Fibronectins in Vascular Morphogenesis (original) (raw)
. Author manuscript; available in PMC: 2009 Jul 27.
Published in final edited form as: Angiogenesis. 2009 Feb 14;12(2):165–175. doi: 10.1007/s10456-009-9136-6
Abstract
Fibronectin is an extracellular matrix protein found only in vertebrate organisms containing endothelium-lined vasculature and is required for cardiovascular development in fish and mice. Fibronectin and its splice variants containing EIIIA and EIIIB domains are highly upregulated around newly developing vasculature during embryogenesis and in pathological conditions including atherosclerosis, cardiac hypertrophy and tumorigenesis, however, their molecular roles in these processes are not entirely understood. We review genetic studies examining functions of fibronectin and its splice variants during embryonic cardiovascular development, and discuss potential roles of fibronectins in vascular disease and tumor angiogenesis.
Keywords: alternative splicing, angiogenesis, cardiovascular development, endothelium, integrins, pericyte, vascular smooth muscle
Blood vessel formation is an essential developmental process required for the survival of all vertebrate organisms [1]. Formation of vascular networks involves extensive interactions of endothelial cells with their environment. These interactions are positively and negatively modulated through the binding of endothelial cell integrins to extracellular matrix proteins, regulating vessel number, size, permeability, vessel sprouting and regression [2, 3]. Timely activation and cessation of vessel formation is necessary to enable vascular networks to grow and change with the developing organism during embryogenesis as well as to respond to physiological and pathological instances when formation of new blood vessels is needed in the adult. One of the key steps in the formation of a functional vascular tree is the recruitment and association of endothelial and perivascular cells (also called pericytes and/or vascular smooth muscle cells) [4–6]. This review will highlight the emerging role of fibronectin in mediating the crosstalk between endothelial and perivascular cells and will discuss functions of fibronectins gleaned from genetic knockout studies.
Functions of fibronectin in vascular morphogenesis
Fibronectin (FN) is an extracellular matrix protein, essential for blood vessel morphogenesis; it is incorporated between endothelial and perivascular cells. Genetic studies demonstrate the requirement for FN in cardiovascular development: absence of FN leads to embryonic lethal vascular defects [7–9]. These defects vary in severity depending on the genetic background indicating the existence of genetic modifier(s) acting in concert with FN during vascular morphogenesis [7]. In FN-null embryos derived from 129S4 strain of mice, endothelial cells are present within the embryo-proper but are not assembled into dorsal aortae, indicating the requirement for FN in vasculogenesis, assembly of blood vessels directly from endothelial precursors. In embryos derived from the C57BL/6J genetic background, dorsal aortae form in the absence of FN; however, the endothelial linings of the dorsal aortae and the heart appear collapsed and separated from the apposing tissue, suggesting defects in vessel lumen formation [7]. This phenotype could also indicate that absence of FN causes defective interactions between endothelial and mesenchymal cells (in the case of dorsal aortae) and endocardial and ventricular myocardial cell layers (in case of the heart). These observations suggest that the role(s) of FN in vascular morphogenesis may be in modulating the formation of vascular lumens and/or modulating interactions between endothelial and perivascular cells. Importantly, formation of vascular networks is also defective in FN-null embryoid bodies (EBs) indicating that vascular defects in FN-null embryos reflect a direct role of FN in vascular morphogenesis and are not simply a consequence of secondary effects resulting from decreased haemodynamic force due to cardiac insufficiency in these embryos [10, 11].
The severity of cardiac defects in FN-null embryos also depends on the genetic background. In FN-null embryos derived from 129S4 genetic background, heart formation is blocked before cardiac progenitors on either side of the embryonic midline fuse to form a tube, resulting in cardia bifida. On a C57BL/6J background, the heart tube forms in the absence of FN [7]. Our genetic mapping studies identified a one-megabase region on chromosome four, which contains modifier(s) regulating the severity of heart defects in FN-null embryos [12]. This modifier(s) may modulate the function of FN during blood vessel morphogenesis as well.
Experiments performed on the zebrafish natter mutant, a null mutation in the FN1 gene, also showed that FN1 is essential for the formation of a single heart tube from the bi-lateral cardiac primordia [13]. These experiments demonstrated that FN is essential for cell polarity within cardiac precursors. In the absence of FN, components of tight junction and adherens junction complexes are downregulated and mislocalized with respect to each other [13]. These defects in cellular polarity presumably lead to defective migration of bi-lateral cardiac progenitors at the midline toward each other to form a single cardiac vessel. It is likely that in FN-null mouse embryos from 129S4 genetic background, the absence of FN leads to altered cardiac cell polarity giving rise to cardia bifida. Interestingly, defects in heart development in natter mutants are also dependent on genetic background, leading to formation of a single heart tube in some natter fish [13]. This suggests that the modifier(s) interacting with FN in heart development may be evolutionarily conserved between species. Since early heart tube in either a zebrafish or a mouse embryo resembles a blood vessel (with endothelial cells forming a tube on the inside and a layer of muscle cells on the outside), determining the identity of genetic modifier(s) interacting with FN during heart development is most likely to provide further insights into the function of FN during vascular morphogenesis.
Analysis of mutations in FN and its main receptor, integrin α5β1, in zebrafish and Xenopus strongly suggested that this receptor-ligand pair functions in establishing and/or maintaining cell polarity [14, 15]. In Xenopus, FN and integrin α5β1 are required for polarized lamellipodial protrusions and for polarized orientation of mitotic spindles during axis elongation through convergent extension [15]. Integrin 〈5-null mutants in zebrafish exhibit loss of polarized somite boundaries and randomized centrosome positioning [14]. Interestingly, recent studies showed that genetic ablation of mouse ZO-1, a component of tight junctions in mature epithelia, leads to severe defects in embryonic and vascular morphogenesis, resembling defects observed in FN-null embryos [16]. ZO-1 is one of the three ZO (zonula occludens) proteins, important for localization and organization of claudins in the process of tight junction formation during establishment/maintainance of apicobasal cell polarity [17, 18]. ZO-1 null mouse mutants exhibit syncytial yolk sac vasculature, defects in vascular remodeling both in yolk sac and head vasculature, shortened anterior-posterior axis and absence of turning. It would be interesting to determine whether FN is required for localization of ZO-1 to endothelial cell-cell junctions and whether FN functions in establishing and/or maintaining endothelial cell polarity during vascular morphogenesis.
Collapsed blood vessel lumens observed in FN-null embryos could potentially be due to defective endothelial cell polarity. Experiments using three-dimensional culture systems in vitro and in zebrafish showed that endothelial lumen formation involves polarized movements of vacuoles followed by their fusion and requires the function of genes involved in cell polarity such as Cdc42, Rac1, Par3, Pak2, Pak4 [19–21]. The functions of these proteins could be activated following the binding of FN to endothelial integrin α5β1 [22]. Taken together, these studies suggest that FN may function in vascular morphogenesis through mediating endothelial cell polarity.
Fibronectin-binding Integrins
Some, and possibly most, of FN function(s) in vascular morphogenesis are mediated by integrin α5β1. Integrin α5β1 is expressed by vascular endothelial cells and binds FN [23]. Genetic studies demonstrated that among all integrin alpha-encoding genes, ablation of integrin 〈5 produces the most severe vascular defects; these defects are slightly milder but overall comparable with defects observed in FN-null embryos [3]. Similar to embryos lacking FN, integrin 〈5-null embryos display dilated and improperly patterned yolk sac vessels [24]. Vascular networks in the heads of integrin 〈5-null embryos are less intricate, exhibiting decreased vessel branching and sprouting suggesting that, similar to FN, integrin 〈5 is required for angiogenesis [10]. In vitro experiments using integrin 〈5-null embryonic stem (ES) cells showed decreased vascular network formation in 〈5-null EBs compared with controls [10]. In addition, teratomas generated using 〈5-null ES cells were largely devoid of ES cell-derived blood vessels suggesting that integrin 〈5 may also play a role in vasculogenesis [25]. Importantly, these experiments demonstrated that, similar to FN, integrin 〈5 is required for vascular morphogenesis.
Integrin α5β1 binds FN by interacting with the RGD sequence within the Xth type III repeat of FN and this binding is greatly facilitated by the synergy site located within the IXth FN type III repeat [26]. The RGD sequence is required for the binding of FN to α5β1, as well as for α5β1-mediated FN matrix assembly and signaling [27]. Mutation of the aspartic acid to glutamic acid in this sequence, (RGD to RGE) abolishes these functions. Generation of mice in which the RGD sequence was replaced with the RGE sequence provided further insights into the function of FN-integrin interactions in vascular morphogenesis [28].
