Psychologic Intervention Improves Survival for Breast Cancer Patients: A Randomized Clinical Trial (original) (raw)
. Author manuscript; available in PMC: 2009 Mar 27.
Published in final edited form as: Cancer. 2008 Dec 15;113(12):3450–3458. doi: 10.1002/cncr.23969
Abstract
BACKGROUND
The question of whether stress poses a risk for cancer progression has been difficult to answer. A randomized clinical trial tested the hypothesis that cancer patients coping with their recent diagnosis but receiving a psychologic intervention would have improved survival compared with patients who were only assessed.
METHODS
A total of 227 patients who were surgically treated for regional breast cancer participated. Before beginning adjuvant cancer therapies, patients were assessed with psychologic and behavioral measures and had a health evaluation, and a 60-mL blood sample was drawn. Patients were randomized to Psychologic Intervention plus assessment or Assessment only study arms. The intervention was psychologist led; conducted in small groups; and included strategies to reduce stress, improve mood, alter health behaviors, and maintain adherence to cancer treatment and care. Earlier articles demonstrated that, compared with the Assessment arm, the Intervention arm improved across all of the latter secondary outcomes. Immunity was also enhanced.
RESULTS
After a median of 11 years of follow-up, disease recurrence was reported to occur in 62 of 212 (29%) women and death was reported for 54 of 227 (24%) women. Using Cox proportional hazards analysis, multivariate comparison of survival was conducted. As predicted, patients in the Intervention arm were found to have a reduced risk of breast cancer recurrence (hazards ratio [HR] of 0.55; P=.034) and death from breast cancer (HR of 0.44; P=.016) compared with patients in the Assessment only arm. Follow-up analyses also demonstrated that Intervention patients had a reduced risk of death from all causes (HR of 0.51; P=.028).
CONCLUSIONS
Psychologic interventions as delivered and studied here can improve survival.
Keywords: breast, cancer, recurrence, survival, psychologic, intervention, Biobehavioral
Psychologic and behavioral variables can have profound effects on health. For cancer patients, a recent meta-analysis revealed stress-related psychosocial factors to be associated with a higher cancer incidence in initially healthy people, poorer survival in patients diagnosed with cancer, and higher cancer mortality.1 In the general case, the putative mechanism for stress/health effects has been stressor-induced activation of the autonomic nervous system and the hypothalamic-pituitary-adrenal axis with their molecular cellular, organ-level, and systemic effects. When stress is chronic, it negatively affects most systems because of prolonged exposure to catecholamines and glucocorticoids. To our knowledge, much of the research concerning stress and cancer has focused on suppressed immune responses.2–4
To conceptualize these relations in adult humans, we proposed that stress accompanying a cancer diagnosis would trigger psychologic and behavioral responses, as well as biologic responses, relevant to subsequent disease outcomes (see Fig. 1).5 A randomized clinical trial (RCT) was designed to test this possibility, reasoning that receipt of a psychologic intervention might serve as a protective mechanism to significantly alter the chain of adverse stress effects, and thereby impact disease endpoints. Now, with a median follow-up of 11 years, we found an altered disease course, a reduced risk of disease recurrence, and improved survival for the breast cancer patients randomized to receive the psychologic intervention.
FIGURE 1.
The biobehavioral model of cancer stress and disease course is shown, noting psychologic (stress and quality of life), behavioral (compliance and health behaviors), and biologic pathways to disease progression. CNS indicates central nervous system. Copyright © 1994 by the American Psychological Association (APA). Reproduced with permission from Andersen BL, Kiecolt-Glaser JK, Glaser R. A biobehavioral model of cancer stress and disease course. Am Psychol. 1994;49:389-404. The use of APA information does not suggest endorsement by APA.
MATERIALS AND METHODS
Patients and Procedures
As previously described,6 women diagnosed with breast carcinoma stage IIA (T0-1 N1 M0/T2 N0 M0), IIB (T2 N1 M0/T3 N0 M0), IIIA (T0-2 N2 M0/T3 N1-2 M0), or IIIB (T4 any N M1/any T N3 M0),7 who were surgically treated and awaiting adjuvant therapy, were eligible. Exclusion criteria included prior cancer diagnosis; refusal of cancer treatment; age <20 years or >85 years; residence >90 miles from the research site; and diagnoses of mental retardation, severe or untreated psychopathology (eg, schizophrenia), neurologic disorders, dementia, or any immunologic condition/disease.
As previously detailed,6 227 patients were accrued and randomized to study arms within the following strata: 1) lymph node status/tumor size: negative lymph nodes but tumor >2 cm, 1 to 3 positive lymph nodes, or >4 positive lymph nodes; 2) hormone receptor status: positive versus negative; 3) menopausal status: premenopausal/perimenopausal versus postmenopausal; and 4) spouse/partner status: spouse/partner versus none. White and Freedman’s 8 minimization method (ie, a biased coin, weighted in favor of the arm with fewer patients) was used to assign patients to Intervention and assessment (n= 114) or Assessment–only (n= 113) study arms. Minimization has been recommended as the method of choice for smaller trials to achieve balance in several prognostic factors. It has also been suggested that adjustment should always be made for the minimization factors when analyzing data from a trial using this method.9 As previously reported,6 there were no significant differences between study arms in site of accrual (university vs community), sociodemographics, disease, prognostic factors, type of surgery received, or adjuvant treatments scheduled to begin or eventually received (all P values >.23) (see Table 1).
TABLE 1.
