Amelioration of the premature ageing-like features of Fgf-23 knockout mice by genetically restoring the systemic actions of FGF-23 (original) (raw)
. Author manuscript; available in PMC: 2009 Nov 11.
Published in final edited form as: J Pathol. 2008 Nov;216(3):345–355. doi: 10.1002/path.2409
Abstract
Genetic ablation of fibroblast growth factor 23 from mice (_Fgf_-_23_−/−) results in a short lifespan with numerous abnormal biochemical and morphological features. Such features include kyphosis, hypogonadism and associated infertility, osteopenia, pulmonary emphysema, severe vascular and soft tissue calcifications, and generalized atrophy of various tissues. To determine whether these widespread anomalies in _Fgf_-_23_−/− mice can be ameliorated by genetically restoring the systemic actions of FGF-23, we generated _Fgf_-_23_−/− mice expressing the human _FGF_-23 transgene in osteoblasts under the control of the 2.3 kb _α_1(I) collagen promoter (_Fgf_-_23_−/−/_hFGF_-_23_-Tg double mutants). This novel mouse model is completely void of all endogenous Fgf-23 activity, but produces human FGF-23 in bone cells that is subsequently released into the circulation. Our results suggest that lack of _Fgf_-23 activities results in extensive premature ageing-like features and early mortality of _Fgf_-_23_−/− mice, while restoring the systemic effects of FGF-23 significantly ameliorates these phenotypes, with the resultant effect being improved growth, restored fertility, and significantly prolonged survival of double mutants. With regard to their serum biochemistry, double mutants reversed the severe hyperphosphataemia, hypercalcaemia, and hypervitaminosis D found in _Fgf_-_23_−/− littermates; rather, double mutants show hypophosphataemia and normal serum 1,25-dihydroxyvitamin D3 levels similar to pure _FGF_-23 Tg mice. These changes were associated with reduced renal expression of NaPi2a and 1_α_-hydroxylase, compared to _Fgf_-_23_−/− mice. FGF-23 acts to prevent widespread abnormal features by acting systemically to regulate phosphate homeostasis and vitamin D metabolism. This novel mouse model provides us with an in vivo tool to study the systemic effects of FGF-23 in regulating mineral ion metabolism and preventing multiple abnormal phenotypes without the interference of native Fgf-23.
Keywords: organ atrophy, mineral ion homeostasis, vitamin D metabolism, transgene, human FGF23
Introduction
FGF-23 plays a crucial role in the regulation of phosphate homeostasis and vitamin D metabolism. Initially identified as the causative factor in autosomal dominant hypophosphataemic rickets [1], FGF-23 has since been implicated in other isolated renal phosphate wasting disorders, including X-linked hypophosphataemic rickets [2,3], autosomal dominant hypophosphataemia [4,5], and tumour-induced osteomalacia [6]. Moreover, inactivating mutations in the _FGF_-23 gene result in familial tumoural calcinosis [7], an autosomal recessive disorder characterized by ectopic calcifications and high serum phosphate levels.
To better define the pathophysiological role of FGF-23 in hypophosphataemic diseases, transgenic mice misexpressing FGF-23 under the control of various promoters have been generated [8–10]. These mice reproduce the typical biochemical and skeletal findings of patients with isolated renal phosphate wasting disorders, including profound hypophosphataemia, low or inappropriately normal levels of 1,25-dihydroxyvitamin D3 [1,25(OH)2D3], and rachitic skeletal changes, despite insignificant changes in serum calcium levels. Fgf -_23_−/− animals show the opposite biochemical phenotype and exhibit hyperphosphataemia and increased 1,25(OH)2D3 activity, recapitulating the phenotype observed in patients with familial tumoural calcinosis [11,12].
Interestingly, genetic ablation of _Fgf_-23 results in a widespread phenotype resembling human premature ageing, including profound growth retardation, kyphosis, muscle wasting, infertility, atherosclerosis, extensive soft tissue calcifications, atrophy of multiple organ systems, biochemical dysregulation of phosphate and vitamin D, pulmonary emphysema, osteoporosis, and a severely shortened lifespan [11,12]. To determine whether lack of circulating FGF-23 is in fact responsible for the widespread systemic ageing-like phenotype observed in Fgf -_23_−/− animals, we investigated whether such features can be ameliorated through the systemic actions of FGF-23. For this purpose, we generated Fgf -_23_−/−/_hFGF_-_23_-Tg double mutants, void of endogenous Fgf-23, with circulating human FGF-23 derived from osteoblasts.
Materials and methods
Generation of _Fgf-23_−/−/hFGF-23-Tg double-mutant mice
We have previously reported the generation of _Fgf_-23 knockout mice (Fgf -_23_−/−) [11] and hFGF-23 transgenic animals under the control of the _α_1(I) collagen promoter (_col 1_-_FGF_-_23_-Tg) [9]. Fgf -_23_−/−/_hFGF_-_23_-Tg double-mutant animals were generated by crossing _col 1_-_FGF_-_23_-Tg animals into the Fgf -_23_−/− mouse background. All mice analysed were littermates and in the C57Bl/6 mouse genetic background. Mice were maintained on a standard diet (PicoLab Mouse Diet 20–5058) and autoclaved water. Experiments were approved by the Institutional Care and Use Committee (IACUC) at HMS and maintained in accordance with the NIH Guide for the Care and Use of Laboratory Animals.
Genotyping of wild-type and Fgf -_23_−/− mice was conducted as previously described [11]. The presence of the human _FGF_-23 transgene was detected using the following primers and PCR conditions: _hFGF_-23 forward 5′-GGCAACATTTTTGGATCA-3′, reverse 5′-CCGGGGCTTCAGCACGTT-3′; 94 °C for 5 min, 94 °C for 50 s, 57 °C for 50 s, 72 °C for 50 s for 35 cycles, and 72 °C for 10 min; expected product size 291 bp.
Macroscopic phenotype
The gross appearance of Fgf -_23_−/−/_hFGF_-_23_-Tg double mutants and littermates was determined. Total body weight was measured weekly from 3 to 12 weeks of age. Survival was recorded until death or 6 months of age. The ability of circulating hFGF-23 to rescue the infertility present in Fgf -_23_−/− animals was evaluated by crossing double mutants with wild-type mice.
Skeletal analyses
The skeletal changes were determined in age-matched littermates by both X-ray analyses and dual-energy X-ray absorptiometery (DEXA), as previously described [11]. Total bone mineral content (BMC) and left hind-limb bone mineral density (BMD) were measured.