The gross appearance and the onset of embryonic lethality in RGE/RGE mutant mice is very similar to 〈5-null embryos suggesting that the binding of FN to integrin α5β1 through the RGD motif mediates most, if not all, of the functions of integrin 〈5β1 during embryogenesis and vascular morphogenesis [28]. Interestingly, similarly to the phenotypic variability in vascular morphogenesis observed in FN-null and integrin 〈5-null mutants, the severity of phenotype in RGE/RGE embryos is also dependent on the genetic background: it is milder in C57BL/6J strain of mice (R. Fassler, personal communication). This suggests that the modifier(s) affecting FN function during vascular morphogenesis is(are) genetically downstream of integrin α5β1-FN interactions or act in a parallel pathway. A more detailed analysis of vascular development in these and 〈5-null embryos is required to determine more precisely the role of integrin α5β1-FN interactions during vascular morphogenesis.
The vascular phenotype of FN-null mutants is more severe than the phenotypes of either 〈5-null or RGE/RGE mutants, suggesting that in addition to signaling through integrin α5β1, FN has other functions required for vascular morphogenesis. Integrins α4β1, αvβ3, and αvβ5 are expressed on endothelium and can bind FN, and while, individually, α4 or αv -containing integrins are not required for early vascular morphogenesis (integrin α4-null and integrin αv-null embryos show defects in head vessels after e10.5) [29, 30], the deletion of FN negates interactions of all of the above integrins on endothelial cells with surrounding FN. The absence of these interactions could give rise to the increased severity of vascular defects in FN-null embryos compared with α5-null mutants. Alternatively, the function of FN in pathways other than integrin signaling could be important for vascular morphogenesis. These functions may include (but are not limited to) binding to and presenting growth factors such as VEGF [31, 32]. Matrix-bound and soluble VEGF plays distinct and important roles both in embryonic and tumor angiogenesis [33–35].
Interestingly, in vitro three-dimensional culture experiments demonstrated that FN fibrillogenesis is required for vascular network formation [36, 37]. However, vascular networks do form in FN-null or integrin α5-null embryos, suggesting that neither FN nor FN fibrillogenesis are essential for this process [7, 10]. Moreover, the presence of FN fibrils is not sufficient for vascular development, since FN fibrils are formed relatively normally in RGE/RGE mutant embryos but these embryos still develop severe cardiovascular defects [28].
Integrins containing the αv subunit also bind FN and play overlapping but distinct roles during embryonic development compared with integrin α5β1 [29, 38, 39]. Mice lacking both integrin αv and integrin 〈5 subunits fail to proceed through gastrulation [39], suggesting that αv and 〈5-containing integrins perform distinct functions in vivo. In vitro, αv-containing integrins can compensate for the absence of α5 in some assays (e.g., FN fibrillogenesis) [24]. However, αv-containing integrins cannot perform all of the α5β1 functions in vivo. Specifically, the presence of αv-containing integrins (these integrins bind and assemble FN fibrils) does not rescue the vascular defects in α5-null embryos [24]. Interestingly, endothelial cells in which all β1-containing integrins have been deleted showed that these cells still bind to FN (while they are unable to bind collagen or laminin, substrates for α1β1 and α2β1 integrins) [40]. The binding of FN to β1-null endothelial cells is mediated by αv-containing integrins αvβ3 and αvβ5; however, the binding of αv-containing integrins to FN is not sufficient for vascular morphogenesis and does not rescue embryonic lethality in these embryos [40]. Taken together these studies indicate that engagement of αv-containing integrins by FN is not sufficient to elicit intracellular signaling pathways orchestrating proper vascular morphogenesis. Phenotype of Tie2-Cre; β1flox/flox mutants, in which all β1-containing integrins are deleted in endothelial cells closely resembles the phenotype of integrin α5-null mutants [40] and suggest that binding of FN to endothelial-expressed integrin α5β1 mediates the function(s) of FN in vascular morphogenesis.
Functions of fibronectin splice variants in vascular morphogenesis
FN is a large modular glycoprotein composed of repeating subunits (Fig. 1) [27]. Biochemical studies in the past three decades have provided us with a general map of functions of some of the individual domains or their combinations [41]. Investigation of the function(s) of FN splice variants containing EIIIA and/or EIIIB alternatively spliced exons has received the most attention and has been recently extensively reviewed [42]. Here we will provide insights into the function of these splice variants from the most recent genetic studies.
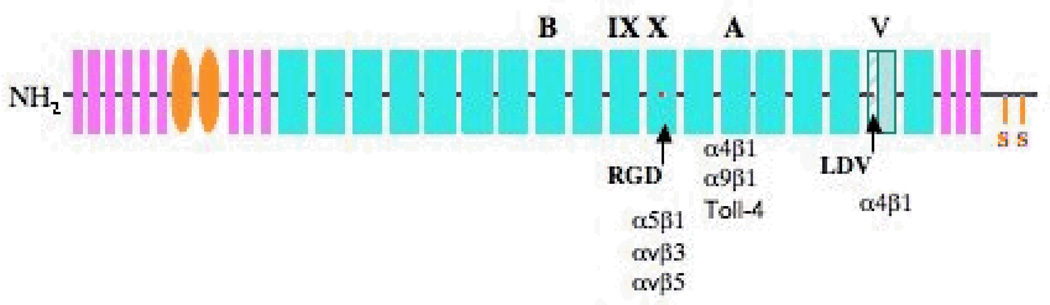
Figure 1. Schematic structure of FN.
FN is composed of structural repeats of three types. Type I repeats are designated in pink, type II repeats are in orange and type III repeats are in cyan (the 9th and 10th repeats are marked). Alternatively spliced EIIIA, EIIIB and V regions are marked. The stippled part of the V region can be included (V120 isoform) or excluded (V95 isoform) in the mouse. Red dots indicate integrin-binding sites in the 10th type III repeat and in the V region. Integrins known to bind these sites and EIIIA are listed.
Analysis of embryonic phenotypes of EIIIA and EIIIB-double-null mutants led to a number of key findings, which will help to decode the function of EIIIA- and EIIIB-containing FNs in vascular morphogenesis. The sequences of EIIIA and EIIIB are only 29% identical to each other, while individual EIIIA and EIIIB sequences are nearly 100% conserved among vertebrates, suggesting that inclusion or exclusion of EIIIA and EIIIB domains gives rise to FN proteins with distinct functions. However, mice lacking either EIIIA or EIIIB exons are viable and fertile, suggesting that embryonic vascular development proceeds fairly normally in the absence of one of the alternatively spliced exons.
Embryos lacking both EIIIA and EIIIB exons die between e9.5 and e10.5 of gestation from severe cardiovascular defects [43]. We observed variability in the severity and the type of vascular defects in the double-null embryos probably due to the fact that the double-null mutation was generated in mouse ES cells derived from a mixed 129P2/C57BL/6J genetic background, and segregation of genetic factors in the EIIIA/EIIIB double-null embryos has contributed to this phenotypic variability [43]. In the most severely affected EIIIA/EIIIB double-null embryos vasculature appeared syncytial in the yolk sac, cephalic and trunk vascular beds, similar to FN-null mutants. Since FN protein is expressed in EIIIA/EIIIB double-nulls to a similar extent as in controls, vascular defects in EIIIA/EIIIB double-null mutants are specifically due to the absence of both EIIIA and EIIIB domains of FN. Staining of embryos and yolk sacs with an antibody recognizing Pecam1 (a marker of vascular endothelial cells) showed that instead of an organized network of small and large vessels, mutant embryos contained sheets of endothelial cells with intervascular spaces that were few in number and diminished in size [43]. A similar phenotype has been observed in numerous other mutants including strains with genetic deletions in VEGF-A, Ang1, TGFβ1, or bFGF [44–47]. Since binding of integrin α5β1 to FN mediates signaling of a number of growth factors including PDGF-BB, EGF, and bFGF [48], it is possible that FN and its splice variants modulate growth factor signaling during vascular morphogenesis. Reagents to detect in vivo activation of some of these growth factor pathways (e.g. TGFβ1) are now available (e.g. antibodies recognizing phosphorylated Smads) and will allow testing of this hypothesis in the future.
Formation of vascular sheets instead of networks in EIIIA/EIIIB double-null embryos could also be due to the possibility that the absence of both EIIIA and EIIIB altered extracellular matrix mechanical properties promoting cell-cell adhesion at the expense of cell-matrix interactions and led to the formation of vascular sheets instead of vessels with distinct diameters [49]. Cell binding and migration cause stretching of extracellular matrix proteins, including FN [50, 51]. The degree of rigidity or elasticity of extracellular matrix alters cellular responses in addition to, and also independent of, chemical composition of the matrix. For example, at identical substrate densities, plating endothelial cells on softer surfaces promotes cell-cell interactions and network formation, whereas plating endothelial cells on rigid surfaces promotes cell-matrix interactions, endothelial cell dispersal and inhibition of vascular network formation. Therefore, it would be interesting to determine whether deletion of both EIIIA and EIIIB leads to altered mechanical properties of vascular extracellular matrix, promoting formation of vascular sheets instead of distinct vessels. Alternatively, inclusion of EIIIA and EIIIB exons into FN proteins may have altered relative orientations between FN domains, leading to suboptimal presentation of the RGD motif (or other domains) to cells, thereby causing diminished integrin activation.