Sociodemographic, Prognostic, Treatment, Performance Status, and Emotional Distress Variables for the Assessment Only and Intervention Study Arms at the Time of Accrual/Randomization
| Total(N5227) | Assessment Only(n5113) | Intervention(n5114) | ||||
|---|---|---|---|---|---|---|
| Variable | Mean (SD) | % | Mean (SD) | % | Mean (SD) | % |
| Sociodemographic | ||||||
| Age, y | ||||||
| <35 | 5 | 4 | 6 | |||
| 5–49 | 44 | 42 | 46 | |||
| 0–69 | 45 | 46 | 44 | |||
| >69 | 6 | 8 | 4 | |||
| Race (white) | 90 | 90 | 90 | |||
| Partner status (partnered) | 74 | 72 | 75 | |||
| Education, y | 14.75 (2.74) | 14.34 (2.57) | 15.16 (2.85) | |||
| Family income ($K/y) | 67.98 (71.41) | 66.30 (84.68) | 69.64 (55.59) | |||
| Prognostic | ||||||
| Stage (II vs III, %II) | 90 | 92 | 89 | |||
| Tumor size, cm | 3.02 (1.77) | 2.91 (1.75) | 3.12 (1.78) | |||
| No. of positive lymph nodes | 3.05 (5.45) | 3.06 (5.27) | 3.04 (5.64) | |||
| ER/PR positive | 68 | 68 | 68 | |||
| Histologic grade | ||||||
| Poorly differentiated | 42 | 43 | 41 | |||
| Moderately differentiated | 51 | 53 | 50 | |||
| Well differentiated | 7 | 4 | 9 | |||
| Histologic type | ||||||
| Ductal | 75 | 77 | 73 | |||
| Lobular | 13 | 11 | 16 | |||
| Other | 12 | 12 | 11 | |||
| Premenopausal status | 54 | 52 | 55 | |||
| Treatment | ||||||
| Surgery (lumpectomy) | 43 | 43 | 43 | |||
| Chemotherapy* | 84 | 85 | 83 | |||
| Radiotherapy* | 54 | 51 | 56 | |||
| Hormonal therapy* | 75 | 80 | 71 | |||
| KPS† | 85.11 (7.95) | 86.55 (6.91) | 83.68 (8.65) | |||
| Mood states (POMS)† | 36.32 (34.26) | 31.38 (32.11) | 41.42 (35.67) |
Because patients with regional disease were studied, the first endpoint to be reached was expected to be breast cancer recurrence, although survival is ultimately relevant. The trial was powered to detect a doubling of time to an endpoint, a standard metric in cancer treatment trials, which was estimated to require 27 events (in this case, recurrences) in each treatment arm (_P_= .05; power= .80). Disease recurrence was defined as the detection of metastatic disease either at the same site (local) or distant from the original site.
Study Arms
Assessment only
After accrual, psychologic and behavioral data were obtained through in-person interviews and questionnaire completion with a research assistant and a research nurse who completed a health assessment, with inspection of patients’ medical records and/or discussion with the treating physician as needed. A 60-mL (milliliter) blood sample was also drawn for immune assays. Patients were paid $25.00 per assessment. At 4 and 12 months later, corresponding to the end of the intervention phases (see below), patients were similarly reassessed. All patients were then observed, with assessments occurring every 6 months during Years 2 to 5 and annually thereafter.
With regard to the patients’ medical follow-up, standards of care in the National Cancer Institute (NCI)-designated comprehensive cancer center and surrounding community practices from which these patients came consisted of annual mammograms and physical examinations every 3 months through Year 2 and every 6 months thereafter. Suspicious signs/symptoms were evaluated with appropriate laboratory tests, radiologic studies, and biopsies.
Intervention and assessment
An identical assessment protocol was used, and medical follow-up occurred. In addition, a psychologic intervention was provided in small cohorts (n= 13) ranging from 8 to 12 patients and led by 2 psychologists, as previously described.6,10,11 The format was 4 months of weekly sessions (Intensive phase) followed by 8 monthly sessions (Maintenance phase). In combination, a total of 26 sessions (39 therapy hours) over 12 months were delivered. As previously described,11 treatment integrity was high, as was patient attendance.
On the basis of the biobehavioral conceptualization for the trial,5 the goal of the intervention was to reduce distress and improve quality of life, improve health behaviors (diet, exercise, smoking cessation), and facilitate cancer treatment compliance and medical follow-up. Strategies included the following: progressive muscle relaxation for stress reduction, problem solving for common difficulties (eg, fatigue), identifying supportive family members or friends capable of providing assistance, using assertive communication to get one’s psychologic and medical needs met, strategies to increase daily activity (eg, walking, exercise), improving dietary habits (eg, lowering fat intake), and finding ways to cope with treatment side effects (eg, nausea) and maintain adherence to medical treatment and follow-up.6,10,11 As previously reported,6,10 statistical analyses at the end of both therapy phases demonstrated that the patients in the Intervention arm had significantly improved across all secondary outcomes (psychologic, behavioral, health) as well as immunity factors (higher levels of phytohemagglutinin (PHA) and concanavalin A (ConA) T cell blastogenesis) compared with patients in the Assessment–only arm.
