Membrane binding of the bacterial signal recognition particle receptor involves two distinct binding sites (original) (raw)
Abstract
Cotranslational protein targeting in bacteria is mediated by the signal recognition particle (SRP) and FtsY, the bacterial SRP receptor (SR). FtsY is homologous to the SRα subunit of eukaryotes, which is tethered to the membrane via its interaction with the membrane-integral SRβ subunit. Despite the lack of a membrane-anchoring subunit, 30% of FtsY in Escherichia coli are found stably associated with the cytoplasmic membrane. However, the mechanisms that are involved in this membrane association are only poorly understood. Our data indicate that membrane association of FtsY involves two distinct binding sites and that binding to both sites is stabilized by blocking its GTPase activity. Binding to the first site requires only the NG-domain of FtsY and confers protease protection to FtsY. Importantly, the SecY translocon provides the second binding site, to which FtsY binds to form a carbonate-resistant 400-kD FtsY–SecY translocon complex. This interaction is stabilized by the N-terminal A-domain of FtsY, which probably serves as a transient lipid anchor.
Introduction
About 30% of all proteins synthesized in an Escherichia coli cell execute their cellular function in extracytoplasmic compartments like the inner membrane, the periplasm, or the outer membrane. The distribution of newly synthesized proteins to their final location is therefore a crucial issue, and bacteria have evolved multiple targeting pathways for selecting and properly directing these extracytoplasmic proteins (Müller et al., 2001). The majority of these proteins engage the SecY translocon for exiting the cytoplasm and are targeted to this protein-conducting channel by two distinct pathways. Secretory proteins, e.g., proteins that are translocated across the inner membrane to reside in the periplasm or in the outer membrane are transported posttranslationally by the bacterial-specific SecA–SecB pathway (de Keyzer et al., 2003). In this pathway, the chaperone SecB binds to most secretory proteins before they are transferred to the ATPase SecA, which is distributed between the cytoplasm and the membrane (Cabelli et al., 1991). Membrane binding of SecA is mediated by its affinity for both phospholipids and the SecY translocon (Lill et al., 1990). Importantly, only the translocon bound SecA is thought to interact with the signal sequence of secretory protein in a highly specific manner (Hartl et al., 1990). Finally, the secretory protein is threaded through the SecY channel by repeated ATP-dependent conformational changes of SecA (Economou and Wickner, 1994; den Blaauwen et al., 1996).
Different to secretory proteins, bacterial inner membrane proteins are recognized cotranslationally by the universally conserved signal recognition particle (SRP; Koch et al., 2003). SRP binds to the signal anchor sequence of a nascent membrane protein when it emerges from the ribosome and subsequently targets the SRP–ribosome-associated nascent chain (RNC) complex to the membrane via a GTP-dependent interaction with FtsY, the bacterial SRP receptor (SR). At the membrane, the RNC is transferred from the SRP–SR complex to the SecY translocon, and the membrane protein is cotranslationally inserted into the lipid bilayer. This step requires the sequential dissociation of SRP from both the signal anchor sequence and SR and is regulated by the GTPase activities of both Ffh, the protein component of the bacterial SRP, and FtsY (Wild et al., 2004; Halic and Beckmann, 2005). In eukaryotic cells, the SRP-interacting SRα subunit of the SR is tethered to the membrane via its specific interaction with the membrane-integral SRβ subunit (Gilmore et al., 1982). As SRβ is suggested to interact with the Sec61 channel, the eukaryotic SRP might target its substrates directly into close vicinity of the Sec61 translocon (Helmers et al., 2003). One particular facet of the bacterial SRP pathway is that the bacterial SR consists of only the FtsY protein, which is homologous to the eukaryotic SRα (Bernstein et al., 1989; Römisch et al., 1989). Although the bacterial SR lacks a membrane integral subunit, ∼30% of the cellular FtsY is stably associated with the membrane. Importantly, only the membrane bound FtsY appears to be able to induce the dissociation of SRP from the signal anchor sequence (Valent et al., 1998). The association of FtsY with the membrane is thought to involve both protein–lipid contacts and protein–protein contacts (Millman et al., 2001). Recent data indicate that FtsY is at least transiently associated with the SecY translocon (Angelini et al., 2005), and it has been proposed that the SecY translocon provides one binding site for FtsY at the membrane.
In this study, we have further analyzed the membrane association of FtsY. Our data indicate that FtsY binds to two discrete binding sites at the E. coli membrane and that both interactions are stabilized by guanosine 5′-[β,γ-imido] triphosphate (GMP-PNP), a nonhydrolysable GTP analogue. Binding to the first site requires only the NG-domain of FtsY and results in a proteinase K–resistant conformation of FtsY. The second binding site is provided by the SecY translocon, to which FtsY binds in a carbonate-resistant manner. This interaction results in the formation of a 400-kD FtsY translocon complex and is stabilized by the N-terminal A-domain of FtsY, which probably serves as a transient lipid anchor.
Results
Membrane association of FtsY is stabilized by blocking its GTPase activity
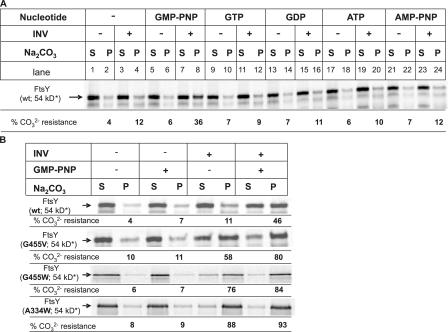
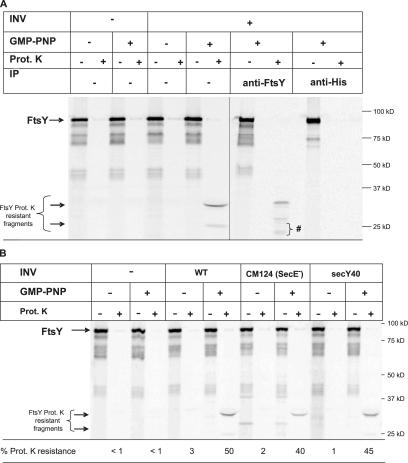
Despite the lack of a transmembrane domain, FtsY has been shown to be partially resistant toward alkaline carbonate extraction (Luirink et al., 1994; de Leeuw et al., 1997), a method that is routinely used to differentiate between membrane-inserted and soluble proteins (Fujiki et al., 1982). We used this method to determine whether membrane binding of FtsY was influenced by nucleotides. When in vitro–synthesized FtsY was incubated with inner membrane vesicles (INVs) and subsequently extracted with 0.2 M Na2CO3, we observed a small but reproducible increase in carbonate resistance (Fig. 1 A, compare lanes 2 and 4). Strikingly, when FtsY was incubated with INVs in the presence of the nonhydrolysable GTP analogue GMP-PNP, a significantly larger portion of FtsY was bound to the membrane in a carbonate-resistant manner (Fig. 1 A, compare lanes 4 and 8). This was not due to a GMP-PNP–induced aggregation of FtsY, because in the absence of INVs, the addition of GMP-PNP did not change the amount of FtsY present in the pellet fraction after carbonate extraction (Fig. 1 A, lane 6). The increase in carbonate resistance in the presence of INVs was strictly dependent on GMP-PNP; the addition of GTP, GDP, ATP, or adenyl-5′-yl imidodiphosphate (AMP-PNP) did not significantly influence the ability of FtsY to associate with the membrane in a carbonate-resistant manner (Fig. 1 A).