EIIIA and EIIIB domains may engage specific receptors on endothelial and/or perivascular cells promoting the functions of these domains in vascular development. The EIIIA domain of FN has been reported to bind integrins α4β1 and α9β1 as well as Toll-4 receptor in vitro [52–54]; However, in vivo, the biological importance of these interactions is not yet clear [55]. It is possible that vascular defects in the heads of EIIIA/EIIIB double-null embryos are due in part to defective interactions of EIIIA with α4β1, since this integrin is required for cephalic vascular morphogenesis - it mediates interactions of neural crest-derived pericytes with the endothelium of cephalic vessels [30]; Since α9β1 is important for the development of lymphatics [55] it remains to be investigated whether embryos in which EIIIA/EIIIB double-null mutation is embryonic lethal have altered specification of lymphatic endothelium. The binding partners of EIIIB are not known. Identification of specific morphogenetic abnormalities in EIIIA/EIIIB double-null embryos will lead to understanding of specific biological properties affected by the absence of EIIIA and/or EIIIB and aid in identification of cell types whose biological function is influenced by EIIIA and/or EIIIB-containing FNs. These studies may lead to identification of new receptors for EIIIA and EIIIB (and/or confirmation of the proposed EIIIA receptors) as well as to a better understanding of the molecular roles of EIIIA- and EIIIB-containing FNs in vascular morphogenesis.
Most of the EIIIA/EIIIB double-null embryos develop hemorrhages, suggesting that the absence of both EIIIA and EIIIB leads to rupture and/or leakiness of vessels. However, not all vessels appear abnormal. For example, while perineural vascular plexuses in the head and trunk appear syncytial, intersomitic vessels seem to form normally. In addition, cephalic vessels in mildly affected double-null mutants appear to end abruptly (giving rise to blunt-ended vessels) and some head vessels appear to be acutely constricted. Such defects were not found in other embryonic locations in these mutants [43]. Currently, it is not clear why the absence of both EIIIA and EIIIB affects only a subset of vessels. A more detailed investigation into expression patterns of EIIIA and EIIIB FN mRNA and protein in e8.5 and e9.5 embryos will aid in the identification of the type and location of defective vessels in EIIIA/EIIIB double-null mutants, and will likely point to potential mechanistic functions for these splice variants.
Interactions of mural and endothelial cells
Immunohistochemical analyses using limited amounts of antibodies directed to either EIIIA or EIIIB, or recognizing all forms of FNs, demonstrated that FN proteins are enriched around dorsal aortae and branchial arch arteries within the anterior trunk of embryos (dorsal to the heart) at e10.5 [43]. Dorsal aortae are the first embryonic vessels to be invested with alpha smooth muscle actin (αSMA)-expressing VSMCs [56]. In addition, prior experiments suggested that EIIIA-containing FNs may be important in facilitating differentiation of VSMC phenotype [57]. Our experiments showed a three-fold decrease in the number of αSMA-expressing VSMCs around dorsal aortae in the mutants [43]. These results suggested that EIIIA and/or EIIIB splice variants of FN are important in a process of formation of aortic VSMC layer. Their role may be in any of the following biological processes: 1) migration of VSMC progenitors to the dorsal aorta; 2) differentiation of perivascular cells to adopt aortic VSMC characteristics; 3) proliferation and/or survival of VSMCs or their precursors. FN and its receptors are known to play a role in all of these types of processes in vitro and in vivo. Interestingly, the absence of all FN forms leads to defective association of aortic endothelial cells with the adjacent mesenchyme [7]. This observation further strengthens the notion that FN and it splice variants are important for endothelial-perivascular cell interactions during morphogenesis of the vascular tree.
In the past few years, there has been a considerable advance in our understanding of mechanisms important for differentiation of pericytes/VSMCs and a number of genetic studies clearly showed the role for Notch and TGFβ signaling in this process [4, 58–60]. Genetic and fate-mapping studies in mice and chicks identified a number of embryonic sites that contain precursors to VSMCs [61]. However, the mechanism of recruitment of VSMC progenitors from their places of origin to blood vessels is not well understood. Recent studies showed that PDGF-B (platelet-derived growth factor B) is important for the recruitment of VSMCs to blood vessels in normal embryos [62] and in a subcutaneous tumor model using T421 fibrosarcoma cells [63]. Nevertheless, whether PDGF-B functions in directed migration of precursor cells toward endothelial tubes remains unknown [4, 63].
Recent studies showed that VSMCs of a segment of aorta located dorsal to the heart are derived from the hypaxial myotome of somites expressing transcription factor Pax3 (but not from Pax3-expressing neural crest cells) [64]. These studies showed that somite-derived cells expressing EGFP knocked into the Pax3 locus migrate to dorsal aortae to form VSMCs expressing αSMA [64]. However, the expression of Pax3 ceases upon the transit of these cells toward the dorsal aortae [64]. Future experiments such as genetic fate-mapping studies using mice expressing Cre recombinase driven by Pax3 promoter (Pax3-Cre) will be necessary to delineate the role(s) of EIIIA and/or EIIIB in the process of recruitment of VSMC progenitors to dorsal aortae. In addition, since EIIIA and EIIIB-containing FNs are also expressed around branchial arch arteries, it would be interesting to determine whether the absence of these splice variants in the double-null mutants leads to defective recruitment of VSMCs to these vessels as well. Unlike VSMCs of dorsal aortae, VSMCs of branchial arches are derived from a subpopulation of neural crest cells termed cardiac neural crest [65]. These cells originate from the dorsal aspect of the neural tube between the otic vesicle and the posterior boundary of the third somite. Following their migration, these neural crest cells are recruited to the branchial arch arteries and form a VSMC layer. Future fate-mapping studies using Wnt1-Cre and/or P0-Cre strains in conjunction with Rosa26-lacZ reporter strain [65] will be necessary to determine the role of EIIIA and/or EIIIB in the formation of VSMC layer of branchial arch arteries. Such fate-mapping studies will also provide insights into the role of FN during various stage(s) of arterial VSMC development.
In situ hybridizations in chicken embryos detecting mRNA of FN and its splice variants showed that endothelial cells of extraembryonic blood vessel secreted FN during embryogenesis [66], however, it remains unclear whether any embryonic vessels express FN mRNA. If endothelial cells of dorsal aortae also secrete FNs, than one could envision that expression of FNs by the aortic endothelial cells may facilitate recruitment of the nearby mesenchymal cells to the aortae or may act upon mesenchymal cells adjacent to dorsal aortae to induce their differentiation. This model implies that perivascular cells and/or their precursors express receptors for FN and/or EIIIA and EIIIB splice variants. However, if instead perivascular cells or their precursors produced FN proteins, one might hypothesize that these proteins are involved in bi-directional communication between perivascular and endothelial cells. Past genetic data showed that binding of growth factors produced by perivascular cells to their receptors expressed on endothelial cells is essential for vascular morphogenesis [67]. The most well-known examples of these interactions are VEGFs and angiopoietins produced by the perivascular cells and their receptors, VEGFRs and Tie2, expressed on endothelial cells, respectively [67]. If mRNAs for FN and its splice variants are produced by the mesenchymal cells adjacent to dorsal aortae, it would be expected that the receptors for FN and its splice variants are expressed on endothelial cells. Indeed, during vascular remodeling following balloon catherization, FN and its splice variants are expressed by the proliferating VSMCs and receptors known or believed to bind FN’s RGD or EIIIA sequences (α5β1 or α4β1 and α9β1) are expressed by endothelial cells [68–70]. Taken together, detailed examination of spatial and temporal expression of FN mRNAs and proteins during embryonic vascular morphogenesis will aid in understanding the functions of these splice variants as well as in determining the identity and functional significance of cellular receptors for EIIIA and EIIIB.
A more detailed analysis of VSMC recruitment to the aortae in mice carrying single-exon deletions of either EIIIA or EIIIB may give further insight into the individual roles of these highly conserved domains in vascular morphogenesis. These analyses are important even though EIIIA-null and EIIIB-null mutants are viable and fertile. We noticed that VSMC recruitment to dorsal aortae is defective even in phenotypically normal EIIIA/EIIIB double-null embryos (these embryos presumably would have survived gestation to produce viable and fertile mice). In addition, in situ hybridization studies (reviewed below) showed that upregulation of EIIIA-containing and EIIIB-containing forms of FN follow a different time course after vascular injury suggesting that EIIIA-FNs and EIIIB-FNs have non-overlapping functions.