In addition, as the trial tests the effects of a specific psychologic intervention, patients in both arms were regularly queried concerning the receipt of any medication for mood (anxiolytic or antidepressant) or counseling (ie, individual or group psychotherapy or formalized cancer support). At no time were there significant differences noted between the study arms. For example, medication use during the period of intervention delivery was examined for the patients most likely to have received it—patients with either significant symptoms of anxiety (ie, Impact of Event Scale12 ≥30) or significant symptoms of depression (ie, Center for Epidemiological Studies Depression Scale13 ≥10). In total there were 56 such patients, and from diagnosis through 12 months, 20% to 27% of them were receiving medication. There were no significant differences noted between study arms with regard to medication at any point (0 months, 4 months, 8 months, and 12 months); P values ranged from .24 to .93. For the entire sample, the frequency of other counseling experiences was low and did not differ between study arms (eg, 7% initially [_P_= .46], 12% at 4 months [_P_= .50], 8% at 60 months [_P_=.29], 5% at 132 months [P =.17]).
Data Analysis
The purpose of the analyses was to contrast study arms in disease outcomes: 1) recurrence free survival, defined as time from randomization to biopsy/study confirming first disease recurrence and 2) breast cancer–specific survival, defined as time from randomization to breast cancer death. Patients who were lost to follow-up had their recurrence data censored at the time of our last contact with them. Although we had no expectation that the intervention would have adverse effects, intent-to-treat analyses were nevertheless conducted (ie, all Intervention patients [n= 114], irrespective of whether they actually participated in the intervention, were included in the analyses).
Multivariate comparison of survival for the study arms was conducted using the Cox proportional hazards analysis.14 The following were considered as potential covariates: disease prognostic factors at diagnosis (tumor size, lymph node status, hormone receptor status, histologic grade, histologic type, menopausal status, and age), cancer treatment received (surgery type, chemotherapy, radiotherapy, and hormonal therapies), and variables with a significant group difference at diagnosis6: patient’s functional performance status (Karnofsky performance status)15 and negative mood (Profile of Mood Status). 16 Estimates for hazards ratios (HRs) and corresponding 95% confidence intervals were obtained for each covariate and for the effect of study arm. By using a backward elimination procedure, any covariates with P < .25 with an endpoint remained in the final model for that endpoint. The proportional hazards assumption was tested using the log minus log test. All statistical tests were 2-sided.
RESULTS
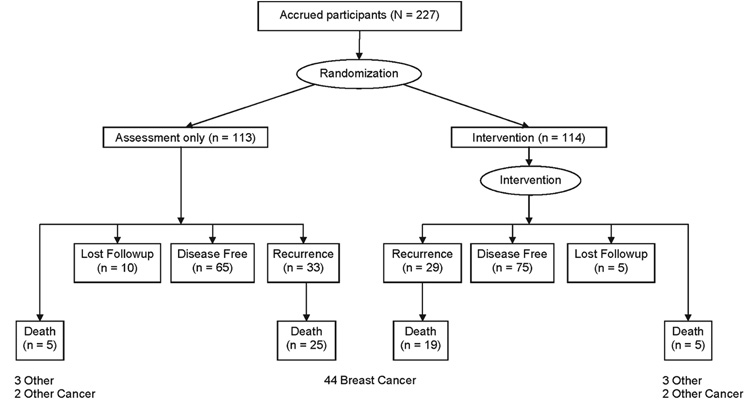
By October 2007, the duration of follow-up ranged from 7 to 13 years (see Fig. 2). Recurrence status was known for 93% (212 of 227 patients) of the patients, and mortality was known for 100%, either through patient contact or, for those lost to medical followup, by using the Social Security Death Index. When deaths occurred, death certificates were routinely sought.
FIGURE 2.
Disease-free, disease recurrence, death, and lost to follow-up status after a median of 11 years of follow-up are shown for 227 breast cancer patients randomized to the Intervention and assessment or Assessment only study arms.
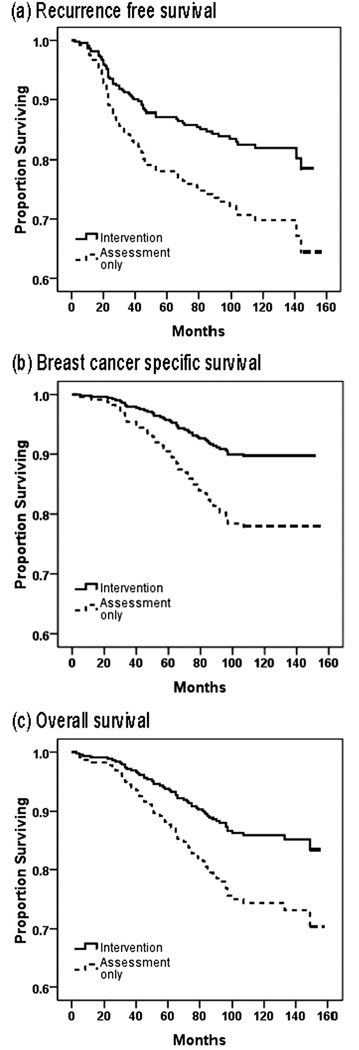
With 11 years median follow-up, disease recurrence had occurred for 62 of 212 (29%) women, 29 in the Intervention arm and 33 in the Assessment–only arm. The observed median time to recurrence for the Intervention arm was 2.8 years (range, 0.9 years–11.8 years), and for the Assessment only arm it was 2.2 years (range, 0.2 years–12.0 years). Multivariate analyses (see Table 2) confirmed that patients randomized to the Intervention arm had a significantly lower risk of disease recurrence (HR of 0.55; P = .034), as shown in Figure 3a.
TABLE 2.