Biochemical analyses
Blood was collected by heart puncture of 3-, 6-, and 9- to 12-week-old animals under anaesthesia. Serum phosphorus and total serum calcium were determined using Stanbio Phosphorus LiquiUV and LiquiColor (Arsenazo III) kits, respectively (Stanbio Laboratory, Boerne, TX, USA). PTH and FGF-23 were measured using the Mouse Intact PTH Kit and the Human FGF-23 (C-terminal) ELISA Kit (Immunotopics, San Clemente, CA, USA), respectively. Serum 1,25(OH)2D3 levels were determined using a radio-receptor assay (Immunodiagnostik, Bensheim, Germany).
Immunohistochemistry
Immunofluorescence staining for NaPi2a was performed on PLP (1%, phosphate/lysine/PFA)-fixed frozen kidney sections, as previously described [13], using a primary polyclonal anti-NaPi2a antibody (Alpha Diagnostic, TX, USA), diluted 1: 100, and FITC-labelled anti-rabbit secondary antibody, diluted 1: 100. Rabbit serum was used as a negative control. Quantification of the area and staining intensity of the fluorescent signal was determined for the various groups of mice, as previously described [14].
Quantitative real-time (qRT) PCR
qRT-PCR was performed to measure the relative expression of renal 1α(OH)ase and klotho mRNA of 9- to 12-week-old mice. Total RNA was isolated from snap-frozen kidneys using the Agilent Total RNA Isolation Mini Kit (Agilent Technologies, Palo Alto, CA, USA) and reverse-transcribed using the QuantiTectR Reverse Transcription Kit (Quiagen). qRT-PCR was performed using the ABI 7300 RT-PCR system (Applied Biosystems): 1α(OH)ase forward 5′-TCAGATGTTTGCCTTTGCCC-3′, reverse 5′-TGGTTCCTCATCGCAGCTTC-3′; klotho forward 5′-TGGCTTTCCTCCTTTACCTG-3′, reverse 5′-GCCGACACTGGGTTTTGT-3′; m36B4 forward 5′-AGATGCAGCAGATCCGCAT-3′, reverse 5′-GTTCTTGCCCATCAGCACC-3′; 95 °C for 2 min, 95 °C for 10 s, 55 °C for 20 s, 68.0 °C for 1 min for 45 cycles. All samples were run in triplicate using SYBR green (Eppendorf, Westbury, NY, USA), with 36B4 used as an internal control [15]. The relative expression of renal 1α(OH)ase and klotho is expressed as RQ values.
Histological evaluation and morphometric analyses
Histological evaluation was performed on sex-matched mice of each genotype at 6 and 9–12 weeks of age (n > 6 for each group, 6–8 sections per tissue per mouse) [11,16]. Paraffin sections were prepared and stained with haematoxylin and eosin (H&E) to determine tissue morphology, and with von Kossa staining to detect ectopic calcifications.
Morphometric analyses were performed on H&E-stained tissue sections from 9- to 12-week-old mice using Adobe Photoshop 7.0. Two representative sections for each of the histological parameters of five mice per genotype were used. Variations in intestinal morphology were assessed by considering measures of intestinal villus height and intestinal villus area on 100× images, as previously described [17,18]. Quantification of changes in hind-limb skin morphology was assessed by measuring skin thickness, from the top of the epidermis to the bottom of the dermis on 50× images, as previously described [19]. The underlying subcutaneous fat layer thickness and muscle thickness were also quantified. Changes in lung morphology were assessed by comparing both the relative number of alveolar air spaces per section and the alveolar air space area on 100× images, as previously described [20,21]. To determine the relative number of alveolar air spaces between the various groups of mice, all alveolar air spaces present on each of the 100× images were counted. The area of each alveolar air space was also determined. For this purpose, areas with large conducting airways, longitudinal sections of alveolar ducts, multiple blood vessels, tears in air space walls, and clumps of extravasated red blood cells were excluded.
Statistical analysis
Student’s _t_-test for comparison between two groups and one-way analysis of variance (ANOVA) followed by the Bonferroni _post_-hoc test for multiple comparisons were performed using SPSS 15.0 for Windows (SPSS Inc, Chicago, IL, USA). All values are presented as mean ± SEM. p < 0.05 was considered statistically significant. The following groups were used for comparison: control (CTRL); Fgf -_23_−/− (KO); double mutant (Fgf -_23_−/−/_hFGF_-_23_-Tg, DM); and transgenic Fgf -23+/+/_col 1_-_FGF_-_23_-Tg (TG) animals.
Results
Generation and characterization _of Fgf-23_−/−/hFGF-23-Tg double-mutant mice
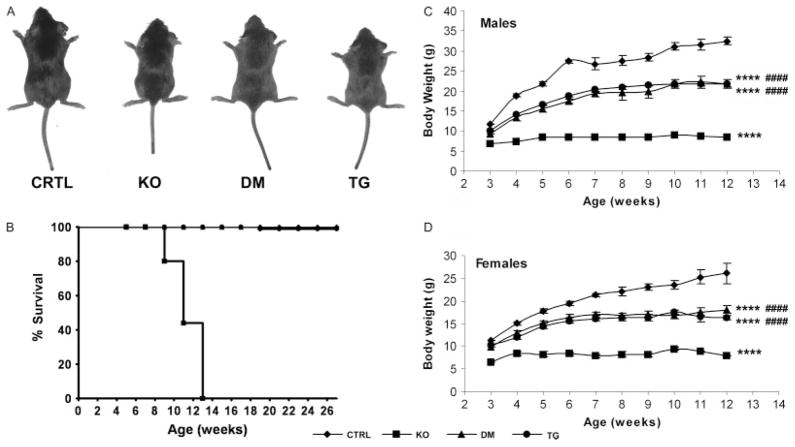
Fgf -_23_−/−/_hFGF_-_23_-Tg double mutants were born at the expected Mendelian frequency. Fgf -_23_−/− animals developed severe growth retardation, including reduced body weight by 3 weeks of age. Kyphosis, gait walk, hypokinesis, sparse hair, and decreased muscle mass were also apparent. Double mutants showed a significant improvement in their gross appearance, body weight, and physical activity compared with Fgf -_23_−/− mice, with the exception of a slight degree of growth retardation after weaning when compared with control littermates (Figures 1A, 1C, and 1D). Moreover, the shortened lifespan and infertility secondary to the hypogonadism documented in Fgf -_23_−/− animals were completely rescued in double-mutant mice, and female double mutants were able to wean their pups successfully. While Fgf -_23_−/− animals died by 13 weeks of age, circulating hFGF-23 was able to extend their lifespan significantly, with all of the double mutants surviving the entire 6-month experimental period and beyond (Figure 1B), as also seen in _col 1_-_FGF_-_23_-Tg littermates (Figures 1A–1D).
