Function and dysfunction of the PI system in membrane trafficking (original) (raw)
Abstract
The phosphoinositides (PIs) function as efficient and finely tuned switches that control the assembly–disassembly cycles of complex molecular machineries with key roles in membrane trafficking. This important role of the PIs is mainly due to their versatile nature, which is in turn determined by their fast metabolic interconversions. PIs can be tightly regulated both spatially and temporally through the many PI kinases (PIKs) and phosphatases that are distributed throughout the different intracellular compartments. In spite of the enormous progress made in the past 20 years towards the definition of the molecular details of PI–protein interactions and of the regulatory mechanisms of the individual PIKs and phosphatases, important issues concerning the general principles of the organisation of the PI system and the coordination of the different PI-metabolising enzymes remain to be addressed. The answers should come from applying a systems biology approach to the study of the PI system, through the integration of analyses of the protein interaction data of the PI enzymes and the PI targets with those of the ‘phenomes' of the genetic diseases that involve these PI-metabolising enzymes.
Keywords: genetic diseases, membrane trafficking, phosphoinositides
Introduction
The phosphoinositides (PIs) derive from reversible phosphorylation in three of the five hydroxyl groups of the inositol headgroup of the ‘parent' PI, phosphatidylinositol (PtdIns). This process operates through the large repertoire of PI kinases (PIKs) and PI phosphatases that are present in practically all cell compartments (Figures 1 and 2). The combined activities of the various isoforms of these PIKs and PI phosphatases provide a dynamic equilibrium between the seven distinct, but interconvertible, PI species (Figure 1). It is now clear that all of these different PIs are ‘active' in their own right, rather than many just serving as intermediates in the synthesis of the higher phosphorylated species.
Figure 1.
The PI kinases and phosphatases and their genetic defects. (A) Schematic representation of the PI metabolic cycle with the PIKs indicated in blue, and the PI phosphatases in green. (B) Listing of the different isoforms of the PIKs and PI phosphatases, together with their domain organisation and their corresponding genetic disease and knockout or knockdown phenotypes in mice. RSBD, regulatory subunit-binding domain; C2, conserved region 2; HD, helical domain; PRD, proline-rich domain, NLS, nuclear localisation signal; LKU, lipid kinase unique domain; PH, pleckstrin-homology domain; SRD, serine-rich domain; CRD, cysteine-rich domain; FYVE, Fab1 YOTB Vac1 EEA1; DEP, domain present in dishevelled, EGL-10 and pleckstrin; TCP1, tailless complex polypeptide-1; SPEC, spectrin repeat; PH-GRAM, pleckstrin homology glucosyltransferases, Rab-like GTPase activators and myotubularins; DENN, differentially expressed in normal versus neoplastic; LZ, leucine zipper; TM, transmembrane domain; ASH, abnormal spindle-like microcephaly-associated protein (ASPM), C. elegans centrosomal protein (SPD-2), hydrocephalus-associated protein (Hydin); RhoGAP, Rho-GTPase-activating protein; SAC, yeast suppressor of actin 1; Skitch, SKIP carboxyl homology domain; SH2, phosphotyrosine-binding module 2; SAM, sterile alpha-motif domain.
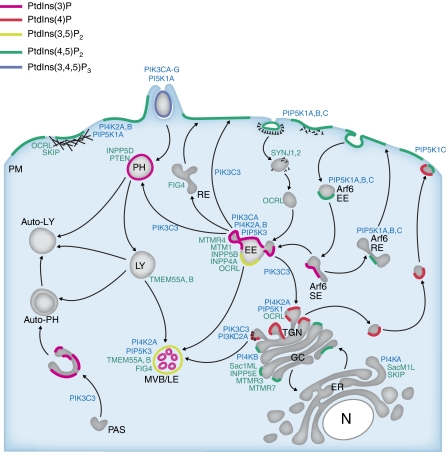
Figure 2.
Subcellular distribution of the PIs and PI-metabolising enzymes. The localisation of the different PIKs (blue) and PI phosphatases (green), as well as the predominant PI species (as visualised by PI-binding protein domains) in the different cell compartments. It is noted that many of the PI-metabolising enzymes are present in more than one cellular compartment, and their overall distributions do not completely fit with the PI map, as indicated using the PI-binding protein probes (see text for details). The PIKs and PI phosphatases are indicated according to the nomenclature given in Figure 1. PM, plasma membrane; EE, early endosome; SE: sorting endosomes; RE, recycling endosome; LY, lysosome; MVB/LE, multivesicular body/late endosome; PAS, pre-autophagosomal structure; PH, phagosome; TGN, trans-Golgi network; GC, Golgi complex; ER: endoplasmic reticulum; N, nucleus.
Although they are quantitatively minor components of cell membranes, the PIs regulate many fundamental processes in the cell, including membrane trafficking, cell growth, cytoskeleton remodelling and nuclear events. These regulatory actions are mainly due to their ability to control the subcellular localisation and activation of various effector proteins that possess PI-binding domains, such as the PH, FYVE, PX, ENTH, PH-GRAM, FERM and GLUE domains (Lemmon, 2008).
Here, we will focus on the role of the PIs in membrane trafficking, where they can function as ‘local' organisers of membrane domains and controllers of membrane sorting and deformation machineries, and as integrators of membrane trafficking within other cell function modules, such as the cytoskeleton, signalling, lipid metabolism and energy control. Although we refer to excellent recent reviews for an update on the enormous progress made towards the definition of the roles, regulation and mechanisms of action of the PIs (Di Paolo and De Camilli, 2006; Engelman et al, 2006; Gamper and Shapiro, 2007; Krauss and Haucke, 2007; Strahl and Thorner, 2007; Yeung and Grinstein, 2007; Marone et al, 2008; Michell, 2008), we will highlight in the following the many open questions that still dominate the field. We will also attempt to extract the general principles of the functioning of the PI system, and finally, we will illustrate how a systems biology approach to the study of the genetic defects of the PI-metabolising enzymes can provide important lessons for the understanding of the PI system itself.
The roles of the PIs in membrane trafficking: organisers of molecular machineries and regulators of membrane lipid composition
The main role of the PIs in membrane trafficking involves the spatially and temporally controlled activation, recruitment and/or assembly of the molecular machineries (see Table I) involved in membrane bending and fission, and in vesicle movement, tethering and fusion. The components of these machineries are mainly peripheral proteins that are targeted to their correct sites of action through their binding to a specific PI species. These PI–protein interactions usually occur with relatively low affinities, and additional stabilising binding sites are often required to engage either membrane-resident proteins or specific-organelle-associated small GTPases (Lemmon, 2008). A similar combinatorial recognition system increases the individuality of the identity code for each organelle and membrane domain, and offers an opportunity for signal integration (Itoh and De Camilli, 2004; Behnia and Munro, 2005). Various examples of such integration are known: many adaptors (e.g. the clathrin adaptors AP1 and AP2) share binding to the same coat protein (clathrin) and the same sorting signals in the cargo proteins (YXXΦ), but they are recruited to different intracellular ‘districts' (trans-Golgi network (TGN) endosomes for AP1, and plasma membrane (Bai and Chapman) for AP2), which is here probably due to their different PI-binding selectivities (Table I).
Table 1.
PI-binding proteins belonging to membrane trafficking machineries
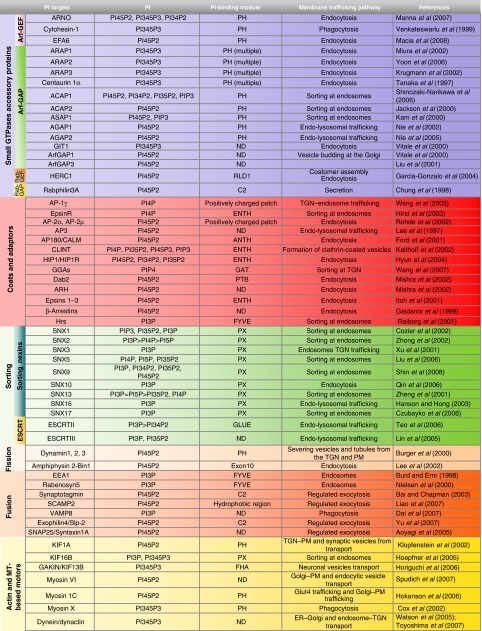 |
|---|
| RLD1, RCC1-like domain; ENTH, epsin N-terminal homology; ANTH, AP180 N-terminal homology; GAT, GGA and Tom1; PTB, phospho-tyrosine-binding domain; PX, Phox homology; FHA, forkhead associated. |
| PI-binding proteins belonging to membrane trafficking machineries are shown according to their specific roles, together with their PI-binding modules and their PI-binding preference. |
The PIs also operate at the interface between membranes and the cytoskeleton, a key site for fundamental membrane trafficking events, such as membrane deformation and fission, and vesicle movements. The importance of actin-based machineries in endocytic processes is well established both in yeast and in mammals (Engqvist-Goldstein and Drubin, 2003). In particular, actin assembly accompanies the internalisation of coated pits at the plasma-membrane (PM) in yeast (Engqvist-Goldstein and Drubin, 2003), and a burst of localised actin polymerisation accompanies the cycling of synaptic vesicles. This cycling also correlates with the cycling of PtdIns 4,5-bisphosphate (PI45P2) production/consumption, and PI45P2 does indeed have an active role in coordinating endocytic and cytoskeleton events (Di Paolo and De Camilli, 2006). The targets involved in this coordinating activity of PI45P2 during endocytosis belong to different classes of proteins. These include the actin network growth machinery proteins, including Cdc42, Arp2/3 and Abp1; some of the so-called endocytic accessory proteins, such as HIP1/HIP1R, which is recruited into growing coated structures and which connects the clathrin coat with actin filaments (Chen and Brodsky, 2005) and the actin-based myosin I and VI motors (Krendel et al, 2007; Spudich et al, 2007), which are believed to provide the required pulling force to drive membrane deformation and fission (Table I). The PIs are also involved in the subsequent cytoskeleton-driven endocytic steps, such as microtubule-dependent motility of endosomes, where the PIs act on the motors (e.g. KIF16B; Hoepfner et al, 2005) that mediate the plus-end-directed motility of early endosomes, and that are required for recycling and degradation pathways. Furthermore, the PIs appear to coordinate the cytoskeleton and membrane trafficking not only at the PM and endosomes but also at the Golgi complex, where they are involved in the assembly of a spectrin-based actin skeleton (De Matteis and Morrow, 1998), in synergising with Cdc42, NWASP and cortactin in Arp2/3-mediated actin nucleation, and in the control of the Golgi pool of myosin VI (Egea et al, 2006).