Association of endothelial and perivascular cells is crucial during embryogenesis as well as after birth, for physiological and pathological processes that require new blood vessel formation or remodeling [4, 67, 71–73]. For example, association of these cell types is required during the physiological process of retinal vascularization in a newborn and disruption of this process leads to endothelial cell death and vessel regression [74]. Similarly, during tumor angiogenesis, association of endothelial and perivascular cells is required for tumor vessel stability; disruption of this process leads to regression of a select subset of tumor vessels [5, 75].
Fibronectin and its Splice Variants in Pathology
Cardiovascular Disease
A number of cardiovascular diseases stem from inappropriate recruitment and proliferation of VSMCs during vascular remodeling [76]. For example, migration and proliferation of VSMCs are key factors in the pathology of atherosclerosis [77]. Accumulation of these cells and secretion of extracellular matrix proteins (including FN and its splice variants [78]) results in the formation of the fibrous cap, which if untreated leads to narrowing and eventual occlusion of the affected arterial blood vessels [77]. Breaking of the fibrous cap causes thrombosis and accounts for 50% of the stroke cases in the United States (the third most common cause of death in this country) [79]. EIIIA+ FNs are expressed within atherosclerotic lesions [78], however their functions are unknown and their effects on the progression of atherosclerotic lesions are controversial since both the absence of EIIIA [80, 81] or its constitutive presence have been shown to have protective effects [80]. The functions of EIIIB within atherosclerotic lesions have not been examined.
Importantly, a large percentage (up to 40%) of current surgical procedures to treat atherosclerosis including balloon angioplasty, bypass surgery, and endarterectomy fail within the first year after the treatment due to re-occlusion (i.e. restenosis) of the treated artery, grafted vein, or implanted stent [79, 82]. This happens as a result of VSMC migration into the site of injury and their excessive proliferation following treatment [77]. Interestingly, while there is little or no expression of EIIIA and EIIIB splice variants in normal adult vessels (mouse or human), mouse models of balloon angioplasty showed upregulation of these splice variants as soon as four days after injury [83].
mRNAs of FN and its splice variants were upregulated as soon as one day following coarctation of the ascending aorta. This injury creates cardiac pressure overload leading to cardiac hypertrophy. Following this injury, FN mRNAs were expressed in smooth muscle cells of coronary arteries but not veins, suggesting that expression of FN and its splice variants plays a role during arterial but not venous vessel remodeling following injury [84]. Aortic injury resulting from balloon catherization (this injury approximates endothelial injury following atherosclerotic plaque rupture) results in 3–4 fold upregulation of EIIIA and EIIIB splice variants compared with uninjured vessels [85]. Interestingly, the inclusion of the EIIIA exon into FN mRNA is detectable by the fourth day post-injury, before the formation of neointima, while the expression of EIIIB is not detectable at that time. By the seventh day, neointima is detected in injured vessels and so is the expression of EIIIB. The expression of both EIIIA and EIIIB seems to occur in the vascular smooth muscle cells, and the delay in the appearance of EIIIB compared with EIIIA suggests that these splice variants have non-overlapping roles in vessel healing and/or pathology of atherosclerosis [85].
Similarly, upregulation of EIIIA-containing FNs precedes inclusion of EIIIB into FN mRNA following transplantation of cardiac allografts or isografts [86]. Interestingly, in both allografts and isografts, these splice variants were upregulated in the epicardium [86], the site containing progenitor cells with the potential to give rise to coronary vascular smooth muscle cells, cardiac fibroblasts and ventricular myocardial cells [87, 88] and in zebrafish, these cells have the capacity to differentiate into coronary vascular endothelial cells during cardiac regeneration [89]. These progenitors are mobilized following injury, and thus EIIIA and/or EIIIB splice variants may play a role in this process by affecting migration, proliferation, differentiation and/or survival of these progenitors. Taken together, the time-delay in the onset of expression of EIIIA and EIIIB-containing FNs implies that these splice variants play non-overlapping functions in vivo. The availability of viable EIIIA/EIIIB double-null mutants will help determine the role of these splice variants in vascular morphogenesis following injury and could point to specific individual roles of EIIIA and EIIIB in this process.
Tumorigenesis
Striking upregulation of EIIIA and EIIIB-containing FNs has been observed around tumor vasculature in both human and mouse tumors [90–103]. These splice variants are sometimes called “oncofetal fibronectin isoforms” to signify their prominent expression in embryos and in tumors [104] but not around normal adult vasculature. Correlation of expression of EIIIB and EIIIA with the presence of tumor angiogenesis led to the development of anti-EIIIB and EIIIA antibodies as tumor-targeting and tumor-imaging reagents [105–108].
Individual deletion of EIIIA or EIIIB exon from the FN gene did not have a significant effect on tumor progression in the transgenic Rip1-Tag2 model of pancreatic islet carcinogenesis [103]. Interestingly, pericytes expressing αSMA were abundant around tumor vasculature in either EIIIA-null or EIIIB-null mice, and RNase protection assay revealed that absence of either EIIIA or EIIIB did not quantitatively affect the levels of αSMA in these tumors [103]. These experiments suggest that the presence of either EIIIA or EIIIB-containing FNs is sufficient for the formation of a layer of pericytes around tumor vasculature. Since some EIIIA/EIIIB double-null mice are viable and fertile, it would be interesting to determine whether the absence of both EIIIA and EIIIB forms of FN impairs tumor angiogenesis.
In the course of tumor progression, a thick sleeve of extracellular matrix, which includes FN and its splice variants, surrounds tumor vessels [103, 109]. Interestingly, a thick layer of pericytes also surrounds those tumor vessels expressing high levels of FN. Tumor vessels not expressing FNs have a single pericyte layer (or no pericytes at all) [103]. The functional distinction between these types of tumor vessels is not clear. Our immunohistochemistry experiments in embryos showed that FN and its splice variants are preferentially assembled around dorsal aortae and not anterior cardinal veins suggesting that tumor vessels surrounded by a coat of pericytes and FN may have characteristics of arterial vessels. Alternatively, upregulation of EIIIA and/or EIIIB FNs may function in recruitment and/or differentiation of pericytes by tumor blood vessels. Understanding the process of recruitment of pericytes
000 to, and their association with, blood vessels is essential for the design of effective drugs to block tumor angiogenesis and tumor progression. Since the absence of both EIIIA and EIIIB led to defective recruitment of vascular smooth muscle cells to embryonic dorsal aortae, we hypothesize that EIIIA and/or EIIIB are required for the recruitment of pericytes to tumor blood vessels as well.
Elimination of tumor vessels has been a major goal of anti-angiogenic therapy [110]. Drugs and antibodies that interfere with VEGFR or PDGFR receptor signaling (these agents affect proliferation and survival of tumor blood vessel endothelial cells and association of endothelial cells with pericytes, respectively), led only to a transient decrease in tumor growth [110]. Interestingly, blockade of VEGFR signaling leads to a transient “normalization” of tumor blood vessels: enrichment in the number of tumor vessels surrounded by pericytes, tighter endothelial-pericyte associations, and decrease in the number of vessels without the pericyte coat [109]. This normalization allows for more efficient delivery of chemotherapeutic drugs to kill tumor cells. This concept has been successfully tested by clinical trials [111].
Drugs interfering with PDGFR signaling (these drugs were hypothesized to inhibit interactions of tumor blood vessels with pericytes) also give rise to “normalized” tumor blood vessels. While vessels in untreated tumors are surrounded by a coat of vascular smooth muscle cells loosely associated with the blood vessel wall, tumors receiving drugs blocking PDGFR signaling contain blood vessels that are tightly associated with the surrounding pericytes [110]. Since EIIIA- and EIIIB-containing FNs are expressed around vessels surrounded by a thick coat of pericytes, and since anti-angiogenic therapy leads to enrichment of tumor blood vessels surrounded by pericytes, it would be interesting to determine whether blood vessels expressing EIIIA and/or EIIIB FNs are especially refractory to anti-angiogenic treatments described above. If found to be the case, further understanding of the role of EIIIA and EIIIB FNs in the process of tumor angiogenesis may lead to the design of drugs targeting all types of tumor vasculature.
Conclusions and Perspectives
FN was discovered over thirty years ago as a protein abundantly secreted by tumor cells [27]. Intense investigation of the function of FN in vitro lead to the discovery of integrins and other proteins involved cell motility and in the bi-directional communication between cells and their environment. However, understanding the molecular function of FN in the context of a developing vertebrate organism has proven to be challenging. Recent genetic studies in fish, frogs and mice have suggested that FN functions in maintaining or establishing cell polarity and highlighted the role of FN and its splice variants in development of vascular smooth muscle cells. Future studies involving an extensive temporal and spatial analysis of FN mRNA and protein expression along with genetic fate-mapping studies and in vivo assessment of growth factor signaling pathways in FN-null mutants will lead to a more detailed understanding on the function of FN in embryonic and cardiovascular development.