Figure 1.
Macroscopic characterization. (A) Macroscopic image of control (CTRL), Fgf -_23_−/− (KO), Fgf -_23_−/−/_hFGF_-_23_-Tg double-mutant (DM), and _col 1_-_FGF_-_23_-Tg (TG) male littermates at 3 weeks of age. (B) Survival curve of mice with various genotypes until death or 6 months of age (n = 7 for CTRL, ◆; n = 10 for KO, ■; n = 5 for DM, ▲; n = 18 for TG, ●). Body weight curve of male (C) and female (D) control (CTRL, ◆), Fgf -_23_−/− (KO, ■), Fgf -_23_−/−/_hFGF_-_23_-Tg double-mutant (DM, ▲) and _col 1_-_FGF_-_23_-Tg (TG, ●) mice from 3 to12 weeks of age; n > 5 for each group of mice. ****p < 0.0001, statistically significant difference compared with controls. ####p < 0.0001, statistically significant difference compared with Fgf -_23_−/− mice
Skeletal analyses
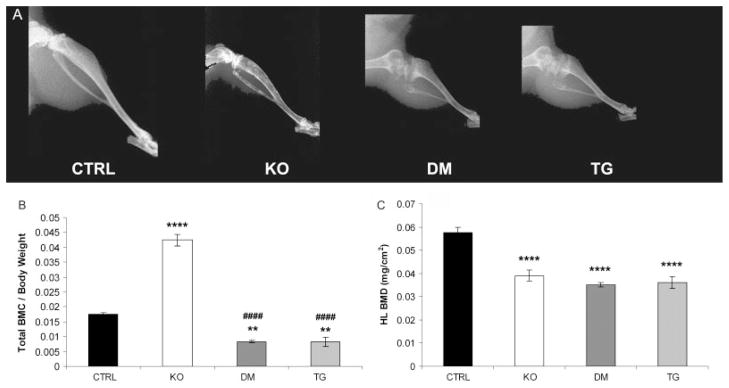
X-ray imaging showed a reduction in BMD in the hind limbs from Fgf -_23_−/−, double mutants, and transgenic mice compared with control littermates. In contrast to the bones of Fgf -_23_−/− animals, the bones of double-mutant and transgenic mice showed typical features of rickets, with widening of the epiphysis and capping of the metaphysis, suggesting that the transgene dominates over the Fgf -_23_−/− phenotype. Long bones of mice bearing the transgene were short and disproportionately wide, with a radiolucent appearance (Figure 2A).
Figure 2.
Skeletal phenotype. (A) X-ray autoradiographs of the hind limbs from male littermates at 9 weeks of age. Graphic display of total body mineral content (BMC) (B) and hind-limb bone mineral density (BMD) (C) of control (CTRL, n = 8), Fgf -_23_−/− (KO, n = 8), Fgf -_23_−/−/_hFGF_-_23_-Tg double-mutant (DM, n = 4), and _col 1_-_FGF_-_23_-Tg (TG, n = 4) mice as measured by PIXImus analysis at 9 weeks of age. Each value obtained for BMC was normalized to the body weight of the corresponding animal. **p < 0.01 and ****p < 0.0001, statistically significant difference compared with controls. ####p < 0.0001, statistically significant difference compared with Fgf -_23_−/− mice
The significant reduction in BMD observed in the hind limbs of Fgf -_23_−/−, double-mutant, and transgenic mice was quantified using PIXImus analysis (Figure 2B). Interestingly, the gross reduction in BMD apparent in Fgf -_23_−/− animals and consistent with senile osteoporosis in humans persisted in mice with high circulating levels of FGF-23. In contrast to the increase in total BMC observed in Fgf -_23_−/− mice [11,12], an overall reduction in BMC was observed in double-mutant and transgenic mice, compared with their control littermates (Figure 2C). This observation indicates that expression of the _hFGF_-23 transgene leads to a reduction in ectopic soft tissue mineralization throughout the body.
Serum biochemistry
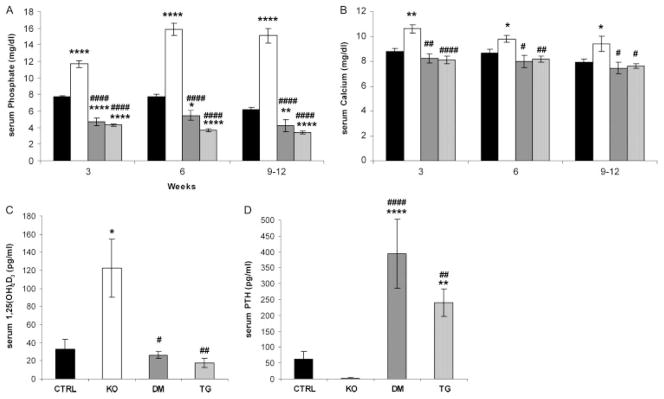
Fgf -_23_−/− mice showed significantly higher serum phosphate, calcium, and 1,25(OH)2D3 levels compared with control littermates. In contrast, the newly generated double mutants and _col 1_-_FGF_-_23_-Tg mice showed hypophosphataemia and inappropriately normal levels of 1,25(OH)2D3. Furthermore, in contrast to the suppressed PTH levels in Fgf -_23_−/− mice, increased serum PTH levels were noted in double-mutant and transgenic mice, despite insignificant changes in serum calcium levels at all the time points investigated (Figures 3A–3D).
Figure 3.
Serum biochemistry. Comparison of serum parameters for control (CTRL), Fgf -_23_−/− (KO), Fgf -_23_−/−/_hFGF_-_23_-Tg double-mutant (DM), and _col 1_-_FGF_-_23_-Tg (TG) littermates; n > 3 for each group of mice. (A) Serum phosphate and (B) serum calcium levels for the various groups of mice at 3, 6, and 9–12 weeks of age. (C) Serum 1,25(OH)2D3 and (D) serum PTH levels of the various groups of mice at 6–9 weeks of age. *p < 0.05, **p < 0.01, and ****p < 0.0001, statistically significant difference compared with controls. #p < 0.05, ##p < 0.01, and ####p < 0.0001, statistically significant difference compared with Fgf -_23_−/− mice
To determine the effect of the _col 1_-_hFGF_-_23_transgene and to confirm that the FGF-23 protein is in fact secreted into the bloodstream, we measured circulating hFGF-23 levels in double mutants at 6–9 weeks of age (10 569.9 ± 203.4 RU/ml). As expected, hFGF-23 was not detectable in the serum of control or Fgf -_23_−/− animals.
