usage by a virus and/or coreceptor expression on pDCs. However, other factors, including dysregulation of pDC development or direct killing by NK cells, could affect the numbers of pDCs in blood and tissues. Regardless of the mechanism(s) involved, loss of a functional pDC population would have detrimental consequences on innate immune functions. Impairment of the innate immune system, in turn, would enhance acquisition of opportunistic infections and affect immunization against pathogens, with or without targeting pDCs, or development of therapeutic strategies (Becker, 2005, Cafaro et al., 2001, Jones et al., 1999, Teleshova et al., 2004, Verthelyi et al., 2003). Furthermore, the differences between SIVmac239 and SHIV-89.6P infection of macaques that we identified here suggest that, when using these models, the choice of an appropriate challenge virus may be critical for the specific hypothesis being tested.
Materials and methods
Animals and cell samples
Juvenile rhesus and pig-tailed macaques of both sexes were used in this study. All of the SIV- and SHIV-infected animals had been or at the time were in other unrelated pathogenesis studies. Uninfected animals were used as normal blood donors. Before collecting blood samples or inoculating virus, macaques were anesthetized with an intramuscular injection of ketamine HCl (10 mg/kg). Macaques were housed in isolation facilities at the University of Alabama at Birmingham in accordance with institutional and Animal Welfare Act guidelines.
Whole blood was collected from macaques and PBMC were isolated from heparinized blood by density gradient centrifugation through lymphocyte separation medium (LSM, ICN Biomedicals, Inc.). To evaluate changes in subsets of peripheral blood cells, cryopreserved PBMCs, obtained during previous studies from three rhesus and three pig-tailed macaques infected with SIVmac239 and six pig-tailed macaques infected with the SHIV-89.6P chimera were used. Cells collected up to 81 weeks post-infection, or for a shorter time when an animal had progressed to AIDS, were assessed. Lymphoid tissues were evaluated using fresh or cryopreserved single-cell suspensions of PLN and MLN and spleens taken at necropsy from uninfected or infected animals in end-stage disease. Animals from which tissue samples were available included: uninfected (n = 9); SHIV-89.6P-infected (n = 10); and those infected with SIVmac239 (n = 5) or one of two SIVmac239 mutants, SIVmac239Y > I (n = 2) or SIVmac239ΔGY(S > P) (n = 2). The two SIVmac239 mutant strains were described previously (Fultz et al., 2001, LaBranche et al., 1995). Since no significant differences were observed among animals infected with either of the two mutant or wild-type SIVmac239 strains with respect to effects on pDCs, for the purposes of this study, the animals were grouped and collectively referred to as being infected with SIVmac239.
Flow cytometric analysis of ex vivo mononuclear cells
Three- and four-color flow cytometry were used to enumerate percentages of various cell types and to characterize phenotypically macaque pDCs in EDTA-treated whole blood, PBMC, or single-cell suspensions of splenic and lymph node tissues. CD123+ pDCs were identified within HLA-DR+Lin− populations using: a FITC-conjugated antibody cocktail to Lin markers CD3ε (SP34), CD14 (MϕP9), CD16 (3G8), and CD20 (2H7); PE-labeled anti-CD123 (7G3); and PerCp-labeled anti-HLA-DR (G46-6). Expression of coreceptor and activation/maturation molecules was evaluated using one of the following antibodies: anti-CD40 (5C3), anti-CD86 (2331), anti-CCR5 (2D7), or anti-CXCR4 (12G5), all of which were conjugated to APC. CD4 expression on pDCs was evaluated by indirect staining using biotin-conjugated anti-CD4 (SK3), and either anti-biotin–APC (Miltenyi Biotec, Germany) or streptavidin–APC. CD4+ T lymphocytes were identified using PE-labeled anti-CD4 (SK3). Isotype-matched controls for each fluorochrome were included in all experiments and were used to calculate percentages of positive cells above background fluorescence. For phenotyping pDCs, mononuclear cells were gated based on forward-and-side-scatter characteristics. All antibodies and reagents were purchased from BD Biosciences Pharmingen (San Diego, CA), unless otherwise noted, and all acquisitions were performed on a BD LSRII flow cytometer.