Figure 1.
FtsY acquires a carbonate-resistant conformation in the presence of INVs and GMP-PNP. (A) Wild-type (wt) FtsY was in vitro synthesized and incubated with buffer or membranes in the presence or absence of nucleotides (final concentration, 2 mM) and a nucleotide-regenerating system (Koch et al., 1999). Subsequently, the samples were extracted with Na2CO3 and ultracentrifuged. Soluble material (S) was neutralized with glacial acetic acid and TCA precipitated. Carbonate-resistant material (P) was directly dissolved in SDS loading buffer. For quantification, the amount of soluble and resistant material was set as 100% and the material present in the individual fractions was quantified. Several independent experiments were performed, and a representative gel is shown. (B) Carbonate resistance of wild-type and mutant FtsY. Analysis was performed as in A. Asterisks indicate that although the calculated molecular mass of FtsY is 54 kD, it migrates in SDS-PAGE as a 90-kD band (Fig. 5).
The data described in the previous paragraph could suggest that blocking the GTPase activity of FtsY by GMP-PNP stabilizes its association with the membrane. We therefore analyzed the carbonate resistance of FtsY mutants that were affected in their GTPase activities. The FtsY mutant FtsY(G455V) has been shown to bind GTP but is unable to hydrolyze it efficiently (Lu et al., 2001). In contrast to wild-type FtsY, in vitro–synthesized FtsY(G455V) exhibited a strong carbonate resistance even in the absence of GMP-PNP (Fig. 1 B). When assayed in the presence of GMP-PNP, the carbonate-resistant interaction with the membrane increased even further (Fig. 1 B). This could reflect the fact that the GTPase activity of this particular mutant is only partially blocked (Lu et al., 2001) but could also suggest that additional GTPases, like SRP, are involved in the GMP-PNP–dependent carbonate-resistant interaction of FtsY with the membrane. This was addressed by analyzing the carbonate resistance of two additional FtsY mutants: FtsY(G455W) and FtsY(A334W). Both mutants exhibit no significant GTP hydrolysis (<5% of wild type; Shan et al., 2004); they differ, however, in their ability to form an FtsY–SRP complex (Shan et al., 2004). FtsY(G455W) is like the aforementioned FtsY(G455V) mutant, defective in SRP–FtsY complex formation, whereas complex formation is not impaired in the FtsY(A334W) mutant (Shan et al., 2004). Nevertheless, the G455W and A334W mutants exhibited the same carbonate-resistant phenotype, which was almost completely independent of GMP-PNP (Fig. 1 B). These data suggest that the GTPase activity of FtsY is directly correlated with its ability to interact with the membrane in a carbonate-resistant manner.
FtsY assembles into a 400-kD FtsY translocon complex upon the addition of GMP-PNP
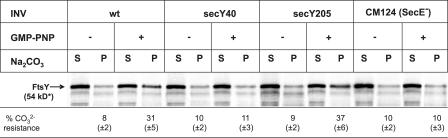
We have recently shown that FtsY transiently interacts with the SecY translocon (Angelini et al., 2005). Whether this interaction was responsible for the GMP-PNP–induced carbonate-resistant membrane binding of FtsY was analyzed by using INVs derived from translocon mutants. The SecY40 mutant has been shown to specifically block the integration of SRP-dependent membrane proteins (Newitt and Bernstein, 1998), and it has been proposed that it is particularly the SecY–FtsY interaction that is impaired in this mutant (Angelini et al., 2005). In agreement with this hypothesis, FtsY did not gain carbonate resistance in the presence of secY40 INVs upon the addition of GMP-PNP (Fig. 2). In contrast, if INVs derived from the secY205 mutant were tested, the GMP-PNP–induced association of FtsY with the membrane was comparable to wild-type INVs. The secY205 mutation blocks a functional SecA–SecY interaction (Matsumoto et al., 1997) without disturbing the SRP/FtsY-dependent steps of membrane protein integration (Koch and Müller, 2000; Neumann-Haefelin et al., 2000; Deitermann et al., 2005). Importantly, no GMP-PNP–induced carbonate resistance of FtsY was observed in SecE-depleted INVs (Fig. 2, CM124), in which SecY is rapidly degraded (Traxler and Murphy, 1996). These data are consistent with the idea that GMP-PNP stabilizes a specific interaction between the SecY translocon and FtsY, which, as a consequence, acquires carbonate resistance.
Figure 2.
Carbonate resistance of FtsY requires the SecY translocon. (A) In vitro–synthesized FtsY was incubated with wild-type (wt) INVs or INVs derived from secY mutants (secY40 and secY205) and a SecE-depletion mutant (CM124). After incubation in the presence or absence of GMP-PNP, samples were carbonate extracted and quantified as described in Fig. 1. A representative gel is shown, and the standard deviation is indicated. The asterisk indicates that although the calculated molecular mass of FtsY is 54 kD, it migrates in SDS-PAGE as a 90-kD band.
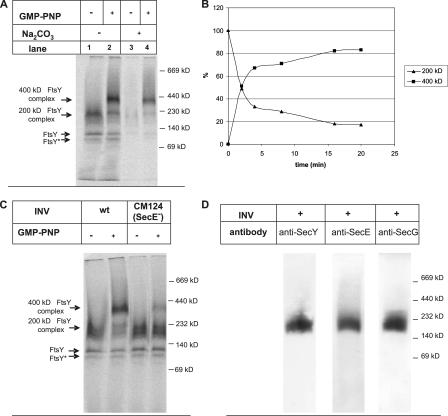
To obtain evidence for a possible complex formation between FtsY and the SecY translocon upon addition of GMP-PNP, we used blue native PAGE (BN-PAGE), a technique that has been widely used for studying membrane bound protein complexes (Schägger, 2001). We first determined whether FtsY would assemble into differently sized complexes in the presence or absence of GMP-PNP. His-tagged FtsY was in vitro synthesized, purified by metal affinity chromatography, and incubated with INVs. Subsequently, the membranes were solubilized with dodecyl-β-d-maltoside and separated by BN-PAGE. In the absence of GMP-PNP, we observed a strong 200-kD radiolabeled FtsY complex (Fig. 3 A, lane 1). However, if FtsY was incubated with INVs in the presence of GMP-PNP, the 200-kD band became significantly weaker and instead a new complex of ∼400 kD appeared (Fig. 3 A, lane 2). The appearance of the 400-kD FtsY complex was GMP-PNP specific and not observed in the presence of GTP, GDP, ATP, or AMP-PNP (unpublished data). To analyze whether the 400-kD FtsY complex represented the carbonate-resistant state of FtsY, membranes were incubated with FtsY in the presence or absence of GMP-PNP and carbonate extracted. Only the carbonate-resistant material was then solubilized and separated on BN-PAGE. Importantly, the 400-kD FtsY complex that was formed in the presence of GMP-PNP was resistant to carbonate extraction (Fig. 3 A, lane 4), whereas the 200-kD FtsY complex was carbonate sensitive, i.e., only barely detectable (Fig. 3 A, lane 3). Thus, the carbonate-resistant interaction of FtsY with the membrane is reflected by the formation of the 400-kD complex. The formation of the 400-kD complex was further analyzed in a time-course experiment. Membrane vesicles were incubated with radiolabeled FtsY and GMP-PNP, and at different time points after GMP-PNP addition, samples were solubilized and separated on BN-PAGE. Fig. 3 B shows that the 200-kD complex was converted within minutes into the 400-kD complex.