Key molecules of mineral homeostasis
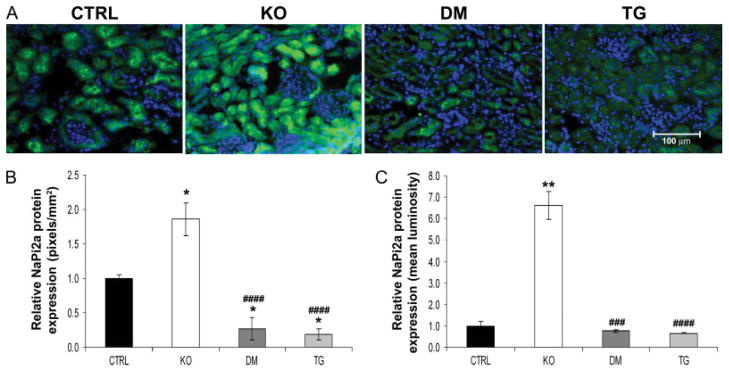
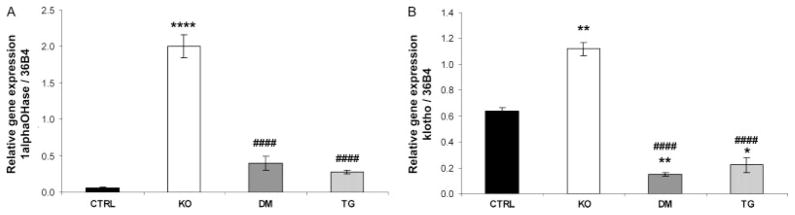
Analyses at the molecular level further clarified the biochemical parameters present in control, Fgf -_23_−/−, Fgf -_23_−/−/_hFGF_-_23_-Tg double-mutant, and _col 1_-_FGF_-_23_-Tg mice. No significant difference in NaPi2a mRNA levels was detected in the various groups of mice at 9 weeks of age, as determined by qRT-PCR (data not shown); however, an increase in NaPi2a protein was consistently detected on the luminal side of the proximal tubules of Fgf -_23_−/− mice when compared with controls. As expected, double-mutant and transgenic animals showed a clear reduction in NaPi2a expression, corresponding to the hypophosphataemia observed in these mice (Figures 4A and 4B). In contrast to the increased expression of renal 1α(OH)ase in Fgf -_23_−/− mice, double-mutant and transgenic mice exhibited normal levels of 1α(OH)ase, indistinguishable from wild-type littermates (Figure 5A), suggesting that FGF-23 acts systemically to regulate phosphate homeostasis and vitamin D metabolism. Moreover, Fgf -_23_−/− animals showed a significant increase in the renal expression of klotho. In contrast, the double-mutant and transgenic mice showed a corresponding and significant reduction in klotho mRNA compared with controls (Figure 5B).
Figure 4.
Immunofluorescence staining for NaPi2a. (A) Immunohistochemistry of renal NaPi2a protein (green) at 9 weeks of age for control (CTRL), Fgf -_23_−/− (KO), Fgf -_23_−/−/_hFGF_-_23_-Tg double-mutant (DM), and _col 1_-_FGF_-_23_-Tg (TG) littermates (n = 3 for each group of mice). In contrast to the increased expression of NaPi2a protein observed in Fgf -_23_−/− mice, reduced expression is observed in the double-mutant and pure transgenic mice compared with their littermate controls. Nuclear counterstaining was performed with DAPI (blue); original magnification × 400. Quantification of protein expression was performed by considering both the area (B) and the staining intensity (C) of the fluorescent signal. All data were quantified on images taken under identical conditions. The relative area of staining was determined by calculating the number of positively stained pixels per mm2 of cells. The relative staining intensity was determined by considering the mean luminosity value on background corrected images. *p < 0.05 and **p < 0.01, statistically significant difference compared with controls. ###p < 0.001 and ####p < 0.0001, statistically significant difference compared with Fgf -_23_−/−
Figure 5.
Quantitative real-time PCR. RT-PCR for renal (A) 1α(OH)ase and (B) klotho mRNA of control (CTRL), Fgf -_23_−/− (KO), Fgf -_23_−/−/_hFGF_-_23_-Tg double-mutant (DM), and _col 1_-_FGF_-_23_-Tg (TG) littermates at 9 weeks of age (n = 3 for each genotype). **p < 0.01 and ****p < 0.0001, statistically significant difference compared with controls. ####p < 0.0001, statistically significant difference compared with Fgf -_23_−/−
Histological evaluation and morphometric analyses
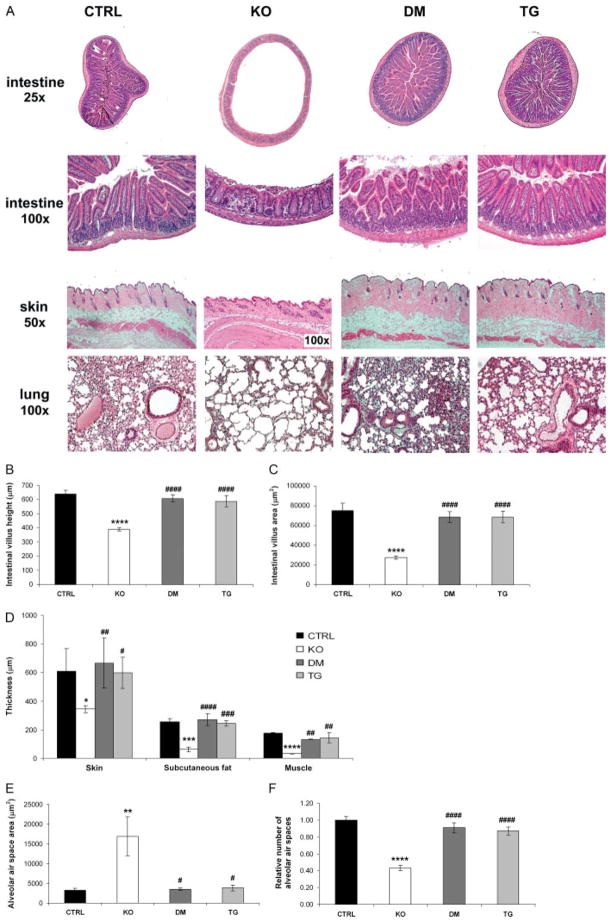
Histological examination of the various groups of mice suggested that many of the soft tissue abnormalities present in Fgf -_23_−/− mice could be ameliorated by circulating hFGF-23 (Figure 6). Fgf -_23_−/− mice showed severe atrophy of the skin, with a significant reduction in the skin thickness and a decrease in the underlying subcutaneous fat and muscle layer thicknesses compared with controls. These atrophic features present in Fgf -_23_−/− animals were dramatically ameliorated in all the double mutants studied (Figures 6A and 6D). Similarly, atrophy of the intestinal mucosa, consistently observed in Fgf -_23_−/− mice, was rescued by bone-derived hFGF-23. Intestinal villus height and villus area were also restored and were comparable to those observed in controls (Figures 6A–6C). Moreover, the emphysemic features noted in the lungs of Fgf -_23_−/− mice were significantly improved in double mutants. Whereas the lungs of Fgf -_23_−/− mice demonstrated destruction of the normal alveolar architecture, including enlargement of the air spaces distal to the terminal bronchioles, double mutants displayed complete amelioration of this phenotype, with pulmonary structures indistinguishable from those of control littermates (Figures 6E and 6F). Fgf -_23_−/− male mice displayed hypogonadism, as demonstrated by the atrophy of the seminiferous tubules, which renders them infertile. In contrast, double-mutant animals showed a normal testicular size (data not shown). In all cases, the histological features of the double mutants were indistinguishable from those of their _col 1_-_FGF_-_23_-Tg littermates (Figures 6A–6F). The soft tissue abnormalities mentioned above were consistently observed in Fgf -_23_−/− mice at all the time points investigated. Normalization of these phenotypes was found in all double-mutant and transgenic animals.