pDC enrichment, isolation, and activation
pDCs were enriched, first, by culturing whole PBMC from naïve pig-tailed macaques for a minimum of 4 h in RPMI 1640 containing 15% FBS to deplete adherent cells and, second, by depleting other Lin+ cells using Pan-IgG Dynabeads (Dynal Biotech, Norway) coated with anti-CD3 (FN-18) (Biosource, Camarillo, CA), anti-CD16 (3G8) and anti-CD20 (2H7) (both from BD Biosciences). The remaining cell fraction was incubated with anti-CD123-PE Ab; all CD123+ cells were sorted and collected using a BD FACS Aria. For activation experiments, aliquots of enriched cells were reanalyzed; greater than 95% of cells were negative for Lin markers, 40 to 70% were HLA-DR+pDCs, and the remaining cells were CD123+HLA-DRdim/−Lin−, most likely basophils (Strunk et al., 2005, Toba et al., 1999). Alternatively, for PCR experiments in which a relatively pure pDC population was necessary, the enriched cell fraction (97% Lin− cells) was stained with anti-CD123-PE and anti-HLA-DR-PerCp or -FITC and only double-positive cells were collected by sorting; these cells were 98.2% CD123+HLA-DR+Lin− (mean, eight experiments). To avoid possible activation or induction of apoptosis by HLA-DR ligation (Nagy and Mooney, 2003), cells enriched only for CD123 were assayed. Approximately 2.5 × 104 cells were cultured in polystyrene tubes in enriched medium (RPMI 1640 supplemented with 10% FBS, 10% normal monkey serum, and 20 ng of IL-3/ml) with either SIVmac239 or SHIV-89.6P at a moi of 0.2. After 48 h, culture supernatants were collected and the cells were washed and reanalyzed by flow cytometry for CD86 (FITC) expression on CD123+HLA-DR+ cells. As another measure of activation, concentrations of IFN-α in the 48-h culture supernatants were determined using a cross-reactive human IFN-α ELISA kit, according to the manufacturer's (Biosource) protocol. The lower limit of detection of IFN-α was 10 pg/ml.
Detection of provirus in pDCs
CD123++HLA-DR+Lin− pDCs were purified from the 97% Lin− population by cell sorting, as described above, using PBMC from SIVmac239- and SHIV-89.6P-infected macaques at various stages of disease. Isolated cells were mixed with uninfected CEMx174 cells (1:10 ratio, pDCs to CEMx174 cells) to provide a source of carrier DNA. Proviral DNA was amplified by nested PCR as a 359-bp fragment of SIVmac239 gag (also encoded by the chimeric SHIV-89.6P). The first-round gag primers were: forward, 5′-CGCAGAAGAGAAAGTGAAACAC-3′, and reverse, 5′-AAGCCGTCAGCATTTCTTC-3′ (spanning nucleotide positions 1319 to 2103). The second-round gag primers were: forward, 5′-TGGTAACTATGTCCACCTGCC-3′, and reverse, 5′-TGCCTACTGGTATGGGGTTC-3′ (spanning nucleotide positions 1475 to 1833). DNA isolated from SIVmac239-infected or uninfected CEMx174 cells served as positive and negative controls, respectively.
Viral load
Plasma virion RNA was quantified (sensitivity, less than 100 copies/ml) for SIVmac239-infected (99P041, AK93, and AD7C) and SHIV-89.6P-infected pig-tailed macaques (98P012, AP1D, and AP1E) at the Quantitative Molecular Diagnostics Core of the AIDS Vaccine Program, SAIC Frederick, NCI (Frederick, MD), as described (Lifson et al., 2001). Viral load data for SIVmac239-infected rhesus (N2V, K7K, and N7K) and SHIV-89.6P-infected pig-tailed macaques (95P001, 95P002, and 95P003) were reported previously (Fultz et al., 2001, Fultz et al., 2003).
Statistical analyses
Comparisons of results were done using Spearman's correlation or, when data from two or more groups were analyzed, the Mann–Whitney U test. Differences were considered significant if p values were less than 0.5. All analyses were performed using InStat 2.0 software (GraphPad, San Diego, CA).
Acknowledgments
The authors thank Jacqueline Stallworth for processing blood samples; Barry Cochran for assistance with cell enrichment; Marion Spell for flow cytometry and FACS acquisitions; Michael Piatak and Jeffrey Lifson for quantification of SIV virion RNA in some plasma samples; and the UAB Center for AIDS Research Flow Cytometry core, supported by NIH grant P30 AI027767. R.K.R. was supported by NIH Basic Mechanisms of Virology training grant T32 AI007150-29. Portions of this work were supported by NIH grants P01 AI028147, R01 AI033854, and R01 AI049784.