Figure 3.
In the presence of GMP-PNP, FtsY assembles into a 400-kD membrane complex. (A) In vitro–synthesized FtsY was purified and subsequently incubated with membranes in the absence or presence of GMP-PNP. The samples were either directly solubilized with 0.2% (wt/vol) n-dodecylmaltoside or only after carbonate extraction. The solubilized material was separated on a 5–13% BN-PAGE, and radioactively labeled samples were visualized by phosphorimaging. Indicated are the positions of the 400- and 200-kD FtsY complexes. FtsY and FtsY* represent as-yet-uncharacterized FtsY variants (see Results). (B) The formation of the 400-kD complex was analyzed in a time-course experiment. Purified FtsY was incubated with INVs, and after the addition of GMP-PNP, samples were withdrawn at the indicated time points and separated on BN-PAGE as in A. The amount of radioactively labeled material present in the 200-kD complex at 0 min was set as 100%. (C) BN-PAGE analyses of FtsY bound to either wild-type (wt) membranes or SecE-depleted INVs (CM124) in the presence or absence of GMP-PNP. (D) Detection of the SecYEG complex in wild-type membranes by immune detection. 100 μg INV was solubilized with 0.2% n-dodecylmaltoside and separated on BN-PAGE. After Western transfer, the membrane was decorated with the indicated polyclonal antibodies.
Because FtsY does not acquire carbonate resistance in SecE-depleted membranes (Fig. 2), one would expect that the 400-kD FtsY complex is not formed in the presence of these membranes. Indeed, the conversion of the 200-kD complex into the 400-kD complex was only barely detectable in the presence of SecE-depleted membranes (Fig. 3 C), suggesting that the formation of the 400-kD complex required the presence of the SecY translocon. The very small amount of the 400-kD complex observed in these membranes is probably due to residual amounts of the Sec translocon, which, as an essential protein, cannot be completely depleted.
Independently of whether GMP-PNP had been added, two weaker radiolabeled bands below the 140-kD protein marker band were detectable (Fig. 3 A, lanes 1 and 2); these were not carbonate resistant (Fig. 3 A, lanes 3 and 4) and were also detected in the SecE-depleted INVs (Fig. 3 C). Like the dominating 200- and 400-kD complexes, they were recognized by α-FtsY antibodies (unpublished data). Despite its predicted size of 54 kD, FtsY migrates in SDS-PAGE as a doublet band of 100 and 75 kD. The 75-kD band represents a truncated FtsY derivative that lacks the first N-terminal 14 amino acids (unpublished data; Luirink et al., 1994). We currently do not know whether the two bands recognized below the 140-kD marker band correspond to these 100 and 75 kD species or whether they reflect additional proteolytic fragments, like the 56-kD fragment observed by Millman and Andrews (1999).
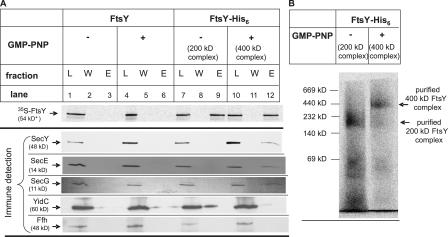
Importantly, these data are all consistent with a GMP-PNP stabilized FtsY translocon complex of 400 kD. Different oligomers of the SecYEG complex have been reported to exist in E. coli membranes, with a preponderance of a SecYEG complex of ∼230 kD (Duong, 2003). We confirmed the presence of this SecYEG complex by performing a BN-PAGE of solubilized wild-type INV proteins. After Western transfer, a single complex of ∼230 kD was immunodetected with antibodies directed against SecY, SecE, or SecG, the core subunits of the bacterial Sec translocon (Fig. 3 D). Thus, the 400-kD FtsY complex observed in the presence of GMP-PNP would be compatible with the association of the 200-kD FtsY complex with an ∼230-kD SecY translocon complex. To verify this, His-tagged FtsY was synthesized in a large-scale in vitro reaction and incubated with INVs in the absence or presence of GMP-PNP. INVs were isolated and solubilized, and the solubilized material was purified by metal affinity chromatography. Independently of whether GMP-PNP had been added, similar amounts of radiolabeled FtsY were eluted from the column (Fig. 4 A, top, lanes 9 and 12). A small aliquot of the eluted material was separated on BN-PAGE to verify that both the 200- and 400-kD complexes were detectable, which was indeed the case (Fig. 4 B). The remaining material was separated on SDS-PAGE for immune detection. SecY, SecE, and SecG were detectable in the eluted 400-kD FtsY complex (Fig. 4 A, lane 12). All three translocon components were, however, undetectable in the eluted 200-kD FtsY complex (Fig. 4 A, lane 9). Neither the 200- nor the 400-kD complex appeared to contain significant amounts of YidC or Ffh, the protein component of the bacterial SRP (Fig. 4 A, lanes 9 and 12), although some YidC was detectable in the wash fraction (Fig. 4 A, lane 8). This is probably due to a partial overloading of the column. To validate the copurification method, we also in vitro synthesized FtsY lacking the His tag (Fig. 4 A, lanes 3 and 6) and repeated the purification procedure, but neither FtsY nor the translocon components were eluted from the column. In conclusion, our data strongly suggest that the 400-kD complex represents an FtsY–SecYEG translocon complex.
Figure 4.
The 400-kD complex represents an FtsY–SecYEG complex. (A) A large-scale FtsY in vitro synthesis was incubated with membranes in the absence or presence of GMP-PNP. After solubilization, the solubilized material was purified via metal affinity chromatography. (top) A small portion of the eluted material was separated on SDS-PAGE, and the radiolabeled FtsY was detected via phosphorimaging (L, 1% of load; W, 5% of final wash; E, 5% of eluted material). (bottom) The remaining material was separated on SDS-PAGE and transferred to nitrocellulose membranes for subsequent immune detection (L, 2% of load; W, 10% of final wash; E, 90% of eluted material). As control, nontagged FtsY was subjected to the same purification procedure (lanes 1–6). (B) 5% of the eluted material shown in lane 9 (without GMP-PNP) and lane 12 (with GMP-PNP) was separated on BN-PAGE, and FtsY complexes were detected by phosphorimaging.
Identification of a second, SecY-independent binding site for FtsY at the membrane
Several reports have suggested that the association of FtsY with the membrane involves two distinct binding sites (de Leeuw et al., 1997, 2000; Millman et al., 2001). Our data suggest that the SecY translocon provides the proteinaceous binding site proposed by Millman et al. (2001). Because FtsY has been shown to bind to liposomes and to insert into lipid monolayers (de Leeuw et al., 2000), lipids probably provide a second binding site for FtsY. For analyzing SecY-independent binding of FtsY to the membrane, we adopted the approach of limited proteolysis, which has been used to detect lipid- or nucleotide-induced conformational changes in FtsY (Kusters et al., 1995; de Leeuw et al., 2000). In the absence of INVs, FtsY was almost completely degraded by proteinase K, independently of whether GMP-PNP had been added (Fig. 5 A). In the presence of INVs, FtsY was also almost completely degraded, unless GMP-PNP had been added. In fact, under these conditions, a strong protease-protected fragment of 33 kD was detected, which over time was further degraded into a 25-kD protease-protected fragment (Fig. 5 A). Both fragments were immunoprecipitated by α-FtsY antibodies, confirming that they were derived from full-size FtsY. Two additional fragments recognizable only after immunoprecipitation (Fig. 5 A, asterisk) are probably the result of an additional cleavage event during the immunoprecipitation procedure. Antibodies directed against the C-terminal (His)6 tag of FtsY immunoprecipitated only full-size FtsY but not the protease-protected fragments, suggesting that in addition to the cleavage at the N-terminal part (Kusters et al., 1995; see Fig. 7), proteinase K also cleaves at the C-terminal part of FtsY. As for the carbonate resistance, the appearance of the proteinase K–resistant fragments was strictly dependent on the addition of GMP-PNP; the addition of GTP, GDP, ATP, or AMP-PNP did not result in proteinase K protection of FtsY (unpublished data).
