Preclinical Pharmacology and Toxicology of Intravenous MV-NIS, an Oncolytic Measles Virus Administered With or Without Cyclophosphamide (original) (raw)
. Author manuscript; available in PMC: 2009 Oct 28.
Published in final edited form as: Clin Pharmacol Ther. 2007 Oct 31;82(6):700–710. doi: 10.1038/sj.clpt.6100409
Abstract
MV-NIS is an oncolytic measles virus encoding the human thyroidal sodium iodide symporter (NIS). Here, we report the results of preclinical pharmacology and toxicology studies conducted in support of our clinical protocol “Phase I Trial of Systemic Administration of Edmonston Strain of Measles Virus, Genetically Engineered to Express NIS, with or without Cyclophosphamide, in Patients with Recurrent or Refractory Multiple Myeloma.” Dose–response studies in the KAS-6/1 myeloma xenograft model demonstrated a minimum effective dose of 4 × 106 TCID50 (tissue culture infectious dose 50)/kg. Toxicity studies in measles-naive squirrel monkeys and measles-susceptible transgenic mice were negative at intravenous doses up to 108 and 4 × 108 TCID50/kg, respectively. Abundant viral mRNA, maximal on day 8, was detected in cheek swabs of squirrel monkeys, more so after pretreatment with cyclophosphamide. On the basis of these data, the safe starting dose of MV-NIS for our clinical protocol was set at 1 – 2 × 104 TCID50/kg (106 TCID50 per patient).
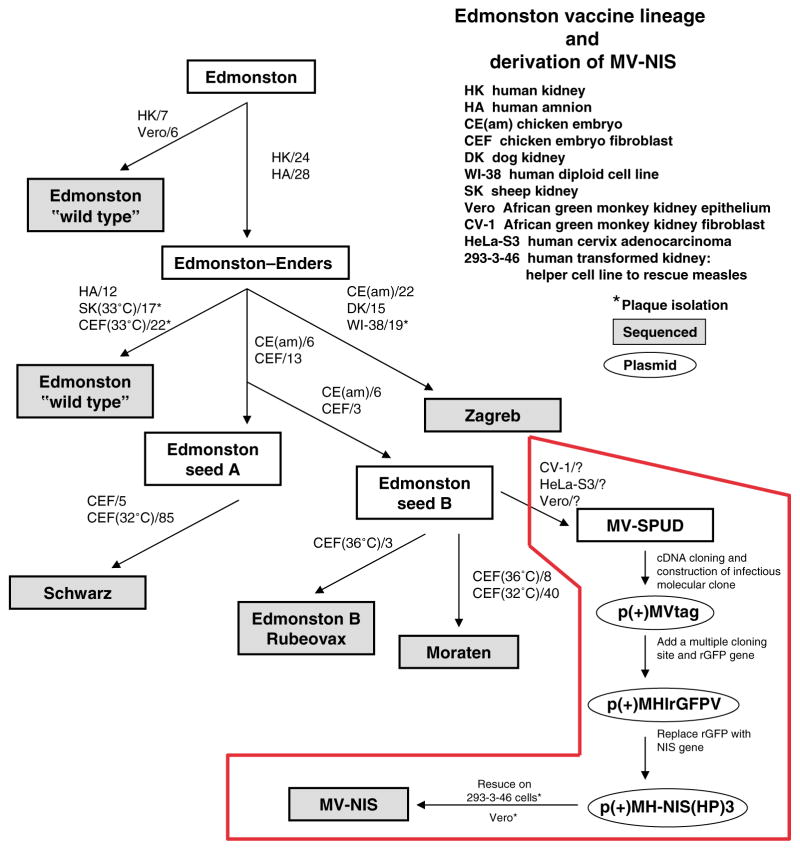
Measles virus (MV, family Paramyxoviridae) was isolated in 1954 from the throat washings of a measles patient, David Edmonston.1 Tissue culture passage resulted in loss of pathogenicity and attenuation of wild-type MV (Figure 1), giving rise to the Edmonston measles vaccines used worldwide today.2 We recently discovered that attenuated Edmonston strain MV has potent antitumor activity in vitro and in vivo.3 Intravenous, intratumoral, or intraperitoneal administration of the virus inhibited tumor growth or induced tumor regression in a variety of human tumor xenograft models.4–7 To tailor the virus for cancer therapy, we have genetically engineered the viral coat protein to display tumor-targeting ligands to enable tumor-specific killing or inserted trackable transgenes into the viral genome to enable non-invasive monitoring of viral gene expression.8–12
Figure 1.
Schematic illustration of the derivation of the infectious molecular clone (MV-Tag) that was used to generate recombinant MV-NIS by reverse genetics. The numbers indicate the number of passages in each of the indicated cellular substrate. (Adapted from ref. 2.)
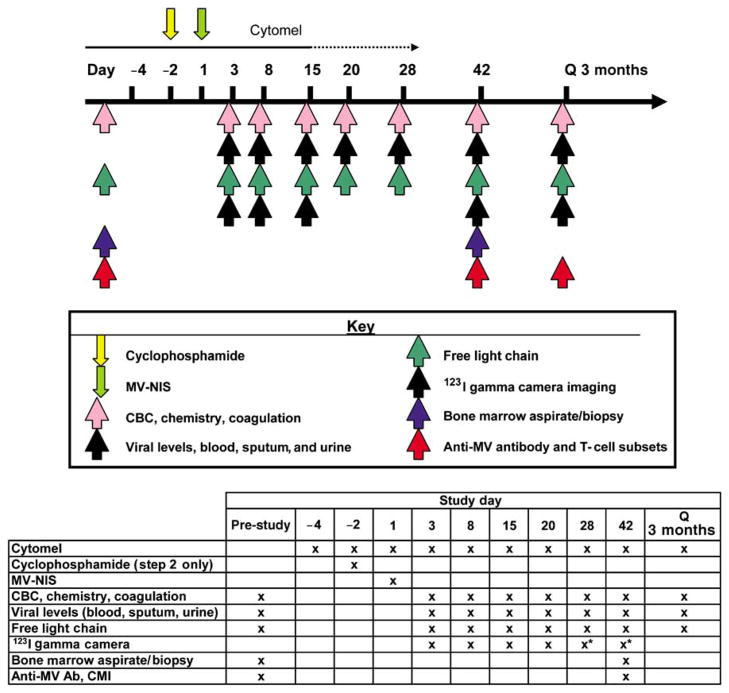
MV-NIS is an Edmonston-lineage MV that expresses the human sodium iodide symporter (hNIS) (Figure 2).12 The NIS protein is normally expressed in the thyroid, mammary glands, stomach, and salivary tissue. Expression of NIS allows cells to actively transport iodide ions into the cell. Thus, patients with thyroid cancer are typically treated with 131I to destroy the NIS-expressing thyroid cancer while leaving most normal tissues undamaged.13 Loss of thyroid function due to the radiotherapy can be treated by replacement therapy with synthetic thyroid hormones. Insertion of NIS into MV facilitates pharmacokinetic evaluation and enhancement of MV oncolytic activity. MV-NIS-infected cells express NIS, and radioiodine uptake provides a basis for in vivo radioiodine imaging studies to reveal the profile of MV-NIS gene expression and the location of MV-NIS-infected cells. Thus, NIS functions as a non-immunogenic marker of viral gene expression. Preclinical studies have demonstrated the efficacy of MV-NIS against human multiple myeloma, ovarian and hepatocellular carcinoma in vivo.7,12,14 A phase I clinical trial is in progress at Mayo Clinic Rochester using MV-NIS in patients with relapsed or refractory myeloma. The trial is designed to determine in a sequential manner the maximal tolerable dose of MV-NIS when administered intravenously with or without cyclophosphamide (Figure 3). In the first phase of the trial, MV-NIS will be administered alone and in the second phase, a single intravenous dose of 10 mg/kg of cyclophosphamide will be administered 2 days before MV-NIS. The purpose of cyclophosphamide therapy is to delay the onset of effective antiviral cell-mediated immunity, thereby slowing immune-mediated killing of MV-NIS-infected tumor cells and prolonging the time available for the virus to spread in the tumor. Cyclophosphamide can also accelerate virus replication by suppressing innate antiviral immune responses.15,16 The biodistribution of MV-NIS-infected cells will be determined non-invasively at different time points after virus administration by whole-body gamma camera imaging of 123I uptake.