References
- Barron M.A., Blyveis N., Palmer B.E., MaWhinney S., Wilson C.C. Influence of plasma viremia on defects in number and immunophenotype of blood dendritic cell subsets in human immunodeficiency virus 1-infected individuals. J. Infect. Dis. 2003;187:26–37. doi: 10.1086/345957. [DOI] [PubMed] [Google Scholar]
- Batten C.J., De Rose R., Wilson K.M., Agy M.B., Chea S., Stratov I., Montefiori D.C., Kent S.J. Comparative evaluation of simian, simian–human, and human immunodeficiency virus infections in the pigtail macaque (Macaca nemestrina) model. AIDS Res. Hum. Retroviruses. 2006;22:580–588. doi: 10.1089/aid.2006.22.580. [DOI] [PubMed] [Google Scholar]
- Bauer M., Redecke V., Ellwart J.W., Scherer B., Kremer J.P., Wagner H., Lipford G.B. Bacterial CpG-DNA triggers activation and maturation of human CD11c−, CD123+ dendritic cells. J. Immunol. 2001;166:5000–5007. doi: 10.4049/jimmunol.166.8.5000. [DOI] [PubMed] [Google Scholar]
- Baumgart D.C., Metzke D., Schmitz J., Scheffold A., Sturm A., Wiedenmann B., Dignass A.U. Patients with active inflammatory bowel disease lack immature peripheral blood plasmacytoid and myeloid dendritic cells. Gut. 2005;54:228–236. doi: 10.1136/gut.2004.040360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker Y. CpG ODNs treatments of HIV-1 infected patients may cause the decline of transmission in high risk populations—A review, hypothesis and implications. Virus Genes. 2005;30:251–266. doi: 10.1007/s11262-004-5632-2. [DOI] [PubMed] [Google Scholar]
- Beignon A.S., McKenna K., Skoberne M., Manches O., DaSilva I., Kavanagh D.G., Larsson M., Gorelick R.J., Lifson J.D., Bhardwaj N. Endocytosis of HIV-1 activates plasmacytoid dendritic cells via Toll-like receptor–viral RNA interactions. J. Clin. Invest. 2005;115:3265–3275. doi: 10.1172/JCI26032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomberg S., Eloranta M.L., Magnusson M., Alm G.V., Ronnblom L. Expression of the markers BDCA-2 and BDCA-4 and production of interferon-α by plasmacytoid dendritic cells in systemic lupus erythematosus. Arthritis Rheum. 2003;48:2524–2532. doi: 10.1002/art.11225. [DOI] [PubMed] [Google Scholar]
- Brenchley J.M., Schacker T.W., Ruff L.E., Price D.A., Taylor J.H., Beilman G.J., Nguyen P.L., Khoruts A., Larson M., Haase A.T., Douek D.C. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J. Exp. Med. 2004;200:749–759. doi: 10.1084/jem.20040874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cafaro A., Titti F., Fracasso C., Maggiorella M.T., Baroncelli S., Caputo A., Goletti D., Borsetti A., Pace M., Fanales-Belasio E., Ridolfi B., Negri D.R., Sernicola L., Belli R., Corrias F., Macchia I., Leone P., Michelini Z., ten Haaft P., Butto S., Verani P., Ensoli B. Vaccination with DNA containing tat coding sequences and unmethylated CpG motifs protects cynomolgus monkeys upon infection with simian/human immunodeficiency virus (SHIV89.6P) Vaccine. 2001;19:2862–2877. doi: 10.1016/s0264-410x(01)00002-0. [DOI] [PubMed] [Google Scholar]
- Cameron P.U., Handley A.J., Baylis D.C., Solomon A.E., Bernard N., Purcell D.F.J., Lewin S.R. Preferential infection of DC during HIV-1 infection of blood leukocytes. J. Virol. 2006 doi: 10.1128/JVI.01795-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cella M., Jarrossay D., Facchetti F., Alebardi O., Nakajima H., Lanzavecchia A., Colonna M. Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type I interferon. Nat. Med. 1999;5:919–923. doi: 10.1038/11360. [DOI] [PubMed] [Google Scholar]
- Chehimi J., Campbell D.E., Azzoni L., Bacheller D., Papasavvas E., Jerandi G., Mounzer K., Kostman J., Trinchieri G., Montaner L.J. Persistent decreases in blood plasmacytoid dendritic cell number and function despite effective highly active antiretroviral therapy and increased blood myeloid dendritic cells in HIV-infected individuals. J. Immunol. 2002;168:4796–4801. doi: 10.4049/jimmunol.168.9.4796. [DOI] [PubMed] [Google Scholar]
- Chung E., Amrute S.B., Abel K., Gupta G., Wang Y., Miller C.J., Fitzgerald-Bocarsly P. Characterization of virus-responsive plasmacytoid dendritic cells in the rhesus macaque. Clin. Diagn. Lab. Immunol. 2005;12:426–435. doi: 10.1128/CDLI.12.3.426-435.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coates P.T., Barratt-Boyes S.M., Zhang L., Donnenberg V.S., O'Connell P.J., Logar A.J., Duncan F.J., Murphey-Corb M., Donnenberg A.D., Morelli A.E., Maliszewski C.R., Thomson A.W. Dendritic cell subsets in blood and lymphoid tissue of rhesus monkeys and their mobilization with Flt3 ligand. Blood. 2003;102:2513–2521. doi: 10.1182/blood-2002-09-2929. [DOI] [PubMed] [Google Scholar]