A further mechanism through which the PIs can affect the properties of cell membranes and have an impact in membrane trafficking has emerged recently with the demonstration that they can control the synthesis of the sphingolipids. This control is mediated through a family of lipid-transfer proteins that share a common domain organisation, and includes CERT and FAPP2 (De Matteis et al, 2007). Thus, these possess a closely homologous PH domain at their N terminus, and a distinct additional lipid-binding/transfer domain at their C terminus. Their homologous PH domains interact with PtdIms4-phosphate (PI4P) and the small GTPase Arf, and are responsible for the association of these lipid-transfer proteins with the Golgi complex. Their distinct lipid-transfer domains bind ceramide and glucosylceramide (GlcCer), for CERT and FAPP2, respectively (D'Angelo et al, 2008).
CERT mediates the non-vesicular transport of ceramide from the endoplasmic reticulum (ER), its site of synthesis, to the Golgi complex, where it is converted into sphingomyelin (SM). Consequently, defects in CERT inhibit SM synthesis (Hanada et al, 2003). Lowering the levels of PI4P also inhibits SM synthesis, through its impact on CERT recruitment/activity (Toth et al, 2006; D'Angelo et al, 2007).
FAPP2 is a GlcCer-transfer protein that is required for complex glycosphingolipid (GSL) synthesis (D'Angelo et al, 2007; Halter et al, 2007) due to its ability to transfer GlcCer from the cytosolic side of the early Golgi compartments to the late-Golgi compartments, where the GSL synthetic enzymes reside. This activity of FAPP2 is regulated by PI4P, and interfering with PI4P production inhibits GSL synthesis (D'Angelo et al, 2007).
The PIs as integrators of signalling and membrane trafficking
The PIs have recognised roles in membrane trafficking also as transducers of PM receptor activation and of the general nutritional and stress status of the cell.
Well-known examples of the ability of the PIs to mediate the effects of PM receptor-initiated signalling cascades on membrane trafficking include clathrin-mediated endocytosis (Irie et al, 2005), phagocytosis (Stephens et al, 2002), macropinocytosis (Lanzetti et al, 2004), translocation of GLUT4 (Thong et al, 2005) and degranulation of mast cells (Ito et al, 2002). A more recent demonstration of how PM receptors regulate trafficking through the PIs relates to the involvement of the PI 4-phosphatase Sac1 in the stimulation of anterograde trafficking in the Golgi complex in response to growth factors (Blagoveshchenskaya et al, 2008). Sac1 accumulates at the Golgi complex in quiescent cells, where it ‘consumes' the Golgi pool of PI4P, and by doing so, it downregulates anterograde trafficking. After stimulation by mitogens, Sac1 is phosphorylated and undergoes a transition from an oligomeric to a monomeric state of aggregation. As a monomer, Sac1 is relocated to the ER, resulting in an increase in PI4P levels at the Golgi complex, and consequently in the promotion of anterograde trafficking (Blagoveshchenskaya et al, 2008).
Local pools of the PIs can undergo significant changes in relation to nutrient availability and cell stress. In yeast, nutrient availability appears to strictly control the pool of PI4P at the Golgi complex, which is a key determinant in anterograde trafficking through and out of the Golgi complex (Strahl and Thorner, 2007). Interestingly, nutrient deprivation reduces the Golgi pool of PI4P (thus inducing a sort of quiescent state of the organelle) by two parallel but synergistic mechanisms: by releasing the PI 4-kinase (PI4K) Pik1p (the major source for PI4P at the Golgi complex) from the Golgi complex (Demmel et al, 2008); and by promoting the translocation of the PI 4-phosphatase Sac1p from the ER to the Golgi complex (Faulhammer et al, 2007).
With the PI response to cell stress, in their seminal paper identifying PtdIms 3,5-bisphosphate (PI35P2) as a novel endogenous PI species, Dove et al (1997) reported that PI35P2 levels increase by up to 30-fold in yeast cells subjected to hyperosmotic stress. This increase is mainly sustained by an increased production of PI35P2 through the PIP5K Fab1p (Cooke et al, 1998), with PI35P2 having a major role in controlling the size and shape of the vacuole (Dove and Johnson, 2007) and in mediating its fragmentation in response to hyperosmotic stress (Bonangelino et al, 2002). A remaining open question concerns the nature of the stress sensors that lead to this increase in PI35P2 levels. An intriguing possibility is that the PIP5K Fab1p might itself function as a stress sensor through its chaperonin-like domain (Dove and Johnson, 2007).
The principles behind the organisation of PI metabolism: compartmentalisation and tight spatio-temporal control
A distinctive feature of the organisation of PI metabolism is its regionalisation, as opposed to the centralisation of most lipid biosynthetic pathways in the ER (and in the Golgi complex). For the PIs, the ER is just the site of synthesis of the common precursor, PtdIns, as all the subsequent steps of phosphorylation (and dephosphorylation) occur in practically all the other cell compartments through the PIKs and PI phosphatases (Figure 2). This regionalised organisation and the isoform specialisation of PI metabolism have manifold implications.
First, the kinds, levels and activities of the PIKs and PI phosphatases are different in each cellular compartment, meaning that at steady state, each of the seven PI species can maintain different concentrations across these compartments. This non-homogeneous distribution of the PI species in the cell has been ‘visualised' using PI-binding protein modules that have distinct PI-binding profiles (De Matteis and Godi, 2004). With due caution deriving from an awareness of the limits of these tools (Roy and Levine, 2004; Lemmon, 2008), which can only detect the free pools of the PIs and which can have protein as well as PI targeting determinants (Godi et al, 2004; Lemmon, 2008), a distribution map of the PIs in the cell has been constructed. In this map, PI45P2 is enriched at the PM, PI4P at the Golgi complex, PI3P at the early endosomes, and PI3P and PI35P2 in the late endosomes. However, many ‘deviations' from this main distribution map have been described as a more assorted series of tools have become available (Roy and Levine, 2004; Lemmon, 2008) and more accurate methods of detection are applied (Downes et al, 2003). Thus, it has been possible to obtain direct visualisation and functional data in favour of the presence of PI45P2 in the Golgi complex (Watt et al, 2002), of PI4P at the PM (Roy and Levine, 2004) and at the micropexophagy-specific membrane apparatus (Yamashita et al, 2007), of PI3P at the PM (Falasca et al, 2007; Lodhi et al, 2008) and of PI35P2 in secretory granules (Osborne et al, 2008).
A question raised by this apparent spatial segregation of the PI species into different and distant compartments is how the global PI homoeostasis is maintained across the cell. Different possibilities can be envisaged here. One is that the PI homoeostasis is maintained due to intense communication of the different PI pools through vesicular trafficking. This would establish a sort of bidirectional profitable relationship, where the PIs are used as organisers of membrane trafficking, and membrane trafficking serves PI metabolism by ensuring the delivery of PI substrates generated in a given compartment to their metabolic enzymes located in a distant, but communicating, one. Another possibility is that in spite of hosting an apparently predominant PI species, each compartment is in fact self sufficient in sustaining an autonomous phosphorylation–dephosphorylation PI cycle and thus contains different PI species at the same time at steady state.
These two possibilities are not mutually exclusive and are indeed both pursued with the differently located PI enzyme isoforms that are involved in the generation of the same PI product in a given cell compartment depending on the cell or environmental conditions. This has been shown recently for the origin of PI4P as a precursor for PI45P2 at the PM: this is different under steady-state and stimulated conditions, whereby for the former it arises from the Golgi-complex-localised PI4Ks, PI4KIIα and IIIβ, whereas in angiotensin-II-stimulated cells it arises mainly from PI4KIIIα (Balla et al, 2008). Interestingly, although it cannot be excluded that a small fraction of PI4KIIIα relocalises to the PM upon receptor stimulation, the majority of this PI4K isoform resides in the ER. This prompts the speculation that at least under these circumstances, the generation of PI4P at the PM might occur at the sites of close apposition between the ER and the PM (the ER–PM contact sites; Levine and Loewen, 2006). This is an additional and intriguing possibility for PI metabolic reactions that has been shown to occur for other lipid metabolic pathways, such as phosphatidylserine–phosphatidylethanolamine conversion at the level of the ER–mitochondria membrane contact sites (Shiao et al, 1998).
The ability of the PIs to serve the multiple and dynamic functions in membrane trafficking mentioned above is due to the tight spatial and temporal control of their generation/consumption, that is, of the PIKs and PI phosphatases. These are usually cytosolic enzymes that are timely and precisely recruited to sites that are actively involved in trafficking events, through their interactions with key components of the molecular machineries that control or carry out specific transport steps. These include the small GTPases, coat/adaptors and the fissioning machinery in particular (Table II). Interestingly, these same classes of molecules are also often targets of the PIs (Table II). Thus, the interactions of the PI-metabolising enzymes with components of the trafficking machinery constitute a way not only for recruiting these enzymes to specific cellular compartments but also to effectively channel specific PIs to their effectors and to sustain positive or negative feedback if the recruited enzymes produce or consume, respectively, the PI species that interacts with the effector. The best studied examples of GTPases that control the PI-metabolising enzymes are those of Rab5 and the Arfs. Rab5 seems to be a key controller and coordinator of the PIKs and PI phosphatases in the endocytic pathway, as it binds and stimulates type III PI3K, type I PI3Kβ, type I PI 4-phosphatase and the INPP5B and OCRL 5-phosphatases (Shin et al, 2005), whereas both Arf1 and Arf6 can recruit and stimulate PIP5K (Santarius et al, 2006), with Arf1 also recruiting and activating PI4KIIIβ on the Golgi complex (Godi et al, 1999).