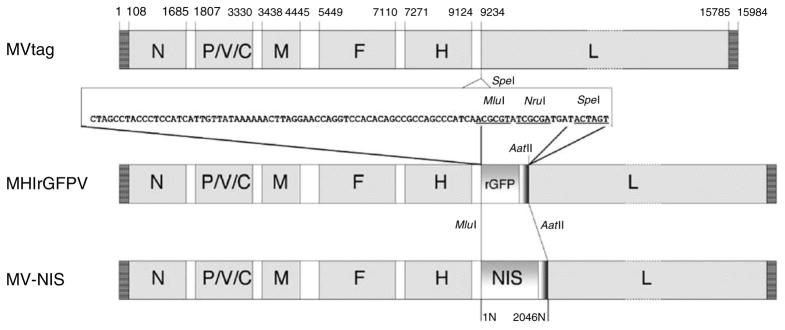
Figure 2.
Schematic representations of the MVs rescued from the plasmid molecular clones used to derive MV-NIS: p(+)MV-tag produced MVtag; p(+)MHlrGFPV produced MHlrGFPV; and p(+)MH-NIS(HP)3 produced the drug substance MV-NIS. To construct p(+)MH-NIS(HP)3, the _Mlu_I–_Aat_II fragment of p(+)MHlrGFPV containing the additional gene encoding rGFP was replaced with a PCR product containing the human NIS gene flanked by _Mlu_I and _Aat_II sites.12 An artificial gene boundary region (nucleotide sequence shown) was inserted into the _Spe_I site (position 9234) using two synthesized oligonucleotides. The p(+)MHlrGFPV plasmid was constructed by inserting the rGFP coding region into the _Mlu_I and _Nru_I cloning sites of the polylinker. The _Aat_II site is in the rGFP 3′-non-coding sequence. The p(+)MH-NIS(HP)3 plasmid was constructed by inserting the human NIS coding region into the _Mlu_I and _Aat_II sites of p(+)MHlrGFPV, replacing the rGFP gene. The position of the gene coding regions is indicated above the MVtag genome. To facilitate the comparison of the MV-NIS genome sequence (17,940 nucleotides) with other MV genomes of the Edmonston lineage (15,894 nucleotides), the 2,046 bp inserted transcription unit containing the NIS coding region is numbered separately (1N–2046N).
Figure 3.
Schema for phase I clinical trial to assess the safety, maximum tolerated dose, and preliminary efficacy of MV-NIS administered intravenously with and without cyclophosphamide in patients with relapsed or refractory multiple myeloma. Correlative studies performed are summarized in the table of this figure.
In support of the investigational new drug (IND) file that was submitted to the Food and Drug Administration (FDA) to justify the clinical evaluation of MV-NIS as an intravenous treatment for multiple myeloma, formal pharmacology/toxicology studies were undertaken in collaboration with the Rapid Access to Intervention Development (RAID) program of the National Cancer Institute to assess the efficacy, biodistribution, and safety of MV-NIS in transgenic, measles-susceptible mice and in non-human primates. The results of these studies are reported below.
RESULTS
Efficacy of intravenous MV-NIS in the KAS-6/1 myeloma xenograft model
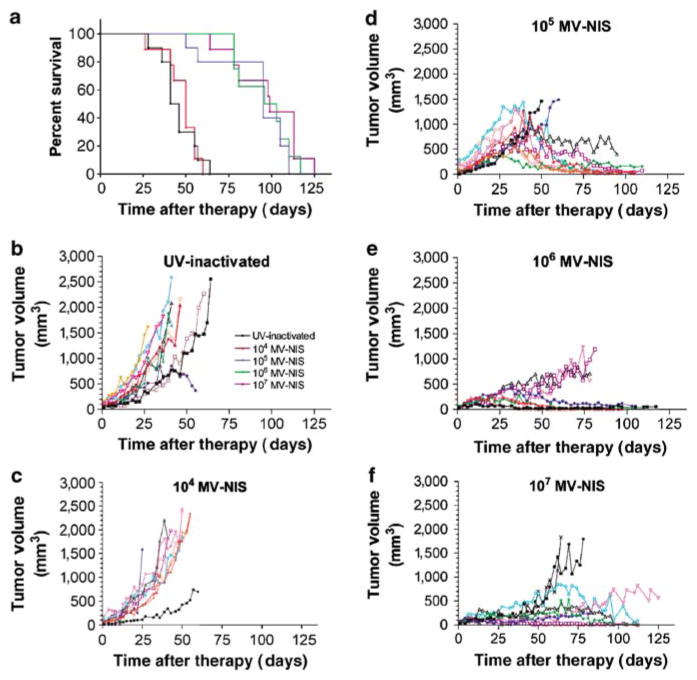
KAS-6/1 cells were implanted subcutaneously in 6-week-old female CB17 ICR severe combined immunodeficiency (SCID) mice. When tumors reached 0.5 cm in diameter, the mice (n = 10 per group) were injected intravenously with 107, 106, 105, or 104 TCID50 MV-NIS or with ultraviolet (UV)-inactivated MV-NIS virus. The KAS-6/1 myeloma xenografts grew rapidly in mice treated with UV-inactivated MV-NIS and at the lowest dose level of 104 TCID50 MV-NIS. All these mice eventually had to be euthanized because of large subcutaneous tumors. Tumors treated with a single intravenous dose of 105 TCID50 MV-NIS grew rapidly at first but then regressed and finally stabilized in size. Mice treated with the higher doses of 106 and 107 TCID50 MV-NIS had stable tumor volume throughout the study (Figure 4). At the time of euthanasia, most of the mice in the higher dose level groups had no detectable viable subcutaneous flank tumors: 4 of 10 (105 TCID50), 3 of 8 (106 TCID50), and 3 of 9 (107 TCID50).
Figure 4.
Survival and individual tumor volume in SCID mice bearing subcutaneous KAS-6/1 multiple myeloma xenografts treated with various doses of MV-NIS. (a) Survival proportions (UV-inactivated, black; 104 TCID50, red; 105 TCID50, blue; 106 TCID50, green; 107 TCID50, purple). Individual tumor volumes plotted for mice treated with (b) UV-inactivated, (c) 104 TCID50, (d) 105 TCID50, (e) 106 TCID50, and (f) 107 TCID50 MV-NIS.