- Colonna M., Trinchieri G., Liu Y.-J. Plasmacytoid dendritic cells in immunity. Nat. Immunol. 2004;5:1219–1226. doi: 10.1038/ni1141. [DOI] [PubMed] [Google Scholar]
- Del Corno M., Gauzzi M.C., Penna G., Belardelli F., Adorini L., Gessani S. Human immunodeficiency virus type 1 gp120 and other activation stimuli are highly effective in triggering alpha interferon and CC chemokine production in circulating plasmacytoid but not myeloid dendritic cells. J. Virol. 2005;79:12597–12601. doi: 10.1128/JVI.79.19.12597-12601.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diebold S.S., Kaisho T., Hemmi H., Akira S., Reis e Sousa C. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science. 2004;303:1529–1531. doi: 10.1126/science.1093616. [DOI] [PubMed] [Google Scholar]
- Donaghy H., Pozniak A., Gazzard B., Qazi N., Gilmour J., Gotch F., Patterson S. Loss of blood CD11c+ myeloid and CD11c− plasmacytoid dendritic cells in patients with HIV-1 infection correlates with HIV-1 RNA virus load. Blood. 2001;98:2574–2576. doi: 10.1182/blood.v98.8.2574. [DOI] [PubMed] [Google Scholar]
- Donaghy H., Gazzard B., Gotch F., Patterson S. Dysfunction and infection of freshly isolated blood myeloid and plasmacytoid dendritic cells in patients infected with HIV-1. Blood. 2003;101:4505–4511. doi: 10.1182/blood-2002-10-3189. [DOI] [PubMed] [Google Scholar]
- Duan X.Z., Wang M., Li H.W., Zhuang H., Xu D., Wang F.S. Decreased frequency and function of circulating plasmacytoid dendritic cells (pDC) in hepatitis B virus infected humans. J. Clin. Immunol. 2004;24:637–646. doi: 10.1007/s10875-004-6249-y. [DOI] [PubMed] [Google Scholar]
- Dzionek A., Fuchs A., Schmidt P., Cremer S., Zysk M., Miltenyi S., Buck D.W., Schmitz J. BDCA-2, BDCA-3, and BDCA-4: three markers for distinct subsets of dendritic cells in human peripheral blood. J. Immunol. 2000;165:6037–6046. doi: 10.4049/jimmunol.165.11.6037. [DOI] [PubMed] [Google Scholar]
- Fanning S.L., George T.C., Feng D., Feldman S.B., Megjugorac N.J., Izaguirre A.G., Fitzgerald-Bocarsly P. Receptor cross-linking on human plasmacytoid dendritic cells leads to the regulation of IFN-α production. J. Immunol. 2006;177:5829–5839. doi: 10.4049/jimmunol.177.9.5829. [DOI] [PubMed] [Google Scholar]
- Farkas L., Beiske K., Lund-Johansen F., Brandtzaeg P., Jahnsen F.L. Plasmacytoid dendritic cells (natural interferon-α/β-producing cells) accumulate in cutaneous lupus erythematosus lesions. Am. J. Pathol. 2001;159:237–243. doi: 10.1016/s0002-9440(10)61689-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman S., Stein D., Amrute S., Denny T., Garcia Z., Kloser P., Sun Y., Megjugorac N., Fitzgerald-Bocarsly P. Decreased interferon-α production in HIV-infected patients correlates with numerical and functional deficiencies in circulating type 2 dendritic cell precursors. Clin. Immunol. 2001;101:201–210. doi: 10.1006/clim.2001.5111. [DOI] [PubMed] [Google Scholar]
- Ferbas J.J., Toso J.F., Logar A.J., Navratil J.S., Rinaldo C.R., Jr. CD4+ blood dendritic cells are potent producers of IFN-α in response to in vitro HIV-1 infection. J. Immunol. 1994;152:4649–4662. [PubMed] [Google Scholar]
- Finke J.S., Shodell M., Shah K., Siegal F.P., Steinman R.M. Dendritic cell numbers in the blood of HIV-1 infected patients before and after changes in antiretroviral therapy. J. Clin. Immunol. 2004;24:647–652. doi: 10.1007/s10875-004-6250-5. [DOI] [PubMed] [Google Scholar]
- Fong L., Mengozzi M., Abbey N.W., Herndier B.G., Engleman E.G. Productive infection of plasmacytoid dendritic cells with human immunodeficiency virus type 1 is triggered by CD40 ligation. J. Virol. 2002;76:11033–11041. doi: 10.1128/JVI.76.21.11033-11041.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonteneau J.F., Gilliet M., Larsson M., Dasilva I., Munz C., Liu Y.J., Bhardwaj N. Activation of influenza virus-specific CD4+ and CD8+ T cells: a new role for plasmacytoid dendritic cells in adaptive immunity. Blood. 2003;101:3520–3526. doi: 10.1182/blood-2002-10-3063. [DOI] [PubMed] [Google Scholar]