Acknowledgements
SA is supported by the Scientist Development grant from the American Heart Association, # 0835556D. Work in the laboratory of ROH is supported by the Howard Hughes Medical Institute and by a grant from the National Heart Lung and Blood Institute (PO1-HL66105).
References
- 1.Coultas L, Chawengsaksophak K, Rossant J. Endothelial cells and VEGF in vascular development. Nature. 2005;438:937–945. doi: 10.1038/nature04479. [DOI] [PubMed] [Google Scholar]
- 2.Hodivala-Dilke K. alphavbeta3 integrin and angiogenesis: a moody integrin in a changing environment. Curr Opin Cell Biol. 2008;20:514–519. doi: 10.1016/j.ceb.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 3.Hynes RO. Cell-matrix adhesion in vascular development. J Thromb Haemost. 2007;5 Suppl 1:32–40. doi: 10.1111/j.1538-7836.2007.02569.x. [DOI] [PubMed] [Google Scholar]
- 4.Armulik A, Abramsson A, Betsholtz C. Endothelial/Pericyte Interactions. Circ Res. 2005;97:512–523. doi: 10.1161/01.RES.0000182903.16652.d7. [DOI] [PubMed] [Google Scholar]
- 5.Bergers G, Song S. The role of pericytes in blood-vessel formation and maintenance. Neuro Oncol. 2005;7:452–464. doi: 10.1215/S1152851705000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.von Tell D, Armulik A, Betsholtz C. Pericytes and vascular stability. Exp Cell Res. 2006;312:623–629. doi: 10.1016/j.yexcr.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 7.George EL, Baldwin HS, Hynes RO. Fibronectins are essential for heart and blood vessel morphogenesis but are dispensable for initial specification of precursor cells. Blood. 1997;90:3073–3081. [PubMed] [Google Scholar]
- 8.George EL, Georges-Labouesse EN, Patel-King RS, Rayburn H, Hynes RO. Defects in mesoderm, neural tube and vascular development in mouse embryos lacking fibronectin. Development. 1993;119:1079–1091. doi: 10.1242/dev.119.4.1079. [DOI] [PubMed] [Google Scholar]
- 9.Georges-Labouesse EN, George EL, Rayburn H, Hynes RO. Mesodermal development in mouse embryos mutant for fibronectin. Dev Dyn. 1996;207:145–156. doi: 10.1002/(SICI)1097-0177(199610)207:2<145::AID-AJA3>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 10.Francis SE, Goh KL, Hodivala-Dilke K, Bader BL, Stark M, Davidson D, Hynes RO. Central roles of alpha5beta1 integrin and fibronectin in vascular development in mouse embryos and embryoid bodies. Arterioscler Thromb Vasc Biol. 2002;22:927–933. doi: 10.1161/01.atv.0000016045.93313.f2. [DOI] [PubMed] [Google Scholar]
- 11.Lucitti JL, Jones EA, Huang C, Chen J, Fraser SE, Dickinson ME. Vascular remodeling of the mouse yolk sac requires hemodynamic force. Development. 2007;134:3317–3326. doi: 10.1242/dev.02883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Astrof S, Kirby A, Linblad-Toh K, Daly M, Hynes RO. Heart development in fibronectin-null mice is goverened by a genetic modifier on chromosome four. Mech. Dev. 2007 doi: 10.1016/j.mod.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 13.Trinh LA, Stainier DY. Fibronectin regulates epithelial organization during myocardial migration in zebrafish. Dev Cell. 2004;6:371–382. doi: 10.1016/s1534-5807(04)00063-2. [DOI] [PubMed] [Google Scholar]
- 14.Koshida S, Kishimoto Y, Ustumi H, Shimizu T, Furutani-Seiki M, Kondoh H, Takada S. Integrinalpha5-dependent fibronectin accumulation for maintenance of somite boundaries in zebrafish embryos. Dev Cell. 2005;8:587–598. doi: 10.1016/j.devcel.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 15.Marsden M, DeSimone DW. Regulation of cell polarity, radial intercalation and epiboly in Xenopus: novel roles for integrin and fibronectin. Development. 2001;128:3635–3647. doi: 10.1242/dev.128.18.3635. [DOI] [PubMed] [Google Scholar]
- 16.Katsuno T, Umeda K, Matsui T, Hata M, Tamura A, Itoh M, Takeuchi K, Fujimori T, Nabeshima Y, Noda T, Tsukita S, Tsukita S. Deficiency of zonula occludens-1 causes embryonic lethal phenotype associated with defected yolk sac angiogenesis and apoptosis of embryonic cells. Mol Biol Cell. 2008;19:2465–2475. doi: 10.1091/mbc.E07-12-1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ikenouchi J, Umeda K, Tsukita S, Furuse M, Tsukita S. Requirement of ZO-1 for the formation of belt-like adherens junctions during epithelial cell polarization. J Cell Biol. 2007;176:779–786. doi: 10.1083/jcb.200612080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Umeda K, Ikenouchi J, Katahira-Tayama S, Furuse K, Sasaki H, Nakayama M, Matsui T, Tsukita S, Furuse M, Tsukita S. ZO-1 and ZO-2 independently determine where claudins are polymerized in tight-junction strand formation. Cell. 2006;126:741–754. doi: 10.1016/j.cell.2006.06.043. [DOI] [PubMed] [Google Scholar]
- 19.Buchner DA, Su F, Yamaoka JS, Kamei M, Shavit JA, Barthel LK, McGee B, Amigo JD, Kim S, Hanosh AW, Jagadeeswaran P, Goldman D, Lawson ND, Raymond PA, Weinstein BM, Ginsburg D, Lyons SE. pak2a mutations cause cerebral hemorrhage in redhead zebrafish. Proc Natl Acad Sci U S A. 2007;104:13996–14001. doi: 10.1073/pnas.0700947104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kamei M, Saunders WB, Bayless KJ, Dye L, Davis GE, Weinstein BM. Endothelial tubes assemble from intracellular vacuoles in vivo. Nature. 2006;442:453–456. doi: 10.1038/nature04923. [DOI] [PubMed] [Google Scholar]
- 21.Koh W, Mahan RD, Davis GE. Cdc42-and Rac1-mediated endothelial lumen formation requires Pak2, Pak4 and Par3, and PKC-dependent signaling. J Cell Sci. 2008;121:989–1001. doi: 10.1242/jcs.020693. [DOI] [PubMed] [Google Scholar]
- 22.Giancotti FG, Tarone G. Positional control of cell fate through joint integrin/receptor protein kinase signaling. Annu Rev Cell Dev Biol. 2003;19:173–206. doi: 10.1146/annurev.cellbio.19.031103.133334. [DOI] [PubMed] [Google Scholar]
- 23.Hynes RO, Lively JC, McCarty JH, Taverna D, Francis SE, Hodivala-Dilke K, Xiao Q. The diverse roles of integrins and their ligands in angiogenesis. Cold Spring Harb Symp Quant Biol. 2002;67:143–153. doi: 10.1101/sqb.2002.67.143. [DOI] [PubMed] [Google Scholar]
- 24.Yang JT, Hynes RO. Fibronectin receptor functions in embryonic cells deficient in alpha 5 beta 1 integrin can be replaced by alpha V integrins. Mol Biol Cell. 1996;7:1737–1748. doi: 10.1091/mbc.7.11.1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taverna D, Hynes RO. Reduced blood vessel formation and tumor growth in alpha5-integrin-negative teratocarcinomas and embryoid bodies. Cancer Res. 2001;61:5255–5261. [PubMed] [Google Scholar]
- 26.Aota S, Nomizu M, Yamada KM. The short amino acid sequence Pro-His-Ser-Arg-Asn in human fibronectin enhances cell-adhesive function. J Biol Chem. 1994;269:24756–24761. [PubMed] [Google Scholar]
- 27.Hynes RO. Fibronectins. New York: Springer-Verlag; 1990. [Google Scholar]
- 28.Takahashi S, Leiss M, Moser M, Ohashi T, Kitao T, Heckmann D, Pfeifer A, Kessler H, Takagi J, Erickson HP, Fassler R. The RGD motif in fibronectin is essential for development but dispensable for fibril assembly. J Cell Biol. 2007;178:167–178. doi: 10.1083/jcb.200703021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bader BL, Rayburn H, Crowley D, Hynes RO. Extensive vasculogenesis, angiogenesis, and organogenesis precede lethality in mice lacking all alpha v integrins. Cell. 1998;95:507–519. doi: 10.1016/s0092-8674(00)81618-9. [DOI] [PubMed] [Google Scholar]
- 30.Grazioli A, Alves CS, Konstantopoulos K, Yang JT. Defective blood vessel development and pericyte/pvSMC distribution in alpha 4 integrin-deficient mouse embryos. Dev Biol. 2006;293:165–177. doi: 10.1016/j.ydbio.2006.01.026. [DOI] [PubMed] [Google Scholar]