Figure 6.
Histological evaluation. (A) Haematoxylin and eosin staining of various tissues from control (CTRL, n = 9), Fgf -_23_−/− (KO, n = 7), Fgf -_23_−/−/_hFGF_-_23_-Tg double-mutant (DM, n = 7), and _col 1_-_FGF_-_23_-Tg (TG, n = 9) littermates at 9–12 weeks of age. The images shown represent typical findings for each genotype. Please note the difference in magnification between the Fgf -_23_−/− and littermates of other genotypes for the images of the skin. Quantification of changes in (B, C) intestinal, (D) skin, and (E, F) lung morphology. Compared with controls, Fgf -_23_−/− mice exhibit extensive atrophy of the intestinal mucosa with a significant decrease in the intestinal villus height and intestinal villus area. A significant reduction in skin, subcutaneous fat layer, and muscle layer thicknesses is also apparent in the Fgf -_23_−/− mice. Moreover, the lungs of Fgf -_23_−/− animals exhibit typical features of emphysema, including significant enlargement of the mean alveolar air space area and a reduction in the number of alveolar air spaces compared with controls. Such features were consistently absent in all double-mutant and transgenic mice examined, with normalization of the intestine, skin, and lung morphology
Soft tissue calcifications
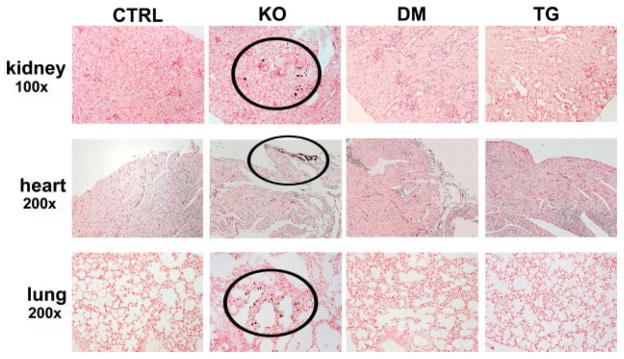
Ectopic calcifications were first detected in the soft tissues of Fgf -_23_−/− mice at 9 weeks of age by von Kossa staining. Mineral deposits were observed in various non-skeletal tissues, including the lung, small arteries in the kidney, and heart. The atherosclerotic lesions, kidney stones, and other extensive soft tissue calcifications present in all Fgf -_23_−/− animals were consistently absent in the double-mutant and _col 1_-_FGF_-_23_-Tg mice (Figure 7).
Figure 7.
Soft tissue calcifications. Von Kossa staining of various tissues from control (CTRL, n = 9), Fgf -_23_−/− (KO, n = 7), Fgf -_23_−/−/_col 1_-_FGF_-_23_-Tg double-mutant (DM, n = 7), and _col 1_-_FGF_-_23_-Tg (TG, n = 9) littermates at 9 weeks of age. Fgf -_23_−/− animals exhibit extensive soft tissue calcifications in multiple tissues; calcifications can be noted within areas depicted by black circles. Kidney calcifications are primarily present in the tubules, while mineral deposition in the heart is mainly localized in the valves. In the lungs, most of the calcifications can be detected in the alveolar septal wall. No soft tissue calcifications were observed in any tissues examined for control, double-mutant, and pure transgenic animals
Discussion
In this study, we have described the generation of a novel mouse model that is completely void of endogenous _Fgf_-23 activity, but misexpresses human FGF-23 in osteoblasts, which is subsequently released into the circulation. This mouse model offers a unique in vivo tool to study the systemic effects of human FGF-23 in a micro-environment without interference of mouse endogenous Fgf-23. Our newly generated Fgf -_23_−/−/_hFGF_-_23_-Tg double mutants are therefore different from conventional transgenic FGF-23 mice; in conventional transgenic mice, it is difficult to differentiate to what extent the biochemical changes are due to human FGF-23 versus mouse endogenous Fgf-23. This unique mouse model allows the study of systemic and potential autocrine/paracrine actions of FGF-23 in vivo and helps to clarify its role in mineral ion homeostasis and the subsequent development of premature ageing-like phenotypes.
Genetic ablation of the _Fgf_-23 gene results in an extensive premature ageing-like phenotype involving multiple organ systems and early mortality of Fgf -23_−/− mice. Circulating FGF-23 was able to rescue the macroscopic and histological features present in these mice, with the newly generated double mutants being largely indistinguishable from their littermate controls. This rescue experiment shows that FGF-23 acts in a systemic manner, rather than as an autocrine/paracrine factor, to regulate these processes in multiple tissues. Specifically, it appears that FGF-23 primarily serves as a systemic factor to regulate phosphate homeostasis and vitamin D metabolism by acting on the renal proximal tubules to down-regulate NaPi2a and 1_α(OH)ase activity, respectively.