Table 2.
Interaction between PI-metabolising enzymes and components of membrane trafficking machineries
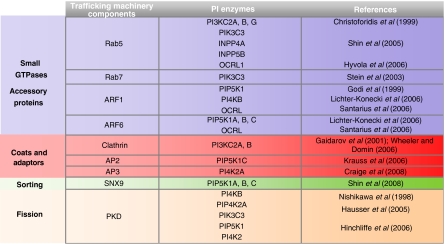 |
|---|
| Components of molecular machineries involved in different steps of membrane trafficking that control the localisation and/or activity of the PI-metabolising enzymes. |
The open questions on substrate channelling, functional redundancy and the general coordination of the PI-metabolising enzymes
A peculiar feature of the PI system is the coexistence of pathways that are both divergent (where the same PI species is subjected to different and alternative modifications) and convergent (where the same PI species is produced through different routes). These features pose two important questions for which only partial answers are at present available: (i) what are the mechanisms through which a given PI species is channelled towards one of its different possible products? and (ii) to what extent are the alternative pathways leading to the same end product redundant or ‘dedicated' to different functions?
The answer to the issue of substrate channelling towards a given product will need to come from a consideration of the regionalised distribution of the different PI-metabolising enzymes that determines that the same PI species can have distinct destinies in different locations (Figure 2). This could thus explain, for instance, why PtdIns is mainly converted to PI4P in the Golgi complex and into PI3P in the endosomes, whereby PI4K and PI3K are differentially enriched in these two compartments.
However, in many instances, different enzymes acting on the same substrate coexist in the same compartment (Figure 2), and together with the highly diffusible nature of the PIs as substrates; this means that the spatial segregation argument cannot be applied here.
A solution that appears to be pursued in some cases is the preassembly of multi-enzyme complexes, which are generally centred on regulatory/scaffold components. One of these complexes controls the turnover of PI3P at the endosomes, and includes a PI3K (Vps34), a PIP 3-phosphatase (MTM1) and a regulatory component (Vps15) (Cao et al, 2007). Another complex controls PI35P2 synthesis and turnover in MVBs and contains the PIP5K PIKfyve, the PIP 5-phosphatase Sac3 (a Fig4 homologue), and the regulatory component ArPIKfyve (homologue of Vac14) (Sbrissa et al, 2007). Other examples of multi-enzyme complexes in this context include a complex isolated from platelets that contains PIP 5-phosphatases and 4-phosphatases and PI 3-kinases (Munday et al, 1999), and a complex orchestrated by Rab5 that includes type I PI3K, PIP 4-phosphatase and PIP 5-phosphatase (Shin et al, 2005).
Interestingly, this latter complex has been shown to sustain one of the two convergent pathways that leads to the production of PI3P in the endosomal membranes: one which proceeds through the two-step dephosphorylation of PI345P3 in positions 5 and 4, as opposed to the one which is based on the direct phosphorylation of PIs by type III PI3K (Munday et al, 1999; Shin et al, 2005). The production of PI45P2 can also proceed through two convergent pathways: either by 5-phosphorylation of PI4P (by PIP5K1; Figure 1) or by 4-phosphorylation of PI5P (by PIP4KII; Figure 1).
A question that arises from the existence of convergent pathways and of different enzyme isoforms (Figure 1) is to what extent these pathways or enzyme isoforms are functionally redundant or generate ‘specialised' and distinct pools of the PIs. This is a question that has not been systematically and quantitatively addressed, and for which the solution will be of extraordinary importance to exploit this, even if limited, functional redundancy as a target in the treatment of diseases linked to genetic defects of single PI-metabolising enzymes (see below).
An aspect that has remained little explored to date relates to the coordination of the PI enzymatic activities at both the local and the global cellular levels. Locally, the small GTPases have key roles, as they function as recruiters and timers for the PI-metabolising enzymes (Table II), and in selected cases (such as for Rab5), they can also coordinate multiple enzymatic activities. Another level of local homoeostatic control appears to be intrinsic to the structures of the enzymes themselves (Figure 1), as in addition to their catalytic domain, some of these possess additional PI-binding domains with good affinities for their substrate. This is the case, for instance, of the 3-phosphatase MTMR4, which has a FYVE domain that binds PI3P and that contributes both to its recruitment and to its release once the substrate has been removed by the enzyme itself (Lorenzo et al, 2006), or of MTMR2, which is recruited, through its PH-GRAM domain, to PI35P2-containing vesicles (Berger et al, 2003). A special case is seen with PI35P2 synthesis in the vacuole in yeast: here, Fig4 not only has 5-phosphatase activity towards PI35P2 but also appears to activate the PIP5K Fab1 kinase that synthesises PI35P2 from PI3P (Duex et al, 2006). Thus, Fig4 regulates both the turnover and the synthesis of PI35P2. A similar dual regulation might be very effective in ensuring that PI35P2 undergoes continual production that is balanced by continual consumption, and thus promoting an elevated flux of PI35P2 through a phosphorylation–dephosphorylation cycle. An elevated PI35P2 flux is an elegant way to increase the local availability (without raising the absolute levels) of PI35P2 and to reduce its diffusion.
Another way to exploit the coordination of the activities of the PIKs and PI phosphatases is to subject them to common regulation through phosphorylation–dephosphorylation cycles through shared protein kinases and phosphatases. This is the case for PI45P2 synthesis and turnover at the synapse, which are both inhibited through Cdk5-mediated phosphorylation and inactivation of PIP5KC and synaptojanin, and are activated by calcineurin-mediated dephosphorylation of both PIP5K and synaptojanin (Lee et al, 2004).
At present, we have scattered examples of the mechanisms that coordinate the local activities of the different PI-metabolising enzymes. However, we have no real clues as to the existence of a more general plan for the coordination and specific combination of the expression and/or interactions in the cell of given PI-metabolising enzyme isoforms that function along the same or alternative PI-metabolising branches. Deciphering such a plan will be important for the unravelling of the ‘real' physiological roles of apparently equivalent pathways that can operate through the coupling of different enzyme isoforms. Similarly, this will enable us to determine the tissue specificities of the different pathways, and to identify the pathopathways underlying the diseases that derive from genetic defects in single PI-metabolising enzymes and the drug targets for the treatment for these diseases (see below).
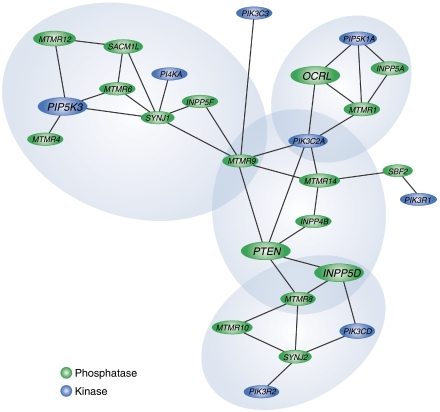
Help towards the answering these questions may well arise from the application of a systems biology approach to the study of the PI-metabolising enzymes. By combining and completing the data for the expression profiles and protein interactions of these enzymes (and of the PI targets), this approach should arrive at the definition of the ‘interactome' of the PI system. An example of an analysis of co-expression profiles of PI-metabolising enzymes is given in Figure 3. By centring the analysis on 3-phosphatases and 5-phosphatases and selecting the genes for PI-metabolising enzymes that are among the most significantly co-expressed genes, a number of PI-enzyme clusters emerge. It turns out, for instance, that the OCRL gene, which is responsible for Lowe syndrome (see below), is part of a highly interconnected gene cluster that comprises PIP5K1A, INPP5A, PI3KC2A and MTMR1. Thus, the expression of two 5-phosphatases, OCRL and INPP5A (a type I 5-phosphatase acting exclusively on soluble inositol phosphates and involved in inositol 1,4,5-trisphosphate removal), seems to be coordinated with that of a PI45P2-synthesising enzyme, specifically PIP5K1A. This co-expression profile might represent the basis for homoeostatic control of PI45P2 and inositol 1,4,5-trisphosphate levels, and might provide an explanation for the calcium-signalling imbalance that occurs when PI45P2 levels increase due to a mutation in OCRL (Suchy et al, 2005). Furthermore, the significant co-expression of OCRL with MTMR1 suggests a possible role for OCRL in the control of the endocytic PI3P pool.
Figure 3.
Co-expression network of the PI-metabolising enzymes. Co-expression network of the PI-metabolising enzymes (PIKs, blue ellipses; PI phosphatases, green ellipses; as listed in Figure 1), focused on the 3- and 5-phosphatases. The analysis was performed using the COXPRESdb database of gene expression profiles from a variety of normal and pathological conditions (from a total of 123 experiments; Obayashi et al, 2008 no. 22). Here, using the 3- and 5-phosphatase genes as individual queries and analysing the most significantly co-expressed genes (according to the weighted Pearson's correlation coefficient between gene probes, and choosing 0.4 as a threshold; Obayashi et al 2008), we selected specifically for the genes for PI-metabolising enzymes. Groups of genes extensively connected in the network represent co-expressed gene clusters that are likely to be involved in common cell functions. The 3- and 5-phosphatase genes responsible for genetic diseases (see Figure 1) are highlighted in bold. Four interconnected enzyme clusters emerge from the analysis, each containing a disease gene (see Figure 1). In particular, OCRL gene is part of a cluster that also comprises PIP5K1A, INPP5A and MTMR1.