Mice receiving 104 TCID50 MV-NIS had equivalent survival (P = 0.8358) to control mice treated with UV-inactivated virus (median survivals: 50 and 41 days post-article administration, respectively). Mice receiving 105 TCID50 and higher doses of MV-NIS had greatly prolonged survivals (median survival: ≥95 days post-article administration; P < 0.0001) as compared to the UV-inactivated control animals (Figure 4). Mice in the lower dose groups were primarily euthanized because of increasing subcutaneous tumor volume, whereas the mice that were euthanized in the 105 TCID50 MV-NIS and higher dose groups had good local control of subcutaneous tumor volume and were most often euthanized because of clinical deterioration due to metastasis of the KAS-6/1 cells to skeletal structures and lymphatic tissues (Table 1).
Table 1.
Primary reason for euthanasia in mice bearing subcutaneous KAS-6/1 xenografts and mean survival of subgroups (_n_=8–10 per group)
| Primary reason for euthanasia (mean survival in days) | |||
|---|---|---|---|
| Treatment (TCID50) | Subcutaneous | Metastatic | Other |
| UV-inactivated | 9 (44.4) | 0 | 1 (55.0) |
| 104 MV-NIS | 8 (46.3) | 0 | 1 (60.0) |
| 105 MV-NIS | 2 (53.5) | 8 (101.3) | 0 |
| 106 MV-NIS | 2 (79.5) | 5 (106.2) | 1 (78.0) |
| 107 MV-NIS | 2 (71.0) | 5 (112.6) | 2 (89.5) |
Biodistribution of MV-NIS in CD46 transgenic mice
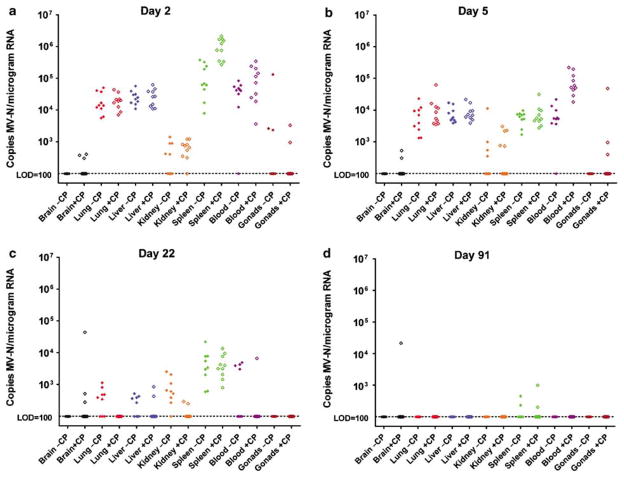
One hundred and sixty (5/sex/group/time point) 6- to 8-week-old measles-susceptible (IfnarKO × CD46 Ge) mice were given intravenous saline (group 1), 125 mg/kg cyclophosphamide followed 2 days later by 107 TCID50 MV-NIS as a single intravenous injection (group 2), 107 TCID50 MV-NIS without cyclophosphamide (group 3), or 105 TCID50 MV-NIS without cyclophosphamide (group 4). Mice from each group were euthanized at days 2, 5, 22, and 91 post-article administration, and their tissues analyzed for content of MV RNA.
Pretreatment with cyclophosphamide changed the biodistribution of MV-NIS-infected cells and changed the kinetics of virus elimination from infected tissues (Figure 5). The brains of cyclophosphamide-pretreated mice were sporadically measles-positive by quantitative real-time (QRT)-PCR throughout the study, whereas those not treated with cyclophosphamide remained negative (P = 0.003). Viremia was higher on day 2 after virus challenge in the mice receiving cyclophosphamide before MV-NIS (_P_≤0.001). Likewise, more virus were detectable in the spleens of cyclophosphamide-treated animals on day 2 after virus challenge (_P_≤0.001); this difference was no longer apparent by day 5 (P = 0.97). In contrast to these observations at early time points after virus challenge, analysis of the data from later time points shows that MV-NIS was cleared faster from the organs of cyclophosphamide-treated mice. By day 22 after MV-NIS challenge, there were significantly fewer copies of the viral RNA detectable in the lungs (P = 0.006) and kidneys (P = 0.006) of the cyclophosphamide-treated animals. MV-NIS RNA was not detected in the majority of tissues tested day 91, indicating a lack of persistence in the mouse tissues, except for spleen and brain.
Figure 5.
Biodistribution of MV-NIS genome in IfnarKO × CD46 Ge mice after intravenous administration of 107 TCID50 MV-NIS day 0 with and without 125 mg/kg cyclophosphamide (CP) pretreatment day −1. (a) Day 2, (b) day 5, (c) day 22, (d) day 91. Data normalized per microgram of total RNA. Closed diamonds, without CP; open diamonds, with CP; black, brain; red, lung; blue, liver; orange, kidney; green, spleen; purple, blood; and brown, gonads. Limit of detection (LOD) of QRT-PCR assay is 100 copies of MV nucleoprotein RNA per microgram of total RNA.
Toxicology studies in CD46 transgenic mice
Twenty-four (3/sex/group/time point) 6- to 8-week-old measles-susceptible (IfnarKO × CD46 Ge) mice received 125 mg/kg cyclophosphamide in a single bolus intravenous injection. Two days later, half of the mice were injected intravenously with 106 TCID50 MV-NIS. Thereafter, they were observed and weighed regularly for signs of health deterioration. Selected mice were euthanized on days 2, 5, 22, and 91 after virus administration, and complete necropsies were performed, at which time blood samples were obtained for complete blood count, clinical chemistry, and coagulation testing. Anti-measles antibody titers were also determined. Multiple tissues were harvested and processed for histological analysis by a board-certified veterinary pathologist.
Male and female mice did equally well on the study. There was no observable clinical deterioration in the health of the mice. Body weights declined transiently after cyclophosphamide treatment, but returned to normal within 2 weeks. There were no changes in coagulation or clinical chemistry parameters that were considered to be related to the administration of MV-NIS. The total white cell count was depressed at early time points in mice receiving both agents compared to those receiving cyclophosphamide alone, suggesting that the additional challenge of MV-NIS to mice pretreated with cyclophosphamide might have further suppressed the white cell count. Mice receiving MV-NIS developed positive anti-MV antibody titers by day 22 (data not shown). Histological analysis of the tissues obtained at necropsy revealed variable lymphocytic depletion changes in the lymphoid organs (mandibular and mesenteric lymph nodes, spleen, and thymus), hypocellularity in the bone marrow, inflammatory changes in the urinary bladder, and degenerative changes in the gonads (data not shown). These acute changes, considered to be solely related to the administration of cyclophosphamide, showed evidence of recovery at day 5 and beyond. None of the pathological changes could be attributed to the administration of MV-NIS.