Final Multivariate Cox Proportional Hazards Models for Recurrence-free Survival, Breast Cancer–specific Survival, and Overall Survival
| Recurrence-Free Survival | Breast Cancer-Specific Survival | Overall Survival | ||||
|---|---|---|---|---|---|---|
| Variable | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P |
| Study arm (Intervention vs Assessment only) | 0.553 (0.320–0.957) | .034 | 0.435 (0.221–0.859) | .016 | 0.510 (0.280–0.930) | .028 |
| Age, y | .091 | .040 | .206 | |||
| <35 | 1 (reference) | 1 (reference) | 1 (reference) | |||
| 35–49 | 0.406 (0.149–1.104) | 0.278 (0.096–0.806) | 0.364 (0.130–1.018) | |||
| 50–69 | 0.379 (0.137–1.050) | 0.266 (0.092–0.767) | 0.332 (0.119–0.928) | |||
| >69 | 0.108 (0.018–0.643) | 0.090 (0.014–0.579) | 0.316 (0.076–1.317) | |||
| Tumor classification* | 0.592 (0.387–0.905) | .016 | 0.662 (0.417–1.052) | .081 | 0.668 (0.440–1.013) | .058 |
| Lymph node classification* | 0.824 (0.713–0.953) | .009 | 0.811 (0.687–0.957) | .013 | 0.844 (0.724–0.985) | .031 |
| ER/PR (positive vs negative) | 0.471 (0.262–0.845) | .012 | 0.349 (0.182–0.669) | .002 | 0.435 (0.236–0.800) | .007 |
| Histologic grade | .116 | |||||
| Poorly differentiated | 1 (reference) | |||||
| Moderately differentiated | 0.548 (0.307–0.977) | |||||
| Well differentiated | 0.812 (0.247–2.675) | |||||
| Type of surgery (mastectomy vs lumpectomy) | 0.490 (0.201–1.196) | .117 | ||||
| Chemotherapy (yes vs no) | 0.377 (0.161–0.883) | .025 | 0.331 (0.125–0.878) | .026 | 0.377 (0.160–0.891) | .026 |
| Radiotherapy (yes vs no) | 0.383 (0.171–0.856) | .019 | 0.498 (0.268–0.926) | .028 | 0.526 (0.296 –0.933) | .028 |
| Baseline KPS | 0.972 0.941–1.004) | .090 | 0.951 (0.917–0.986) | .006 | 0.963 (0.930–0.997) | .032 |
| Baseline mood states (POMS) | 0.984 (0.973–0.995) | .004 | 0.992 (0.979–1.004) | .172 |
FIGURE 3.
Predicted cumulative survival of 227 breast cancer patients is shown according to study arm, Intervention and assessment arm versus Assessment–only arm. (a) Cumulative survival without breast cancer recurrence. (b) Cumulative survival without breast cancer-specific death. (c) Cumulative survival from all-cause death.
With regard to survival, 54 of 227 (24%) women had died, 24 in the Intervention arm and 30 in the Assessment only arm. Breast cancer was the primary cause of death for 44 of the 54 patients (81%; 19 patients from the Intervention arm and 25 from the Assessment only arm). Among patients who died of breast cancer, the observed median survival time for the Intervention arm patients was 6.1 years (range, 1.0 years–8.1 years) versus 4.8 years (range, 0.4 years–8.9 years) for patients in the Assessment only arm. Multivariate analyses confirm that patients randomized to the Intervention arm had a significantly lower risk of breast cancer death (HR of 0.44; P = .016) (Table 2) (Fig. 3b).
Two post hoc analyses were conducted. The first tested for survival differences in all-cause mortality. Overall survival was defined as the time from randomization to death from any cause. Nonbreast cancer causes of death were because of other cancers (n= 4) or other diseases/illnesses (eg, cardiac; n= 6). For those who died, the observed median survival time was 6.0 years (range, 0.3 years–11.1 years) for the Intervention arm and 5.0 years (range, 0.4 years–12.4 years) for the Assessment–only arm. Multivariate analyses confirm that women randomized to the Intervention arm had significantly lower risk of all-cause mortality (HR of 0.51; _P_=.028) (Table 2) (Fig. 3c).
The second post hoc analyses were conducted with evaluable patients. Clinical trials analysts may commonly exclude patients who received little or none of the treatment being studied, with the perspective that a more accurate view of the specific efficacy of the treatment is then provided. Thus, the data were reanalyzed excluding 16 of 114 (14%) of the patients in the Intervention arm who attended <20% of the intervention sessions (ie, ≤5 of 26 sessions). With a total of 211 patients, the same analyses were conducted, and the findings were consistent. Patients in the Intervention arm were found to have reduced risk of breast cancer recurrence (HR of 0.50; P =.021), death from breast cancer (HR of 0.32; P = .004), and death from all causes (HR of 0.38; P = .005) compared with patients in the Assessment–only arm. For comparison, these analyses suggest a 68% reduced risk of breast cancer death for patients in the Intervention arm, whereas a 56% risk reduction was estimated with the intent-to-treat analyses.