Figure 5.
A protease-resistant conformation of FtsY is observed in the presence of membranes and GMP-PNP. (A) In vitro–synthesized FtsY was incubated with buffer or INVs in the presence of absence of GMP-PNP. Half of the reaction was directly TCA precipitated and the other half only after treatment with 0.5 mg/ml proteinase K for 20 min at 25°C. Indicated are full-size FtsY and its proteinase K–protected fragments. Immunoprecipitation (IP) experiments were performed with antibodies covalently bound to protein A–Sepharose beads. For proteinase K–treated samples, the protease inhibitor PMSF was added before the addition of the antibody beads. The asterisk indicates unspecific cleavage products of FtsY, which were observed only after immunoprecipitations. (B) Proteinase K protection was analyzed as in A in the presence of wild-type (WT), SecE-depleted (CM124), and secY40 INVs. The percentage of protease protection was calculated by quantifying the ratio of radioactivity present in the proteinase K–treated sample and the directly TCA precipitated sample. The protease-protected fragment of FtsY comprises the major parts of the NG-domain (Fig. 7), and the percentage of protease protection was corrected for the loss of methionine residues as a result of cleaving off the A-domain (see Fig 6 for the position of methionine residues in FtsY). However, as the exact boundaries of the 33-kD fragment are not known, the values are estimates only.
Figure 7.
The A-domain is not essential for the proteinase K–resistant interaction of FtsY with the membrane. (A) Proteinase K protection of full-size FtsY and FtsY(NG+1) was analyzed as in Fig. 5. (B) Carbonate resistance of full-size FtsY was analyzed after a proteinase K treatment (lanes 3 and 4). As control, carbonate extraction without prior proteinase K treatment is shown (lanes 1 and 2). S, soluble material; P, carbonate-resistant material.
Finally, we tested whether the proteinase K protection of FtsY reflected a SecY-independent interaction by using SecE-depleted INVs. In contrast to the carbonate-resistance data, protease protection of FtsY in the presence of GMP-PNP was observed for both the SecE-depleted and the SecY40 INVs, albeit at a slightly reduced level in comparison to wild-type INVs (Fig. 5 B). In summary, our data strongly suggest that the carbonate- and proteinase K–resistant states of FtsY reflect two different types of FtsY membrane interactions: to achieve carbonate resistance, FtsY has to be in close contact with the SecY translocon, whereas the proteinase K–resistant state of FtsY is independent of the SecY translocon.
The A-domain of FtsY stabilizes its interaction with the SecY translocon
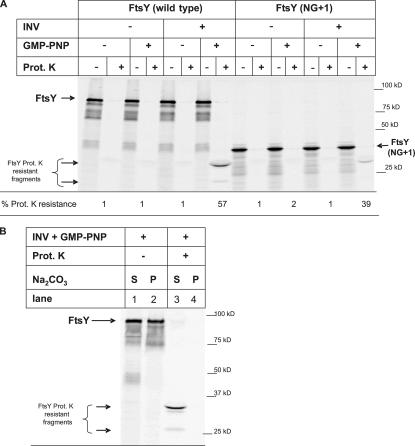
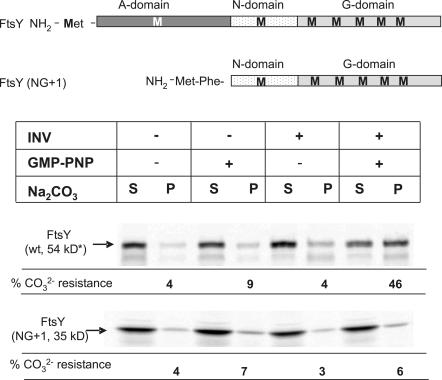
Membrane binding of the E. coli FtsY is thought to require at least its N-terminal A-domain (Powers and Walter, 1997; Zelazny et al., 1997; Herskovits et al., 2001) but probably also involves the N-domain (Millman and Andrews, 1999). Surprisingly, it has recently been shown that adding a single phenylalanine residue to the N terminus of a nonfunctional NG derivative of FtsY is sufficient to support the growth of E. coli (Eitan and Bibi, 2004). To analyze the role of the A-domain in more detail, we analyzed membrane binding of the FtsY(NG+1) derivative both by carbonate-resistance and proteinase K–protection assays. Different from full-size FtsY, FtsY(NG+1) did not become carbonate resistant in the presence of GMP-PNP (Fig. 6).
Figure 6.
The A-domain of FtsY is required for carbonate-resistant interaction with the membrane. (A) Wild-type (wt) FtsY and FtsY(NG+1), lacking the A-domain, were in vitro synthesized and incubated with INV or buffer in the presence or absence of GMP-PNP. Treatment of the samples and quantification was performed as described in Fig. 1. The asterisk indicates that although the calculated molecular mass of FtsY is 54 kD, it migrates in SDS-PAGE as a 90-kD band. S, soluble material; P, carbonate-resistant material.
Although FtsY(NG+1) did not gain carbonate resistance in the presence of GMP-PNP, the 33-kD proteinase K–protected fragment was clearly visible (Fig. 7 A). The presence of identical protease-protected fragments of full-size FtsY and FtsY(NG+1) suggests that proteinase K predominantly cleaves off the A-domain of FtsY. Importantly, our data suggest that in the absence of the A-domain, FtsY is still able to interact with the membrane and to acquire a proteinase K–resistant conformation in the presence of GMP-PNP, although protease protection is slightly less pronounced than for full-size FtsY. On the other hand, the lack of the A-domain seems to completely diminish the carbonate-resistant interaction with the SecY translocon. To verify this, we made use of the observation that the A-domain of membrane bound FtsY is accessible to proteinase K. When full-size FtsY was incubated with GMP-PNP and INVs, a large portion was carbonate resistant (Fig. 7 B, lane 2). When membrane-associated full-size FtsY was first treated with proteinase K and carbonate extracted, the 33- and 25-kD FtsY fragments were exclusively found in the supernatant. This suggests that cleaving off the A-domain prevents a carbonate-resistant interaction of FtsY with the SecY translocon. We also analyzed the membrane association of FtsY(NG+1) by BN-PAGE analyses. However, because the electrophoretic mobility of FtsY lacking the A-domain in SDS-PAGE (de Leeuw et al., 1997) and BN-PAGE (unpublished data) is completely different from full-size FtsY, it is difficult to draw internally consistent conclusions from this particular experiment.
