Analysis of Ipl1-Mediated Phosphorylation of the Ndc80 Kinetochore Protein in Saccharomyces cerevisiae (original) (raw)
Abstract
Phosphorylation of the Ndc80 kinetochore protein by the Ipl1/Aurora B kinase reduces its microtubule binding activity in vitro. We found that kinetochore-bound Ndc80 is phosphorylated on Ipl1 sites in vivo, but this phosphorylation is not essential. Instead, we show that additional Ipl1 targets contribute to segregation and the spindle checkpoint.
THE faithful transmission of genetic material requires chromosomes to biorient such that sister kinetochores attach to spindle microtubules from opposite poles. In the absence of biorientation, cells activate the spindle checkpoint to halt the cell cycle and give cells time to fix errors. The conserved Ipl1 protein kinase is essential for both error correction and checkpoint activation because it destabilizes aberrant interactions between kinetochores and microtubules, creating unattached kinetochores that trigger the checkpoint (Biggins et al. 1999; Biggins and Murray 2001; Tanaka et al. 2002; Pinsky et al. 2006b). The Ndc80 outer kinetochore protein that directly binds to microtubules has been proposed to be a key target of Ipl1 for biorientation because phosphorylation of its unstructured N terminus reduces its affinity toward microtubules in vitro (Cheeseman et al. 2006; DeLuca et al. 2006; Wei et al. 2007; Ciferri et al. 2008; Guimaraes et al. 2008; Miller et al. 2008). However, it is not known whether Ndc80 is phosphorylated on kinetochores in vivo. In addition, the relative contributions of Ipl1-mediated phosphorylation of Ndc80 to segregation and the spindle checkpoint have not been analyzed.
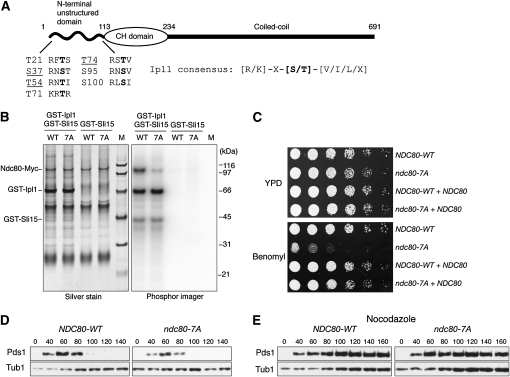
We recently developed a method to detect phosphorylation on centromere-bound kinetochore proteins in vivo by isolating minichromosomes and then performing mass spectrometry (MS) (B. Akiyoshi, C. R. Nelson, J. A. Ranish, S. Biggins, unpublished data). We applied this method to identify Ipl1 phosphorylation on kinetochores by purifying minichromosomes from conditions where the activity of the Glc7 protein phosphatase 1 (PP1), which opposes Ipl1, was decreased (Francisco et al. 1994). We overexpressed the Gip4 regulatory subunit that titrates Glc7 from the nucleus to enrich for cells arrested in mitosis with increased phosphorylation on Ipl1 targets (Pinsky et al. 2006a). When we analyzed the minichromosomes purified from this condition by MS, we detected three phosphorylated peptides corresponding to Ipl1 consensus sites in the N terminus of Ndc80 (S37, T54, and T74, Figure 1A and data not shown), providing the first evidence that kinetochore-bound Ndc80 is phosphorylated in vivo on its N-terminal domain. In addition, it was previously reported that S100 on Ndc80 is phosphorylated in vivo (Cheeseman et al. 2002). All of these residues map to the Ndc80 unstructured N-terminal domain that is phosphorylated by Ipl1/Aurora in vitro and implicated in regulating microtubule attachments to kinetochores in multicellular eukaryotes (Cheeseman et al. 2006; DeLuca et al. 2006; Ciferri et al. 2008; Guimaraes et al. 2008; Miller et al. 2008). We therefore examined the potential relevance of phosphorylation of the budding yeast Ndc80 N-terminal region (1–112 residues) on the Ipl1 consensus sites in this domain by mutating all of them to create the Ndc80-7A mutant (Figure 1A). We confirmed that phosphorylation of the Ndc80-7A protein by Ipl1 is decreased in vitro (Figure 1B). The residual phosphorylation of Ndc80-7A is likely due to the presence of other putative Ipl1 sites outside of the N terminus. However, these sites are not conserved in multicellular eukaryotes and do not map to the microtubule binding region of Ndc80, and phosphorylation on these residues in vivo has not been reported.