- 31.Wijelath ES, Murray J, Rahman S, Patel Y, Ishida A, Strand K, Aziz S, Cardona C, Hammond WP, Savidge GF, Rafii S, Sobel M. Novel vascular endothelial growth factor binding domains of fibronectin enhance vascular endothelial growth factor biological activity. Circ Res. 2002;91:25–31. doi: 10.1161/01.res.0000026420.22406.79. [DOI] [PubMed] [Google Scholar]
- 32.Wijelath ES, Rahman S, Namekata M, Murray J, Nishimura T, Mostafavi-Pour Z, Patel Y, Suda Y, Humphries MJ, Sobel M. Heparin-II domain of fibronectin is a vascular endothelial growth factor-binding domain: enhancement of VEGF biological activity by a singular growth factor/matrix protein synergism. Circ Res. 2006;99:853–860. doi: 10.1161/01.RES.0000246849.17887.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee S, Jilani SM, Nikolova GV, Carpizo D, Iruela-Arispe ML. Processing of VEGF-A by matrix metalloproteinases regulates bioavailability and vascular patterning in tumors. J Cell Biol. 2005;169:681–691. doi: 10.1083/jcb.200409115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carmeliet P, Ng YS, Nuyens D, Theilmeier G, Brusselmans K, Cornelissen I, Ehler E, Kakkar VV, Stalmans I, Mattot V, Perriard JC, Dewerchin M, Flameng W, Nagy A, Lupu F, Moons L, Collen D, D'Amore PA, Shima DT. Impaired myocardial angiogenesis and ischemic cardiomyopathy in mice lacking the vascular endothelial growth factor isoforms VEGF164 and VEGF188. Nat Med. 1999;5:495–502. doi: 10.1038/8379. [DOI] [PubMed] [Google Scholar]
- 35.Maes C, Stockmans I, Moermans K, Van Looveren R, Smets N, Carmeliet P, Bouillon R, Carmeliet G. Soluble VEGF isoforms are essential for establishing epiphyseal vascularization and regulating chondrocyte development and survival. J Clin Invest. 2004;113:188–199. doi: 10.1172/JCI19383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou X, Rowe RG, Hiraoka N, George JP, Wirtz D, Mosher DF, Virtanen I, Chernousov MA, Weiss SJ. Fibronectin fibrillogenesis regulates three-dimensional neovessel formation. Genes Dev. 2008;22:1231–1243. doi: 10.1101/gad.1643308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bayless KJ, Salazar R, Davis GE. RGD-dependent vacuolation and lumen formation observed during endothelial cell morphogenesis in three-dimensional fibrin matrices involves the alpha(v)beta(3) and alpha(5)beta(1) integrins. Am J Pathol. 2000;156:1673–1683. doi: 10.1016/s0002-9440(10)65038-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McCarty JH, Monahan-Earley RA, Brown LF, Keller M, Gerhardt H, Rubin K, Shani M, Dvorak HF, Wolburg H, Bader BL, Dvorak AM, Hynes RO. Defective associations between blood vessels and brain parenchyma lead to cerebral hemorrhage in mice lacking alphav integrins. Mol Cell Biol. 2002;22:7667–7677. doi: 10.1128/MCB.22.21.7667-7677.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang JT, Bader BL, Kreidberg JA, Ullman-Cullere M, Trevithick JE, Hynes RO. Overlapping and independent functions of fibronectin receptor integrins in early mesodermal development. Dev Biol. 1999;215:264–277. doi: 10.1006/dbio.1999.9451. [DOI] [PubMed] [Google Scholar]
- 40.Carlson TR, Hu H, Braren R, Kim YH, Wang RA. Cell-autonomous requirement for beta1 integrin in endothelial cell adhesion, migration and survival during angiogenesis in mice. Development. 2008;135:2193–2202. doi: 10.1242/dev.016378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wierzbicka-Patynowski I, Schwarzbauer JE. The ins and outs of fibronectin matrix assembly. J Cell Sci. 2003;116:3269–3276. doi: 10.1242/jcs.00670. [DOI] [PubMed] [Google Scholar]
- 42.White ES, Baralle FE, Muro AF. New insights into form and function of fibronectin splice variants. J Pathol. 2008;216:1–14. doi: 10.1002/path.2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Astrof S, Crowley D, Hynes RO. Multiple cardiovascular defects caused by the absence of alternatively spliced segments of fibronectin. Dev Biol. 2007 doi: 10.1016/j.ydbio.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dickson MC, Martin JS, Cousins FM, Kulkarni AB, Karlsson S, Akhurst RJ. Defective haematopoiesis and vasculogenesis in transforming growth factor-beta 1 knock out mice. Development. 1995;121:1845–1854. doi: 10.1242/dev.121.6.1845. [DOI] [PubMed] [Google Scholar]
- 45.Ferrara N, Carver-Moore K, Chen H, Dowd M, Lu L, O'Shea KS, Powell-Braxton L, Hillan KJ, Moore MW. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature. 1996;380:439–442. doi: 10.1038/380439a0. [DOI] [PubMed] [Google Scholar]
- 46.Lee SH, Schloss DJ, Swain JL. Maintenance of vascular integrity in the embryo requires signaling through the fibroblast growth factor receptor. J Biol Chem. 2000;275:33679–33687. doi: 10.1074/jbc.M004994200. [DOI] [PubMed] [Google Scholar]
- 47.Suri C, Jones PF, Patan S, Bartunkova S, Maisonpierre PC, Davis S, Sato TN, Yancopoulos GD. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell. 1996;87:1171–1180. doi: 10.1016/s0092-8674(00)81813-9. [DOI] [PubMed] [Google Scholar]
- 48.Miyamoto S, Teramoto H, Gutkind JS, Yamada KM. Integrins can collaborate with growth factors for phosphorylation of receptor tyrosine kinases and MAP kinase activation: roles of integrin aggregation and occupancy of receptors. J Cell Biol. 1996;135:1633–1642. doi: 10.1083/jcb.135.6.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reinhart-King CA, Dembo M, Hammer DA. The dynamics and mechanics of endothelial cell spreading. Biophys J. 2005;89:676–689. doi: 10.1529/biophysj.104.054320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Davidson LA, Dzamba BD, Keller R, Desimone DW. Live imaging of cell protrusive activity, and extracellular matrix assembly and remodeling during morphogenesis in the frog, Xenopus laevis. Dev Dyn. 2008;237:2684–2692. doi: 10.1002/dvdy.21600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Erickson HP. Reversible unfolding of fibronectin type III and immunoglobulin domains provides the structural basis for stretch and elasticity of titin and fibronectin. Proc Natl Acad Sci U S A. 1994;91:10114–10118. doi: 10.1073/pnas.91.21.10114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liao YF, Gotwals PJ, Koteliansky VE, Sheppard D, Van De Water L. The EIIIA segment of fibronectin is a ligand for integrins alpha 9beta 1 and alpha 4beta 1 providing a novel mechanism for regulating cell adhesion by alternative splicing. J Biol Chem. 2002;277:14467–14474. doi: 10.1074/jbc.M201100200. [DOI] [PubMed] [Google Scholar]
- 53.Okamura Y, Watari M, Jerud ES, Young DW, Ishizaka ST, Rose J, Chow JC, Strauss JF., 3rd The extra domain A of fibronectin activates Toll-like receptor 4. J Biol Chem. 2001;276:10229–10233. doi: 10.1074/jbc.M100099200. [DOI] [PubMed] [Google Scholar]
- 54.Shinde AV, Bystroff C, Wang C, Vogelezang MG, Vincent PA, Hynes RO, Van De Water L. Identification of the peptide sequences within the EIIIA (EDA) segment of fibronectin that mediate integrin alpha9beta1-dependent cellular activities. J Biol Chem. 2008;283:2858–2870. doi: 10.1074/jbc.M708306200. [DOI] [PubMed] [Google Scholar]