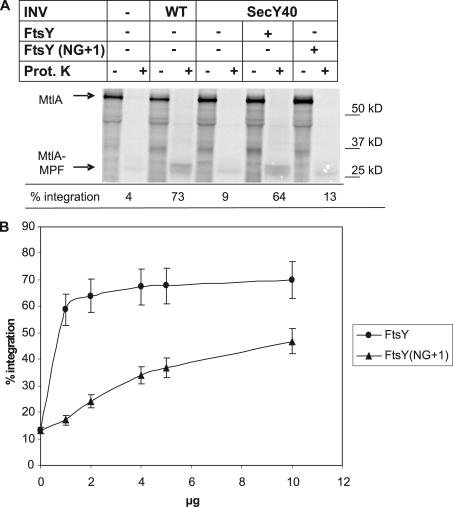
In light of the recent observation that FtsY(NG+1) is at least partially functional in vivo (Eitan and Bibi, 2004), we used a complementation assay (Angelini et al., 2005) to test the functionality of FtsY(NG+1) in vitro. The integration of the SRP-dependent membrane protein mannitol permease (MtlA) was drastically reduced in the presence of SecY40 INVs (Fig. 8 A) but was almost completely restored by the addition of 1 μg wild-type FtsY. In contrast, the same amount of FtsY(NG+1) did not significantly improve the integration of MtlA. It was only by increasing the concentrations of FtsY(NG+1) that a measurable integration of MtlA in secY40 INVs was observed (Fig. 8 B). This suggests that although the A-domain is not essential for the interaction between FtsY and the SecY translocon, it improves the efficiency of this interaction. In light of the fact that carbonate resistance is only observed in the presence of the A-domain, the A-domain probably serves as a transient lipid anchor that stabilizes the FtsY–SecY interaction.
Figure 8.
FtsY(NG+1) is less active than full-size FtsY. (A) The activity of FtsY and FtsY(NG+1) was analyzed in an in vitro complementation assay using INVs derived from the secY40 mutant (Angelini et al., 2005). MtlA was in vitro synthesized in the presence of wild-type (WT) or secY40 INV. When indicated, 1 μg of purified FtsY or FtsY(NG+1) was added. Translation products were precipitated either directly with TCA or only after incubation with proteinase K as described in Fig. 5. The percentage of integration was calculated as in Fig. 5, and the values were corrected for the loss of methionine by cleaving off the 30-kD cytoplasmic domain of MtlA. (B) Integration of MtlA into secY40 INVs was tested as in A but with increasing concentrations of FtsY and FtsY(NG+1).
Discussion
Although the bacterial SR lacks a membrane-integral SRβ homologue, the SRα homologue FtsY is still found partly associated with the cytoplasmic membrane (Luirink et al., 1994). Importantly, only the membrane-associated FtsY appears to mediate the transfer of the RNC from the SRP to the SecY translocon (Valent et al., 1998), suggesting that FtsY requires the context of the membrane to be functional in the targeting reaction. This raises the important question of how FtsY is tethered to the cytoplasmic membrane and how this is coordinated with the targeting reaction.
Our data provide clear evidence that the SecY translocon provides one binding site for FtsY, which supports our recent hypothesis (Angelini et al., 2005) that SecY is most likely the proteinaceous factor proposed to be involved in membrane binding of FtsY (de Leeuw et al., 2000; Millman et al., 2001). Binding of FtsY to the SecY translocon is reflected by its carbonate resistance and the formation of a 400-kD FtsY–SecY translocon complex. It involves most likely the surface-exposed cytoplasmic loop C5 of SecY, which is suggested to provide an important interface for cytosolic factors during protein transport (van den Berg et al., 2004). A single amino acid exchange in this loop, like in the secY40 mutant, blocks the carbonate-resistant interaction between SecY and FtsY. This nicely explains why, in this mutant, the SRP- but not the SecA-dependent protein transport is impaired (Newitt and Bernstein, 1998; Angelini et al., 2005). The A-domain of FtsY appears to be involved in stabilizing the FtsY–SecY interaction. The important function of the A-domain is also reflected by the observation that FtsY derivatives lacking the complete A-domain are nonfunctional in E. coli (Zelazny et al., 1997). Surprisingly, however, it has been shown recently that adding a single phenylalanine residue to the N terminus of an otherwise nonfunctional NG-domain is sufficient to support growth of E. coli in vivo (Eitan and Bibi, 2004), although its ability to bind to the membrane is reduced. In agreement with this observation, our analyses show that this (NG+1) derivative is partly functional in an in vitro–complementation assay using secY40 mutant INVs. However, to suppress the secY40 phenotype, significantly higher concentrations of FtsY(NG+1) are required in comparison to wild-type FtsY. In addition, unlike wild-type FtsY, FtsY(NG+1) does not associate in a carbonate-resistant manner with the SecY translocon. These data could indicate that the primary contact between FtsY and SecY is mediated via the NG-domain of FtsY, which is then further stabilized by the A-domain. This would also be in agreement with the observation that some bacterial FtsY lack the A-domain (Bibi et al., 2001). Because carbonate resistance is considered to primarily reflect protein–lipid contact, the A-domain could serve as a transient lipid anchor in E. coli.
It has been shown that even in the absence of the SecY translocon, FtsY can still bind to the membrane and is able to induce with low efficiency SRP release from a nascent chain (Scotti et al., 1999). However, in the absence of the SecY translocon, SRP release is not coupled to membrane insertion (Scotti et al., 1999). To prevent such a futile targeting cycle, it has been suggested that FtsY needs to be primed for coordinating the efficient SRP–RNC targeting with the subsequent insertion process (Lu et al., 2001; Shan et al., 2004). Consistently, our data could suggest that it is the interaction with SecY that primes FtsY for efficient binding of the SRP–RNC complex. In this scenario, the targeting reaction would be directly coupled to the accessibility of the translocon. In eukaryotes, a role of SRβ in sensing the availability of the translocon has been suggested (Helmers et al., 2003), which is in agreement with data showing that SRβ plays an important role in the transfer of the RNC from SRP to the translocon (Fulga et al., 2001). A recent model suggests that only in the presence of an empty translocon does SRα assemble with SRβ, thus forming a functional receptor for SRP–RNCs in close vicinity to an accessible translocon (Schwartz and Blobel, 2003). The recent cryo-EM structure of an SR–SRP–RNC complex does not reveal any direct contact between SRα and the ribosome (Halic et al., 2006) but confirms cross-linking data suggesting a direct contact between SRβ and ribosomal proteins (Fulga et al., 2001). Even though biochemical data indicate a role also of SRα in ribosome binding (Mandon et al., 2003), it is evident that SRβ is an important factor for membrane tethering of the SRP–RNC complex. In bacteria, the absence of SRβ might necessitate the formation of an FtsY translocon complex to provide binding sites for both SRP (via FtsY) and the ribosome (via SecY), thereby allowing the efficient and highly selective delivery of an SRP–RNC complex to the protein-conducting channel.
