Wnt signalling in development and disease. Max Delbrück Center for Molecular Medicine meeting on Wnt Signaling in Development and Disease (original) (raw)

A meeting on Wnt Signaling in Development and Disease took place between 12 and 15 September 2007, in the Max Delbrück Center for Molecular Medicine in Berlin-Buch, Germany, and was organized by W. Birchmeier (Berlin, Germany) and T. Holstein (Heidelberg, Germany).
Introduction
Wnt signalling is implicated in numerous aspects of development, cell biology and physiology. This meeting brought together several leading scientists in the Wnt signalling field, who discussed their latest research in this rapidly growing discipline. The expanding number of diseases that are the consequence of mutations in components of the Wnt signalling pathway also attracted researchers with a more clinical interest. Many new insights were presented into the mechanics of Wnt signalling and the role of Wnts in development, regeneration, stem cells and cancer, which we have attempted to summarize in this meeting report.
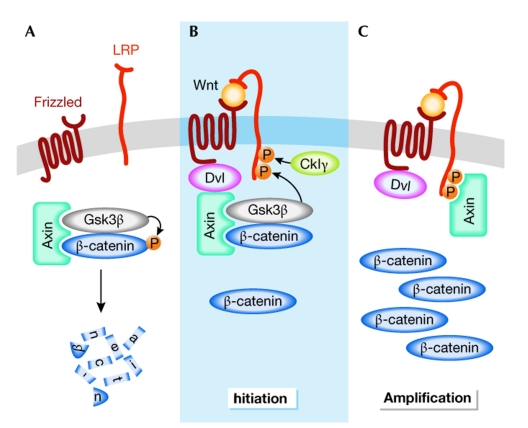
In the absence of Wnt ligands, β-catenin is phosphorylated by a destruction complex that contains the scaffolding proteins axin and adenomatous polyposis coli (Apc), and the glycogen synthase kinase 3β (Gsk3β). Phosphorylated β-catenin is recognized by the E3 ubiquitin ligase β-TrCP and targeted for proteasomal degradation. Wnt signalling is initiated at the plasma membrane by the binding of Wnt ligands to the receptors Frizzled (Fz) and Arrow/LRP. The signal is then relayed in the cytoplasm through Dishevelled (Dsh/Dvl) and leads to inhibition of the destruction complex. Unphosphorylated β-catenin can then localize to the nucleus where it binds to Tcf/Lef proteins and activates transcription through the recruitment of various cofactors (see Clevers (2006) and the Wnt homepage (http://www.stanford.edu/~rnusse/wntwindow.html) for reviews on Wnt signalling).
Wnt/β-catenin signalling at the membrane
The binding of Wnt to Fz/LRP leads to the phosphorylation of LRP6 by Gsk3β and casein kinase Iγ (CkIγ), which is necessary for correct Wnt signalling, presumably because axin can then dock to the phosphorylated residues. At the same time, Dvl is recruited to Fz upon ligand binding. Although the formation of a trimeric complex between Wnt, Fz and LRP is widely accepted as a prerequisite to trigger signalling, the molecular nature of this complex, the precise events that lead to its formation and the way the signal is relayed further in the cytoplasm are subject to debate. C. Niehrs (Heidelberg, Germany), X. He (Boston, MA, USA) and A. Kikuchi (Hiroshima, Japan) shed light on these intricate events. Niehrs showed that phosphorylated LRP6, Dvl, Fz, axin and Gsk3β co-localized in LRP6 signalosomes and that Dvl was required for LRP6 phosphorylation (Bilic et al, 2007). Furthermore, He showed that Fz, Dvl and axin were all required for LRP6 phosphorylation by Gsk3β (Zeng et al, 2008). Altogether, this provides support for a model in which Wnt binding to Fz/LRP recruits a complex formed by Dvl–axin–Gsk3β to Fz. Gsk3β can then phosphorylate the axin docking residues of LRP6, which allows binding of this scaffolding protein to LRP6 and inactivation of the destruction complex (Fig 1). This model is in agreement with the initiation–amplification hypothesis, in which the stepwise assembly of Wnt pathway components leads to sequestration of axin in regions of high LRP concentration (Baig-Lewis et al, 2007). Kikuchi showed that part of the signalling complex was found at lipid rafts and internalized through caveolin (Yamamoto et al, 2006), and that this could disrupt the axin–β-catenin interaction further.