- Fonteneau J.F., Larsson M., Beignon A.S., McKenna K., Dasilva I., Amara A., Liu Y.J., Lifson J.D., Littman D.R., Bhardwaj N. Human immunodeficiency virus type 1 activates plasmacytoid dendritic cells and concomitantly induces the bystander maturation of myeloid dendritic cells. J. Virol. 2004;78:5223–5232. doi: 10.1128/JVI.78.10.5223-5232.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fultz P.N., Vance P.J., Endres M.J., Tao B., Dvorin J.D., Davis I.C., Lifson J.D., Montefiori D.C., Marsh M., Malim M.H., Hoxie J.A. In vivo attenuation of simian immunodeficiency virus by disruption of a tyrosine-dependent sorting signal in the envelope glycoprotein cytoplasmic tail. J. Virol. 2001;75:278–291. doi: 10.1128/JVI.75.1.278-291.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fultz P.N., Stallworth J., Porter D., Novak M., Anderson M.J., Morrow C.D. Immunogenicity in pig-tailed macaques of poliovirus replicons expressing HIV-1 and SIV antigens and protection against SHIV-89.6P disease. Virology. 2003;315:425–437. doi: 10.1016/s0042-6822(03)00546-4. [DOI] [PubMed] [Google Scholar]
- Gerosa F., Gobbi A., Zorzi P., Burg S., Briere F., Carra G., Trinchieri G. The reciprocal interaction of NK cells with plasmacytoid or myeloid dendritic cells profoundly affects innate resistance functions. J. Immunol. 2005;174:727–734. doi: 10.4049/jimmunol.174.2.727. [DOI] [PubMed] [Google Scholar]
- Gill M.A., Palucka A.K., Barton T., Ghaffar F., Jafri H., Banchereau J., Ramilo O. Mobilization of plasmacytoid and myeloid dendritic cells to mucosal sites in children with respiratory syncytial virus and other viral respiratory infections. J. Infect. Dis. 2005;191:1105–1115. doi: 10.1086/428589. [DOI] [PubMed] [Google Scholar]
- Goutagny N., Vieux C., Decullier E., Ligeoix B., Epstein A., Trepo C., Couzigou P., Inchauspe G., Bain C. Quantification and functional analysis of plasmacytoid dendritic cells in patients with chronic hepatitis C virus infection. J. Infect. Dis. 2004;189:1646–1655. doi: 10.1086/383248. [DOI] [PubMed] [Google Scholar]
- Gurney K.B., Colantonio A.D., Blom B., Spits H., Uittenbogaart C.H. Endogenous IFN-α production by plasmacytoid dendritic cells exerts an antiviral effect on thymic HIV-1 infection. J. Immunol. 2004;173:7269–7276. doi: 10.4049/jimmunol.173.12.7269. [DOI] [PubMed] [Google Scholar]
- Harouse J.M., Gettie A., Tan R.C.H., Blanchard J., Cheng-Meyer C. Distinct pathogenic sequela in rhesus macaques infected with CCR5 or CXCR4 utilizing SHIVs. Science. 1999;284:816–819. doi: 10.1126/science.284.5415.816. [DOI] [PubMed] [Google Scholar]
- Herbeuval J.-P., Hardy A.W., Boasso A., Anderson S.A., Dolan M.J., Dy M., Shearer G.M. Regulation of TNF-related apoptosis-inducing ligand on primary CD4+ T cells by HIV-1: role of type I IFN-producing plasmacytoid dendritic cells. Proc. Natl. Acad. Sci. U.S.A. 2005;102:13974–13979. doi: 10.1073/pnas.0505251102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hishizawa M., Imada K., Kitawaki T., Ueda M., Kadowaki N., Uchiyama T. Depletion and impaired interferon-alpha-producing capacity of blood plasmacytoid dendritic cells in human T-cell leukaemia virus type I-infected individuals. Br. J. Haematol. 2004;125:568–575. doi: 10.1111/j.1365-2141.2004.04956.x. [DOI] [PubMed] [Google Scholar]
- Ibegbu C., Brodie-Hill A., Kourtis A.P., Carter A., McClure H., Chen Z.W., Nahmias A.J. Use of human CD3 monoclonal antibody for accurate CD4+ and CD8+ lymphocyte determinations in macaques: phenotypic characterization of the CD3−CD8+ cell subset. J. Med. Primatol. 2001;30:291–298. doi: 10.1034/j.1600-0684.2001.300601.x. [DOI] [PubMed] [Google Scholar]
- Jahnsen F.L., Lund-Johansen F., Dunne J.F., Farkas L., Haye R., Brandtzaeg P. Experimentally induced recruitment of plasmacytoid (CD123high) dendritic cells in human nasal allergy. J. Immunol. 2000;165:4062–4068. doi: 10.4049/jimmunol.165.7.4062. [DOI] [PubMed] [Google Scholar]
- Jones T.R., Obaldia N., Gramzinski R.A., Charoenvit Y., Kolodny N., Kitov S., Davis H.L., Krieg A.M., Hoffman S.L. Synthetic oligodeoxynucleotides containing CpG motifs enhance immunogenicity of a peptide malaria vaccine in Aotus monkeys. Vaccine. 1999;17:3065–3071. doi: 10.1016/s0264-410x(99)00145-0. [DOI] [PubMed] [Google Scholar]