Toxicology and biodistribution studies in squirrel monkeys
Twelve adult male measles-naive squirrel monkeys (Saimiri sciureus) were divided into four groups and received a single intravenous dose of saline (groups 1 and 2) or MV-NIS (groups 3 and 4) on day 0. Monkeys in groups 2 and 4 were also given cyclophosphamide intravenously (31 mg/kg) 2 days before therapy. Body weights, temperatures, and clinical signs were monitored, and blood was collected for hematology and clinical chemistry at baseline and on days 3, 10, 15, 29, and 91. Thyroid-stimulating hormone and T4 levels were checked at baseline and on days 29 and 91. Interleukin (IL)-1, IL-6, IL-12, and tumor necrosis factor levels were monitored at baseline and on days 1, 4, 8, 29, and 91. Serum was obtained at baseline and on days 8, 15, 29, and 91 for determination of anti-MV antibody levels. Samples containing epithelial cells and saliva were obtained on days 1, 2, 8, 15, 29, and 91 by swabbing or scraping the oral mucosa with cotton-tipped swabs. Complete necropsies and histological analyses were performed on two monkeys from each group on day 29 and the remaining monkey on day 91. Multiple tissues were fixed at the time of necropsy, embedded in paraffin, sectioned at approximately 5 μm, stained with hematoxylin and eosin, and then examined microscopically by a board-certified veterinary pathologist.
There were no premature or unscheduled deaths during the study. No adverse clinical signs or meaningful effects on body weight or temperature were seen. No MV-NIS treatment-related effects were observed for any hematology, clinical chemistry, thyroid hormone, or cytokine parameter, but reticulocyte counts were transiently suppressed in the cyclophosphamide-treated animals. Anti-MV antibody titers increased in the MV-NIS-treated groups after day 15 (Table 2). RNA was extracted from buccal swabs, and QRT-PCR for measles virus nucleoprotein (MV-N) mRNA was performed. MV-N mRNA was detected at day 1 post-virus infusion in the epithelial cells in the buccal cavity with levels declining on day 2, then surging on day 8, suggesting that the virus had replicated at this site between days 2 and 8 (Table 3). Monkeys receiving cyclophosphamide had higher viral RNA copy numbers in buccal swabs compared to monkeys that did not receive the cyclophosphamide. Also, viral RNA remained detectable in cheek swabs for a longer period of time, out to day 29 in the cyclophosphamide-treated animals. By day 91, no viral RNA was detected in the cheek swabs from the remaining monkeys. No treatment-related lesions were seen in any of the animals at the time of necropsy, and no histopathological changes were observed in any of the organs tested.
Table 2.
Anti-measles antibody response in squirrel monkeys receiving 108 TCID50 MV-NIS±31 mg/kg cyclophosphamide
| Study day | |||||
|---|---|---|---|---|---|
| MV-NIS | CP | 8 | 15 | 29 | 91 |
| + | − | <30 | 2,430 | 2,430 | |
| + | − | <30 | 810 | 270 | |
| + | − | <30 | 7,290 | 2,430 | 810 |
| + | + | <30 | 2,430 | 2,430 | |
| + | + | <30 | 2,430 | 1,620 | |
| + | + | 30 | 2,430 | 1,620 | 810 |
Table 3.
Presence of MV-N RNA in buccal swabs
| Study day | |||||||
|---|---|---|---|---|---|---|---|
| MV-NIS | CP | 1 | 2 | 8 | 15 | 29 | 91 |
| + | − | 1,660 | <100 | 1,455 | <100 | <100 | |
| + | − | <100 | <100 | <100 | <100 | <100 | |
| + | − | 66,950 | <100 | 896,500 | 8,830 | <100 | <100 |
| + | + | 2,270 | <100 | 988,500 | 5,880 | <100 | |
| + | + | 53,850 | 955 | 2,465,000 | 2,379,870 | 960 | |
| + | + | 150,250 | 2,520 | 516,000 | 4,590 | 690 | <100 |
DISCUSSION
A single intravenous administration of MV-NIS into SCID mice bearing subcutaneous human myeloma xenografts resulted in tumor regression and extended survival at doses of 105 TCID50 and above. A dose of 104 TCID50 MV-NIS was ineffective. The minimum effective dose in this model is therefore 105 TCID50 or 4 × 106 TCID50 MV-NIS/kg. It should be noted that the tumors in this study were well established and of substantial size, measuring 0.5 cm in diameter before treatment. In most cases, subcutaneous tumor eradication was not complete and the responding mice eventually had to be euthanized because of myeloma dissemination. Administration of 105 TCID50 MV-NIS to a mouse with a blood volume of 2 ml will result in a circulating concentration of 5 × 104 TCID50/ml. In an adult human with a blood volume of approximately 5,000 ml, it would take a dose of 2.5 × 108 TCID50 to achieve a similar concentration of virus in the bloodstream. However, the half-life of the circulating viruses may be considerably shorter in human myeloma patients than in tumor-bearing SCID mice, as many myeloma patients (approximately 70%) have significant circulating titers of neutralizing anti-measles antibodies.17 In contrast, the stromal elements of the myeloma xenografts established subcutaneously in SCID mice, including the endothelial cells lining the tumor blood vessels, do not express the measles receptor CD46. The virus must therefore extravasate from the blood vessel lumen before it can gain access to the human myeloma cells in this mouse model. It is quite possible that in human myeloma patients, where the tumor stromal elements do express abundant CD46, myeloma deposits may be efficiently transduced at significantly lower circulating concentrations of MV-NIS. Moreover, MV-NIS infection of circulating monocytes and T lymphocytes in myeloma patients might provide an additional route by which virus could gain access to myeloma deposits if these cells were to migrate into the tumor parenchyma. Thus, it is impossible to reliably extrapolate from the mouse to the human system in predicting the likely minimum effective dose of MV-NIS for myeloma patients.
A major consideration for the toxicology/biodistribution studies was the choice of animal species. Ideally, the model should be permissive to MV replication and express the relevant receptor(s) (CD46 and/or SLAM (signaling lymphocyte activation molecule)) in a tissue distribution that is comparable to that of humans. Wild-type MV infects cells through SLAM found on activated T cells, B cells, and monocytes.18 In contrast, the attenuated lab-adapted Edmonston MV strains have evolved to also use CD46 as a receptor.19 Thus, the host-range properties of MV-NIS are quite distinct from those of wild-type pathogenic MV. MV-NIS efficiently uses CD46, which is highly expressed on myeloma cells, as a receptor for binding, cell entry, and cell–cell fusion.20 In contrast, wild-type MVs interact poorly with CD46. Thus, CD46 receptor usage by MV-NIS is not only the key to its oncolytic activity21 but is also a critical factor influencing the choice of an appropriate animal model for toxicology and biodistribution studies. Several CD46 transgenic mouse models have been created to facilitate the study of MV pathogenesis.22 MV-Tag (corresponding to the original infectious molecular cDNA clone of MV-Edmonston), MV-NIS, and other engineered MVs derivative of MV-Tag have been shown to infect cells (predominantly macrophages) in CD46 transgenic mice lacking the interferon-α/β receptor (Ifnrko × CD46 Ge).23,24 These mice express human CD46 in a tissue distribution that recapitulates the pattern of CD46 expression in humans, including low to absent expression on erythrocytes.25 Intraperitoneal and intravenous administration of MV-green fluorescent protein (GFP) into these mice resulted predominantly in infection of cells of the monocyte/macrophage lineage in the milky spots of the peritoneal cavity, as well as the greater omentum, lymph nodes, and spleen.26 However, there is an intracellular restriction to MV replication in murine tissues with minimal (if any) progeny production.27,28 On the basis of above considerations, the CD46 transgenic Ifnrko × CD46 Ge mice were considered to be an appropriate rodent model for biodistribution and toxicology studies.