Figure 1.
Model for the activation of the Wnt/β-catenin pathway. (A) In the absence of a Wnt signal, β-catenin is phosphorylated and targeted for proteasome-mediated degradation by a destruction complex that contains axin and Gsk3β among other proteins. (B) On binding of Wnt to the receptors Fz and LRP, Dvl binds to Fz and recruits the destruction complex through interaction with axin. Subsequently, Gsk3β phosphorylates critical sites on LRP, which, together with residues phosphorylated by CkIγ, act as docking sites for axin. (C) Binding of axin to LRP leads to inhibition of the destruction complex and stabilization of β-catenin. CkIγ, casein kinase Iγ; Dvl, dishevelled; Fz, Frizzled; Gsk3β, glycogen synthase kinase 3β.
Many signalling components of the Wnt pathway bind to multiple partners or dimerize, and could thus favour aggregation. In particular, a scaffolding role has been proposed for axin as it can bind to all other components of the destruction complex. M. Wehrli (Portland, OR, USA) showed that ubiquitously expressed axin fully rescues axin-null mutants and that axin proteins with large internal deletions are able to rescue the patterning defects observed in axin-null fly embryos. This suggests that disruption of single protein–protein interactions between axin and its partners—Apc, Dsh, β-catenin, Gsk3β—is not sufficient to prevent the assembly of the destruction complex. Nevertheless, the rescue did not give rise to viable flies. By contrast, co-expression of two axin mutants—one unable to bind to Apc and the other β-catenin—led to viable animals. This not only suggests that axin dimers can form in vivo, but also that they are necessary for correct signal transduction. It is tempting to speculate that axin dimers might have a crucial role in the establishment of receptor complex aggregates.
Wnt signalling in the nucleus: multiple pathways
Although the precise mechanisms that control the transcription of Wnt target genes are not known, β-catenin has been shown to recruit many cofactors—such as p300/CBP, Brg1, parafibromin/Hyrax and, through Bcl9/Legless, the PHD-finger-containing protein Pygopus—to promoters.
M. Bienz (Cambridge, UK) showed that Pygopus co-localized with Tcf on the DNA and that this interaction was mediated by the N-box of Pygopus and was independent of a Wnt signal. In addition, she presented the crystal structure of the Pygopus PHD domain in complex with the Bcl9 HD1 domain, and showed that HD1 promoted the interaction of the PHD with modified histone tails. She hypothesized that the role of Pygopus is to capture β-catenin through its interaction with Bcl9 (de la Roche & Bienz, 2007).
R. Grosschedl (Freiburg, Germany) showed that both Bcl9 and β-catenin were essential to activate transcription in lymphoid cells, and that the carboxyl terminus of Bcl9 was important for this function in a manner that was independent of Pygopus but dependent on the C-terminus of β-catenin. Interestingly, this region of β-catenin has been implicated in the activation of transcription through binding of parafibromin/Hyrax in a Pygopus-dependent manner (Mosimann et al, 2006).
Several talks focused on β-catenin-independent responses to Wnt signalling. S. Sokol (New York, NY, USA) presented a model in which Wnt8 activates the Vent2 promoter through the induction of homeodomain-interacting protein kinase 2 (Hipk2) binding to and phosphorylation of Tcf3, leading to relief of Tcf3-dependent repression. This is in contrast to a recent study that proposed—using a different experimental setup—a negative role for Hipk2 in Tcf/β-catenin-mediated signalling (Wei et al, 2007). Grosschedl showed that the mammary gland and hair follicle defects found in _Lef1_-knockout mice were rescued by a Lef1 allele that was unable to bind to β-catenin but still bound DNA (Lef1m5). Wnt-reporter activity was absent in the rescued tissues, suggesting a β-catenin-independent role for Lef1. Grosschedl proposed that Tcf/Lef are dependent on β-catenin for the initiation of hair follicle formation, but later perform β-catenin-independent functions. This was supported by the identification of Lef1 target genes in the hair follicle, a subset of which was also regulated by Lef1m5.