Recent investigations suggest that the renal effects of FGF-23 are mediated by the cofactor klotho [22,23]. Since 1,25(OH)2D3 is an active stimulator of klotho expression in the kidney, the relatively higher renal expression of klotho in Fgf -_23_−/− mice could be a 1,25(OH)2D3-mediated process. It may also represent an attempt at compensation for the lack of Fgf-23 activity in these mice, similar to the up-regulation of _Fgf_-23 observed in _klotho_−/− mice [23]. Interestingly, the level of klotho expression in the kidney of double mutants was less than that of their control littermates and may represent a similar attempt to compensate for the onslaught of high circulating FGF-23 levels. This would suggest the presence of a negative feedback mechanism, which may act under physiological conditions to fine tune FGF-23 bioactivity through the alteration of klotho expression. Needless to say, the expression of klotho in Fgf -_23_−/− mice may differ with age as a result of compensatory effects from associated altered mineral ion and 1,25(OH)2D3 homeostasis [16,24,25].
Both Fgf -_23_−/− and klotho_−/− mutant mice exhibited a similar and striking biochemical phenotype consisting of hyperphosphataemia, hypercalcaemia, and high serum 1,25(OH)2D3 levels [12,26]. In contrast, both double-mutant and pure transgenic mice exhibited inappropriately normal levels of serum 1,25(OH)2D3 for their degree of hypophosphataemia. Our in vivo results suggest that FGF-23 primarily serves as a hormone to regulate phosphate homeostasis and vitamin D metabolism by acting on the renal proximal tubules to down-regulate NaPi2a and 1_α(OH)ase activity, respectively. Interestingly, reduction or ablation of vitamin D activity in Fgf -_23_−/− and _klotho_−/− mutants can rescue much of their abnormal ageing-like phenotype [16,27–29]. The secondary hyperparathyroidism exhibited by the Fgf -_23_−/−/_hFGF_-23_-Tg double mutants may contribute to the hypophosphataemia and skeletal anomalies observed in these mice. Moreover, high serum PTH may account for the normal serum 1,25(OH)2D3 levels in double mutants, since PTH is a potent stimulator of 1_α(OH)ase and may act to counterbalance the suppressive effects of FGF-23 on 1,25(OH)2D3 in double mutants. The exact mechanism for the high PTH levels and relatively normal 1,25(OH)2D3 level in double mutants, however, remains to be determined. It is well known that PTH production can be up-regulated in response to both hypocalcaemia and low serum 1,25(OH)2D3 [30], neither of which is present in our studied Fgf -_23_−/−/_hFGF_-_23_-Tg double-mutant mice. Given the normal serum calcium and 1,25(OH)2D3 levels observed, the severe hypophosphataemia apparent in these mice is expected to cause a reduction, not induction, of PTH production. Since PTH is up-regulated in double-mutant mice, despite normal calcium and 1,25(OH)2D3, and hypophosphataemia, the possibility that FGF-23 acts directly on the parathyroid gland to induce PTH production cannot be ignored, since klotho has been shown to be expressed in the parathyroid gland [31,32]. It is worth mentioning, however, that a recent study suggested an inhibitory effect of FGF-23 on PTH production [33,34]. In such a scenario, theoretically, our double-mutant mouse model should exhibit a low rather than a high serum PTH level. Clinically, a corresponding increase in the FGF-23 and PTH levels is observed in patients with chronic kidney disease (CKD) [35,36]. Moreover, patients with increased FGF-23 levels are more likely to develop severe secondary hyperparathyroidism, and a positive correlation between serum FGF-23 and PTH levels has been detected in CKD patients receiving dialysis treatment [37–40]. Additional studies will resolve the exact molecular interrelationship among FGF-23, 1,25(OH)2D3, and PTH, and our newly generated mice will help in studying such relationships.
Earlier in vivo genetic manipulation approaches suggest a possible local effect of FGF-23 in bone [2,11,41]. In the current investigation, the osteopenia present in Fgf -_23_−/− mice persists in the double-mutant mice despite an increase in circulating FGF-23 levels. The current findings are similar to those observed in klotho rescue experiments, in which osteopenia persists despite klotho supplementation following weaning [27,31,42]. While the mouse model developed for the current study does not permit the separation of systemic versus potential autocrine/paracrine actions of FGF-23 in skeletal tissues, the possibility that FGF-23 has a cell autonomous effect in bone cannot be ignored. Another potential limitation of this model is that FGF-23 is being expressed from a transgene promoter, rather than the native Fgf-23 locus, which may prevent possible transcriptional feedback mechanisms from operating. Thus, while the role of FGF-23 as a principal player in the bone–kidney axis regulating skeletal mineralization through systemic phosphate homeostasis and vitamin D metabolism is undisputable, future in vivo and in vitro studies will help to clarify the potential autocrine/paracrine effects of FGF-23 in bone and the molecular interplay between FGF-23 and other mineralization factors produced by bone cells.
The results of this study suggest the hormonal effects of FGF-23 in systemic regulation of phosphate homeostasis by coordinating the effects of the bone–kidney–parathyroid gland axis. Moreover, we show that circulating FGF-23 prevents the development of multiple abnormal phenotypes observed in Fgf -_23_−/− mice by acting systemically in a non-cell-autonomous manner. FGF-23 specifically targets the kidney to cause an increase in phosphate excretion; moreover, it serves as a potent regulator of both 1,25(OH)2D3 and PTH. The therapeutic potential of FGF-23 in diseases of mineral ion dysregulation [43], including the multitude of patients with CKD, and its involvement in the prevention of multiple age-related disorders, including senile osteoporosis and vascular calcifications, are exciting and require further investigation.
In conclusion, our newly generated _Fgf 23_−/−/_col 1_-_FGF23_-Tg double-mutant mice provide a unique in vivo tool to study as yet unknown systemic and local effects of human FGF-23 without interference of endogenous mouse Fgf-23. These transgenic mice offer a cleaner mouse model system to study the molecular mechanisms of human FGF-23 in the regulation of phosphate homeostasis, vitamin D metabolism, and skeletogenesis.
Acknowledgments
This work was partly supported by grants provided by NIDDK (R01-073944 to BL and R01-077276 to MSR). We would also like to thank Somi Kim for her technical contribution.
Footnotes
No conflicts of interest were declared.