The maximum tolerated dose for MV-NIS in combination with cyclophosphamide was not reached in the mouse toxicology study. Male and female mice did equally well. As expected, the cyclophosphamide was toxic to the bone marrow, urinary bladder, lymphoid tissue, and to a lesser extent, the sex organs. However, the addition of MV-NIS did not exacerbate the toxicity of the cyclophosphamide treatment, except for a slightly greater suppression of the white cell count. This observation is not altogether unexpected, as MV, including attenuated strains, is known to cause transient lymphopenia in humans.29,30 In the biodistribution study, pretreatment with cyclophosphamide resulted in some alterations in the kinetics of MV-NIS elimination and in its biodistribution. At early time points after virus administration (days 2 and 5), there were significantly more copies of virus RNA detectable in the blood and spleen of the cyclophosphamide-treated mice. By day 22 after virus administration, the situation had reversed such that there were fewer copies of viral RNA in the lungs, liver, kidneys, and blood of the cyclophosphamide-treated animals. Thus, cyclophosphamide appears to accelerate virus replication at early time points and (counterintuitively) to enhance the efficiency of virus elimination at later time points. This observation is borne out by additional unpublished studies that were performed using higher doses of cyclophosphamide. Thus, when mice were challenged intraperitoneally with MVCEA (a virus closely related to MV-NIS), there was quite striking persistence of MV RNA in their splenic tissue out to day 59, although they appeared healthy throughout the study. Surprisingly, in mice treated with 250 mg/kg cyclophosphamide 2 days before MVCEA administration, the spleens were completely negative for viral RNA by day 59 (data not shown). Unexpectedly, the brains of cyclophosphamide-pretreated mice were sporadically measles-positive by QRT-PCR throughout the study, whereas those not treated with cyclophosphamide remained negative, suggesting that cyclophosphamide may be capable of interfering with the integrity of the blood–brain barrier.
Because of the previously discussed deficiencies of the Ifnrko × CD46 Ge transgenic mouse model (absence of SLAM receptors and intracellular restrictions to virus replication), we chose to perform toxicology/biodistribution studies in an additional animal model that is known to be permissive to wild-type MV replication. Non-human primates have traditionally been used in measles vaccine studies. Old World primates usually develop a measles-like illness after challenge with wild-type MV but not after exposure to attenuated Edmonston vaccine strains.31–33 Even “reactogenic” vaccines that cause a high frequency of measles-like illnesses in humans are innocuous in these animals. This may be because CD46 is expressed abundantly on macaque but not human red blood cells.34 MV-Edmonston strains therefore agglutinate macaque but not human erythrocytes, an interaction that most likely impedes virus dissemination in this primate model, thereby negating its relevance for human virulence testing. In contrast, New World monkeys, such as squirrel monkeys, express a truncated version of CD46 that lacks the extracellular domain repeat short consensus repeat1 (SCR1) and does not bind MV.34 Squirrel monkeys were therefore considered to be an ideal species to assess the likelihood that an attenuated MV such as MV-NIS will cause a measles-like illness (via SLAM interaction) in human subjects.
Toxicology studies in the squirrel monkeys indicated that the combination of cyclophosphamide and MV-NIS did not appear to cause any greater toxicity as compared to cyclophosphamide alone. At the dose that was selected for this study (discussed below), the cyclophosphamide did cause modest bone marrow suppression, and squirrel monkeys receiving the drug pretreatment showed increased reticulocyte counts in the first 2 weeks after administration over squirrel monkeys that had not received the cyclophosphamide. The biphasic increase in the copy numbers of viral RNA detected by QRT-PCR in the buccal epithelial swabs indicates that the virus was able to travel to a distant site via the bloodstream and subsequently to undergo at least one round of replication before being cleared by the immune system. The cell type that was infected remains to be identified. The higher level of virus RNA in the buccal epithelium in monkeys receiving the combination of virus and cyclophosphamide supports the view that this drug will enhance the propagation of MV-NIS in myeloma patients. Moreover, systemic administration of MV-NIS, with or without cyclophosphamide, in these measles-naive monkeys did not result in any observable toxicity. All monkeys that received MV-NIS developed robust anti-MV antibody titers by day 15. The anti-MV titers in the monkeys that received MV-NIS and cyclophosphamide were not significantly different from monkeys that received virus alone.
Regarding the dose of cyclophosphamide that was used in our preclinical toxicology studies, the pharmacokinetics of cyclophosphamide has been studied in several animal species. When the dose is expressed in mg/m2, the area under the plasma concentration–time curve (AUC) for cyclophosphamide has been demonstrated to be essentially the same in all species tested, including mouse, hamster, dog, rat, monkey, and human.35 This allows for reasonably accurate prediction of interspecies toxicity. On the basis of the above considerations, we calculated that a mouse dose of 125 mg/kg and a squirrel monkey dose of 31 mg/kg would correspond approximately to the proposed human dose of 10 mg/kg cyclophosphamide. These are the doses of cyclophosphamide that were therefore employed in the corresponding biodistribution and toxicology studies.
As to the timing of the cyclophosphamide dose relative to administration of MV-NIS, cyclophosphamide is known to be highly toxic to proliferating lymphocytes and can modulate both primary and anamnestic immune responses to a variety of antigenic challenges, including virus infection. Precise effects vary with the dose of antigen, the dose of cyclophosphamide, and the timing of cyclophosphamide administration relative to the antigenic challenge. Suppression of B and T-cell responses is most pronounced when cyclophosphamide is administered at the same time or within 3 days following antigen challenge but is also seen when the drug is administered 1 or 2 days before exposure to antigen, particularly when higher doses (>100 mg/kg in a mouse) of cyclophosphamide are used.36
The results of our squirrel monkey toxicology study in which monkeys were pretreated with 31 mg/kg of cyclophosphamide, administered 2 days before intravenous MV-NIS have provided strong support for the proposed dose and dose schedule. The data from this study show that the cyclophosphamide did not cause significant toxicity to the animals but weakly suppressed the antiviral immune response sufficient to facilitate more extensive spread of the virus before its elimination.
In conclusion, the no observable adverse effect level, that is, the highest dose that can be given without causing toxicity, for MV-NIS was not reached in these toxicology and biodistribution studies in either of the animal models that were used, even in animals pretreated with cyclophosphamide. The minimum effective dose was 4 × 106 TCID50/kg MV-NIS administered as a single intravenous injection in SCID mice bearing subcutaneous KAS-6/1 myeloma xenografts. The maximum dose of MV-NIS administered intravenously to CD46 transgenic rodents was 107 TCID50/mouse, equivalent to 4 × 108 TCID50/kg. Administration of cyclophosphamide 2 days before MV-NIS therapy was safe in these animals. In the measles-naive monkeys, it is noteworthy that the virus was well tolerated (at a dose of 2 × 108 TCID50/kg), despite the replication of the virus in the buccal swabs of the animals. Anti-MV antibody titers increased in all animals tested. On the basis of these data, the starting dose of MV-NIS for intravenous therapy in myeloma patients was set at 106 TCID50 per patient (approximately 1.3 × 104 TCID50/kg) with 1 log dose increments up to a maximum dose level until 109 TCID50 (1.4 × 107 TCID50/kg).