P. McCrea (Houston, TX, USA) showed that Wnts can signal through p120-catenin to relieve Kaiso-mediated repression, and that Wnt effects on p120-catenin seems to occur through Dact1, a protein that binds to Dvl (Park et al, 2006). As the promoters of several Wnt target genes contain both Tcf and Kaiso response elements, it seems that two distinct catenins additively co-regulate their activity. The questions now being addressed include the possible participation of additional members of the p120-catenin sub-family—such as Arvcf- and δ-catenins—in Wnt signalling.
The dual role of Apc
Apc proteins not only have a role in β-catenin degradation, but also have been proposed to perform cytoskeletal functions. However, M. Peifer (Chapel Hill, NC, USA) showed that the loss of both apc genes in fly embryos does not lead to defects in cell adhesion, spindle morphology or orientation of the division plane (McCartney et al, 2006), and that the defects observed in axon outgrowth are entirely due to increased β-catenin levels (Hayden et al, 2007; N. Rusan and M. Peifer, unpublished data).
T. Akiyama (Tokyo, Japan) showed that Asef—an Apc-binding protein involved in the migratory phenotype of colorectal cancer cells—is important for adenoma formation. Sequential deletion of Asef and Asef2 alleles leads to a marked decrease of polyp formation in the Apcmin background. Endothelial cells from Asef−/− mice show a lower motility, and have fewer protrusions, sprouting and invasion capacities than wild-type cells. Consequently, Asef−/− mutant mice are less permissive for the growth of tumour xenografts owing to reduced tumour neovascularization.
Wnt in planar polarity and morphogenetic movements
Planar cell polarity (PCP; Seifert & Mlodzik, 2007) refers to the polarization of cells in an epithelial sheet and occurs, for example, during the orientation of hairs or cilia. Planar polarity is transmitted locally from cell to cell and mediated by signalling through Fz and Dvl independently of β-catenin. The PCP pathway also regulates cell polarity in non-epithelial contexts, for example, during convergent extension in gastrulation, and can control the orientation of groups of cells such as ommatidia in the Drosophila eye.
M. Mlodzik (Mount Sinai, NY, USA) discussed how, during the establishment of PCP in the Drosophila eye, antagonistic interactions between Fz/Dsh and Van Gogh (Vang)/Prickle complexes cause them to distribute on opposite sides of the cell. A crucial element in this process is the phosphorylation of Dsh, and an RNA interference (RNAi) screening identified Ck1ε and Nemo as candidate kinases responsible for this modification. Ck1ε is required for both PCP and β-catenin signalling, although its kinase activity is only necessary for β-catenin signalling. J. Axelrod (Stanford, CA, USA) showed that intercellular interactions between different conformations of the Flamingo transmembrane protein maintain cell polarity in the fly wing. He hypothesized that the native ‘V' conformation of Flamingo converts to an ‘F' conformation on interaction with Fz, but remains unchanged on interaction with Vang. This would ensure the correct distribution of Vang and Fz throughout the tissue by preferential transcellular interactions between the ‘V' and ‘F' forms of Flamingo.
In the vertebrate embryo, morphogenetic movements are regulated by β-catenin-dependent and -independent Wnt signalling, and a recurring theme at the meeting was the coordinated regulation of these pathways. C. Houart (London, UK), D. Wedlich (Karlsruhe, Germany) and H. Steinbeisser (Heidelberg, Germany) explored how Wnt signalling controls involution and convergent extension during Xenopus and zebrafish gastrulation. Houart described that Dkk1 (dickkopf 1) regulates these movements by coordinated modulation of the β-catenin and PCP pathways, through its interaction with Knypek—a heparan sulphate proteoglycan involved in PCP signalling (Caneparo et al, 2007). Wedlich showed that Wnt5a and paraxial protocadherin (papc) are required for correct cell migration, and that Wnt5a signals through Ror2 (receptor tyrosine kinase-like orphan receptor 2) to phosphorylate Atf2 (activating transcription factor 2), which activates papc expression (Schambony & Wedlich, 2007). Furthermore, Steinbeisser showed that papc recruits the receptor tyrosine kinase inhibitor sprouty1 to the membrane, preventing it from repressing PCP signalling by Fz7. The formation of the chick myotome presents another example of PCP and β-catenin pathways acting in concert. C. Marcelle (Marseille, France) described how the neural tube controls expression of Wnt11 in the somites through a Wnt/β-catenin signalling cascade, and how Wnt11 then aligns the myocytes along the embryonic axis through the PCP pathway.