DISCUSSION
Survival analyses from an RCT confirmed that breast cancer patients randomized to receive a psychologic intervention had reduced risk for breast cancer recurrence and death compared with patients who did not receive the intervention. These effects were observed above and beyond the contribution of known predictors of disease progression in breast cancer, such as lymph node status, receptor status, histology, and others. Furthermore, treatment quality was high for patients in both arms because the majority (83%) of patients received cancer treatment and follow-up at an NCI-designated comprehensive cancer center, and community care and follow-up were comparable. The benefits to the intervention patients were evidenced both in the reduced likelihood of an event occurring and in extended event-free time (ie, fewer patients randomized to the Intervention arm were diagnosed with a recurrence, and if they were, they had been cancer-free an average of 6 months longer than the patients in the Assessment only arm [a 45% reduced risk]. In addition, fewer patients in the Intervention arm died of breast cancer, and those who did survived >1 year longer after disease recurrence than did the patients in the Assessment only arm [a 56% reduced risk]).
As is possible, these patients will continue to be observed. The 5-year survival rate for the stage II patients in the current trial was 90%, which compares favorably with the same estimates for the state of Ohio (84%) and the surrounding county (Franklin) for Columbus, Ohio (83%).17 The 30-year risk of death from breast cancer is in the range of .47.18 However, the majority of the risk of recurrence (.37) occurs in the first 10 years of follow-up18; this is a time point already passed by greater than half of the sample. Because of the potential implications of these findings, the methods used require replication by our and other laboratories. At the current time, the prior findings from the trial provide a complimentary scientific context within which the survival data stand. We will also make comparisons with previous RCTs testing psychotherapy interventions and breast cancer survival19–22 and identify mechanism(s) and moderators of the survival finding. We discuss 3 possible mechanisms here.
The significant psychologic improvements and positive behavioral changes observed for the patients in the Intervention arm may have been critical. Previously, we reported that the significant distress reduction achieved for the Intervention arm at 4 months subsequently predicted significant, positive improvements in health at 12 months.6,10 Recent analyses shed light on this particular effect as well as others.11 Specifically, we found that patients in the Intervention arm with the greatest reductions in distress and physical symptoms were also those who practiced progressive muscle relaxation frequently (daily) and those who understood and remembered (daily) that continued stress could adversely affect their health and that it could be controlled/reduced by using the intervention techniques. Moreover, the benefits from these techniques were greatest for patients vulnerable to poor outcomes (ie, those with the highest levels of cancerspecific stress) (unpublished data). These data suggest that the relation between patients’ use of particular intervention strategies and their subsequent heath was important.
A recent meta-analysis implicates stress in poorer survival and higher cancer mortality.1 Considering biologic mechanisms, stress may have impacted disease processes via endocrine and immune pathways. For the first path, studies using animal models (often with mammary cell tumor lines) have provided evidence of the effects of stress on tumor proliferation/angiogenesis,23 invasion,24 embolism/circulation,25 transport,26 and adhesion in organs or vessel wall structures,27 and there is evidence that these effects are directly mediated by stress hormones (eg, catecholamines).3 For example, administration of epinephrine mimicked the tumorpromoting effects produced by behavioral stress (eg, restraint, isolation), whereas administration of β-adrenergic antagonists blocked the effects of behavioral stress. For humans, an emerging literature notes the effects of stress hormones on tumor growth.28,29 To our knowledge there are few data, but β-adrenergic receptors have been observed on human breast and ovarian tumor cells,24,30 suggesting similar pathways to those observed in animals.
Immune changes secondary to stress hormones may promote cancer growth or metastasis. Neuroendocrine effects on immune dysregulation include impaired diurnal cortisol rhythms, which have been associated with impairments of immune cell function, inflammation, and poorer survival.31–34 Similarly, tumor progression via stress modulation of the immune system (eg, compromised natural killer [NK] cell or T cell function) has been shown and replicated. 35,36
We hypothesized immunity as a mechanism that might covary with psychologic and behavioral variables and disease progression. We first demonstrated that as patients entered the trial, their high stress covaried with lowered immunity, found with multiple indices of NK cell cytotoxicity and lower T lymphocyte proliferative responses.37 Next, we observed that as the patients in the Intervention arm reported significant declines in emotional distress and were found to have reduced symptoms and treatmentrelated toxicities, simultaneously their T cell blastogenesis was stabilized or improving, but this was on a downward trajectory for patients in the Assessment –only arm.6,10 Thus, added immune control of disease processes, particularly early—when patients were recovering from surgery and receiving adjuvant cancer therapies—may have occurred with the declining stress. If so, the impact appears after approximately 20 months, the time at which the groups began to diverge (Fig. 3a).
A related possibility is that arising from the stress-related release of proinflammatory cytokines. 38,39 Inflammatory processes appear to promote tumor growth both in clinical studies and in murine models.38,40 This may be because of the ability of proinflammatory factors to induce angiogenesis, stimulate the accumulation of myeloid suppressor cells, or promote an antiapoptotic tumor phenotype.41–43 As a period of tumor growth precedes its clinical detection, we hypothesized that inflammatory processes might be operative during this period. Enabled by the continuous data collection, we explored the possibility that patients in the trial who were to develop disease recurrence might demonstrate reliable biobehavioral alterations beforehand.44 At the time of the latter analysis, the 48 trial patients who had recurred (R; n= 48) were compared with trial patients remaining disease-free (DF; n= 48), with the 2 groups matched on demographic characteristics, prognostic factors and cancer treatments received when initially diagnosed, study arm, and duration of disease-free follow-up. Data were examined from 3 assessments, occurring on average 17 months, 11 months, and 4 months before the recurrence was detected clinically, with equivalent time points for the disease-free group.