- Karlsson G.B., Halloran M., Schenten D., Lee J., Racz P., Tenner-Racz K., Manola J., Gelman R., Etemad-Moghadam B., Desjardins E., Wyatt R., Gerard N.P., Marcon L., Margolin D., Fanton J., Axthelm M.K., Letvin N.L., Sodroski J. The envelope glycoprotein ectodomains determine the efficiency of CD4+ T lymphocyte depletion in simian–human immunodeficiency virus-infected macaques. J. Exp. Med. 1998;188:1159–1171. doi: 10.1084/jem.188.6.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krug A., Rothenfusser S., Hornung V., Jahrsdorfer B., Blackwell S., Ballas Z.K., Endres S., Krieg A.M., Hartmann G. Identification of CpG oligonucleotide sequences with high induction of IFN-α/β in plasmacytoid dendritic cells. Eur. J. Immunol. 2001;31:2154–2163. doi: 10.1002/1521-4141(200107)31:7<2154::aid-immu2154>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- LaBranche C.C., Sauter M.M., Haggarty B.S., Vance P.J., Romano J., Hart T.K., Bugelski P.J., Marsh M., Hoxie J.A. A single amino acid change in the cytoplasmic domain of the simian immunodeficiency virus transmembrane molecule increases envelope glycoprotein expression on infected cells. J. Virol. 1995;69:5217–5227. doi: 10.1128/jvi.69.9.5217-5227.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtner M., Rossi R., Mengoni F., Vignoli S., Colacchia B., Massetti A.P., Kamga I., Hosmalin A., Vullo V., Mastroianni C.M. Circulating dendritic cells and interferon-α production in patients with tuberculosis: correlation with clinical outcome and treatment response. Clin. Exp. Immunol. 2006;143:329–337. doi: 10.1111/j.1365-2249.2005.02994.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lifson J.D., Rossio J.L., Piatak M., Jr., Parks T., Li L., Kiser R., Coalter V., Fisher B., Flynn B.M., Czajak S., Hirsch V.M., Reimann K.A., Schmitz J.E., Ghrayeb J., Bischofberger N., Nowak M.A., Desrosiers R.C., Wodarz D. Role of CD8+ lymphocytes in control of simian immunodeficiency virus infection and resistance to rechallenge after transient early antiretroviral treatment. J. Virol. 2001;75:10187–10199. doi: 10.1128/JVI.75.21.10187-10199.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lore K., Smed-Sorensen A., Vasudevan J., Mascola J.R., Koup R.A. Myeloid and plasmacytoid dendritic cells transfer HIV-1 preferentially to antigen-specific CD4+ T cells. J. Exp. Med. 2005;201:2023–2033. doi: 10.1084/jem.20042413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund J., Sato A., Akira S., Medzhitov R., Iwasaki A. Toll-like receptor 9-mediated recognition of Herpes simplex virus-2 by plasmacytoid dendritic cells. J. Exp. Med. 2003;198:513–520. doi: 10.1084/jem.20030162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund J.M., Alexopoulou L., Sato A., Karow M., Adams N.C., Gale N.W., Iwasaki A., Flavell R.A. Recognition of single-stranded RNA viruses by Toll-like receptor 7. Proc. Natl. Acad. Sci. U. S. A. 2004;101:5598–5603. doi: 10.1073/pnas.0400937101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund J.M., Linehan M.M., Iijima N., Iwasaki A. Cutting edge: plasmacytoid dendritic cells provide innate immune protection against mucosal viral infection in situ. J. Immunol. 2006;177:7510–7514. doi: 10.4049/jimmunol.177.11.7510. [DOI] [PubMed] [Google Scholar]
- Mattapallil J.J., Douek D.C., Hill B., Nishimura Y., Martin M., Roederer M. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature. 2005;434:1093–1097. doi: 10.1038/nature03501. [DOI] [PubMed] [Google Scholar]
- Megjugorac N.J., Young H.A., Amrute S.B., Olshalsky S.L., Fitzgerald-Bocarsly P. Virally stimulated plasmacytoid dendritic cells produce chemokines and induce migration of T and NK cells. J. Leukocyte Biol. 2004;75:504–514. doi: 10.1189/jlb.0603291. [DOI] [PubMed] [Google Scholar]
- Moore J.P., Kitchen S.G., Pugach P., Zack J.A. The CCR5 and CXCR4 coreceptors—Central to understanding the transmission and pathogenesis of human immunodeficiency virus type 1 infection. AIDS Res. Hum. Retroviruses. 2004;20:111–126. doi: 10.1089/088922204322749567. [DOI] [PubMed] [Google Scholar]
- Moseman E.A., Liang X., Dawson A.J., Panoskaltsis-Mortari A., Krieg A.M., Liu Y.J., Blazar B.R., Chen W. Human plasmacytoid dendritic cells activated by CpG oligodeoxynucleotides induce the generation of CD4+ CD25+ regulatory T cells. J. Immunol. 2004;173:4433–4442. doi: 10.4049/jimmunol.173.7.4433. [DOI] [PubMed] [Google Scholar]