A. Wynshaw-Boris (San Diego, CA, USA) showed that Dvl genes have redundant and unique functions: a Dvl1 transgene rescues the loss of Dvl3, but different combinations of _Dvl_-null alleles result in cardiac outflow defects such as double outlet right ventricle (Dorv) and persistent truncus arteriosus. Both the secondary heart field and the cardiac neural crest contribute to the outflow tract, and Wynshaw-Boris pinpointed a PCP signalling defect in the secondary heart field as responsible for the Dorv phenotype.
Wnt in development and regeneration
Wnt3a controls posterior development of the mouse embryo by regulating mesoderm specification, axial patterning, production of presomitic mesoderm and its segmentation into somites. To elucidate the role of Wnt3a in these processes, T. Yamaguchi (Frederick, MD, USA) performed transcriptional profiling of _Wnt3a_-knockout primitive streaks, and identified mesogenin and ripply2 as Wnt3a target genes. Yamaguchi and B. Herrmann (Berlin, Germany) found that mesogenin expression is controlled directly by Wnt/β-catenin signalling and by Tbx6, a factor downstream from Wnt3a (Wittler et al, 2007). In mesogenin-knockout mice, unsegmented mesodermal cells accumulate in the tail bud, a phenotype that is opposite to that of the Wnt3a knockout. Yamaguchi presented a model in which mesogenin acts as a Wnt3a feedback inhibitor, its loss allowing Wnt3a to maintain presomitic cells in an immature state. Wnt3a also has a role in controlling the expression of ripply2 in the posterior presomitic mesoderm through transcriptional repression (Biris et al, 2007; Dunty et al, 2008).
C. Hartmann (Vienna, Austria) showed that Wnts are required to suppress the chondrogenic potential of joint interzone cells during synovial joint formation. In the absence of Wnt9a and Wnt4, ectopic cartilage forms in elbow, ankle and knee joints, and there is fusion of carpal and tarsal elements. Joint initiation seems normal, but subsequent chondrogenesis in the developing joints is not inhibited (Spater et al, 2006). In adult mice lacking Wnt9a, the articular cartilage is lost, the joint is mineralized and osteoarthritis develops. Therefore, continuous Wnt signalling is required to maintain a functional joint.
Wnt signalling has a crucial role in many regenerative processes. R. Moon (Seattle, WA, USA) and G. Weidinger (Dresden, Germany) showed that Dkk-mediated inhibition of the Wnt/β-catenin pathway severely impairs regeneration of the zebrafish tail fin and heart (Stoick-Cooper et al, 2007). Furthermore, Wnt signalling is required for the formation of heart precursors in pregastrula embryos, but Wnt antagonism is required at later stages for continued development.
Both R. Nusse (Stanford, CA, USA) and D. Eckardt (Freiburg, Germany) described roles for Wnt signalling in preventing stem-cell differentiation. Nusse showed that the Wnt signalling pathway is active in stem-cell compartments such as the mammary gland and the subventricular zone of the brain, and that these cells can only be propagated in culture in the presence of both a mitogen and a Wnt signal. These results—together with other experiments performed with embryonic stem cells—led him to conclude that Wnt3a prevents stem-cell differentiation, whereas a receptor tyrosine kinase ensures cell proliferation. Eckardt showed that derivation of embryonic stem-cell lines from mouse blastocysts is markedly improved by genetic or pharmacological stabilization of β-catenin.