Figure 1.—
An N-terminal Ndc80 mutant lacking Ipl1 phosphorylation sites is benomyl sensitive but spindle checkpoint proficient. (A) A schematic of Ndc80 shows that it contains seven putative Ipl1 phosphorylation Ser/Thr sites (shown in boldface type) in the unstructured N terminus. Phosphorylation sites detected by MS are underlined. Minichromosomes were purified from cells expressing Gip4 from the galactose promoter (SBY6247) for 3 hr and subjected to MS analysis (Kim et al. 2007 and unpublished data). Yeast strains used in this study are listed in supporting information, Table S1. (B) Phosphorylation of the Ndc80-7A phospho-deficient mutant by Ipl1 is reduced in vitro. Ndc80-WT-Myc (WT, SBY7258) and Ndc80-7A-Myc (7A, SBY7259) proteins were immunoprecipitated with anti-Myc antibodies and used as substrates in vitro for an Ipl1 kinase assay as previously described (Buvelot et al. 2003). M, molecular weight markers. (C) Ndc80-7A mutant cells are viable, but show hypersensitivity to benomyl that is rescued by endogenous NDC80. Serial dilutions (fivefold) of NDC80-WT and ndc80-7A cells in the presence or absence of an endogenous NDC80 were plated on YPD plates with or without 7.5 μg/ml benomyl (SBY7258, SBY7259, SBY6859, and SBY6860). (D) Ndc80-7A cells do not exhibit defects in cell-cycle progression. Cells containing Pds1-Myc and either NDC80-WT (SBY7120) or ndc80-7A (SBY7123) were released from G1 and lysates were prepared at the indicated time points and monitored for Pds1-Myc by immunoblot as previously described (Biggins et al. 1999). (E) Ndc80-7A cells activate the spindle checkpoint in response to microtubule depolymerization. The experiment in D was repeated by releasing cells into 10 μg/ml nocodazole.
We tested whether Ndc80-7A is functional in vivo by testing whether it could complement an _ndc80_Δ mutant. Strikingly, ndc80-7A cells were viable and did not exhibit any obvious growth defects at various temperatures (Figure 1C and data not shown), or defects in bipolar spindle assembly (data not shown). These data are consistent with the report that cells are viable when this domain is completely deleted (Kemmler et al. 2009). In addition, there was no delay in cell cycle progression when the levels of the anaphase inhibitor Pds1 were monitored as cells were released from G1 (Figure 1D). However, although Ndc80-7A protein levels are similar to Ndc80 (data not shown), we found that ndc80-7A mutant cells exhibited hypersensitivity to the microtubule depolymerizing agent, benomyl (Figure 1C). Spindle checkpoint mutants are benomyl sensitive because the cell cycle continues when microtubules are depolymerized (Hoyt et al. 1991; Li and Murray 1991). However, ndc80-7A cells activate the checkpoint and arrest in metaphase with high levels of Pds1 when treated with the microtubule destabilizing drug, nocodazole (Figure 1E). Therefore, the ndc80-7A mutant cells likely have altered microtubule dynamics that result in a benomyl-sensitive phenotype.
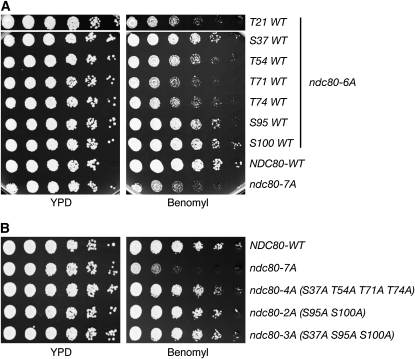
We next examined the importance of individual phosphorylation sites by reversing each site in ndc80-7A back to wild type, creating seven different ndc80-6A mutants. These mutants exhibited various degrees of sensitivity to benomyl and revealed that S37, S95, and S100 are more important than other residues (Figure 2A). To test whether specific sites are sufficient to lead to benomyl sensitivity, we constructed ndc80-2A (S95A S100A), ndc80-3A (S37A S95A S100A), and ndc80-4A (S37A T54A T71A T74A) mutants. However, none of these mutants were hypersensitive to benomyl (Figure 2B). Taken together, these results demonstrate that the presence of multiple sites is important, but that no single specific site is critical for Ndc80 function. The interaction between Ndc80 and microtubules is primarily electrostatic (the former being positively charged and the latter negatively charged), and the N-terminal unstructured domain is highly positively charged to strengthen the interaction (Cheeseman et al. 2006; DeLuca et al. 2006; Wei et al. 2007; Ciferri et al. 2008; Guimaraes et al. 2008; Miller et al. 2008). Therefore, it is most likely that the overall charge state of the N-terminal domain, but not any specific site, is important for the function.
Figure 2.—
Multiple phosphorylations on Ndc80 are important for proper microtubule dynamics. (A) Ndc80-6A mutants exhibit varying degrees of sensitivity to benomyl. Cells containing six mutations were assayed for benomyl sensitivity as in Figure 1C (SBY7257, SBY7255, SBY7252, SBY7328, SBY7329, SBY7326, SBY7327, SBY7258, and SBY7259). (B) Ndc80-4A, ndc80-2A, and ndc80-3A mutants do not exhibit benomyl hypersensitivity, indicating that there is no specific site required for the phenotype (SBY7258, SBY7259, SBY7296, SBY7325, and SBY8597).