References
- 1.Consortium A. Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23. Nature Genet. 2000;26:345–348. doi: 10.1038/81664. [DOI] [PubMed] [Google Scholar]
- 2.Liu S, Guo R, Simpson LG, Xiao ZS, Burnham CE, Quarles LD. Regulation of fibroblastic growth factor 23 expression but not degradation by PHEX. J Biol Chem. 2003;278:37419–37426. doi: 10.1074/jbc.M304544200. [DOI] [PubMed] [Google Scholar]
- 3.Yamazaki Y, Okazaki R, Shibata M, Hasegawa Y, Satoh K, Tajima T, et al. Increased circulatory level of biologically active full-length FGF-23 in patients with hypophosphatemic rickets/osteomalacia. J Clin Endocrinol Metab. 2002;87:4957–4960. doi: 10.1210/jc.2002-021105. [DOI] [PubMed] [Google Scholar]
- 4.Lorenz-Depiereux B, Bastepe M, Benet-Pages A, Amyere M, Wagenstaller J, Muller-Barth U, et al. DMP1 mutations in autosomal recessive hypophosphatemia implicate a bone matrix protein in the regulation of phosphate homeostasis. Nature Genet. 2006;38:1248–1250. doi: 10.1038/ng1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feng JQ, Ward LM, Liu S, Lu Y, Xie Y, Yuan B, et al. Loss of DMP1 causes rickets and osteomalacia and identifies a role for osteocytes in mineral metabolism. Nature Genet. 2006;38:1310–1315. doi: 10.1038/ng1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shimada T, Mizutani S, Muto T, Yoneya T, Hino R, Takeda S, et al. Cloning and characterization of FGF23 as a causative factor of tumor-induced osteomalacia. Proc Natl Acad Sci U S A. 2001;98:6500–6505. doi: 10.1073/pnas.101545198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benet-Pages A, Orlik P, Strom TM, Lorenz-Depiereux B. An FGF23 missense mutation causes familial tumoral calcinosis with hyperphosphatemia. Hum Mol Genet. 2005;14:385–390. doi: 10.1093/hmg/ddi034. [DOI] [PubMed] [Google Scholar]
- 8.Shimada T, Urakawa I, Yamazaki Y, Hasegawa H, Hino R, Yoneya T, et al. FGF-23 transgenic mice demonstrate hypophosphatemic rickets with reduced expression of sodium phosphate cotransporter type IIa. Biochem Biophys Res Commun. 2004;314:409–414. doi: 10.1016/j.bbrc.2003.12.102. [DOI] [PubMed] [Google Scholar]
- 9.Larsson T, Marsell R, Schipani E, Ohlsson C, Ljunggren O, Tenenhouse HS, et al. Transgenic mice expressing fibroblast growth factor 23 under the control of the alpha1(I) collagen promoter exhibit growth retardation, osteomalacia, and disturbed phosphate homeostasis. Endocrinology. 2004;145:3087–3094. doi: 10.1210/en.2003-1768. [DOI] [PubMed] [Google Scholar]
- 10.Bai X, Miao D, Li J, Goltzman D, Karaplis AC. Transgenic mice overexpressing human fibroblast growth factor 23 (R176Q) delineate a putative role for parathyroid hormone in renal phosphate wasting disorders. Endocrinology. 2004;145:5269–5279. doi: 10.1210/en.2004-0233. [DOI] [PubMed] [Google Scholar]
- 11.Sitara D, Razzaque MS, Hesse M, Yoganathan S, Taguchi T, Erben RG, et al. Homozygous ablation of fibroblast growth factor-23 results in hyperphosphatemia and impaired skeletogenesis, and reverses hypophosphatemia in Phex-deficient mice. Matrix Biol. 2004;23:421–432. doi: 10.1016/j.matbio.2004.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shimada T, Kakitani M, Yamazaki Y, Hasegawa H, Takeuchi Y, Fujita T, et al. Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J Clin Invest. 2004;113:561–568. doi: 10.1172/JCI19081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Razzaque MS, Foster CS, Ahmed AR. Role of collagen-binding heat shock protein 47 and transforming growth factor-beta1 in conjunctival scarring in ocular cicatricial pemphigoid. Invest Ophthalmol Vis Sci. 2003;44:1616–1621. doi: 10.1167/iovs.02-0644. [DOI] [PubMed] [Google Scholar]
- 14.Kirkeby S, Thomsen CE. Quantitative immunohistochemistry of fluorescence labelled probes using low-cost software. J Immunol Methods. 2005;301:102–113. doi: 10.1016/j.jim.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 15.Sonoda J, Mehl IR, Chong LW, Nofsinger RR, Evans RM. PGC-1beta controls mitochondrial metabolism to modulate circadian activity, adaptive thermogenesis, and hepatic steatosis. Proc Natl Acad Sci U S A. 2007;104:5223–5228. doi: 10.1073/pnas.0611623104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Razzaque MS, Sitara D, Taguchi T, St-Arnaud R, Lanske B. Premature aging-like phenotype in fibroblast growth factor 23 null mice is a vitamin D-mediated process. FASEB J. 2006;20:720–722. doi: 10.1096/fj.05-5432fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sakamoto K, Hirose H, Onizuka A, Hayashi M, Futamura N, Kawamura Y, et al. Quantitative study of changes in intestinal morphology and mucus gel on total parenteral nutrition in rats. J Surg Res. 2000;94:99–106. doi: 10.1006/jsre.2000.5937. [DOI] [PubMed] [Google Scholar]
- 18.Ribeiro SR, Pinto PE, Jr, de Miranda AC, Bromberg SH, Lopasso FP, Irya K. Weight loss and morphometric study of intestinal mucosa in rats after massive intestinal resection: influence of a glutamine-enriched diet. Rev Hosp Clin Fac Med Sao Paulo. 2004;59:349–356. doi: 10.1590/s0041-87812004000600007. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y, McCormick LL, Gilliam AC. Latency-associated peptide prevents skin fibrosis in murine sclerodermatous graft-versus-host disease, a model for human scleroderma. J Invest Dermatol. 2003;121:713–719. doi: 10.1046/j.1523-1747.2003.12517.x. [DOI] [PubMed] [Google Scholar]
- 20.Sampath V, Davis K, Senft AP, Richardson TR, Kitzmiller JA, Berclaz PY, et al. Altered postnatal lung development in C3H/HeJ mice. Pediatr Res. 2006;60:663–668. doi: 10.1203/01.pdr.0000246071.50268.51. [DOI] [PubMed] [Google Scholar]