Although limited, the example given shows the power of such an approach for the uncovering of unsuspected couplings between specific isoforms of the PI-metabolising enzymes, for the provision of candidate interactors and for the delineation of novel pathways of communication between different branches of PI metabolism, for which their relevance can and will have to be explored experimentally.
What we can learn from the genetic diseases of the PI system?
The importance of maintaining a tight balance between the activities of the various PI-metabolising enzymes is highlighted by the severe consequences arising from defects in PI metabolism. The pivotal roles of PI345P3 dysmetabolism in cancer, inflammation and diabetes are well established (Cantley, 2004; Wymann and Marone, 2005), to the point where the PI3Ks and the PI345P3 phosphatases PTEN and SHIP have become attractive targets for the development of novel pharmacological agents (Workman, 2004; Lazar and Saltiel, 2006; Ruckle et al, 2006; Zhao, 2007).
For monogenic diseases that have been linked to defects in PI-metabolising enzymes, these represent a heterogeneous group of conditions, many of which affect the nervous system (both central and peripheral; such as lethal contractural syndrome, Lowe syndrome and Marie–Charcot–Tooth), with others affecting muscle (myopathy), eye (fleck corneal dystrophy, Lowe syndrome) and kidney (Lowe syndrome) (Figure 1). In the majority of cases, we do not understand in depth the links connecting the genetic defects with the clinical manifestations of these diseases. A lot of help in this direction should come from consideration of pathological states with similar clinical pictures, as it has now been shown that diseases with overlapping clinical manifestations can be caused by mutations in different genes that are part of the same functional module. In such instances, the clinical overlap can be attributed to defects in individual genes that render the entire module dysfunctional. Analyses involving model organisms, and more recently humans, have also shown that direct and indirect interactions often occur between protein pairs that are responsible for similar phenotypes (Oti, 2007). Recently, this concept has been successfully applied and exploited to identify and experimentally confirm the relationships between genes involved in various inherited ataxias that all share a dysfunctional state of the Purkinje cells (Lim et al, 2006).
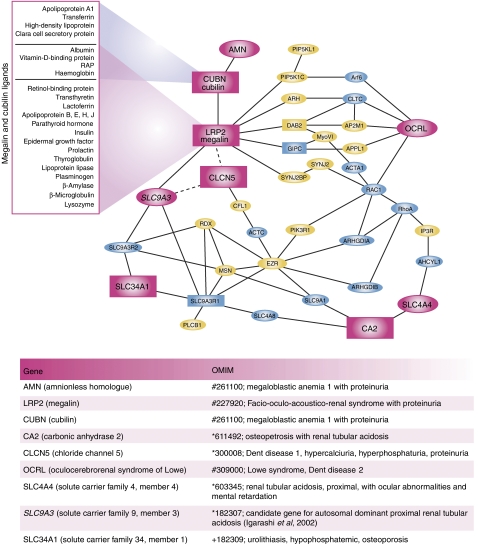
Figure 4 shows the results of a similar approach (based on the available data) as it might be applied to one of the main manifestations of Lowe syndrome: a dysfunction of the kidney proximal tubule cells (PTCs) that is responsible for the loss of salts (including bicarbonates) and low molecular weight (LMW) proteins with the urine (Igarashi et al, 2002; Christensen and Gburek, 2004). This proximal tubular acidosis and LMW proteinuria are pathognomic of the renal Fanconi syndrome, which is common to a series of genetic defects that involve intracellular chloride channels (ClC5 in Dent syndrome) and ion transporters or exchangers (SLC9A3, SLC34A1 and SLC4A4). In some cases, the extent of the overlap of clinical signs is such that the conditions cannot be distinguished: this is the case for patients who have been clinically diagnosed as affected by Dent disease, but have then been found to carry mutations in OCRL (Attree et al, 1992; Hoopes et al, 2005). Building up a comprehensive network including the mouse genes where a knockout causes protein and/or salt urinary loss (Figure 4, rectangles), and the interactors of the human gene products that show the defects causing renal Fanconi syndrome (Figure 4, large ellipses) highlight the molecular pathways that are involved in protein and salt reabsorption by PTCs and provide a range of testable candidates as possible OCRL interactors and/or PI45P2-binding proteins (yellow ellipses) with proven physiological roles in PTCs (Figure 4). A key node in the network illustrated in Figure 4 is megalin (LRP2), a member of the LDL receptor family that is highly expressed in the brush border of PTCs, and that functions together with cubilin (CBN) as a multiligand receptor that mediates the capture and resorption of the LMW proteins present in the ultrafiltrate (indicated as megalin ligands in Figure 4) (Igarashi et al, 2002; Christensen and Gburek, 2004). Megalin and cubilin continuously cycle between the apical PM, and the early/recycling endosomes, with delivery of luminal ligands to the lysosomal compartments, escorted by PI-binding proteins, such as Dab2 (Disabled-2) and ARH (autosomal recessive hypercholesterolaemia). A defect in megalin recycling has been shown to be at the origin of Dent disease (Piwon et al, 2000), and has been hypothesised to contribute to urinary protein loss also in Lowe syndrome (Norden et al, 2002; Lowe, 2005). This hypothesis has been experimentally reinforced recently by the demonstration that OCRL interacts with APPL1, which in turn binds GIPC, an interactor of megalin (Erdmann et al, 2007).
Figure 4.
Interaction map of the products of the genes responsible or candidate for disorders of kidney proximal tubule cells (PTCs). The genes responsible or strong candidate for diseases due to dysfunction of kidney PTCs are shown (large red ellipses), together with selected interactors, including genes for which the knock out causes proteinuria and tubular acidosis in mice (rectangles). The interaction map was generated using Osprey (powered by Human GRID, General Repository of Interaction Datasets). The components of the network that are active on or sensitive to PI45P2 (the substrate of the 5-phosphatase OCRL) are marked in yellow. LRP2 encodes for megalin, a multiligand receptor that, together with cubilin (CBN), is responsible for the resorption of the indicated LMW proteins by PTCs (Igarashi et al, 2002; Christensen and Gburek, 2004). ARH, autosomal recessive hypercholesterolaemia; DAB2, disabled homologue 2; GIPC, GAIP C terminus interacting protein; PIP5K1C, phosphatidylinositol-4-phosphate 5-kinase type I gamma; SYNJ2BP, synaptojanin 2-binding protein; CLTC, clathrin heavy chain 1; AP2M1, clathrin coat assembly protein AP50, Myo VI, myosin 6, APPL1, adapter protein containing PH domain, PTB domain and leucine zipper motif 1; Arf6, ADP ribosylation factor 6; PIP5KL1, phosphatidylinositol-4-phosphate 5-kinase-like 1; SYNJ2, synaptojanin-2; RAC1, Ras-related C3 botulinum toxin substrate 1; RhoA, transforming protein RhoA; IP3R, inositol 1,4,5-trisphosphate receptor type 1; AHCYL1, putative adenosylhomocysteinase 2; SLC9A1, Na/H exchanger 1 (NHE-1) (solute carrier family 9 member 1); ARHGDIA, Rho GDP dissociation inhibitor 1 (Rho GDI 1); ARHGDIB, Rho GDP dissociation inhibitor 2 (Rho GDI 2); PIK3R1, phosphatidylinositol 3-kinase regulatory alpha subunit; EZR, Ezrin; SLC4A8, solute carrier family 4, sodium bicarbonate cotransporter, member 8; SLC9A3R1, Na(+)/H(+) exchange regulatory cofactor NHE-RF) (NHERF-1); SLC9A3R2, Na(+)/H(+) exchange regulatory cofactor NHE-RF2 (NHERF-2); PLCB1, phospholipase C-beta-1; ACTA1, alpha actin 1; ACTC, alpha cardiac actin.
Finally, introducing a similar phenome analysis in our PI studies will not only allow us to delineate candidate pathopathways for pharmacological intervention in the genetic defects of the PI-metabolising enzymes, but when integrated with the interaction network of these PI-metabolising enzymes, it will also greatly improve our overall understanding of the global organisation of PI metabolism and of the real physio-pathological relevance of its numerous branches.
Conclusions and perspectives
Through work carried out over the last decade on yeast and mammalian cell models in many laboratories, we have now reached a deep state of knowledge of the molecular details of the regulation and roles of several of the PI-metabolising enzymes. However, we are still missing the overall picture of the global functional organisation and coordination of the PI system. An answer to this problem should arise from the computational integration of phenotypic data (derived from genetic diseases and knockout mice) with a high-confidence interaction network of the PI-metabolising enzymes and their regulators and effectors; thus from a combined PI-phenome-interactome network.
Despite these many residual uncertainties, we are, however, also convinced that it is time to translate the many research discoveries of these past years towards the development of pharmacological treatments for genetic diseases that involve the PI-metabolising enzymes. The main considerations here are three-fold: first, powerful drug discovery technologies are now available; second, the diseases that are related to the PI-metabolising enzymes fulfil the general criteria for drug discovery as they originate from defects within molecular pathways where it is possible to identify ‘drugable' targets and third, some of these PI-metabolising enzymes (e.g. PI3K, SHIP and PTEN) are indeed already drug targets and have inspired the development of highly specific inhibitors. The legitimate conclusion that we reach is that the time for a pharmacological approach to genetic diseases involving the PI cycle is ripe. Therefore, greater efforts now need to be put into the exploitation of our basic knowledge for the identification and validation of drug targets.
Acknowledgments
We thank CP Berrie for editorial assistance and E Fontana and R Le Donne for artwork. MADM acknowledges the support of Telethon and AIRC, and GDA is the recipient of a fellowship from FIRC.