The ability of FtsY to bind to the membrane in the absence of the Sec translocon suggests the presence of a second, SecY-independent binding site, which could be provided by phospholipids (de Leeuw et al., 1997, 2000; Millman et al., 2001) or by an as-yet-unidentified protein component. We show here that in the absence of the SecY translocon, FtsY forms a 200-kD membrane bound complex, which in contrast to the 400-kD FtsY translocon complex, is sensitive to carbonate extraction. So far, we have been unable to detect any additional protein besides FtsY in this complex; in particular YidC, which has been shown in chloroplasts to interact with FtsY (Moore et al., 2003), does not seem to be present. This could indicate that the 200-kD FtsY complex reflects just lipid bound FtsY and does not involve any additional protein. This would be in agreement with gel filtration data showing that purified native FtsY elutes in a single peak of 200 kD (Luirink et al., 1994). However, because of the aberrant mobility of FtsY in SDS-PAGE and in gel filtration studies, it is currently not possible to unambiguously determine whether the 200-kD FtsY species corresponds to an FtsY monomer with aberrant mobility, an FtsY oligomer, or a complex between FtsY and an as-yet-unknown component. In any case, the presence of a second, SecY-independent binding site for FtsY is also suggested by our observation that the NG-domain of FtsY acquires protease protection upon membrane contact, which does not require the presence of the SecY translocon. In the absence of the SecY translocon, only the 200-kD complex is observed, suggesting that the protease-protected fragments most likely derive from this complex. Protease protection of FtsY is also largely independent of the A-domain, suggesting that this domain is not essential for the SecY-independent membrane binding of FtsY.
One striking result of our experiments is that binding of FtsY to both the SecY translocon and the SecY-independent binding site is greatly stabilized by blocking the GTP hydrolysis of FtsY. This was achieved by using either GMP-PNP or by analyzing GTPase mutants of FtsY. In solution, GMP-PNP has been shown to stabilize the complex between FtsY and SRP (Shan et al., 2004; Egea et al., 2005); however, we found no indication for the presence of SRP in the 200- or 400-kD FtsY complexes. In addition, the FtsY mutants used in this study show distinct phenotypes as to their ability to bind to SRP. The FtsY(G455W) and FtsY(G455V) mutants are not only defective in GTP hydrolysis but also impair the FtsY–SRP complex formation (Lu et al., 2001; Shan et al., 2004). Thus, although their interaction with SRP is impaired, they are still carbonate resistant. The same carbonate-resistant phenotype is also observed for the FtsY(A334W) mutant, in which the GTPase activity is blocked but not its ability to interact with SRP (Shan et al., 2004). Thus, it appears that the formation of the 400-kD FtsY–SecY translocon complex does not depend on SRP but that FtsY and SecY probably associate before the delivery of the SRP–RNC.
Membranes have been shown to stimulate the GTPase activity of FtsY in the absence of any SRP–RNC complex (de Leeuw et al., 2000). Thus, the strongly increased membrane binding of FtsY, induced by blocking its GTPase activity, could in principle indicate that GTP hydrolysis is involved in dissociating not only the SRP–FtsY interaction but also the FtsY membrane–translocon interaction. A precedent for such an interaction is found in the ATPase SecA, which, like FtsY, shows a low basal hydrolysis activity that is stimulated by membranes (Lill et al., 1990). Likewise, the membrane association of SecA is enhanced in mutants with reduced ATPase activity (Rajapandi and Oliver, 1996) or in the presence of AMP-PNP (Economou and Wickner, 1994). Thus, it appears that FtsY, the receptor for bacterial membrane proteins, and SecA, the receptor for bacterial secretory proteins, have adapted a similar nucleotide-dependent mechanism for membrane association. Because SecA and FtsY are suggested to bind to the same cytoplasmic loops of SecY (Mori and Ito, 2001; Angelini et al., 2005), they probably cannot simultaneously bind to the Sec translocon. A possible dissociation of FtsY from the Sec translocon upon GTP hydrolysis would ensure that FtsY only transiently occupies the Sec translocon and would thereby also allow SRP/FtsY-independent substrates to access it. Binding to lipids would be advantageous because it would locally increase the FtsY concentration at the membrane and subsequently increase the chances to rebind to the limited number of SecYEG translocons.
In summary, our data suggest that FtsY binds to the membrane via two distinct contact sites: the Sec translocon and probably the lipids. Both interactions are stabilized by blocking the GTPase activity of FtsY. In many respects, the features of FtsY described in this study and in previous reports are reminiscent of the features of SecA, which is considered to be a peripheral subunit of the SecY translocon during the transport of secretory proteins (de Keyzer et al., 2003). Thus, it is possible that the dual ability of the SecY translocon to translocate secretory proteins as well as to integrate membrane proteins is determined by its interaction with two peripheral subunits, either SecA or FtsY.
Materials and methods
Strains and plasmids
The strains and plasmids used for in vitro synthesis were described previously (Powers and Walter, 1997; Angelini et al., 2005). The ftsY gene containing the G455V mutation was amplified by PCR from the plasmid pJH15 (provided by H. Bernstein, National Institutes of Health, Bethesda, MD; Lu et al., 2001) using the NcoI–FtsY (5′-GATAACCATGGCGAAAGAAAAAAAACG-3′) and the FtsY–HindIII (5′-GATAAAGCTTATCCTCTCGGGC-3′) primers. FtsY(G455V) PCR product was then digested by NcoI and HindIII and cloned in place of ftsY (wild type) gene in the pTD37 (Powers and Walter, 1997), resulting in the pSAFtsY(G455V) plasmid. The FtsY(NG+1) construct was provided by E. Bibi (Weizmann Institute of Science, Rehovot, Israel) but reconstructed by PCR using the plasmid pTD37 and the two respective primers, NdeI–NG+1–FtsY (5′-GGAATTCCATATGTTCGCGCGCCTG-3′) and FtsY–HindIII. FtsY(NG+1) PCR product was digested by NdeI and HindIII and introduced in the pET22b (Novagen), generating the pSAFtsY(NG+1) plasmid. The FtsY mutants G455W and A334W were constructed by whole plasmid PCR amplification of pTD37 using the mutagenic primers FtsY-455Wf (5′-ACGGCGAAAGGCTGGGTAATTTTCTCGGTGGCT-3′) and FtsY-455r (5′-AGCCACCGAGAAAATTACCCAGCCTTTCGCCGT-3′) and FtsY-334Wf (5′-GGTGATACTTTCCGTTGGGCTGCGGTTGAACAG-3′) and FtsY-334Wr (5′-CTGTTCAACCGCAGCCCAACGGAAAGTATCACC-3′), respectively.
In vitro synthesis and purification of 35S-FtsY
In vitro protein synthesis and the composition of the reconstituted transcription/translation system of E. coli was described previously (Koch et al., 1999). For purification of 35S-FtsY, the protein was first in vitro synthesized and then applied to a Talon metal affinity resin (BD Biosciences). After a 30-min incubation at 4°C, the resin was filled into a 2-ml gravity-flow column (QIAGEN) and washed with 20 bed volume of buffer A (30 mM TeaOAc, pH 7.5, 50 mM KOAc, 9 mM Mg[OAc]2, and 10% glycerol). 35S-FtsY was eluted with buffer A supplemented with 200 mM imidazole/HCl, pH 7. Fractions were analyzed on SDS gel, and FtsY-containing fractions were directly used for the subsequent experiments.
Carbonate extraction, proteinase K treatment, and immunoprecipitation
For carbonate extraction, samples were incubated with 0.18 M Na2CO3, pH 11.3, for 15 min at 4°C and subsequently centrifuged for 30 min at 70,000 rpm in rotor (TLA 100.2; Beckman Coulter). The supernatants were neutralized with glacial acetic acid, precipitated with 1 vol 10% TCA, and resuspended in SDS loading buffer. Pellets were directly dissolved in SDS loading buffer and, like supernatants, separated on SDS-PAGE. For the protease treatment, samples were incubated with 0.5 mg/ml proteinase K for 20 min at 25°C and subsequently TCA precipitated. Immunoprecipitations with α-FtsY and α-His antibodies were performed as reported in Angelini et al. (2005). For proteinase K–treated samples, 1 mM PMSF was added before immunoprecipitations.