- Nagy Z.A., Mooney N.A. A novel, alternative pathway of apoptosis triggered through class II major histocompatibility complex molecules. J. Mol. Med. 2003;81:757–765. doi: 10.1007/s00109-003-0489-9. [DOI] [PubMed] [Google Scholar]
- Nishimura Y., Igarashi T., Donau O.K., Buckler-White A., Buckler C., Lafont B.A., Goeken R.M., Goldstein S., Hirsch V.M., Martin M.A. Highly pathogenic SHIVs and SIVs target different CD4+ T cell subsets in rhesus monkeys, explaining their divergent clinical courses. Proc. Natl. Acad. Sci. U.S.A. 2004;101:12324–12329. doi: 10.1073/pnas.0404620101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura Y., Brown C.R., Mattapallil J.J., Igarashi T., Buckler-White A., Lafont B.A., Hirsch V.M., Roederer M., Martin M.A. Resting naive CD4+ T cells are massively infected and eliminated by X4-tropic simian–human immunodeficiency viruses in macaques. Proc. Natl. Acad. Sci. U.S.A. 2005;102:8000–8005. doi: 10.1073/pnas.0503233102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacanowski J., Kahi S., Baillet M., Lebon P., Deveau C., Goujard C., Meyer L., Oksenhendler E., Sinet M., Hosmalin A. Reduced blood CD123+ (lymphoid) and CD11c+ (myeloid) dendritic cell numbers in primary HIV-1 infection. Blood. 2001;98:3016–3021. doi: 10.1182/blood.v98.10.3016. [DOI] [PubMed] [Google Scholar]
- Pashenkov M., Teleshova N., Kouwenhoven M., Smirnova T., Jin Y.P., Kostulas V., Huang Y.M., Pinegin B., Boiko A., Link H. Recruitment of dendritic cells to the cerebrospinal fluid in bacterial neuroinfections. J. Neuroimmunol. 2002;122:106–116. doi: 10.1016/s0165-5728(01)00451-9. [DOI] [PubMed] [Google Scholar]
- Patterson S., Rae A., Hockey N., Gilmour J., Gotch F. Plasmacytoid dendritic cells are highly susceptible to human immunodeficiency virus type 1 infection and release infectious virus. J. Virol. 2001;75:6710–6713. doi: 10.1128/JVI.75.14.6710-6713.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichyangkul S., Endy T.P., Kalayanarooj S., Nisalak A., Yongvanitchit K., Green S., Rothman A.L., Ennis F.A., Libraty D.H. A blunted blood plasmacytoid dendritic cell response to an acute systemic viral infection is associated with increased disease severity. J. Immunol. 2003;171:5571–5578. doi: 10.4049/jimmunol.171.10.5571. [DOI] [PubMed] [Google Scholar]
- Pichyangkul S., Yongvanitchit K., Kum-arb U., Hemmi H., Akira S., Krieg A.M., Heppner D.G., Stewart V.A., Hasegawa H., Looareesuwan S., Shanks G.D., Miller R.S. Malaria blood stage parasites activate human plasmacytoid dendritic cells and murine dendritic cells through a Toll-like receptor 9-dependent pathway. J. Immunol. 2004;172:4926–4933. doi: 10.4049/jimmunol.172.8.4926. [DOI] [PubMed] [Google Scholar]
- Reimann K.A., Li J.T., Veazey R., Halloran M., Park I.W., Karlsson G.B., Sodroski J., Letvin N.L. A chimeric simian/human immunodeficiency virus expressing a primary patient human immunodeficiency virus type 1 isolate env causes an AIDS-like disease after in vivo passage in rhesus monkeys. J. Virol. 1996;70:6922–6928. doi: 10.1128/jvi.70.10.6922-6928.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimann K.A., Parker R.A., Seaman M.S., Beaudry K., Beddall M., Peterson L., Williams K.C., Veazey R.S., Montefiori D.C., Mascola J.R., Nabel G.J., Letvin N.L. Pathogenicity of simian–human immunodeficiency virus SHIV-89.6P and SIVmac is attenuated in cynomolgus macaques and associated with early T-lymphocyte responses. J. Virol. 2005;79:8878–8885. doi: 10.1128/JVI.79.14.8878-8885.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt B., Scott I., Whitmore R.G., Foster H., Fujimura S., Schmitz J., Levy J.A. Low-level HIV infection of plasmacytoid dendritic cells: onset of cytopathic effects and cell death after PDC maturation. Virology. 2004;329:280–288. doi: 10.1016/j.virol.2004.08.016. [DOI] [PubMed] [Google Scholar]
- Schmidt B., Ashlock B.M., Foster H., Fujimura S.H., Levy J.A. HIV-infected cells are major inducers of plasmacytoid dendritic cell interferon production, maturation, and migration. Virology. 2005;343:256–266. doi: 10.1016/j.virol.2005.09.059. [DOI] [PubMed] [Google Scholar]
- Schmidt B., Fujimura S.H., Martin J.N., Levy J.A. Variations in plasmacytoid dendritic cell (PDC) and myeloid dendritic cell (MDC) levels in HIV-infected subjects on and off antiretroviral therapy. J. Clin. Immunol. 2006;26:55–64. doi: 10.1007/s10875-006-8401-3. [DOI] [PubMed] [Google Scholar]