B. Bowerman and S. Schneider (both from Eugene, OR, USA) uncovered a β-catenin-mediated mechanism that determines cell fate after asymmetrical cell divisions in a lophotrochozoan, the polychaete Platynereis dumerilii (Schneider & Bowerman, 2007). During animal/vegetal-oriented cell divisions, β-catenin consistently accumulates in the vegetal daughter, and ectopic β-catenin expressed in animal-pole cells causes them to adopt the vegetal fate. The animal/vegetal β-catenin asymmetries in Platynereis resemble the anterior–posterior asymmetries of β-catenin that control cell-fate specification in the ecdysozoan Caenorhabditis elegans, suggesting the existence of a β-catenin-mediated ‘asymmetry module' for binary cell-fate specification that has a pre-bilaterian evolutionary origin. Bowerman challenged the audience to find β-catenin asymmetries in asymmetrical stem-cell divisions or during vertebrate organogenesis.
Wnt signals maintain the proliferative compartment of the intestinal crypts and constitutive activation of the pathway causes cancer. H. Clevers (Utrecht, the Netherlands) identified a Wnt target gene, Lgr5, that is expressed in certain slender cells at the bottom of the crypt. Genetic labelling showed that these cells are long-lived proliferative progenitors that give rise to all cell types of the intestine, and are therefore crypt stem cells. Lgr5 is also expressed in stem cells of the stomach, hair follicles and mammary glands, and might therefore be a universal adult stem-cell marker (Barker et al, 2007).
The function of β-catenin in the self-renewal of haematopoietic stem cells (HSCs) remains unclear. Although Wnt signalling promotes HSC self-renewal, β-catenin is dispensable for haematopoiesis (Cobas et al, 2004). A. Leutz (Berlin, Germany) reported that deletion of both β-catenin and γ-catenin in HSCs has no major adverse effects on HSC function and multi-lineage differentiation (Jeannet et al, 2007), although constitutive activation of β-catenin in murine HSCs blocks differentiation and causes rapid death of the animals (Scheller et al, 2006). On the other hand, F. Staal (Rotterdam, the Netherlands) showed that HSCs from _Wnt3a_-knockout mutants have decreased self-renewal and defects in development of the T-lymphoid, myeloid and erythroid lineages, providing evidence that Wnts regulate self-renewal as well as lineage decisions during haematopoiesis. Leutz also identified the interferon regulatory factor 8 (Irf8) as a haematopoietic Wnt target gene involved in myeloid differentiation arrest, and showed that chronic myelogenous leukaemia caused by Irf8 deficiency advances into fatal blast crisis when β-catenin becomes constitutively activated.
Wnt in cancer
Several talks highlighted the role of β-catenin in tumour progression and metastasis. R. Fodde (Rotterdam, the Netherlands) presented two mouse models in which the Wnt signalling pathway is activated by targeted Apc mutations. _Apc_1572T causes multifocal mammary tumours and lung metastases, whereas concomitant expression of _Apc_1638N and activated Ras leads to gastrointestinal tumours and liver metastases (Janssen et al, 2006). In both cases, cells that presented nuclear β-catenin accumulation were distributed heterogeneously throughout the primary tumours and in the metastases, and might represent migrating cancer stem cells. However, gene expression was virtually identical between these potential tumour stem cells and normal stem cells, highlighting the difficulty in distinguishing these populations.
Chromosomal instability is common in colon tumours and might aid tumour progression by promoting genetic abnormalities. J. Behrens (Erlangen, Germany) showed a role for axin2 in suppressing the spindle checkpoint and, therefore, in causing chromosomal instability (Hadjihannas et al, 2006). He identified a specific domain in the axin2 protein that mediates its localization to the centrosome and mitotic spindle, in addition to two spindle microtubule dispersal sequences. Behrens also showed that axin2 was rapidly degraded by the cyclosome after mitosis, and suggested that this would upregulate β-catenin signalling and stimulate a new round of the cell cycle.