Full details are available, but we briefly note here that in the 17 months before detection, patients who were to develop disease recurrence were found to have significantly higher white blood cell, neutrophil, lymphocyte, and natural killer cell counts compared with DF patients. R patients also demonstrated higher cortisol, worse physical functioning, fatigue, and quality of life during this period. Although the immune and behavioral effects may be independent, they may also arise from a common mechanism. It has been hypothesized that fatigue among cancer patients can result from a “cytokine cascade,” triggered by post-treatment elevation of the proinflammatory cytokines tumor necrosis factor-α, interleukin-1β (IL-1β), and IL-6.45 IL-2, also a proinflammatory cytokine, is known to produce fatigue, depressed mood, and other “sickness behaviors” in addition to the enhanced immune function (particularly NK cytotoxicity).46,47 A psychologic intervention that reduces stress could conceivably interrupt the inflammatory process, thereby mediating the intervention effect to limit disease progression.42
In conclusion, an RCT accruing women with regional breast cancer found that a 1-year, 26-session psychologic intervention was associated with improved survival 11 years later. Considered in context, in the last 30 years, hundreds of randomized psychologic intervention trials have shown mental health improvements for cancer patients in comparison with those in control conditions,48,49 although dissemination of interventions to the 1.4 million cancer patients diagnosed annually remains a goal rather than reality. Indeed, policy makers and oncology professionals in the US and around the world (eg, Committee of the Institute of Medicine,50 Central European Cooperative Group51) recommend making efforts to treat cancer patients, and in particular those diagnosed with breast cancer,52 for their psychologic distress. If efficacious psychologic interventions to reduce stress are delivered early, they will improve mental health, health and treatment-relevant behaviors, and potentially, biologic outcomes. If so, there is the possibility for improved survivorship and survival for cancer patients.
Acknowledgments
Supported by the National Institute of Mental Health (RO1MH51487) and the National Cancer Institute (R01CA92704, K05 CA098133, KA24 CA93670, and P01 CA95426), with additional support from the American Cancer Society (PBR-89), the Longaberger Company-American Cancer Society (PBR-89A), the US Army Medical Research Acquisition Activity (DAMD17-94-J-4165, DAMD17-96-1-6294, and DAMD17-97-1-7062), the Ohio State University Comprehensive Cancer Center (P30 CA16058), and the Walther Cancer Institute.
We thank the patients for their participation and continued commitment, the research and graduate staff of the Stress and Immunity Breast Cancer Project for their many contributions, and colleagues for their helpful comments on the article.
REFERENCES
- 1.Chida Y, Hamer M, Wardle J, Steptoe A. Do stress-related psychological factors contribute to cancer incidence and survival? Nat Clin Pract Oncol. 2008;5:466–475. doi: 10.1038/ncponc1134. [DOI] [PubMed] [Google Scholar]
- 2.Dhabhar FS, McEwen BS. Bi-directional effects of stress on immune function: Possible explanations for salubrious as well as harmful effects. In: Ader R, editor. Psychoneuroimmunology. New York, NY: Elsevier; 2007. pp. 723–760. [Google Scholar]
- 3.Antoni MH, Lutgendorf SK, Cole SW, et al. The influence of bio-behavioural factors on tumour biology: pathways and mechanisms. Nat Rev Cancer. 2006;6:240–248. doi: 10.1038/nrc1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller AH, Ancoli-Israel S, Bower JE, Capuron L, Irwin MR. Neuroendocrine-immune mechanisms of behavioral comorbidities in patients with cancer. J Clin Oncol. 2008;26:971–982. doi: 10.1200/JCO.2007.10.7805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andersen BL, Kiecolt-Glaser JK, Glaser R. A biobehavioral model of cancer stress and disease course. Am Psychol. 1994;49:389–404. doi: 10.1037//0003-066x.49.5.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andersen BL, Farrar WB, Golden-Kreutz DM, et al. Psychological, behavioral, and immune changes after a psychological intervention: a clinical trial. J Clin Oncol. 2004;22:3570–3580. doi: 10.1200/JCO.2004.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fleming ID, Cooper JS, Murphy GP, Sullivan BO, Sobin LH, Yarbro JW, editors. AJCC Cancer Staging Manual. 5th ed. Philadelphia: Lippincott-Raven; 1997. [Google Scholar]
- 8.White SJ, Freedman LS. Allocation of patients to treatment group in a controlled clinical trial. Br J Cancer. 1978;37:849–857. doi: 10.1038/bjc.1978.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scott NW, McPherson GC, Ramsay CR, Campbell MK. The method of minimization for allocation to clinical trials: a review. Control Clin Trials. 2002;23:662–674. doi: 10.1016/s0197-2456(02)00242-8. [DOI] [PubMed] [Google Scholar]