- 55.Huang XZ, Wu JF, Ferrando R, Lee JH, Wang YL, Farese RV, Jr, Sheppard D. Fatal bilateral chylothorax in mice lacking the integrin alpha9beta1. Mol Cell Biol. 2000;20:5208–5215. doi: 10.1128/mcb.20.14.5208-5215.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li S, Wang DZ, Wang Z, Richardson JA, Olson EN. The serum response factor coactivator myocardin is required for vascular smooth muscle development. Proc Natl Acad Sci U S A. 2003;100:9366–9370. doi: 10.1073/pnas.1233635100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Serini G, Bochaton-Piallat ML, Ropraz P, Geinoz A, Borsi L, Zardi L, Gabbiani G. The fibronectin domain ED-A is crucial for myofibroblastic phenotype induction by transforming growth factor-beta1. J Cell Biol. 1998;142:873–881. doi: 10.1083/jcb.142.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.High FA, Zhang M, Proweller A, Tu L, Parmacek MS, Pear WS, Epstein JA. An essential role for Notch in neural crest during cardiovascular development and smooth muscle differentiation. J Clin Invest. 2007;117:353–363. doi: 10.1172/JCI30070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hirschi KK, Burt JM, Hirschi KD, Dai C. Gap Junction Communication Mediates Transforming Growth Factor-{beta} Activation and Endothelial-Induced Mural Cell Differentiation. Circ Res %R 10.1161/01.RES.0000091259.84556.D5. 2003;93:429–437. doi: 10.1161/01.RES.0000091259.84556.D5. [DOI] [PubMed] [Google Scholar]
- 60.Wurdak H, Ittner LM, Lang KS, Leveen P, Suter U, Fischer JA, Karlsson S, Born W, Sommer L. Inactivation of TGF{beta} signaling in neural crest stem cells leads to multiple defects reminiscent of DiGeorge syndrome. Genes Dev. %R 10.1101/gad.317405. 2005;19:530–535. doi: 10.1101/gad.317405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Majesky MW. Developmental basis of vascular smooth muscle diversity. Arterioscler Thromb Vasc Biol. 2007;27:1248–1258. doi: 10.1161/ATVBAHA.107.141069. [DOI] [PubMed] [Google Scholar]
- 62.Lindblom P, Gerhardt H, Liebner S, Abramsson A, Enge M, Hellstrom M, Backstrom G, Fredriksson S, Landegren U, Nystrom HC, Bergstrom G, Dejana E, Ostman A, Lindahl P, Betsholtz C. Endothelial PDGF-B retention is required for proper investment of pericytes in the microvessel wall. Genes Dev. 2003;17:1835–1840. doi: 10.1101/gad.266803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Abramsson A, Lindblom P, Betsholtz C. Endothelial and nonendothelial sources of PDGF-B regulate pericyte recruitment and influence vascular pattern formation in tumors. J Clin Invest. 2003;112:1142–1151. doi: 10.1172/JCI18549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Esner M, Meilhac SM, Relaix F, Nicolas JF, Cossu G, Buckingham ME. Smooth muscle of the dorsal aorta shares a common clonal origin with skeletal muscle of the myotome. Development. 2006;133:737–749. doi: 10.1242/dev.02226. [DOI] [PubMed] [Google Scholar]
- 65.Snider P, Olaopa M, Firulli AB, Conway SJ. Cardiovascular development and the colonizing cardiac neural crest lineage. ScientificWorldJournal. 2007;7:1090–1113. doi: 10.1100/tsw.2007.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ffrench-Constant C, Hynes RO. Patterns of fibronectin gene expression and splicing during cell migration in chicken embryos. Development. 1988;104:369–382. doi: 10.1242/dev.104.3.369. [DOI] [PubMed] [Google Scholar]
- 67.Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9:653–660. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- 68.Cascone I, Napione L, Maniero F, Serini G, Bussolino F. Stable interaction between alpha5beta1 integrin and Tie2 tyrosine kinase receptor regulates endothelial cell response to Ang-1. J Cell Biol. 2005;170:993–1004. doi: 10.1083/jcb.200507082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sheppard AM, Onken MD, Rosen GD, Noakes PG, Dean DC. Expanding roles for alpha 4 integrin and its ligands in development. Cell Adhes Commun. 1994;2:27–43. doi: 10.3109/15419069409014200. [DOI] [PubMed] [Google Scholar]
- 70.Vlahakis NE, Young BA, Atakilit A, Sheppard D. The lymphangiogenic vascular endothelial growth factors VEGF-C and -D are ligands for the integrin alpha9beta1. J Biol Chem. 2005;280:4544–4552. doi: 10.1074/jbc.M412816200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bergers G, Javaherian K, Lo KM, Folkman J, Hanahan D. Effects of angiogenesis inhibitors on multistage carcinogenesis in mice. Science. 1999;284:808–812. doi: 10.1126/science.284.5415.808. [DOI] [PubMed] [Google Scholar]
- 72.Bergers G, Song S, Meyer-Morse N, Bergsland E, Hanahan D. Benefits of targeting both pericytes and endothelial cells in the tumor vasculature with kinase inhibitors. J Clin Invest. 2003;111:1287–1295. doi: 10.1172/JCI17929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Morikawa S, Baluk P, Kaidoh T, Haskell A, Jain RK, McDonald DM. Abnormalities in pericytes on blood vessels and endothelial sprouts in tumors. Am J Pathol. 2002;160:985–1000. doi: 10.1016/S0002-9440(10)64920-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Benjamin LE, Keshet E. Conditional switching of vascular endothelial growth factor (VEGF) expression in tumors: induction of endothelial cell shedding and regression of hemangioblastoma-like vessels by VEGF withdrawal. Proc Natl Acad Sci U S A. 1997;94:8761–8766. doi: 10.1073/pnas.94.16.8761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sennino B, Falcon BL, McCauley D, Le T, McCauley T, Kurz JC, Haskell A, Epstein DM, McDonald DM. Sequential loss of tumor vessel pericytes and endothelial cells after inhibition of platelet-derived growth factor B by selective aptamer AX102. Cancer Res. 2007;67:7358–7367. doi: 10.1158/0008-5472.CAN-07-0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004;84:767–801. doi: 10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- 77.Dzau VJ, Braun-Dullaeus RC, Sedding DG. Vascular proliferation and atherosclerosis: new perspectives and therapeutic strategies. Nat Med. 2002;8:1249–1256. doi: 10.1038/nm1102-1249. [DOI] [PubMed] [Google Scholar]
- 78.Glukhova MA, Frid MG, Shekhonin BV, Vasilevskaya TD, Grunwald J, Saginati M, Koteliansky VE. Expression of extra domain A fibronectin sequence in vascular smooth muscle cells is phenotype dependent. J Cell Biol. 1989;109:357–366. doi: 10.1083/jcb.109.1.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rosamond W, Flegal K, Friday G, Furie K, Go A, Greenlund K, Haase N, Ho M, Howard V, Kissela B, Kittner S, Lloyd-Jones D, McDermott M, Meigs J, Moy C, Nichol G, O'Donnell CJ, Roger V, Rumsfeld J, Sorlie P, Steinberger J, Thom T, Wasserthiel-Smoller S, Hong Y. Heart disease and stroke statistics--2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;115:e69–e171. doi: 10.1161/CIRCULATIONAHA.106.179918. [DOI] [PubMed] [Google Scholar]
- 80.Babaev VR, Porro F, Linton MF, Fazio S, Baralle FE, Muro AF. Absence of regulated splicing of fibronectin EDA exon reduces atherosclerosis in mice. Atherosclerosis. 2007 doi: 10.1016/j.atherosclerosis.2007.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tan MH, Sun Z, Opitz SL, Schmidt TE, Peters JH, George EL. Deletion of the alternatively spliced fibronectin EIIIA domain in mice reduces atherosclerosis. Blood. 2004;104:11–18. doi: 10.1182/blood-2003-09-3363. [DOI] [PubMed] [Google Scholar]
- 82.Dangas G, Kuepper F. Cardiology patient page. Restenosis: repeat narrowing of a coronary artery: prevention and treatment. Circulation. 2002;105:2586–2587. doi: 10.1161/01.cir.0000019122.00032.df. [DOI] [PubMed] [Google Scholar]
- 83.Dubin D, Peters JH, Brown LF, Logan B, Kent KC, Berse B, Berven S, Cercek B, Sharifi BG, Pratt RE, Dzau VJ, Van De Water L. Balloon Catheterization Induces Arterial Expression of Embryonic Fibronectins. Arterioscler Thromb Vasc Biol. 1995;15:1958–1967. doi: 10.1161/01.atv.15.11.1958. [DOI] [PubMed] [Google Scholar]