METHODS
Source of MV-NIS
MV-NIS was generated as described previously.12 Passage 5 preclinical lots of MV-NIS were provided by the Mayo Clinic Viral Vector Production Facility (Rochester, MN). Titer was defined by TCID50/ml. Sterile normal saline served as diluent where applicable. A portion of the MV-NIS was inactivated (confirmed by titration assay) with UV irradiation for certain control groups.
In-life studies
All procedures involving animals were approved and performed according to the guidelines of the Institutional Animal Care and Use Committee of either the Illinois Institute of Technology Research Institute (IIT Research Institute, Chicago, IL) (squirrel monkey toxicity study) or the Mayo Foundation (all remaining studies).
Efficacy study
Human myeloma KAS-6/1 cells were grown at 37 °C in RPMI media supplemented with 10% fetal bovine serum and 1 ng/ml IL-6. Six-week-old female CB17 ICR SCID mice (n = 10 per group; Taconic Farms, Germantown, NY) were weighed, ear-notched, and microchipped with AVID MUSICchips and randomized into groups by weight. Mice were given total body irradiation (250 cGy) 24 h before subcutaneous injection of KAS-6/1 tumor cells (107/100 μl) into the right flank. When tumors reached 0.5 cm in diameter, mice received an intravenous 200 μl injection of MV-NIS (test article) at 107, 106, 105, or 104 TCID50, or control article (UV-inactivated MV-NIS). Tumors were measured in two dimensions, and tumor volume was calculated as V = 0.5_a_2_b_, where a is the shorter of the two diameters measured. The mice were euthanized if the tumors reached more than 10% of mouse body weight, if the tumors ulcerated, or caused obvious discomfort. The statistical significance of difference between the survival proportions of mice in the treatment groups was compared using the log-rank test in the Prism program. A _P_-value <0.05 was considered to be significantly different.
Mouse toxicity study
Twenty-four (3/sex/group/time point) 6- to 8-week-old IfnarKO × CD46 Ge mice23 (Mayo Clinic) were weighed, ear notched, and microchipped with AVID MUSICchips and randomized into groups by weight. Two days before study start, mice received 125 mg/kg cyclophosphamide in a single bolus intravenous injection. Half the mice subsequently received 106 TCID50 MV-NIS in a single bolus intravenous injection. Mice were weighed and observed daily through the first week and weekly thereafter. Mice were euthanized at days 2, 5, 22, and 91 post-article administration, and complete necropsies were performed, at which time, blood samples were obtained by retro-orbital plexus blood collection. Blood samples were analyzed for complete blood count with differential (hemoglobin, red blood cell count, hematocrit, mean corpuscular volume, mean corpuscular hemoglobin concentration, red cell distribution width, white blood cell count, lymphocytes, monocytes, granulocytes, platelet count, mean platelet volume, and platelet distribution width), clinical chemistry (albumin, alkaline phosphatase, alanine aminotransferase, amylase, total bilirubin, blood urea nitrogen, calcium, phosphorus, creatinine, glucose, sodium, potassium, total protein, and globulin), and coagulation (prothrombin and activated partial thromboplastin times). For analysis, the VetScan HMT Hematology System (Abaxis, Union City, CA), VetScan Chemistry Analyzer (Abaxis, Union City, CA), and an SCA2000 Veterinary Coagulation Analyzer (Synbiotics, San Diego, CA) were used according to the manufacturer’s instructions. The calibration was verified before each analysis day for quality control. Adrenal glands, bone (femur), bone marrow (femur), brain, cecum, colon, duodenum, epididymides, esophagus, eyes, gall bladder, gonads (testes or ovaries), gross lesions, heart, ileum, jejunum, kidneys, liver, lungs, lymph nodes (mandibular and mesenteric), mammary gland, pituitary gland, salivary glands (mandibular, sublingual, and parotid), sciatic nerve, skeletal muscle, skin (ventral abdomen), spinal cord, spleen, stomach, thymus, thyroid glands, trachea, urinary bladder, and uterine horn were harvested for analysis. The tissues were preserved in Davidson’s solution (eyes only) or 10% neutral-buffered formalin, embedded in paraffin, sectioned at approximately 5 μm, stained with hematoxylin and eosin, and examined microscopically by a board-certified veterinary pathologist.
Mouse biodistribution study
One hundred and sixty (5/sex/group/time point) 6- to 8-week-old IfnarKO × CD46 Ge mice23 (Mayo Clinic) were weighed, ear notched, and microchipped with AVID MUSICchips and randomized into groups by weight. Two days before test or control article administration, group 2 received 125 mg/kg cyclophosphamide in a single bolus intravenous injection. Group 1 received control article (saline) and groups 2–4 received MV-NIS (107, 107, and 105 TCID50 respectively) in a single bolus intravenous injection. Mice were euthanized at days 2, 5, 22, and 91 post-article administration. Blood, bronchoalveolar lavage, urine, thyroid, brain, lung, liver, kidney, pancreas, spleen, gonads (ovaries or testes), heart, quadriceps, stomach, and small intestine were preserved in by freezing (liquids) or RNALater (Ambion, Austin, TX).
RNA isolation for mouse biodistribution study
RNA was isolated from blood samples with the QIAamp RNA Blood Mini Kit, and urine and bronchoalveolar lavage RNA was isolated with the QIAamp Viral RNA Mini Kit, according to the manufacturer’s instructions (Qiagen, Valencia, CA). RNA was isolated from organs using either the RNeasy Tissue Mini Kit (thyroid, brain, lung, liver, kidney, pancreas, spleen, and gonads) or the RNeasy Fibrous Tissue Mini Kit (heart, quadriceps, stomach, and small intestine), according to the manufacturer’s instructions (Qiagen). All samples were treated with DNA-free (Ambion) according to the manufacturer’s instructions to remove DNA before being quantitated by UV spectroscopy.
MV-N QRT-PCR for mouse biodistribution study
A 61-base product of the MV-N gene was amplified using the following procedure: The iScript One-Step RT-PCR kit for probes (Bio-Rad, Hercules, CA) was used as per the manufacturer’s instructions. The final 25 μl reaction contained 300 nM forward primer 5′-GAAGCCAGGGAG AGCTACAG-3′, 200 nM 5′-56-FAM/AAACCGGGCCCAGCAGAG CAA/3BHQ_1/-3′ dual-labeled probe, 300 nM reverse primer 5′-GG GCAGCTCTCGCATCAC-3′, 1 × reverse transcription-PCR reaction mix for probes, nuclease-free H2O, iScript Reverse Transcriptase, and RNA template. One cycle of reverse transcription reaction (10 min at 50 °C) was applied, followed by a denaturation step (5 min at 95 °C) and 45 cycles of amplification (15 s at 95 °C and 30 s at 60 °C) with fluorescence measured at the anneal/extension step on an iCycler Real Time Instrument (Bio-Rad). Tissue RNA samples were diluted as needed to keep the RNA concentration below 1 μg per reaction and were quantitated by comparison to a linear standard curve generated from amplification of known quantities of a synthetic 61-base RNA strand (Dharmacon, Lafayette, CO).