- 10.Andersen BL, Farrar WB, Golden-Kreutz D, et al. Distress reduction from a psychological intervention contributes to improved health for cancer patients. Brain Behav Immun. 2007;21:953–961. doi: 10.1016/j.bbi.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andersen BL, Shelby RA, Golden-Kreutz DM. Results from an RCT of a psychological intervention for patients with cancer: I. Mechanisms of change. J Consult Clin Psychol. 2007;75:927–938. doi: 10.1037/0022-006X.75.6.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Horowitz M, Wilner N, Alvarez W. Impact of Event Scale: a measure of subjective stress. Psychosom Med. 1979;41:209–218. doi: 10.1097/00006842-197905000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Kohout FJ, Berkman LF, Evans DA, Cornoni-Huntley J. Two shorter forms of the CES-D Depression Symptoms Index. J Aging Health. 1993;5:179–193. doi: 10.1177/089826439300500202. [DOI] [PubMed] [Google Scholar]
- 14.Cox DR. Regression models and life-tables. J R Stat Soc. 1972;34:187–220. [Google Scholar]
- 15.Karnofsky DA, Burchenal JH. The clinical evaluation of chemotherapeutic agents in cancer. In: Macleod CM, editor. Evaluation of Chemotherapeutic Agents. New York, NY: Columbia University Press; 1949. pp. 199–205. [Google Scholar]
- 16.McNair DM, Lorr M, Droppleman LF. EITS Manual for the Profile of Mood States. San Diego, CA: Educational and Industrial Testing Service; 1971. [Google Scholar]
- 17.Confer PD. The James Cancer Registry. Columbus, OH: The Ohio State University Comprehensive Cancer Center; 2008. [Google Scholar]
- 18.Schairer C, Mink PJ, Carroll L, Devesa SS. Probabilities of death from breast cancer and other causes among female breast cancer patients. J Natl Cancer Inst. 2004;96:1311–1321. doi: 10.1093/jnci/djh253. [DOI] [PubMed] [Google Scholar]
- 19.Kissane DW, Bloch S, Smith GC, et al. Cognitive-existential group psychotherapy for women with primary breast cancer: a randomised controlled trial. Psychooncology. 2003;12:532–546. doi: 10.1002/pon.683. [DOI] [PubMed] [Google Scholar]
- 20.Kissane DW, Grabsch B, Clarke DM, et al. Supportive-expressive group therapy for women with metastatic breast cancer: survival and psychosocial outcome from a randomized controlled trial. Psychooncology. 2007;16:277. doi: 10.1002/pon.1185. [DOI] [PubMed] [Google Scholar]
- 21.Goodwin PJ, Leszcz M, Ennis M. The effect of group psychosocial support on survival in metastatic breast cancer. N Engl J Med. 2001;345:1719–1726. doi: 10.1056/NEJMoa011871. [DOI] [PubMed] [Google Scholar]
- 22.Spiegel D, Butler LD, Giese-Davis J, et al. Effects of supportive- expressive group therapy on survival of patients with metastatic breast cancer: a randomized prospective trialq. Cancer. 2007;110:1130–1138. doi: 10.1002/cncr.22890. [DOI] [PubMed] [Google Scholar]
- 23.Vandewalle B, Revillion F, Lefebvre J. Functional beta-adrenergic receptors in breast cancer cells. J Cancer Res Clin Oncol. 1990;116:303–306. doi: 10.1007/BF01612908. [DOI] [PubMed] [Google Scholar]
- 24.Sood AK, Bhatty R, Kamat AA, et al. Stress hormone-mediated invasion of ovarian cancer cells. Clin Cancer Res. 2006;12:369–375. doi: 10.1158/1078-0432.CCR-05-1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stefanski V, Ben-Eliyahu S. Social confrontation and tumor metastasis in rats: defeat and beta-adrenergic mechanisms. Physiol Behav. 1996;60:277–282. doi: 10.1016/0031-9384(96)00014-5. [DOI] [PubMed] [Google Scholar]
- 26.Masur K, Niggemann B, Zanker KS, Entschladen F. Norepinephrine-induced migration of SW 480 colon carcinoma cells is inhibited by beta-blockers. Cancer Res. 2001;61:2866–2869. [PubMed] [Google Scholar]
- 27.Rangarajan S, Enserink JM, Kuiperij H, et al. Cyclic AMP induces integrin-mediated cell adhesion through Epac and Rap1 upon stimulation of the I22-adrenergic receptor. J Cell Biol. 2003;160:487–493. doi: 10.1083/jcb.200209105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thaker PH, Lutgendorf SK, Sood AK. The neuroendocrine impact of chronic stress on cancer. Cell Cycle. 2007;6:430–433. doi: 10.4161/cc.6.4.3829. [DOI] [PubMed] [Google Scholar]
- 29.Raison CL, Miller AH. Depression in cancer: new developments regarding diagnosis and treatment. Biol Psychol. 2003;54:283–294. doi: 10.1016/s0006-3223(03)00413-x. [DOI] [PubMed] [Google Scholar]
- 30.Badino GR, Novelli A, Girardi C, Di Carlo F. Evidence for functional β-adrenoceptor subtypes in CG-5 breast cancer cells. Pharmacol Res. 1996;33:255–260. doi: 10.1006/phrs.1996.0036. [DOI] [PubMed] [Google Scholar]
- 31.Kronfol Z, Nair M, Zhang Q, Hil EE, Brown MB. Circadian immune measures in healthy volunteers: relationship to hypothalamic-pituitary-adrenal axis hormones and sympathetic neurotransmitters. Psychosom Med. 1997;59:42–50. doi: 10.1097/00006842-199701000-00006. [DOI] [PubMed] [Google Scholar]
- 32.Rich T, Innominato PF, Boerner J, et al. Elevated serum cytokines correlated with altered behavior, serum cortisol rhythm, and dampened 24-hour rest-activity patterns in patients with metastatic colorectal cancer. Clin Cancer Res. 2005;11:1757–1764. doi: 10.1158/1078-0432.CCR-04-2000. [DOI] [PubMed] [Google Scholar]