References
- Aoyagi K, Sugaya T, Umeda M, Yamamoto S, Terakawa S, Takahashi M (2005) The activation of exocytotic sites by the formation of phosphatidylinositol 4,5-bisphosphate microdomains at syntaxin clusters. J Biol Chem 280: 17346–17352 [DOI] [PubMed] [Google Scholar]
- Attree O, Olivos IM, Okabe I, Bailey LC, Nelson DL, Lewis RA, McInnes RR, Nussbaum RL (1992) The Lowe's oculocerebrorenal syndrome gene encodes a protein highly homologous to inositol polyphosphate-5-phosphatase. Nature 358: 239–242 [DOI] [PubMed] [Google Scholar]
- Bai J, Chapman ER (2003) Application of fluorescent probes to study mechanics and dynamics of Ca2+-triggered synaptotagmin C2 domain–membrane interactions. Methods Enzymol 360: 238–258 [DOI] [PubMed] [Google Scholar]
- Balla A, Kim YJ, Varnai P, Szentpetery Z, Knight Z, Shokat KM, Balla T (2008) Maintenance of hormone-sensitive phosphoinositide pools in the plasma membrane requires phosphatidylinositol 4-kinase III{alpha}. Mol Biol Cell 19: 711–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behnia R, Munro S (2005) Organelle identity and the signposts for membrane traffic. Nature 438: 597–604 [DOI] [PubMed] [Google Scholar]
- Berger P, Schaffitzel C, Berger I, Ban N, Suter U (2003) Membrane association of myotubularin-related protein 2 is mediated by a pleckstrin homology-GRAM domain and a coiled-coil dimerization module. Proc Natl Acad Sci USA 100: 12177–12182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blagoveshchenskaya A, Cheong FY, Rohde HM, Glover G, Knodler A, Nicolson T, Boehmelt G, Mayinger P (2008) Integration of Golgi trafficking and growth factor signaling by the lipid phosphatase SAC1. J Cell Biol 180: 803–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonangelino CJ, Nau JJ, Duex JE, Brinkman M, Wurmser AE, Gary JD, Emr SD, Weisman LS (2002) Osmotic stress-induced increase of phosphatidylinositol 3,5-bisphosphate requires Vac14p, an activator of the lipid kinase Fab1p. J Cell Biol 156: 1015–1028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burd CG, Emr SD (1998) Phosphatidylinositol(3)-phosphate signaling mediated by specific binding to RING FYVE domains. Mol Cell 2: 157–162 [DOI] [PubMed] [Google Scholar]
- Burger KN, Demel RA, Schmid SL, de Kruijff B (2000) Dynamin is membrane-active: lipid insertion is induced by phosphoinositides and phosphatidic acid. Biochemistry 39: 12485–12493 [DOI] [PubMed] [Google Scholar]
- Cantley LC (2004) The role of phosphoinositide 3-kinase in human disease. Harvey Lect 100: 103–122 [PubMed] [Google Scholar]
- Cao C, Laporte J, Backer JM, Wandinger-Ness A, Stein MP (2007) Myotubularin lipid phosphatase binds the hVPS15/hVPS34 lipid kinase complex on endosomes. Traffic 8: 1052–1067 [DOI] [PubMed] [Google Scholar]
- Chen CY, Brodsky FM (2005) Huntingtin-interacting protein 1 (Hip1) and Hip1-related protein (Hip1R) bind the conserved sequence of clathrin light chains and thereby influence clathrin assembly in vitro and actin distribution in vivo. J Biol Chem 280: 6109–6117 [DOI] [PubMed] [Google Scholar]
- Christensen EI, Gburek J (2004) Protein reabsorption in renal proximal tubule––function and dysfunction in kidney pathophysiology. Pediatr Nephrol 19: 714–721 [DOI] [PubMed] [Google Scholar]
- Christoforidis S, Miaczynska M, Ashman K, Wilm M, Zhao L, Yip SC, Waterfield MD, Backer JM, Zerial M (1999) Phosphatidylinositol-3-OH kinases are Rab5 effectors. Nat Cell Biol 1: 249–252 [DOI] [PubMed] [Google Scholar]
- Chung SH, Song WJ, Kim K, Bednarski JJ, Chen J, Prestwich GD, Holz RW (1998) The C2 domains of Rabphilin3A specifically bind phosphatidylinositol 4,5-bisphosphate containing vesicles in a Ca2+-dependent manner. In vitro characteristics and possible significance. J Biol Chem 273: 10240–10248 [DOI] [PubMed] [Google Scholar]
- Cooke FT, Dove SK, McEwen RK, Painter G, Holmes AB, Hall MN, Michell RH, Parker PJ (1998) The stress-activated phosphatidylinositol 3-phosphate 5-kinase Fab1p is essential for vacuole function in S. cerevisiae. Curr Biol 8: 1219–1222 [DOI] [PubMed] [Google Scholar]
- Cox D, Berg JS, Cammer M, Chinegwundoh JO, Dale BM, Cheney RE, Greenberg S (2002) Myosin X is a downstream effector of PI(3)K during phagocytosis. Nat Cell Biol 4: 469–477 [DOI] [PubMed] [Google Scholar]
- Cozier GE, Carlton J, McGregor AH, Gleeson PA, Teasdale RD, Mellor H, Cullen PJ (2002) The phox homology (PX) domain-dependent, 3-phosphoinositide-mediated association of sorting nexin-1 with an early sorting endosomal compartment is required for its ability to regulate epidermal growth factor receptor degradation. J Biol Chem 277: 48730–48736 [DOI] [PubMed] [Google Scholar]
- Craige B, Salazar G, Faundez V (2008) Phosphatidylinositol-4-kinase type II alpha contains an AP-3-sorting motif and a kinase domain that are both required for endosome traffic. Mol Biol Cell 19: 1415–1426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czubayko M, Knauth P, Schluter T, Florian V, Bohnensack R (2006) Sorting nexin 17, a non-self-assembling and a PtdIns(3)P high class affinity protein, interacts with the cerebral cavernous malformation related protein KRIT1. Biochem Biophys Res Commun 345: 1264–1272 [DOI] [PubMed] [Google Scholar]
- D'Angelo G, Polishchuk E, Di Tullio G, Santoro M, Di Campli A, Godi A, West G, Bielawski J, Chuang CC, van der Spoel AC, Platt FM, Hannun YA, Polishchuk R, Mattjus P, De Matteis MA (2007) Glycosphingolipid synthesis requires FAPP2 transfer of glucosylceramide. Nature 449: 62–67 [DOI] [PubMed] [Google Scholar]
- D'Angelo G, Vicinanza M, De Matteis MA (2008) Lipid-transfer proteins in biosynthetic pathways. Curr Opin Cell Biol 20: 360–370 [DOI] [PubMed] [Google Scholar]
- Dai S, Zhang Y, Weimbs T, Yaffe MB, Zhou D (2007) Bacteria-generated PtdIns(3)P recruits VAMP8 to facilitate phagocytosis. Traffic 8: 1365–1374 [DOI] [PubMed] [Google Scholar]
- De Matteis MA, Di Campli A, D'Angelo G (2007) Lipid-transfer proteins in membrane trafficking at the Golgi complex. Biochim Biophys Acta 1771: 761–768 [DOI] [PubMed] [Google Scholar]
- De Matteis MA, Godi A (2004) PI-loting membrane traffic. Nat Cell Biol 6: 487–492 [DOI] [PubMed] [Google Scholar]
- De Matteis MA, Morrow JS (1998) The role of ankyrin and spectrin in membrane transport and domain formation. Curr Opin Cell Biol 10: 542–549 [DOI] [PubMed] [Google Scholar]
- Demmel L, Beck M, Klose C, Schlaitz AL, Gloor Y, Hsu PP, Havlis J, Shevchenko A, Krause E, Kalaidzidis Y, Walch-Solimena C (2008) Nucleocytoplasmic shuttling of the Golgi phosphatidylinositol 4-kinase pik1 is regulated by 14-3-3 proteins and coordinates Golgi function with cell growth. Mol Biol Cell 19: 1046–1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Paolo G, De Camilli P (2006) Phosphoinositides in cell regulation and membrane dynamics. Nature 443: 651–657 [DOI] [PubMed] [Google Scholar]
- Dove SK, Cooke FT, Douglas MR, Sayers LG, Parker PJ, Michell RH (1997) Osmotic stress activates phosphatidylinositol-3,5-bisphosphate synthesis. Nature 390: 187–192 [DOI] [PubMed] [Google Scholar]
- Dove SK, Johnson ZE (2007) Our FABulous VACation: a decade of phosphatidylinositol 3,5-bisphosphate. Biochem Soc Symp 74: 129–139 [DOI] [PubMed] [Google Scholar]
- Downes CP, Gray A, Watt SA, Lucocq JM (2003) Advances in procedures for the detection and localization of inositol phospholipid signals in cells, tissues, and enzyme assays. Methods Enzymol 366: 64–84 [DOI] [PubMed] [Google Scholar]
- Duex JE, Tang F, Weisman LS (2006) The Vac14p–Fig4p complex acts independently of Vac7p and couples PI3,5P2 synthesis and turnover. J Cell Biol 172: 693–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egea G, Lazaro-Dieguez F, Vilella M (2006) Actin dynamics at the Golgi complex in mammalian cells. Curr Opin Cell Biol 18: 168–178 [DOI] [PubMed] [Google Scholar]
- Engelman JA, Luo J, Cantley LC (2006) The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet 7: 606–619 [DOI] [PubMed] [Google Scholar]
- Engqvist-Goldstein AE, Drubin DG (2003) Actin assembly and endocytosis: from yeast to mammals. Annu Rev Cell Dev Biol 19: 287–332 [DOI] [PubMed] [Google Scholar]