Solubilization of FtsY-containing membrane complexes
50 μg INV was incubated for 20 min at 37°C with the purified 35S-FtsY in the presence or absence of GMP-PNP (final concentration, 2 mM). After incubation, INVs were solubilized for 15 min at 4°C in lysis buffer (0.2% [wt/vol] dodecylmaltoside [Roche], 5 mM 6-aminohexanoic acid, 50 mM imidazol, pH 7, 50 mM NaCl, 10% glycerol, and 2 mM PMSF). After solubilization, samples were centrifuged at 4°C for 15 min at 30 000 g. Soluble material was analyzed by BN-PAGE as described in Schägger (2001). For large-scale in vitro synthesis of FtsY, a 100-fold in vitro reaction mixture was incubated for 45 min at 37°C and subsequently incubated for 20 min with 3 mg INV in the presence or absence of GMP-PNP. INVs were isolated and solubilized in 500 μl lysis buffer for 30 min at 4°C. After centrifugation, the supernatant was applied to a 3-ml Talon column. Washing and elution was performed as described above.
Sample analysis and quantification
All samples were analyzed on 15% SDS-PAGE gels or 4–13% or 5–13% BN-PAGE gels. Radiolabeled proteins were visualized by phosphorimaging using a phosphorimager (Molecular Dynamics) and quantified using the Imagequant software (Molecular Dynamics).
Acknowledgments
We gratefully acknowledge Dr. Harris Bernstein and Dr. Eitan Bibi for E. coli strains and plasmids and Dr. Matthias Müller for support and discussion.
This work was supported by grants from Sonderforschungsbereich 388 (A12) and the German-Israeli Foundation for Scientific Research and Development.
Abbreviations used in this paper: AMP-PNP, adenyl-5′-yl imidodiphosphate; BN-PAGE, blue native PAGE; GMP-PNP, guanosine 5′-[β,γ-imido] triphosphate; INV, inner membrane vesicle; MtlA, mannitol permease; RNC, ribosome-associated nascent chain; SR, SRP receptor; SRP, signal recognition particle.
References
- Angelini, S., S. Deitermann, and H.G. Koch. 2005. FtsY, the bacterial signal-recognition particle receptor, interacts functionally and physically with the SecYEG translocon. EMBO Rep. 6:476–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein, H.D., M.A. Poritz, K. Strub, P.J. Hoben, S. Brenner, and P. Walter. 1989. Model for signal sequence recognition from amino-acid sequence of 54K subunit of signal recognition particle. Nature. 340:482–486. [DOI] [PubMed] [Google Scholar]
- Bibi, E., A.A. Herskovitz, E.S. Bockkareva, and A. Zelazny. 2001. Putative integral membrane SRP receptors. Trends Biochem. Sci. 26:15–16. [DOI] [PubMed] [Google Scholar]
- Cabelli, R.J., K.M. Dolan, L.P. Qian, and D.B. Oliver. 1991. Characterization of membrane-associated and soluble states of SecA protein from wild-type and SecA51 (TS) mutant strains of _Escherichia coli._J. Biol. Chem. 266:24420–24427. [PubMed] [Google Scholar]
- Deitermann, S., G.S. Sprie, and H.G. Koch. 2005. A dual function for SecA in the assembly of single spanning membrane proteins in _Escherichia coli._J. Biol. Chem. 280:39077–39085. [DOI] [PubMed] [Google Scholar]
- de Keyzer, J., C. van der Does, and A.J.M. Driessen. 2003. The bacterial translocase: a dynamic protein channel complex. Cell. Mol. Life Sci. 60:2034–2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Leeuw, E., D. Poland, O. Mol, I. Sinning, C. ten Hagen-Jongman, B. Oudega, and J. Luirink. 1997. Membrane association of FtsY, the E. coli SRP receptor. FEBS Lett. 416:225–229. [DOI] [PubMed] [Google Scholar]
- de Leeuw, E., K. te Kaat, C. Moser, G. Menestrina, R. Demel, B. de Kruijff, B. Oudega, J. Luirink, and I. Sinning. 2000. Anionic phospholipids are involved in membrane association of FtsY and stimulate its GTPase activity. EMBO J. 19:531–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Blaauwen, T., P. Fekkes, J.G. De Wit, W. Kuiper, and A.J.M. Driessen. 1996. Domain interactions of the peripheral preprotein translocase subunit SecA. Biochemistry. 35:11994–12004. [DOI] [PubMed] [Google Scholar]
- Duong, F. 2003. Binding, activation and dissociation of the dimeric SecA ATPase at the dimeric SecYEG translocase. EMBO J. 22:4375–4383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Economou, A., and W. Wickner. 1994. SecA promotes preprotein translocation by undergoing ATP-driven cycles of membrane insertion and deinsertion. Cell. 78:835–843. [DOI] [PubMed] [Google Scholar]
- Egea, P.F., R.M. Stroud, and P. Walter. 2005. Targeting proteins to membranes: structure of the signal recognition particle. Curr. Opin. Struct. Biol. 15:213–220. [DOI] [PubMed] [Google Scholar]
- Eitan, A., and E. Bibi. 2004. The core Escherichia coli signal recognition particle receptor contains only the N and G domains of FtsY. J. Bacteriol. 186:2492–2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiki, Y., A.L. Hubbard, S. Fowler, and P.B. Lazarow. 1982. Isolation of intracellular membranes by means of sodium treatment: application to endoplasmic reticulum. J. Cell Biol. 93:97–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulga, T.A., I. Sinning, B. Dobberstein, and M.R. Pool. 2001. SRβ coordinates signal sequence release from SRP with ribosome binding to the translocon. EMBO J. 20:2338–2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore, R., P. Walter, and G. Blobel. 1982. Protein translocation across the endoplasmic reticulum. II. Isolation and characterization of the signal recognition particle receptor. J. Cell Biol. 95:470–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halic, M., and R. Beckmann. 2005. The signal recognition particle and its interactions during protein targeting. Curr. Opin. Struct. Biol. 15:116–125. [DOI] [PubMed] [Google Scholar]
- Halic, M., M. Gartmann, O. Schlenker, T. Mielke, M. Pool, I. Sinning, and R. Beckmann. 2006. Signal recognition particle receptor exposes the ribosomal translocon binding site. Science. 312:745–747. [DOI] [PubMed] [Google Scholar]
- Hartl, F.U., S. Lecker, E. Schiebel, J.P. Hendrick, and W. Wickner. 1990. The binding cascade of SecB to SecA to SecY/E mediates preprotein targeting to the E. coli plasma membrane. Cell. 63:269–279. [DOI] [PubMed] [Google Scholar]
- Helmers, J., D. Schmidt, J.S. Glavy, G. Blobel, and T. Schwartz. 2003. The β-subunit of the protein-conducting channel of the endoplasmic reticulum functions as the guanine nucleotide exchange factor for the β-subunit of the signal recognition particle receptor. J. Biol. Chem. 278:23686–23690. [DOI] [PubMed] [Google Scholar]