Given the importance of the Wnt signalling pathway in tumour progression, it is a logical target for therapeutic intervention. Wnt ligands are expressed in many cancers—for example, Wnt5a in lung, Wnt4 in pancreas and Wnt10a in ovarian tumours—and might drive tumour progression in an autocrine manner. P. Polakis (South San Francisco, CA, USA) reported that intraperitoneal administration of the Wnt antagonist Fz8CRD inhibited the progression of mammary and teratoma tumour models. Screenings for small molecule inhibitors of the pathway are also underway. T. Dale (Cardiff, UK) described several compounds that act at the level of β-catenin or Lef1 and that are now being tested in in vivo assays; Polakis described a compound that inhibits the response to purified exogenous Wnts but is ineffective in autocrine or even paracrine situations—suggesting that purified Wnts differ from endogenous Wnts; and Moon presented a pilot RNAi-based screening for new modulators of the pathway. A large-scale screening is now underway and might expand the catalogue of potential therapeutic targets.
Concluding remarks
This meeting gave an extensive overview of the foremost advances in Wnt signalling, with talks ranging from molecular mechanisms to developmental biology, stem cells and cancer. Similar to many other meetings on Wnt signalling, the Berlin meeting was exciting and lively, inspiring the attendants to go back to their laboratories with new ideas.

From left: Derk ten Berge, Roel Nusse & Christophe Fuerer
Acknowledgments
We thank Dr W. Birchmeier and Dr T. Holstein for putting together such an interesting meeting. The authors thank the speakers for critically reading the manuscript, and acknowledge those speakers whose presentations could not be featured in this report. C.F. is supported by the Swiss National Science Foundation.
References
- Baig-Lewis S, Peterson-Nedry W, Wehrli M (2007) Wingless/Wnt signal transduction requires distinct initiation and amplification steps that both depend on Arrow/LRP. Dev Biol 306: 94–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker N et al. (2007) Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449: 1003–1007 [DOI] [PubMed] [Google Scholar]
- Bilic J, Huang YL, Davidson G, Zimmermann T, Cruciat CM, Bienz M, Niehrs C (2007) Wnt induces LRP6 signalosomes and promotes dishevelled-dependent LRP6 phosphorylation. Science 316: 1619–1622 [DOI] [PubMed] [Google Scholar]
- Biris KK, Dunty WC Jr, Yamaguchi TP (2007) Mouse Ripply2 is downstream of Wnt3a and is dynamically expressed during somitogenesis. Dev Dyn 236: 3167–3172 [DOI] [PubMed] [Google Scholar]
- Caneparo L, Huang YL, Staudt N, Tada M, Ahrendt R, Kazanskaya O, Niehrs C, Houart C (2007) Dickkopf-1 regulates gastrulation movements by coordinated modulation of Wnt/β catenin and Wnt/PCP activities, through interaction with the Dally-like homolog Knypek. Genes Dev 21: 465–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevers H (2006) Wnt/β-catenin signaling in development and disease. Cell 127: 469–480 [DOI] [PubMed] [Google Scholar]
- Cobas M, Wilson A, Ernst B, Mancini SJ, MacDonald HR, Kemler R, Radtke F (2004) β-Catenin is dispensable for hematopoiesis and lymphopoiesis. J Exp Med 199: 221–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Roche M, Bienz M (2007) Wingless-independent association of Pygopus with dTCF target genes. Curr Biol 17: 556–561 [DOI] [PubMed] [Google Scholar]
- Dunty WC Jr, Biris KK, Chalamalasetty RB, Taketo MM, Lewandoski M, Yamaguchi TP (2008) Wnt3a/β-catenin signaling controls posterior body development by coordinating mesoderm formation and segmentation. Development 135: 85–9418045842 [Google Scholar]