- Erdmann KS, Mao Y, McCrea HJ, Zoncu R, Lee S, Paradise S, Modregger J, Biemesderfer D, Toomre D, De Camilli P (2007) A role of the Lowe syndrome protein OCRL in early steps of the endocytic pathway. Dev Cell 13: 377–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falasca M, Hughes WE, Dominguez V, Sala G, Fostira F, Fang MQ, Cazzolli R, Shepherd PR, James DE, Maffucci T (2007) The role of phosphoinositide 3-kinase C2alpha in insulin signaling. J Biol Chem 282: 28226–28236 [DOI] [PubMed] [Google Scholar]
- Faulhammer F, Kanjilal-Kolar S, Knodler A, Lo J, Lee Y, Konrad G, Mayinger P (2007) Growth control of Golgi phosphoinositides by reciprocal localization of Sac1 lipid phosphatase and Pik1 4-kinase. Traffic 8: 1554–1567 [DOI] [PubMed] [Google Scholar]
- Ford MG, Pearse BM, Higgins MK, Vallis Y, Owen DJ, Gibson A, Hopkins CR, Evans PR, McMahon HT (2001) Simultaneous binding of PtdIns(4,5)P2 and clathrin by AP180 in the nucleation of clathrin lattices on membranes. Science 291: 1051–1055 [DOI] [PubMed] [Google Scholar]
- Gaidarov I, Krupnick JG, Falck JR, Benovic JL, Keen JH (1999) Arrestin function in G protein-coupled receptor endocytosis requires phosphoinositide binding. EMBO J 18: 871–881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaidarov I, Smith ME, Domin J, Keen JH (2001) The class II phosphoinositide 3-kinase C2alpha is activated by clathrin and regulates clathrin-mediated membrane trafficking. Mol Cell 7: 443–449 [DOI] [PubMed] [Google Scholar]
- Gamper N, Shapiro MS (2007) Regulation of ion transport proteins by membrane phosphoinositides. Nat Rev Neurosci 8: 921–934 [DOI] [PubMed] [Google Scholar]
- Garcia-Gonzalo FR, Munoz P, Gonzalez E, Casaroli-Marano RP, Vilaro S, Bartrons R, Ventura F, Rosa JL (2004) The giant protein HERC1 is recruited to aluminum fluoride-induced actin-rich surface protrusions in HeLa cells. FEBS Lett 559: 77–83 [DOI] [PubMed] [Google Scholar]
- Godi A, Di Campli A, Konstantakopoulos A, Di Tullio G, Alessi DR, Kular GS, Daniele T, Marra P, Lucocq JM, De Matteis MA (2004) FAPPs control Golgi-to-cell-surface membrane traffic by binding to ARF and PtdIns(4)P. Nat Cell Biol 6: 393–404 [DOI] [PubMed] [Google Scholar]
- Godi A, Pertile P, Meyers R, Marra P, Di Tullio G, Iurisci C, Luini A, Corda D, De Matteis MA (1999) ARF mediates recruitment of PtdIns-4-OH kinase-beta and stimulates synthesis of PtdIns(4,5)P2 on the Golgi complex. Nat Cell Biol 1: 280–287 [DOI] [PubMed] [Google Scholar]
- Halter D, Neumann S, van Dijk SM, Wolthoorn J, de Maziere AM, Vieira OV, Mattjus P, Klumperman J, van Meer G, Sprong H (2007) Pre- and post-Golgi translocation of glucosylceramide in glycosphingolipid synthesis. J Cell Biol 179: 101–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanada K, Kumagai K, Yasuda S, Miura Y, Kawano M, Fukasawa M, Nishijima M (2003) Molecular machinery for non-vesicular trafficking of ceramide. Nature 426: 803–809 [DOI] [PubMed] [Google Scholar]
- Hanson BJ, Hong W (2003) Evidence for a role of SNX16 in regulating traffic between the early and later endosomal compartments. J Biol Chem 278: 34617–34630 [DOI] [PubMed] [Google Scholar]
- Hausser A, Storz P, Martens S, Link G, Toker A, Pfizenmaier K (2005) Protein kinase D regulates vesicular transport by phosphorylating and activating phosphatidylinositol-4 kinase IIIbeta at the Golgi complex. Nat Cell Biol 7: 880–886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinchliffe KA, Irvine RF (2006) Regulation of type II PIP kinase by PKD phosphorylation. Cell Signal 18: 1906–1913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst J, Motley A, Harasaki K, Peak Chew SY, Robinson MS (2003) EpsinR: an ENTH domain-containing protein that interacts with AP-1. Mol Biol Cell 14: 625–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoepfner S, Severin F, Cabezas A, Habermann B, Runge A, Gillooly D, Stenmark H, Zerial M (2005) Modulation of receptor recycling and degradation by the endosomal kinesin KIF16B. Cell 121: 437–450 [DOI] [PubMed] [Google Scholar]
- Hokanson DE, Laakso JM, Lin T, Sept D, Ostap EM (2006) Myo1c binds phosphoinositides through a putative pleckstrin homology domain. Mol Biol Cell 17: 4856–4865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoopes RR Jr, Shrimpton AE, Knohl SJ, Hueber P, Hoppe B, Matyus J, Simckes A, Tasic V, Toenshoff B, Suchy SF, Nussbaum RL, Scheinman SJ (2005) Dent disease with mutations in OCRL1. Am J Hum Genet 76: 260–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiguchi K, Hanada T, Fukui Y, Chishti AH (2006) Transport of PIP3 by GAKIN, a kinesin-3 family protein, regulates neuronal cell polarity. J Cell Biol 174: 425–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyun TS, Rao DS, Saint-Dic D, Michael LE, Kumar PD, Bradley SV, Mizukami IF, Oravecz-Wilson KI, Ross TS (2004) HIP1 and HIP1r stabilize receptor tyrosine kinases and bind 3-phosphoinositides via epsin N-terminal homology domains. J Biol Chem 279: 14294–14306 [DOI] [PubMed] [Google Scholar]
- Hyvola N, Diao A, McKenzie E, Skippen A, Cockcroft S, Lowe M (2006) Membrane targeting and activation of the Lowe syndrome protein OCRL1 by rab GTPases. EMBO J 25: 3750–3761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi T, Sekine T, Inatomi J, Seki G (2002) Unraveling the molecular pathogenesis of isolated proximal renal tubular acidosis. J Am Soc Nephrol 13: 2171–2177 [DOI] [PubMed] [Google Scholar]
- Irie F, Okuno M, Pasquale EB, Yamaguchi Y (2005) EphrinB-EphB signalling regulates clathrin-mediated endocytosis through tyrosine phosphorylation of synaptojanin 1. Nat Cell Biol 7: 501–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito N, Yokomizo T, Sasaki T, Kurosu H, Penninger J, Kanaho Y, Katada T, Hanaoka K, Shimizu T (2002) Requirement of phosphatidylinositol 3-kinase activation and calcium influx for leukotriene B4-induced enzyme release. J Biol Chem 277: 44898–44904 [DOI] [PubMed] [Google Scholar]
- Itoh T, De Camilli P (2004) Membrane trafficking: dual-key strategy. Nature 429: 141–143 [DOI] [PubMed] [Google Scholar]
- Itoh T, Koshiba S, Kigawa T, Kikuchi A, Yokoyama S, Takenawa T (2001) Role of the ENTH domain in phosphatidylinositol-4, 5-bisphosphate binding and endocytosis. Science 291: 1047–1051 [DOI] [PubMed] [Google Scholar]
- Jackson TR, Brown FD, Nie Z, Miura K, Foroni L, Sun J, Hsu VW, Donaldson JG, Randazzo PA (2000) ACAPs are arf6 GTPase-activating proteins that function in the cell periphery. J Cell Biol 151: 627–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalthoff C, Groos S, Kohl R, Mahrhold S, Ungewickell EJ (2002) Clint: a novel clathrin-binding ENTH-domain protein at the Golgi. Mol Biol Cell 13: 4060–4073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kam JL, Miura K, Jackson TR, Gruschus J, Roller P, Stauffer S, Clark J, Aneja R, Randazzo PA (2000) Phosphoinositide-dependent activation of the ADP-ribosylation factor GTPase-activating protein ASAP1. Evidence for the pleckstrin homology domain functioning as an allosteric site. J Biol Chem 275: 9653–9663 [DOI] [PubMed] [Google Scholar]
- Klopfenstein DR, Tomishige M, Stuurman N, Vale RD (2002) Role of phosphatidylinositol(4,5)bisphosphate organization in membrane transport by the Unc104 kinesin motor. Cell 109: 347–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauss M, Haucke V (2007) Phosphoinositide-metabolizing enzymes at the interface between membrane traffic and cell signalling. EMBO Rep 8: 241–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauss M, Kukhtina V, Pechstein A, Haucke V (2006) Stimulation of phosphatidylinositol kinase type I-mediated phosphatidylinositol (4,5)-bisphosphate synthesis by AP-2mu-cargo complexes. Proc Natl Acad Sci USA 103: 11934–11939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krendel M, Osterweil EK, Mooseker MS (2007) Myosin 1E interacts with synaptojanin-1 and dynamin and is involved in endocytosis. FEBS Lett 581: 644–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krugmann S, Anderson KE, Ridley SH, Risso N, McGregor A, Coadwell J, Davidson K, Eguinoa A, Ellson CD, Lipp P, Manifava M, Ktistakis N, Painter G, Thuring JW, Cooper MA, Lim ZY, Holmes AB, Dove SK, Michell RH, Grewal A et al. (2002) Identification of ARAP3, a novel PI3K effector regulating both Arf and Rho GTPases, by selective capture on phosphoinositide affinity matrices. Mol Cell 9: 95–108 [DOI] [PubMed] [Google Scholar]