- Herskovits, A.A., A. Seluanov, R. Rajsbaum, C.M. ten Hagen-Jongman, T. Henrichs, E.S. Bochkareva, G.J. Phillips, F.J. Probst, T. Nakae, M. Ehrmann, et al. 2001. Evidence for coupling of membrane targeting and function of the signal recognition particle (SRP) receptor FtsY. EMBO Rep. 2:1040–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch, H.G., and M. Müller. 2000. Dissecting the translocase and integrase functions of the Escherichia coli SecYEG translocon. J. Cell Biol. 150:689–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch, H.G., T. Hengelage, C. Neumann-Haefelin, J. MacFarlane, H.K. Hoffschulte, K.L. Schimz, B. Mechler, and M. Müller. 1999. In vitro studies with purified components reveal signal recognition particle (SRP) and SecA/SecB as constituents of two independent protein targeting pathways of _Escherichia coli._Mol. Biol. Cell. 10:2163–2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch, H.G., M. Moser, and M. Müller. 2003. Signal recognition particle-dependent protein targeting, universal to all kingdoms of life. Rev. Physiol. Biochem. Pharmacol. 146:55–94. [DOI] [PubMed] [Google Scholar]
- Kusters, R., G. Lentzen, E. Eppens, A. van Geel, C.C. van der Weijden, W. Wintermeyer, and J. Luirink. 1995. The functioning of the SRP receptor FtsY in protein targeting in E. coli is correlated with its ability to bind and hydrolyse GTP. FEBS Lett. 372:253–258. [DOI] [PubMed] [Google Scholar]
- Lill, R., W. Dowhan, and W. Wickner. 1990. The ATPase activity of SecA is regulated by acidic phospholipids, SecY, and the leader and mature domains of precursor proteins. Cell. 60:271–280. [DOI] [PubMed] [Google Scholar]
- Lu, Y., H.Y. Qi, J.B. Hyndman, N.D. Ulbrandt, A. Teplyakov, N. Tomasevic, and H.D. Bernstein. 2001. Evidence for a novel GTPase priming step in the SRP protein targeting pathway. EMBO J. 20:6724–6734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luirink, J., C.M. ten Hagen-Jongman, C.C. van der Weijden, B. Oudega, S. High, B. Dobberstein, and R. Kusters. 1994. An alternative protein targeting pathway in Escherichia coli: studies on the role of FtsY. EMBO J. 13:2289–2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandon, E.C., Y. Jiang, and R. Gilmore. 2003. Dual recognition of the ribosome and the signal recognition particle by the SRP receptor during protein targeting to the endoplasmic reticulum. J. Cell Biol. 162:575–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto, G., T. Yoshihisa, and K. Ito. 1997. SecY and SecA interact to allow SecA insertion and protein translocation across the Escherichia coli plasma membrane. EMBO J. 16:6384–6393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millman, J.S., and D.W. Andrews. 1999. A site-specific, membrane-dependent cleavage event defines the membrane binding domain of FtsY. J. Biol. Chem. 274:33227–33234. [DOI] [PubMed] [Google Scholar]
- Millman, J.S., H.Y. Qi, F. Vulcu, H.D. Berstein, and D.W. Andrews. 2001. FtsY binds to the Escherichia coli inner membrane via interactions with phosphatidylethanolamine and membrane proteins. J. Biol. Chem. 276:25982–25989. [DOI] [PubMed] [Google Scholar]
- Moore, M., R.L. Goforth, H. Mori, and R. Henry. 2003. Functional interaction of chloroplast SRP/FtsY with the ALB3 translocase in thylakoids: substrate not required. J. Cell Biol. 162:1245–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori, H., and K. Ito. 2001. The Sec protein-translocation pathway. Trends Microbiol. 9:494–500. [DOI] [PubMed] [Google Scholar]
- Müller, M., H.G. Koch, K. Beck, and U. Schäfer. 2001. Protein traffic in bacteria: multiple routes from the ribosome to and across the membrane. Prog. Nucleic Acid Res. Mol. Biol. 66:107–157. [DOI] [PubMed] [Google Scholar]
- Neumann-Haefelin, C., U. Schafer, M. Müller, and H.G. Koch. 2000. SRP-dependent co-translational targeting and SecA-dependent translocation analyzed as individual steps in the export of a bacterial protein. EMBO J. 19:6419–6426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newitt, J.A., and H.D. Bernstein. 1998. A mutation in the E. coli secY gene that produces distinct effects on inner membrane protein insertion and protein export. J. Biol. Chem. 273:12451–12456. [DOI] [PubMed] [Google Scholar]
- Powers, T., and P. Walter. 1997. Co-translational protein targeting catalyzed by the Escherichia coli signal recognition particle and its receptor. EMBO J. 16:4880–4886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajapandi, T., and D. Oliver. 1996. Integration of SecA protein into the Escherichia coli inner membrane is regulated by its amino-terminal ATP-binding domain. Mol. Microbiol. 20:43–51. [DOI] [PubMed] [Google Scholar]
- Römisch, K., J. Webb, J. Herz, S. Prehn, R. Frank, M. Vingron, and B. Dobberstein. 1989. Homology of 54K protein of signal-recognition particle, docking protein and two E. coli proteins with putative GTP-binding domains. Nature. 340:478–482. [DOI] [PubMed] [Google Scholar]
- Schägger, H. 2001. Blue-native gels to isolate protein complexes from mitochondria. Methods Cell Biol. 65:231–244. [DOI] [PubMed] [Google Scholar]
- Schwartz, T., and G. Blobel. 2003. Structural basis for the function of the β subunit of the eukaryotic signal recognition particle receptor. Cell. 112:793–803. [DOI] [PubMed] [Google Scholar]
- Scotti, P.A., Q.A. Valent, E.H. Manting, M.L. Urbanus, A.J.M. Driessen, B. Oudega, and J. Luirink. 1999. SecA is not required for signal recognition particle-mediated targeting and initial membrane insertion of a nascent inner membrane protein. J. Biol. Chem. 274:29883–29888. [DOI] [PubMed] [Google Scholar]
- Shan, S.-O., R.M. Stroud, and P. Walter. 2004. Mechanism of association and reciprocal activation of two GTPases. PLoS Biol. 2:e320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traxler, B., and C. Murphy. 1996. Insertion of the polytopic membrane protein MalF is dependent on the bacterial secretion machinery. J. Biol. Chem. 271:12394–12400. [DOI] [PubMed] [Google Scholar]
- Valent, Q.A., P.A. Scotti, S. High, J.-W.L. de Gier, G. von Heine, G. Lentzen, W. Wintermeyer, B. Oudega, and J. Luirink. 1998. The Escherichia coli SRP and SecB targeting pathways converge at the translocon. EMBO J. 17:2504–2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berg, B., W.M. Clermon Jr., I. Collison, Y. Modis, E. Hartmann, S.C. Harrison, and T.A. Rapoport. 2004. X-ray structure of a protein-conducting channel. Nature. 427:36–44. [DOI] [PubMed] [Google Scholar]
- Wild, K., K.R. Rosendal, and I. Sinning. 2004. A structural step into the SRP cycle. Mol. Microbiol. 53:357–363. [DOI] [PubMed] [Google Scholar]
- Zelazny, A., A. Seluanov, A. Cooper, and E. Bibi. 1997. The NG domain of the prokaryotic signal recognition particle receptor, FtsY, is fully functional when fused to an unrelated integral membrane polypeptide. Proc. Natl. Acad. Sci. USA. 94:6025–6029. [DOI] [PMC free article] [PubMed] [Google Scholar]