Squirrel monkey toxicity study
This study was performed in full good laboratory practice compliance by the IIT Research Institute. Twelve (3 per group) measles-naive adult male squirrel monkeys (S. sciureus) were divided into four groups. Two days before test or control article administration, groups 2 and 4 received 31 mg/kg cyclophosphamide in a single bolus intravenous injection. Groups 1 and 2 received control article (saline) and groups 3 and 4 received 108 TCID50 MV-NIS in a slow push intravenous infusion. Body weights, temperatures, and clinical signs were monitored, and blood was collected for hematology and clinical chemistry at baseline and on days 3, 10, 15, 29, and 91. Thyroid-stimulating hormone and T4 levels were checked at baseline and on days 29 and 91. IL-1, IL-6, IL-12, and tumor necrosis factor levels were monitored at baseline and on days 1, 4, 8, 29, and 91. Serum was obtained at baseline and on days 8, 15, 29, and 91 for determination of anti-MV antibody levels. Samples containing epithelial cells and saliva were obtained on days 1, 2, 8, 15, 29, and 91 by swabbing or scraping the oral mucosa with cotton-tipped swabs. Statistical analysis of continuous data through day 29 was performed using analysis of variance, with post hoc comparisons made using Dunnett’s test. A minimum significance level of P ≤ 0.05 was used for all comparisons. Complete necropsies and histological analyses were performed on two monkeys from each group on day 29 and the remaining monkey on day 91. The following tissues were examined, sampled, and fixed in 10% neutral buffered formalin: adrenals, aorta, bone, bone marrow, brain, cecum, colon, duodenum, epididymides, esophagus, eyes, gall bladder, gonads, gross lesions, heart, ileum, jejunum, kidneys, lip, liver, lungs, lymph nodes, mammary gland, pancreas, parathyroid gland, pituitary, prostate, salivary gland, sciatic nerve, skeletal muscle, skin (including injection site), spinal cord, spleen, stomach, thymus, thyroid, tongue, tonsils, trachea, and urinary bladder. Samples of all fixed tissues were embedded in paraffin, sectioned at approximately 5 μm, stained with hematoxylin and eosin, and then examined microscopically by a board-certified veterinary pathologist.
Anti-MV Ab assay for mouse and squirrel monkey plasma or sera
The immunofluorescence assay was based on BION measles virus antigen substrate slides (BION Enterprises, Des Plaines, IL) and was performed according to the manufacturer’s instructions.10 The protocol was modified using goat anti-mouse IgG fluorescein isothiocyanate-conjugated antibodies for detection of murine IgG or goat anti-human IgG-conjugated antibodies shown to have cross-reactivity with squirrel monkey IgG.
RNA isolation of buccal swab samples
Samples were collected on sterile OmniSwabs (Whatman International, UK) by 10 firm back and forth swabs of the buccal cavity, and the tip was ejected into a sterile microcentrifuge tube containing 0.4 ml buffer RLT and β-mercaptoethanol. Sample was vortexed for 1 min and transferred to a QIAShredder, where it was spun at top speed in a tabletop centrifuge for 5 min. Liquid was frozen below −65 °C until processing with the RNeasy Total RNA Kit (Qiagen).
MV-N QRT-PCR of buccal swab RNA
An 81-base product of the MV-N gene was amplified using the following procedure: the TaqMan One-step RT-PCR Master Mix Reagents Kit (Applied Biosystems, Foster City, CA) was used as per the manufacturer’s instructions. The final 25 μl reaction contained 300 nM forward primer 5′-GGGTGTGCCGGTTGGA-3′, 200 nM 5′-56-FAM/TGGGCAGCTCTCGCATCACTTGC/3BHQ_1/-3′ dual-labeled probe, 300 nM reverse primer 5′-AGAAGCCAGGGAGAGCTACAGA-3′, 1 × mix without UNG, nuclease-free H2O, 1 × multiscribe/RNAse inhibitor, and RNA template. One cycle of reverse transcription reaction (30 min at 48 °C) was applied, followed by a denaturation step (10 min at 95 °C) and 40 cycles of amplification (15 s at 95 °C and 1 min at 60 °C) with fluorescence measured at the anneal/extension step on a MX4000 Multiplex Quantitative PCR System (Stratagene, La Jolla, CA). Tissue RNA samples were diluted as needed to keep the RNA concentration below 0.2 μg per reaction and were quantitated by comparison to a linear standard curve generated from in vitro transcription of known target copy quantity.
Acknowledgments
This work was supported by the National Cancer Institute (NCI) Rapid Access to Intervention Development (RAID) program, NCI R01 Grants (CA100634 and CA118488) and the JARI Research Foundation. We thank the Mayo Clinic Comparative Medicine Department (Drs Michael Blanco and Naomi Gades) and Diane Soeffker, James Krempski, Sarah Buhrow, and Mary Kuffel for their expert technical assistance.
Footnotes
CONFLICT OF INTEREST
The authors declared no conflict of interest.