- 33.Sephton SE, Sapolsky RM, Kraemer HC, Spiegel D. Diurnal cortisol rhythm as a predictor of breast cancer survival. J Natl Cancer Inst. 2000;92:994–1000. doi: 10.1093/jnci/92.12.994. [DOI] [PubMed] [Google Scholar]
- 34.Mormont MC, Waterhouse J, Bleuzen P, et al. Marked 24-h rest/activity rhythms are associated with better quality of life, better response, and longer survival in patients with metastatic colorectal cancer and good performance status. Clin Cancer Res. 2000;6:3038–3045. [PubMed] [Google Scholar]
- 35.Ben-Eliyahu S, Yirmiya R, Liebeskind JC, Taylor AN, Gale RP. Stress increases metastatic spread of a mammary tumor in rats: evidence for mediation by the immune system. Brain Behav Immun. 1991;5:193–205. doi: 10.1016/0889-1591(91)90016-4. [DOI] [PubMed] [Google Scholar]
- 36.Kalinichenko VV, Mokyr MB, Graf LH Jr, Cohen RL, Chambers DA. Norepinephrine-mediated inhibition of antitumor cytotoxic T lymphocyte generation involves a beta-adrenergic adrenergic receptor mechanism and decreased TNF-alpha gene expression. J Immunol. 1999;163:2492–2499. [PubMed] [Google Scholar]
- 37.Andersen BL, Farrar WB, Golden-Kreutz D, et al. Stress and immune responses after surgical treatment for regional breast cancer. J Natl Cancer Inst. 1998;90:30–36. doi: 10.1093/jnci/90.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim S, Keku TO, Martin C, et al. Circulating levels of inflammatory cytokines and risk of colorectal adenomas. Cancer Res. 2008;68:323–328. doi: 10.1158/0008-5472.CAN-07-2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Elenkov IJ, Chrousos GP. Stress hormones, proinflammatory and anti-inflammatory cytokines, and autoimmunity. Ann N Y Acad Sci. 2002;966:290–303. doi: 10.1111/j.1749-6632.2002.tb04229.x. [DOI] [PubMed] [Google Scholar]
- 40.Merritt WM, Lin YG, Spannuth WA, et al. Effect of interleukin- 8 gene silencing with liposome-encapsulated small interfering RNA on ovarian cancer cell growth. J Natl Cancer Inst. 2008;100:359–372. doi: 10.1093/jnci/djn024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thaker PH, Han LY, Kamat AA, et al. Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat Med. 2006;12:939–944. doi: 10.1038/nm1447. [DOI] [PubMed] [Google Scholar]
- 42.Bunt SK, Sinha P, Clements VK, Leips J, Ostrand-Rosenberg S. Reduced inflammation in the tumor microenvironment delays the accumulation of myeloid-derived suppressor cells and limits tumor progression. Cancer Res. 2007;67:10019–10026. doi: 10.1158/0008-5472.CAN-07-2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu W, Chaudhuri S, Brickley DR, Pang D, Karrison T, Conzen SD. Microarray analysis reveals glucocorticoid-regulated survival genes that are associated with inhibition of apoptosis in breast epithelial cells. Cancer Res. 2004;64:1757–1764. doi: 10.1158/0008-5472.can-03-2546. [DOI] [PubMed] [Google Scholar]
- 44.Thornton LM, Andersen BL, Carson WE., III Immune, endocrine, and behavioral precursors to breast cancer recurrence: a case-control analysis. Cancer Immunol Immunother. 2008;57:1471–1481. doi: 10.1007/s00262-008-0485-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morrow GR, Andrews PL, Hickok JT, Roscoe JA, Matteson S. Fatigue associated with cancer and its treatment. Support Care Cancer. 2002;10:389–398. doi: 10.1007/s005200100293. [DOI] [PubMed] [Google Scholar]
- 46.Anisman H, Hayley S, Turrin N, Merali Z. Cytokines as a stressor: implications for depressive illness. Int J Neuropsychopharmacol. 2002;5:357–373. doi: 10.1017/S1461145702003097. [DOI] [PubMed] [Google Scholar]
- 47.Ruka W, Rutkowski P, Kaminska J, Rysinska A, Steffen J. Alterations of routine blood tests in adult patients with soft tissue sarcomas: relationships to cytokine serum levels and prognostic significance. Ann Oncol. 2001;12:1423–1432. doi: 10.1023/a:1012527006566. [DOI] [PubMed] [Google Scholar]
- 48.Redd WH, Montgomery GH, DuHamel KN. Behavioral intervention for cancer treatment side effects. J Natl Cancer Inst. 2001;93:810–823. doi: 10.1093/jnci/93.11.810. [DOI] [PubMed] [Google Scholar]
- 49.Sheard T, Maguire P. The effect of psychological interventions on anxiety and depression in cancer patients: results of 2 meta-analyses. Br J Cancer. 1999;80:1770–1780. doi: 10.1038/sj.bjc.6690596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Adler NE, Page AEK. Cancer Care for the Whole Patient: Meeting Psychosocial Health Needs. Washington, DC: The National Academies Press; 2008. [PubMed] [Google Scholar]
- 51.Beslija SEA, Bonneterre J, Burstein HJ, et al. For the Central European Cooperative Group. Consensus on the medical treatment of metastatic breast cancer. Breast Cancer Res. 2003;81:1–7. [Google Scholar]
- 52.Hewitt M, Herdman R, Holland J, editors. Meeting Psychosocial Needs of Women with Breast Cancer. Washington, DC: The National Academies Press; 2004. The effectiveness of psychosocial interventions for women with breast cancer; pp. 95–132. [PubMed] [Google Scholar]