- Lanzetti L, Palamidessi A, Areces L, Scita G, Di Fiore PP (2004) Rab5 is a signalling GTPase involved in actin remodelling by receptor tyrosine kinases. Nature 429: 309–314 [DOI] [PubMed] [Google Scholar]
- Lazar DF, Saltiel AR (2006) Lipid phosphatases as drug discovery targets for type 2 diabetes. Nat Rev Drug Discov 5: 333–342 [DOI] [PubMed] [Google Scholar]
- Lee C, Kang HS, Chung JK, Sekiya F, Kim JR, Han JS, Kim SR, Bae YS, Morris AJ, Rhee SG (1997) Inhibition of phospholipase D by clathrin assembly protein 3 (AP3). J Biol Chem 272: 15986–15992 [DOI] [PubMed] [Google Scholar]
- Lee E, Marcucci M, Daniell L, Pypaert M, Weisz OA, Ochoa GC, Farsad K, Wenk MR, De Camilli P (2002) Amphiphysin 2 (Bin1) and T-tubule biogenesis in muscle. Science 297: 1193–1196 [DOI] [PubMed] [Google Scholar]
- Lee SY, Wenk MR, Kim Y, Nairn AC, De Camilli P (2004) Regulation of synaptojanin 1 by cyclin-dependent kinase 5 at synapses. Proc Natl Acad Sci USA 101: 546–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemmon MA (2008) Membrane recognition by phospholipid-binding domains. Nat Rev Mol Cell Biol 9: 99–111 [DOI] [PubMed] [Google Scholar]
- Levine T, Loewen C (2006) Inter-organelle membrane contact sites: through a glass, darkly. Curr Opin Cell Biol 18: 371–378 [DOI] [PubMed] [Google Scholar]
- Liao H, Ellena J, Liu L, Szabo G, Cafiso D, Castle D (2007) Secretory carrier membrane protein SCAMP2 and phosphatidylinositol 4,5-bisphosphate interactions in the regulation of dense core vesicle exocytosis. Biochemistry 46: 10909–10920 [DOI] [PubMed] [Google Scholar]
- Lichter-Konecki U, Farber LW, Cronin JS, Suchy SF, Nussbaum RL (2006) The effect of missense mutations in the RhoGAP-homology domain on ocrl1 function. Mol Genet Metab 89: 121–128 [DOI] [PubMed] [Google Scholar]
- Lim J, Hao T, Shaw C, Patel AJ, Szabo G, Rual JF, Fisk CJ, Li N, Smolyar A, Hill DE, Barabasi AL, Vidal M, Zoghbi HY (2006) A protein–protein interaction network for human inherited ataxias and disorders of Purkinje cell degeneration. Cell 125: 801–814 [DOI] [PubMed] [Google Scholar]
- Lin Y, Kimpler LA, Naismith TV, Lauer JM, Hanson PI (2005) Interaction of the mammalian endosomal sorting complex required for transport (ESCRT) III protein hSnf7-1 with itself, membranes, and the AAA+ ATPase SKD1. J Biol Chem 280: 12799–12809 [DOI] [PubMed] [Google Scholar]
- Liu H, Liu ZQ, Chen CX, Magill S, Jiang Y, Liu YJ (2006) Inhibitory regulation of EGF receptor degradation by sorting nexin 5. Biochem Biophys Res Commun 342: 537–546 [DOI] [PubMed] [Google Scholar]
- Liu X, Zhang C, Xing G, Chen Q, He F (2001) Functional characterization of novel human ARFGAP3. FEBS Lett 490: 79–83 [DOI] [PubMed] [Google Scholar]
- Lodhi IJ, Bridges D, Chiang SH, Zhang Y, Cheng A, Geletka LM, Weisman LS, Saltiel AR (2008) Insulin stimulates phosphatidylinositol 3-phosphate production via the activation of Rab5. Mol Biol Cell 19: 2718–2728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo O, Urbe S, Clague MJ (2006) Systematic analysis of myotubularins: heteromeric interactions, subcellular localisation and endosome related functions. J Cell Sci 119: 2953–2959 [DOI] [PubMed] [Google Scholar]
- Lowe M (2005) Structure and function of the Lowe syndrome protein OCRL1. Traffic 6: 711–719 [DOI] [PubMed] [Google Scholar]
- Macia E, Partisani M, Favard C, Mortier E, Zimmermann P, Carlier MF, Gounon P, Luton F, Franco M (2008) The Pleckstrin homology domain of the Arf6-specific exchange factor EFA6 localizes to the plasma membrane by interacting with phosphatidylinositol 4,5-bisphosphate and F-actin. J Biol Chem 283: 19836–19844 [DOI] [PubMed] [Google Scholar]
- Manna D, Albanese A, Park WS, Cho W (2007) Mechanistic basis of differential cellular responses of phosphatidylinositol 3,4-bisphosphate- and phosphatidylinositol 3,4,5-trisphosphate-binding pleckstrin homology domains. J Biol Chem 282: 32093–32105 [DOI] [PubMed] [Google Scholar]
- Marone R, Cmiljanovic V, Giese B, Wymann MP (2008) Targeting phosphoinositide 3-kinase: moving towards therapy. Biochim Biophys Acta 1784: 159–185 [DOI] [PubMed] [Google Scholar]
- Michell RH (2008) Inositol derivatives: evolution and functions. Nat Rev Mol Cell Biol 9: 151–161 [DOI] [PubMed] [Google Scholar]
- Mishra SK, Keyel PA, Hawryluk MJ, Agostinelli NR, Watkins SC, Traub LM (2002a) Disabled-2 exhibits the properties of a cargo-selective endocytic clathrin adaptor. EMBO J 21: 4915–4926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra SK, Watkins SC, Traub LM (2002b) The autosomal recessive hypercholesterolemia (ARH) protein interfaces directly with the clathrin-coat machinery. Proc Natl Acad Sci USA 99: 16099–16104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura K, Jacques KM, Stauffer S, Kubosaki A, Zhu K, Hirsch DS, Resau J, Zheng Y, Randazzo PA (2002) ARAP1: a point of convergence for Arf and Rho signaling. Mol Cell 9: 109–119 [DOI] [PubMed] [Google Scholar]
- Munday AD, Norris FA, Caldwell KK, Brown S, Majerus PW, Mitchell CA (1999) The inositol polyphosphate 4-phosphatase forms a complex with phosphatidylinositol 3-kinase in human platelet cytosol. Proc Natl Acad Sci USA 96: 3640–3645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie Z, Fei J, Premont RT, Randazzo PA (2005) The Arf GAPs AGAP1 and AGAP2 distinguish between the adaptor protein complexes AP-1 and AP-3. J Cell Sci 118: 3555–3566 [DOI] [PubMed] [Google Scholar]
- Nie Z, Stanley KT, Stauffer S, Jacques KM, Hirsch DS, Takei J, Randazzo PA (2002) AGAP1, an endosome-associated, phosphoinositide-dependent ADP-ribosylation factor GTPase-activating protein that affects actin cytoskeleton. J Biol Chem 277: 48965–48975 [DOI] [PubMed] [Google Scholar]
- Nielsen E, Christoforidis S, Uttenweiler-Joseph S, Miaczynska M, Dewitte F, Wilm M, Hoflack B, Zerial M (2000) Rabenosyn-5, a novel Rab5 effector, is complexed with hVPS45 and recruited to endosomes through a FYVE finger domain. J Cell Biol 151: 601–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa K, Toker A, Wong K, Marignani PA, Johannes FJ, Cantley LC (1998) Association of protein kinase Cmu with type II phosphatidylinositol 4-kinase and type I phosphatidylinositol-4-phosphate 5-kinase. J Biol Chem 273: 23126–23133 [DOI] [PubMed] [Google Scholar]
- Norden AG, Lapsley M, Igarashi T, Kelleher CL, Lee PJ, Matsuyama T, Scheinman SJ, Shiraga H, Sundin DP, Thakker RV, Unwin RJ, Verroust P, Moestrup SK (2002) Urinary megalin deficiency implicates abnormal tubular endocytic function in Fanconi syndrome. J Am Soc Nephrol 13: 125–133 [DOI] [PubMed] [Google Scholar]
- Obayashi T, Hayashi S, Shibaoka M, Saeki M, Ohta H, Kinoshita K (2008) COXPRESdb: a database of coexpressed gene networks in mammals. Nucleic Acids Res 36**:** D77–D82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne SL, Wen PJ, Boucheron C, Nguyen HN, Hayakawa M, Kaizawa H, Parker PJ, Vitale N, Meunier FA (2008) PIKfyve negatively regulates exocytosis in neurosecretory cells. J Biol Chem 283: 2804–2813 [DOI] [PubMed] [Google Scholar]
- Oti M, Brunner HG (2007) The modular nature of genetic diseases. Clin Genet 71: 1–11 [DOI] [PubMed] [Google Scholar]
- Piwon N, Gunther W, Schwake M, Bosl MR, Jentsch TJ (2000) ClC-5 Cl−-channel disruption impairs endocytosis in a mouse model for Dent's disease. Nature 408: 369–373 [DOI] [PubMed] [Google Scholar]
- Qin B, He M, Chen X, Pei D (2006) Sorting nexin 10 induces giant vacuoles in mammalian cells. J Biol Chem 281: 36891–36896 [DOI] [PubMed] [Google Scholar]
- Raiborg C, Bremnes B, Mehlum A, Gillooly DJ, D'Arrigo A, Stang E, Stenmark H (2001) FYVE and coiled-coil domains determine the specific localisation of Hrs to early endosomes. J Cell Sci 114: 2255–2263 [DOI] [PubMed] [Google Scholar]
- Rohde G, Wenzel D, Haucke V (2002) A phosphatidylinositol (4,5)-bisphosphate binding site within mu2-adaptin regulates clathrin-mediated endocytosis. J Cell Biol 158: 209–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy A, Levine TP (2004) Multiple pools of phosphatidylinositol 4-phosphate detected using the pleckstrin homology domain of Osh2p. J Biol Chem 279: 44683–44689 [DOI] [PubMed] [Google Scholar]