- 21.Hardie WD, Bruno MD, Huelsman KM, Iwamoto HS, Carrigan PE, Leikauf GD, et al. Postnatal lung function and morphology in transgenic mice expressing transforming growth factor-alpha. Am J Pathol. 1997;151:1075–1083. [PMC free article] [PubMed] [Google Scholar]
- 22.Kuro-o M. Klotho as a regulator of fibroblast growth factor signaling and phosphate/calcium metabolism. Curr Opin Nephrol Hypertens. 2006;15:437–441. doi: 10.1097/01.mnh.0000232885.81142.83. [DOI] [PubMed] [Google Scholar]
- 23.Urakawa I, Yamazaki Y, Shimada T, Iijima K, Hasegawa H, Okawa K, et al. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature. 2006;444:770–774. doi: 10.1038/nature05315. [DOI] [PubMed] [Google Scholar]
- 24.Lanske B, Razzaque MS. Mineral metabolism and aging: the fibroblast growth factor 23 enigma. Curr Opin Nephrol Hypertens. 2007;16:311–318. doi: 10.1097/MNH.0b013e3281c55eca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Razzaque MS, St-Arnaud R, Taguchi T, Lanske B. FGF-23, vitamin D and calcification: the unholy triad. Nephrol Dial Transplant. 2005;20:2032–2035. doi: 10.1093/ndt/gfh991. [DOI] [PubMed] [Google Scholar]
- 26.Sitara D, Razzaque MS, St-Arnaud R, Huang W, Taguchi T, Erben RG, et al. Genetic ablation of vitamin D activation pathway reverses biochemical and skeletal anomalies in Fgf-23-null animals. Am J Pathol. 2006;169:2161–2170. doi: 10.2353/ajpath.2006.060329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Razzaque MS, Lanske B. Hypervitaminosis D and premature aging: lessons learned from Fgf23 and Klotho mutant mice. Trends Mol Med. 2006;12:298–305. doi: 10.1016/j.molmed.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 28.Lanske B, Razzaque MS. Premature aging in klotho mutant mice: cause or consequence? Ageing Res Rev. 2007;6:73–79. doi: 10.1016/j.arr.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hesse M, Frohlich LF, Zeitz U, Lanske B, Erben RG. Ablation of vitamin D signaling rescues bone, mineral, and glucose homeostasis in Fgf-23 deficient mice. Matrix Biol. 2007;26:75–84. doi: 10.1016/j.matbio.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 30.Silver J. Molecular mechanisms of secondary hyperparathyroidism. Nephrol Dial Transplant. 2000;15(Suppl 5):2–7. doi: 10.1093/ndt/15.suppl_5.2. [DOI] [PubMed] [Google Scholar]
- 31.Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- 32.Li SA, Watanabe M, Yamada H, Nagai A, Kinuta M, Takei K. Immunohistochemical localization of Klotho protein in brain, kidney, and reproductive organs of mice. Cell Struct Funct. 2004;29:91–99. doi: 10.1247/csf.29.91. [DOI] [PubMed] [Google Scholar]
- 33.Krajisnik T, Bjorklund P, Marsell R, Ljunggren O, Akerstrom G, Jonsson KB, et al. Fibroblast growth factor-23 regulates parathyroid hormone and 1alpha-hydroxylase expression in cultured bovine parathyroid cells. J Endocrinol. 2007;195:125–131. doi: 10.1677/JOE-07-0267. [DOI] [PubMed] [Google Scholar]
- 34.Ben-Dov IZ, Galitzer H, Lavi-Moshayoff V, Goetz R, Kuro OM, Mohammadi M, et al. The parathyroid is a target organ for FGF23 in rats. J Clin Invest. 2007;117:4003–4008. doi: 10.1172/JCI32409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Westerberg PA, Linde T, Wikstrom B, Ljunggren O, Stridsberg M, Larsson TE. Regulation of fibroblast growth factor-23 in chronic kidney disease. Nephrol Dial Transplant. 2007;22:3202–3207. doi: 10.1093/ndt/gfm347. [DOI] [PubMed] [Google Scholar]
- 36.Kazama JJ, Gejyo F, Shigematsu T, Fukagawa M. Role of circulating fibroblast growth factor 23 in the development of secondary hyperparathyroidism. Ther Apher Dial. 2005;9:328–330. doi: 10.1111/j.1744-9987.2005.00291.x. [DOI] [PubMed] [Google Scholar]
- 37.Larsson T, Nisbeth U, Ljunggren O, Juppner H, Jonsson KB. Circulating concentration of FGF-23 increases as renal function declines in patients with chronic kidney disease, but does not change in response to variation in phosphate intake in healthy volunteers. Kidney Int. 2003;64:2272–2279. doi: 10.1046/j.1523-1755.2003.00328.x. [DOI] [PubMed] [Google Scholar]
- 38.Nakanishi S, Kazama JJ, Nii-Kono T, Omori K, Yamashita T, Fukumoto S, et al. Serum fibroblast growth factor-23 levels predict the future refractory hyperparathyroidism in dialysis patients. Kidney Int. 2005;67:1171–1178. doi: 10.1111/j.1523-1755.2005.00184.x. [DOI] [PubMed] [Google Scholar]
- 39.Imanishi Y, Inaba M, Nakatsuka K, Nagasue K, Okuno S, Yoshihara A, et al. FGF-23 in patients with end-stage renal disease on hemodialysis. Kidney Int. 2004;65:1943–1946. doi: 10.1111/j.1523-1755.2004.00604.x. [DOI] [PubMed] [Google Scholar]
- 40.Kazama JJ, Sato F, Omori K, Hama H, Yamamoto S, Maruyama H, et al. Pretreatment serum FGF-23 levels predict the efficacy of calcitriol therapy in dialysis patients. Kidney Int. 2005;67:1120–1125. doi: 10.1111/j.1523-1755.2005.00178.x. [DOI] [PubMed] [Google Scholar]
- 41.Liu S, Zhou J, Tang W, Jiang X, Rowe DW, Quarles LD. Pathogenic role of Fgf23 in Hyp mice. Am J Physiol Endocrinol Metab. 2006;291:E38–E49. doi: 10.1152/ajpendo.00008.2006. [DOI] [PubMed] [Google Scholar]
- 42.Kawaguchi H. Molecular backgrounds of age-related osteoporosis from mouse genetics approaches. Rev Endocr Metab Disord. 2006;7:17–22. doi: 10.1007/s11154-006-9011-3. [DOI] [PubMed] [Google Scholar]
- 43.Bringhurst FR, Demay MB, Kronenberg HM. Williams Textbook of Endocrinology. 9. WB Saunders; Philadelphia: 1998. Hormones and disorders of mineral metabolism; pp. 1155–1210. [Google Scholar]