References
- 1.Enders JF, Peebles TC. Propagation in tissue cultures of cytopathogenic agents from patients with measles. Proc Soc Exp Biol Med. 1954;86:277–286. doi: 10.3181/00379727-86-21073. [DOI] [PubMed] [Google Scholar]
- 2.Rota JS, Wang ZD, Rota PA, Bellini WJ. Comparison of sequences of the H, F, and N coding genes of measles virus vaccine strains. Virus Res. 1994;31:317–330. doi: 10.1016/0168-1702(94)90025-6. [DOI] [PubMed] [Google Scholar]
- 3.Nakamura T, Russell SJ. Oncolytic measles viruses for cancer therapy. Expert Opin Biol Ther. 2004;4:1685–1692. doi: 10.1517/14712598.4.10.1685. [DOI] [PubMed] [Google Scholar]
- 4.Peng KW, et al. Systemic therapy of myeloma xenografts by an attenuated measles virus. Blood. 2001;98:2002–2007. doi: 10.1182/blood.v98.7.2002. [DOI] [PubMed] [Google Scholar]
- 5.Peng KW, et al. Intraperitoneal therapy of ovarian cancer using an engineered measles virus. Cancer Res. 2002;62:4656–4662. [PubMed] [Google Scholar]
- 6.Phuong LK, et al. Use of a vaccine strain of measles virus genetically engineered to produce carcinoembryonic antigen as a novel therapeutic agent against glioblastoma multiforme. Cancer Res. 2003;63:2462–2469. [PubMed] [Google Scholar]
- 7.Blechacz B, et al. Engineered measles virus as a novel oncolytic viral therapy system for hepatocellular carcinoma. Hepatology. 2006;44:1465–1477. doi: 10.1002/hep.21437. [DOI] [PubMed] [Google Scholar]
- 8.Nakamura T, et al. Rescue and propagation of fully retargeted oncolytic measles viruses. Nat Biotechnol. 2005;23:209–214. doi: 10.1038/nbt1060. [DOI] [PubMed] [Google Scholar]
- 9.Hasegawa K, et al. The use of a tropism-modified measles virus in folate receptor-targeted virotherapy of ovarian cancer. Clin Cancer Res. 2006;12:6170–6178. doi: 10.1158/1078-0432.CCR-06-0992. [DOI] [PubMed] [Google Scholar]
- 10.Peng KW, Facteau S, Wegman T, O’Kane D, Russell SJ. Non-invasive in vivo monitoring of trackable viruses expressing soluble marker peptides. Nat Med. 2002;8:527–531. doi: 10.1038/nm0502-527. [DOI] [PubMed] [Google Scholar]
- 11.Peng KW, et al. Pharmacokinetics of oncolytic measles virotherapy: eventual equilibrium between virus and tumor in an ovarian cancer xenograft model. Cancer Gene Ther. 2006;13:732–738. doi: 10.1038/sj.cgt.7700948. [DOI] [PubMed] [Google Scholar]
- 12.Dingli D, et al. Image-guided radiovirotherapy for multiple myeloma using a recombinant measles virus expressing the thyroidal sodium iodide symporter. Blood. 2004;103:1641–1646. doi: 10.1182/blood-2003-07-2233. [DOI] [PubMed] [Google Scholar]
- 13.Mazzaferri EL, Kloos RT. Clinical review 128: current approaches to primary therapy for papillary and follicular thyroid cancer. J Clin Endocrinol Metab. 2001;86:1447–1463. doi: 10.1210/jcem.86.4.7407. [DOI] [PubMed] [Google Scholar]
- 14.Hasegawa K, et al. Dual therapy of ovarian cancer using measles viruses expressing carcinoembryonic antigen and sodium iodide symporter. Clin Cancer Res. 2006;12:1868–1875. doi: 10.1158/1078-0432.CCR-05-1803. [DOI] [PubMed] [Google Scholar]
- 15.Fulci G, et al. Cyclophosphamide enhances glioma virotherapy by inhibiting innate immune responses. Proc Natl Acad Sci USA. 2006;103:12873–12878. doi: 10.1073/pnas.0605496103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kambara H, Saeki Y, Chiocca EA. Cyclophosphamide allows for in vivo dose reduction of a potent oncolytic virus. Cancer Res. 2005;65:11255–11258. doi: 10.1158/0008-5472.CAN-05-2278. [DOI] [PubMed] [Google Scholar]
- 17.Dingli D, et al. Interaction of measles virus vectors with Auger electron emitting radioisotopes. Biochem Biophys Res Commun. 2005;337:22–29. doi: 10.1016/j.bbrc.2005.08.261. [DOI] [PubMed] [Google Scholar]
- 18.Tatsuo H, Ono N, Tanaka K, Yanagi Y. SLAM (CDw150) is a cellular receptor for measles virus. Nature. 2000;406:893–897. doi: 10.1038/35022579. [DOI] [PubMed] [Google Scholar]
- 19.Dorig RE, Marcil A, Chopra A, Richardson CD. The human CD46 molecule is a receptor for measles virus (Edmonston strain) Cell. 1993;75:295–305. doi: 10.1016/0092-8674(93)80071-l. [DOI] [PubMed] [Google Scholar]
- 20.Ong HT, et al. Oncolytic measles virus targets high CD46 expression on multiple myeloma cells. Exp Hematol. 2006;34:713–720. doi: 10.1016/j.exphem.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 21.Anderson BD, Nakamura T, Russell SJ, Peng KW. High CD46 receptor density determines preferential killing of tumor cells by oncolytic measles virus. Cancer Res. 2004;64:4919–4926. doi: 10.1158/0008-5472.CAN-04-0884. [DOI] [PubMed] [Google Scholar]
- 22.Manchester M, Rall GF. Model systems: transgenic mouse models for measles pathogenesis. Trends Microbiol. 2001;9:19–23. doi: 10.1016/s0966-842x(00)01903-x. [DOI] [PubMed] [Google Scholar]
- 23.Mrkic B, et al. Measles virus spread and pathogenesis in genetically modified mice. J Virol. 1998;72:7420–7427. doi: 10.1128/jvi.72.9.7420-7427.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mrkic B, et al. Lymphatic dissemination and comparative pathology of recombinant measles viruses in genetically modified mice. J Virol. 2000;74:1364–1372. doi: 10.1128/jvi.74.3.1364-1372.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kemper C, et al. Membrane cofactor protein (MCP; CD46) expression in transgenic mice. Clin Exp Immunol. 2001;124:180–189. doi: 10.1046/j.1365-2249.2001.01458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peng KW, et al. Biodistribution of oncolytic measles virus after intraperitoneal administration into Ifnar-CD46Ge transgenic mice. Hum Gene Ther. 2003;14:1565–1577. doi: 10.1089/104303403322495070. [DOI] [PubMed] [Google Scholar]
- 27.Evlashev A, et al. Differential permissivity to measles virus infection of human and CD46-transgenic murine lymphocytes. J Gen Virol. 2001;82:2125–2129. doi: 10.1099/0022-1317-82-9-2125. [DOI] [PubMed] [Google Scholar]
- 28.Vincent S, et al. Restriction of measles virus RNA synthesis by a mouse host cell line: trans-complementation by polymerase components or a human cellular factor(s) J Virol. 2002;76:6121–6130. doi: 10.1128/JVI.76.12.6121-6130.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Okada H, et al. Comparative analysis of host responses related to immunosuppression between measles patients and vaccine recipients with live attenuated measles vaccines. Arch Virol. 2001;146:859–874. doi: 10.1007/s007050170121. [DOI] [PubMed] [Google Scholar]
- 30.Okada H, et al. Extensive lymphopenia due to apoptosis of uninfected lymphocytes in acute measles patients. Arch Virol. 2000;145:905–920. doi: 10.1007/s007050050683. [DOI] [PubMed] [Google Scholar]
- 31.Kobune F, et al. Nonhuman primate models of measles. Lab Anim Sci. 1996;46:315–320. [PubMed] [Google Scholar]
- 32.van Binnendijk RS, van der Heijden RW, van Amerongen G, UytdeHaag FG, Osterhaus AD. Viral replication and development of specific immunity in macaques after infection with different measles virus strains. J Infect Dis. 1994;170:443–448. doi: 10.1093/infdis/170.2.443. [DOI] [PubMed] [Google Scholar]
- 33.Auwaerter PG, et al. Measles virus infection in rhesus macaques: altered immune responses and comparison of the virulence of six different virus strains. J Infect Dis. 1999;180:950–958. doi: 10.1086/314993. [DOI] [PubMed] [Google Scholar]
- 34.Hsu EC, et al. Artificial mutations and natural variations in the CD46 molecules from human and monkey cells define regions important for measles virus binding. J Virol. 1997;71:6144–6154. doi: 10.1128/jvi.71.8.6144-6154.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hill DL. Pharmacology. In: Thomas CC, editor. A Review of Cyclophosphamide. Springfield; 1975. pp. 60–85. [Google Scholar]
- 36.Aisenberg AC. Studies on cyclophosphamide-induced tolerance to sheep erythrocytes. J Exp Med. 1967;125:833–845. doi: 10.1084/jem.125.5.833. [DOI] [PMC free article] [PubMed] [Google Scholar]