- Schmitt N., Nugeyre M.T., Scott-Algara D., Cumont M.C., Barre-Sinoussi F., Pancino G., Israel N. Differential susceptibility of human thymic dendritic cell subsets to X4 and R5 HIV-1 infection. AIDS. 2006;20:533–542. doi: 10.1097/01.aids.0000210607.63138.bc. [DOI] [PubMed] [Google Scholar]
- Siegal F.P., Kadowaki N., Shodell M., Fitzgerald-Bocarsly P.A., Shah K., Ho S., Antonenko S., Liu Y.J. The nature of the principal type 1 interferon-producing cells in human blood. Science. 1999;284:1835–1837. doi: 10.1126/science.284.5421.1835. [DOI] [PubMed] [Google Scholar]
- Smed-Sorensen A., Lore K., Vasudevan J., Louder M.K., Andersson J., Mascola J.R., Spetz A.L., Koup R.A. Differential susceptibility to human immunodeficiency virus type 1 infection of myeloid and plasmacytoid dendritic cells. J. Virol. 2005;79:8861–8869. doi: 10.1128/JVI.79.14.8861-8869.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soumelis V., Scott I., Gheyas F., Bouhour D., Cozon G., Cotte L., Huang L., Levy J.A., Liu Y.-J. Depletion of circulating natural type 1 interferon-producing cells in HIV-infected AIDS patients. Blood. 2001;98:906–912. doi: 10.1182/blood.v98.4.906. [DOI] [PubMed] [Google Scholar]
- Strunk D., Rohde E., Lanzer G., Linkesch W. Phenotypic characterization and preclinical production of human lineage-negative cells for regenerative stem cell therapy. Transfusion. 2005;45:315–326. doi: 10.1111/j.1537-2995.2005.04056.x. [DOI] [PubMed] [Google Scholar]
- Teleshova N., Kenney J., Jones J., Marshall J., Van Nest G., Dufour J., Bohm R., Lifson J.D., Gettie A., Pope M. CpG-C immunostimulatory oligodeoxyribonucleotide activation of plasmacytoid dendritic cells in rhesus macaques to augment the activation of IFN-γ-secreting simian immunodeficiency virus-specific T cells. J. Immunol. 2004;173:1647–1657. doi: 10.4049/jimmunol.173.3.1647. [DOI] [PubMed] [Google Scholar]
- Teleshova N., Kenney J., Van Nest G., Marshall J., Lifson J.D., Sivin I., Dufour J., Bohm R., Gettie A., Robbiani M. Local and systemic effects of intranodally injected CpG-C immunostimulatory–oligodeoxyribonucleotides in macaques. J. Immunol. 2006;177:8531–8541. doi: 10.4049/jimmunol.177.12.8531. [DOI] [PubMed] [Google Scholar]
- Toba K., Koike T., Shibata A., Hashimoto S., Takahashi M., Masuko M., Azegami T., Takahashi H., Aizawa Y. Novel technique for the direct flow cytofluorometric analysis of human basophils in unseparated blood and bone marrow, and the characterization of phenotype and peroxidase of human basophils. Cytometry. 1999;35:249–259. [PubMed] [Google Scholar]
- Verthelyi D., Gursel M., Kenney R.T., Lifson J.D., Liu S., Mican J., Klinman D.M. CpG oligodeoxynucleotides protect normal and SIV-infected macaques from Leishmania infection. J. Immunol. 2003;170:4717–4723. doi: 10.4049/jimmunol.170.9.4717. [DOI] [PubMed] [Google Scholar]
- Yoneyama H., Matsuno K., Zhang Y., Nishiwaki T., Kitabatake M., Ueha S., Narumi S., Morikawa S., Ezaki T., Lu B., Gerard C., Ishikawa S., Matsushima K. Evidence for recruitment of plasmacytoid dendritic cell precursors to inflamed lymph nodes through high endothelial venules. Int. Immunol. 2004;16:915–928. doi: 10.1093/intimm/dxh093. [DOI] [PubMed] [Google Scholar]
- Yonezawa A., Morita R., Takaori-Kondo A., Kadowaki N., Kitawaki T., Hori T., Uchiyama T. Natural alpha interferon-producing cells respond to human immunodeficiency virus type 1 with alpha interferon production and maturation into dendritic cells. J. Virol. 2003;77:3777–3784. doi: 10.1128/JVI.77.6.3777-3784.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Xu D., Li Y., Jin L., Shi M., Wang M., Zhou X., Wu H., Gao G.F., Wang F.S. Longitudinal alteration of circulating dendritic cell subsets and its correlation with steroid treatment in patients with severe acute respiratory syndrome. Clin. Immunol. 2005;116:225–235. doi: 10.1016/j.clim.2005.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Fu J., Zhao Q., He Y., Jin L., Zhang H., Yao J., Zhang L., Wang F.S. Differential restoration of myeloid and plasmacytoid dendritic cells in HIV-1-infected children after treatment with highly active antiretroviral therapy. J. Immunol. 2006;176:5644–5651. doi: 10.4049/jimmunol.176.9.5644. [DOI] [PubMed] [Google Scholar]