- Hadjihannas MV, Bruckner M, Jerchow B, Birchmeier W, Dietmaier W, Behrens J (2006) Aberrant Wnt/β-catenin signaling can induce chromosomal instability in colon cancer. Proc Natl Acad Sci USA 103: 10747–10752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden MA, Akong K, Peifer M (2007) Novel roles for APC family members and Wingless/Wnt signaling during Drosophila brain development. Dev Biol 305: 358–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen KP et al. (2006) APC and oncogenic KRAS are synergistic in enhancing Wnt signaling in intestinal tumor formation and progression. Gastroenterology 131: 1096–1109 [DOI] [PubMed] [Google Scholar]
- Jeannet G et al. (2007) Long-term, multilineage hematopoiesis occurs in the combined absence of β-catenin and γ-catenin. Blood [doi:10.1182/blood-2007-07-102558] [DOI] [PubMed] [Google Scholar]
- McCartney BM, Price MH, Webb RL, Hayden MA, Holot LM, Zhou M, Bejsovec A, Peifer M (2006) Testing hypotheses for the functions of APC family proteins using null and truncation alleles in Drosophila. Development 133: 2407–2418 [DOI] [PubMed] [Google Scholar]
- Mosimann C, Hausmann G, Basler K (2006) Parafibromin/Hyrax activates Wnt/Wg target gene transcription by direct association with β-catenin/Armadillo. Cell 125: 327–341 [DOI] [PubMed] [Google Scholar]
- Park JI, Ji H, Jun S, Gu D, Hikasa H, Li L, Sokol SY, McCrea PD (2006) Frodo links Dishevelled to the p120-catenin/Kaiso pathway: distinct catenin subfamilies promote Wnt signals. Dev Cell 11: 683–695 [DOI] [PubMed] [Google Scholar]
- Schambony A, Wedlich D (2007) Wnt-5A/Ror2 regulate expression of XPAPC through an alternative noncanonical signaling pathway. Dev Cell 12: 779–792 [DOI] [PubMed] [Google Scholar]
- Scheller M, Huelsken J, Rosenbauer F, Taketo MM, Birchmeier W, Tenen DG, Leutz A (2006) Hematopoietic stem cell and multilineage defects generated by constitutive β-catenin activation. Nat Immunol 7: 1037–1047 [DOI] [PubMed] [Google Scholar]
- Schneider SQ, Bowerman B (2007) β-Catenin asymmetries after all animal/vegetal-oriented cell divisions in Platynereis dumerilii embryos mediate binary cell-fate specification. Dev Cell 13: 73–86 [DOI] [PubMed] [Google Scholar]
- Seifert JR, Mlodzik M (2007) Frizzled/PCP signalling: a conserved mechanism regulating cell polarity and directed motility. Nat Rev Genet 8: 126–138 [DOI] [PubMed] [Google Scholar]
- Spater D, Hill TP, O'Sullivan RJ, Gruber M, Conner DA, Hartmann C (2006) Wnt9a signaling is required for joint integrity and regulation of Ihh during chondrogenesis. Development 133: 3039–3049 [DOI] [PubMed] [Google Scholar]
- Stoick-Cooper CL, Weidinger G, Riehle KJ, Hubbert C, Major MB, Fausto N, Moon RT (2007) Distinct Wnt signaling pathways have opposing roles in appendage regeneration. Development 134: 479–489 [DOI] [PubMed] [Google Scholar]
- Wei G, Ku S, Ma GK, Saito S, Tang AA, Zhang J, Mao JH, Appella E, Balmain A, Huang EJ (2007) HIPK2 represses β-catenin-mediated transcription, epidermal stem cell expansion, and skin tumorigenesis. Proc Natl Acad Sci USA 104: 13040–13045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittler L, Shin EH, Grote P, Kispert A, Beckers A, Gossler A, Werber M, Herrmann BG (2007) Expression of Msgn1 in the presomitic mesoderm is controlled by synergism of WNT signalling and Tbx6. EMBO Rep 8: 784–789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto H, Komekado H, Kikuchi A (2006) Caveolin is necessary for Wnt-3a-dependent internalization of LRP6 and accumulation of β-catenin. Dev Cell 11: 213–223 [DOI] [PubMed] [Google Scholar]
- Zeng X et al. (2008) Initiation of Wnt signaling: control of Wnt coreceptor LRP6 phosphorylation/activation via frizzled, dishevelled and axin functions. Development 135: 367–375 [DOI] [PMC free article] [PubMed] [Google Scholar]