- 84.Samuel JL, Barrieux A, Dufour S, Dubus I, Contard F, Koteliansky V, Farhadian F, Marotte F, Thiery JP, Rappaport L. Accumulation of fetal fibronectin mRNAs during the development of rat cardiac hypertrophy induced by pressure overload. J Clin Invest. 1991;88:1737–1746. doi: 10.1172/JCI115492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dubin D, Peters JH, Brown LF, Logan B, Kent KC, Berse B, Berven S, Cercek B, Sharifi BG, Pratt RE, et al. Balloon catheterization induced arterial expression of embryonic fibronectins. Arterioscler Thromb Vasc Biol. 1995;15:1958–1967. doi: 10.1161/01.atv.15.11.1958. [DOI] [PubMed] [Google Scholar]
- 86.Coito AJ, Brown LF, Peters JH, Kupiec-Weglinski JW, van de Water L. Expression of fibronectin splicing variants in organ transplantation: a differential pattern between rat cardiac allografts and isografts. Am J Pathol. 1997;150:1757–1772. [PMC free article] [PubMed] [Google Scholar]
- 87.Cai CL, Martin JC, Sun Y, Cui L, Wang L, Ouyang K, Yang L, Bu L, Liang X, Zhang X, Stallcup WB, Denton CP, McCulloch A, Chen J, Evans SM. A myocardial lineage derives from Tbx18 epicardial cells. Nature. 2008;454:104–108. doi: 10.1038/nature06969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhou B, Ma Q, Rajagopal S, Wu SM, Domian I, Rivera-Feliciano J, Jiang D, von Gise A, Ikeda S, Chien KR, Pu WT. Epicardial progenitors contribute to the cardiomyocyte lineage in the developing heart. Nature. 2008;454:109–113. doi: 10.1038/nature07060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lepilina A, Coon AN, Kikuchi K, Holdway JE, Roberts RW, Burns CG, Poss KD. A dynamic epicardial injury response supports progenitor cell activity during zebrafish heart regeneration. Cell. 2006;127:607–619. doi: 10.1016/j.cell.2006.08.052. [DOI] [PubMed] [Google Scholar]
- 90.Castellani P, Viale G, Dorcaratto A, Nicolo G, Kaczmarek J, Querze G, Zardi L. The fibronectin isoform containing the ED-B oncofetal domain: a marker of angiogenesis. Int J Cancer. 1994;59:612–618. doi: 10.1002/ijc.2910590507. [DOI] [PubMed] [Google Scholar]
- 91.Castellani P, Borsi L, Carnemolla B, Biro A, Dorcaratto A, Viale GL, Neri D, Zardi L. Differentiation between high-and low-grade astrocytoma using a human recombinant antibody to the extra domain-B of fibronectin. Am J Pathol. 2002;161:1695–1700. doi: 10.1016/S0002-9440(10)64446-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.D'Ovidio MC, Mastracchio A, Marzullo A, Ciabatta M, Pini B, Uccini S, Zardi L, Ruco LP. Intratumoral microvessel density and expression of ED-A/ED-B sequences of fibronectin in breast carcinoma. Eur J Cancer. 1998;34:1081–1085. doi: 10.1016/s0959-8049(98)00041-0. [DOI] [PubMed] [Google Scholar]
- 93.Inufusa H, Nakamura M, Adachi T, Nakatani Y, Shindo K, Yasutomi M, Matsuura H. Localization of oncofetal and normal fibronectin in colorectal cancer. Correlation with histologic grade, liver metastasis, and prognosis. Cancer. 1995;75:2802–2808. doi: 10.1002/1097-0142(19950615)75:12<2802::aid-cncr2820751204>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 94.Kaczmarek J, Castellani P, Nicolo G, Spina B, Allemanni G, Zardi L. Distribution of oncofetal fibronectin isoforms in normal, hyperplastic and neoplastic human breast tissues. Int J Cancer. 1994;59:11–16. doi: 10.1002/ijc.2910590104. [DOI] [PubMed] [Google Scholar]
- 95.Lohi J, Tani T, Laitinen L, Kangas L, Lehto VP, Virtanen I. Tenascin and fibronectin isoforms in human renal cell carcinomas, renal cell carcinoma cell lines and xenografts in nude mice. Int J Cancer. 1995;63:442–449. doi: 10.1002/ijc.2910630324. [DOI] [PubMed] [Google Scholar]
- 96.Kosmehl H, Berndt A, Strassburger S, Borsi L, Rousselle P, Mandel U, Hyckel P, Zardi L, Katenkamp D. Distribution of laminin and fibronectin isoforms in oral mucosa and oral squamous cell carcinoma. Br J Cancer. 1999;81:1071–1079. doi: 10.1038/sj.bjc.6690809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Matsumoto E, Yamada T, Kawarada Y, Sakakuro T. Expression of Fibronectin Isoforms in Human Breast Tissue: Production of Extra Domain A+/Extra Domain B+ by Cancer Cells and Extra Domain A+ by Stromal Cell. Japanese Journal of Cancer Research. 1999;90:320–325. doi: 10.1111/j.1349-7006.1999.tb00750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Oyama F, Hirohashi S, Shimosato Y, Titani K, Sekiguchi K. Deregulation of alternative splicing of fibronectin pre-mRNA in malignant human liver tumors. J Biol Chem. 1989;264:10331–10334. [PubMed] [Google Scholar]
- 99.Oyama F, Hirohashi S, Shimosato Y, Titani K, Sekiguchi K. Oncodevelopmental regulation of the alternative splicing of fibronectin pre-messenger RNA in human lung tissues. Cancer Res. 1990;50:1075–1078. [PubMed] [Google Scholar]
- 100.Pujuguet P, Hammann A, Moutet M, Samuel JL, Martin F, Martin M. Expression of fibronectin ED-A+ and ED-B+ isoforms by human and experimental colorectal cancer. Contribution of cancer cells and tumor-associated myofibroblasts. Am J Pathol. 1996;148:579–592. [PMC free article] [PubMed] [Google Scholar]
- 101.Scarpino S, Stoppacciaro A, Pellegrini C, Marzullo A, Zardi L, Tartaglia F, Viale G, Ruco LP. Expression of EDA/EDB isoforms of fibronectin in papillary carcinoma of the thyroid. J Pathol. 1999;188:163–167. doi: 10.1002/(SICI)1096-9896(199906)188:2<163::AID-PATH335>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 102.Ohnishi T, Hiraga S, Izumoto S, Matsumura H, Kanemura Y, Arita N, Hayakawa T. Role of fibronectin-stimulated tumor cell migration in glioma invasion in vivo: clinical significance of fibronectin and fibronectin receptor expressed in human glioma tissues. Clin Exp Metastasis. 1998;16:729–741. doi: 10.1023/a:1006532812408. [DOI] [PubMed] [Google Scholar]
- 103.Astrof S, Crowley D, George EL, Fukuda T, Sekiguchi K, Hanahan D, Hynes RO. Direct test of potential roles of EIIIA and EIIIB alternatively spliced segments of fibronectin in physiological and tumor angiogenesis. Mol Cell Biol. 2004;24:8662–8670. doi: 10.1128/MCB.24.19.8662-8670.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ruoslahti E. Specialization of tumour vasculature. Nat Rev Cancer. 2002;2:83–90. doi: 10.1038/nrc724. [DOI] [PubMed] [Google Scholar]
- 105.Borsi L, Balza E, Bestagno M, Castellani P, Carnemolla B, Biro A, Leprini A, Sepulveda J, Burrone O, Neri D, Zardi L. Selective targeting of tumoral vasculature: comparison of different formats of an antibody (L19) to the ED-B domain of fibronectin. Int J Cancer. 2002;102:75–85. doi: 10.1002/ijc.10662. [DOI] [PubMed] [Google Scholar]
- 106.Nilsson F, Kosmehl H, Zardi L, Neri D. Targeted delivery of tissue factor to the ED-B domain of fibronectin, a marker of angiogenesis, mediates the infarction of solid tumors in mice. Cancer Res. 2001;61:711–716. [PubMed] [Google Scholar]
- 107.Kaspar M, Zardi L, Neri D. Fibronectin as target for tumor therapy. Int J Cancer. 2006;118:1331–1339. doi: 10.1002/ijc.21677. [DOI] [PubMed] [Google Scholar]
- 108.Villa A, Trachsel E, Kaspar M, Schliemann C, Sommavilla R, Rybak JN, Rosli C, Borsi L, Neri D. A high-affinity human monoclonal antibody specific to the alternatively spliced EDA domain of fibronectin efficiently targets tumor neo-vasculature in vivo. Int J Cancer. 2008;122:2405–2413. doi: 10.1002/ijc.23408. [DOI] [PubMed] [Google Scholar]
- 109.Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 110.Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer. 2008;8:592–603. doi: 10.1038/nrc2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Batchelor TT, Sorensen AG, di Tomaso E, Zhang WT, Duda DG, Cohen KS, Kozak KR, Cahill DP, Chen PJ, Zhu M, Ancukiewicz M, Mrugala MM, Plotkin S, Drappatz J, Louis DN, Ivy P, Scadden DT, Benner T, Loeffler JS, Wen PY, Jain RK. AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell. 2007;11:83–95. doi: 10.1016/j.ccr.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]