- Ruckle T, Schwarz MK, Rommel C (2006) PI3Kgamma inhibition: towards an ‘aspirin of the 21st century'? Nat Rev Drug Discov 5: 903–918 [DOI] [PubMed] [Google Scholar]
- Santarius M, Lee CH, Anderson RA (2006) Supervised membrane swimming: small G-protein lifeguards regulate PIPK signalling and monitor intracellular PtdIns(4,5)P2 pools. Biochem J 398: 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sbrissa D, Ikonomov OC, Fu Z, Ijuin T, Gruenberg J, Takenawa T, Shisheva A (2007) Core protein machinery for mammalian phosphatidylinositol 3,5-bisphosphate synthesis and turnover that regulates the progression of endosomal transport. Novel Sac phosphatase joins the ArPIKfyve–PIKfyve complex. J Biol Chem 282: 23878–23891 [DOI] [PubMed] [Google Scholar]
- Shiao YJ, Balcerzak B, Vance JE (1998) A mitochondrial membrane protein is required for translocation of phosphatidylserine from mitochondria-associated membranes to mitochondria. Biochem J 331 (Part 1): 217–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin HW, Hayashi M, Christoforidis S, Lacas-Gervais S, Hoepfner S, Wenk MR, Modregger J, Uttenweiler-Joseph S, Wilm M, Nystuen A, Frankel WN, Solimena M, De Camilli P, Zerial M (2005) An enzymatic cascade of Rab5 effectors regulates phosphoinositide turnover in the endocytic pathway. J Cell Biol 170: 607–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin N, Ahn N, Chang-Ileto B, Park J, Takei K, Ahn SG, Kim SA, Di Paolo G, Chang S (2008) SNX9 regulates tubular invagination of the plasma membrane through interaction with actin cytoskeleton and dynamin 2. J Cell Sci 121: 1252–1263 [DOI] [PubMed] [Google Scholar]
- Shinozaki-Narikawa N, Kodama T, Shibasaki Y (2006) Cooperation of phosphoinositides and BAR domain proteins in endosomal tubulation. Traffic 7: 1539–1550 [DOI] [PubMed] [Google Scholar]
- Spudich G, Chibalina MV, Au JS, Arden SD, Buss F, Kendrick-Jones J (2007) Myosin VI targeting to clathrin-coated structures and dimerization is mediated by binding to Disabled-2 and PtdIns(4,5)P2. Nat Cell Biol 9: 176–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein MP, Feng Y, Cooper KL, Welford AM, Wandinger-Ness A (2003) Human VPS34 and p150 are Rab7 interacting partners. Traffic 4: 754–771 [DOI] [PubMed] [Google Scholar]
- Stephens L, Ellson C, Hawkins P (2002) Roles of PI3Ks in leukocyte chemotaxis and phagocytosis. Curr Opin Cell Biol 14: 203–213 [DOI] [PubMed] [Google Scholar]
- Strahl T, Thorner J (2007) Synthesis and function of membrane phosphoinositides in budding yeast, Saccharomyces cerevisiae. Biochim Biophys Acta 1771: 353–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suchy SF, Cronin J, Nussbaum RL (2005) Effect of a chronic deficiency of a PIP2 5-phosphatase on cell signaling. ASCB 45th Annual Meeting San Francisco 10–14 December 2005. NHGRI/GDRB, NIH, Bethesda, MD, USA. Abstract no. 1911
- Tanaka K, Imajoh-Ohmi S, Sawada T, Shirai R, Hashimoto Y, Iwasaki S, Kaibuchi K, Kanaho Y, Shirai T, Terada Y, Kimura K, Nagata S, Fukui Y (1997) A target of phosphatidylinositol 3,4,5-trisphosphate with a zinc finger motif similar to that of the ADP-ribosylation-factor GTPase-activating protein and two pleckstrin homology domains. Eur J Biochem 245: 512–519 [DOI] [PubMed] [Google Scholar]
- Teo H, Gill DJ, Sun J, Perisic O, Veprintsev DB, Vallis Y, Emr SD, Williams RL (2006) ESCRT-I core and ESCRT-II GLUE domain structures reveal role for GLUE in linking to ESCRT-I and membranes. Cell 125: 99–111 [DOI] [PubMed] [Google Scholar]
- Thong FS, Dugani CB, Klip A (2005) Turning signals on and off: GLUT4 traffic in the insulin-signaling highway. Physiology (Bethesda) 20: 271–284 [DOI] [PubMed] [Google Scholar]
- Toth B, Balla A, Ma H, Knight ZA, Shokat KM, Balla T (2006) Phosphatidylinositol 4-kinase IIIbeta regulates the transport of ceramide between the endoplasmic reticulum and Golgi. J Biol Chem 281: 36369–36377 [DOI] [PubMed] [Google Scholar]
- Toyoshima F, Matsumura S, Morimoto H, Mitsushima M, Nishida E (2007) PtdIns(3,4,5)P3 regulates spindle orientation in adherent cells. Dev Cell 13: 796–811 [DOI] [PubMed] [Google Scholar]
- Venkateswarlu K, Gunn-Moore F, Tavare JM, Cullen PJ (1999) EGF-and NGF-stimulated translocation of cytohesin-1 to the plasma membrane of PC12 cells requires PI 3-kinase activation and a functional cytohesin-1 PH domain. J Cell Sci 112 (Part 12): 1957–1965 [DOI] [PubMed] [Google Scholar]
- Vitale N, Patton WA, Moss J, Vaughan M, Lefkowitz RJ, Premont RT (2000) GIT proteins, a novel family of phosphatidylinositol 3,4, 5-trisphosphate-stimulated GTPase-activating proteins for ARF6. J Biol Chem 275: 13901–13906 [DOI] [PubMed] [Google Scholar]
- Wang J, Sun HQ, Macia E, Kirchhausen T, Watson H, Bonifacino JS, Yin HL (2007) PI4P promotes the recruitment of the GGA adaptor proteins to the trans-Golgi network and regulates their recognition of the ubiquitin sorting signal. Mol Biol Cell 18: 2646–2655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YJ, Wang J, Sun HQ, Martinez M, Sun YX, Macia E, Kirchhausen T, Albanesi JP, Roth MG, Yin HL (2003) Phosphatidylinositol 4 phosphate regulates targeting of clathrin adaptor AP-1 complexes to the Golgi. Cell 114: 299–310 [DOI] [PubMed] [Google Scholar]
- Watson P, Forster R, Palmer KJ, Pepperkok R, Stephens DJ (2005) Coupling of ER exit to microtubules through direct interaction of COPII with dynactin. Nat Cell Biol 7: 48–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt SA, Kular G, Fleming IN, Downes CP, Lucocq JM (2002) Subcellular localization of phosphatidylinositol 4,5-bisphosphate using the pleckstrin homology domain of phospholipase C delta1. Biochem J 363: 657–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler M, Domin J (2006) The N-terminus of phosphoinositide 3-kinase-C2beta regulates lipid kinase activity and binding to clathrin. J Cell Physiol 206: 586–593 [DOI] [PubMed] [Google Scholar]
- Workman P (2004) Inhibiting the phosphoinositide 3-kinase pathway for cancer treatment. Biochem Soc Trans 32: 393–396 [DOI] [PubMed] [Google Scholar]
- Wymann MP, Marone R (2005) Phosphoinositide 3-kinase in disease: timing, location, and scaffolding. Curr Opin Cell Biol 17: 141–149 [DOI] [PubMed] [Google Scholar]
- Xu Y, Hortsman H, Seet L, Wong SH, Hong W (2001) SNX3 regulates endosomal function through its PX-domain-mediated interaction with PtdIns(3)P. Nat Cell Biol 3: 658–666 [DOI] [PubMed] [Google Scholar]
- Yamashita S, Oku M, Sakai Y (2007) Functions of PI4P and sterol glucoside are necessary for the synthesis of a nascent membrane structure during pexophagy. Autophagy 3: 35–37 [DOI] [PubMed] [Google Scholar]
- Yeung T, Grinstein S (2007) Lipid signaling and the modulation of surface charge during phagocytosis. Immunol Rev 219: 17–36 [DOI] [PubMed] [Google Scholar]
- Yoon HY, Miura K, Cuthbert EJ, Davis KK, Ahvazi B, Casanova JE, Randazzo PA (2006) ARAP2 effects on the actin cytoskeleton are dependent on Arf6-specific GTPase-activating-protein activity and binding to RhoA-GTP. J Cell Sci 119: 4650–4666 [DOI] [PubMed] [Google Scholar]
- Yu M, Kasai K, Nagashima K, Torii S, Yokota-Hashimoto H, Okamoto K, Takeuchi T, Gomi H, Izumi T (2007) Exophilin4/Slp2-a targets glucagon granules to the plasma membrane through unique Ca2+-inhibitory phospholipid-binding activity of the C2A domain. Mol Biol Cell 18: 688–696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M (2007) PTEN: a promising pharmacological target to enhance epithelial wound healing. Br J Pharmacol 152: 1141–1144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng B, Ma YC, Ostrom RS, Lavoie C, Gill GN, Insel PA, Huang XY, Farquhar MG (2001) RGS-PX1, a GAP for GalphaS and sorting nexin in vesicular trafficking. Science 294: 1939–1942 [DOI] [PubMed] [Google Scholar]
- Zhong Q, Lazar CS, Tronchere H, Sato T, Meerloo T, Yeo M, Songyang Z, Emr SD, Gill GN (2002) Endosomal localization and function of sorting nexin 1. Proc Natl Acad Sci USA 99: 6767–6772 [DOI] [PMC free article] [PubMed] [Google Scholar]