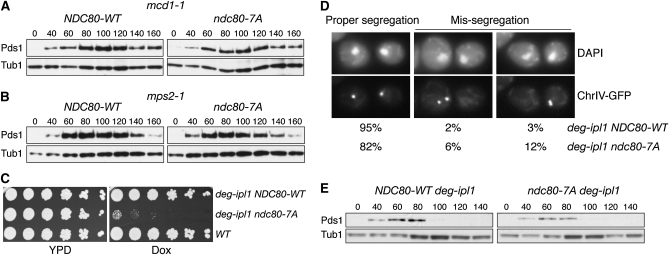
We previously found that Ipl1 is essential for checkpoint activation when kinetochores are not under tension, possibly due to its role in generating unattached kinetochores that signal the checkpoint (Biggins and Murray 2001; Pinsky et al. 2006b). We therefore tested whether the ndc80-7A mutant is defective in the tension checkpoint by analyzing Pds1 levels in two conditions that create tension defects (a cohesion defect and a bipolar spindle assembly defect). Cells containing ndc80-7A activated the checkpoint in both conditions (Figure 3, A and B), suggesting that Ipl1 phosphorylates one or more additional targets or sites in Ndc80 to activate the spindle checkpoint.
Figure 3.—
The Ndc80 N-terminal domain is not the sole Ipl1 downstream target for segregation and spindle checkpoint activation. (A and B) Ndc80-7A mutant cells do not bypass the checkpoint in response to a defect in cohesion (A; mcd1-1) or bipolar spindle assembly (B; mps2-1). Cells containing either NDC80-WT (SBY7131, SBY7118) or ndc80-7A (SBY7133, SBY7121) were arrested in G1, released to 37°, and analyzed as in Figure 1D. (C) Ndc80-7A is synthetically lethal with deg-ipl1. Serial dilutions (fivefold) of deg-ipl1 NDC80-WT (SBY8325), deg-ipl1 ndc80-7A (SBY8241), and WT (SBY818) cells were plated onto YPD with or without 25 μg/ml Dox. (D and E) The ndc80-7A deg-ipl1 mutant exhibits chromosome missegregation but does not activate the spindle checkpoint. Cells containing deg-ipl1 NDC80-WT or deg-ipl1 ndc80-7A were arrested in G1 for 2 hr and then 25 μg/ml Dox was added for an additional 1.5 hr. Cells were released into media containing Dox and either fixed at 140 min for microscopy (D), as previously described (Biggins et al. 1999) [examples of proper segregation (left), missegregation to the mother cell (middle), or daughter cell (right) are shown] or monitored for Pds1 levels at the indicated time points (E).
If Ndc80 contributes to Ipl1 functions in vivo, we expected that the ndc80-7A mutant would exhibit genetic interactions with ipl1 mutants. Consistent with this, we found that ndc80-7A is synthetically lethal with the ipl1-321 temperature sensitive mutant (data not shown). It is noteworthy that an ndc80-1 temperature-sensitive allele does not exhibit lethal interactions with ipl1-321 (Pinsky et al. 2006b), suggesting that ndc80-7A is more specifically defective in an Ipl1-related function than the ndc80-1 mutant. This result also suggested that ipl1-321 cells retain enough activity for survival when Ndc80 sites can be phosphorylated by Ipl1, but becomes lethal when Ndc80 has reduced capacity to be phosphorylated by Ipl1. Taken together, these data strongly suggest that there are unidentified targets of Ipl1. Interestingly, ndc80-7A is also synthetically lethal with the mps1-1 temperature-sensitive mutant (data not shown). Because Mps1 targets some of the Ipl1 sites of Ndc80 (Kemmler et al. 2009), this result may suggest that Ipl1 and Mps1 have overlapping substrate specificity in vivo. Alternatively, Mps1 may be required for activating Ipl1 or vice versa (Jelluma et al. 2008).
To directly analyze whether Ndc80 phosphorylation contributes to the biorientation function of Ipl1, we created a conditional ndc80-7A deg-ipl1 double mutant to analyze chromosome segregation. We previously found that although deg-ipl1 has reduced function, it retains viability even when transcription is repressed by the addition of doxycycline (Dox) (Ng et al. 2009). Consistent with this, deg-ipl1 ndc80-7A double mutant cells grew in the absence of Dox, but deg-ipl1 function was sufficiently reduced to be inviable when combined with ndc80-7A in the presence of Dox (Figure 3C). This allowed us to analyze segregation in the double mutant cells by releasing them from G1 into media containing Dox and monitoring the fate of a fluorescently marked chromosome (Straight et al. 1996). Strikingly, 18% of cells missegregated Chr IV to the same pole instead of opposite poles (Figure 3D), strongly suggesting that the lethality of the double mutant is due to a biorientation defect. If every chromosome exhibited a similar biorientation defect, few cells would remain viable. The chromosome segregation defect in these cells should lead to activation of the spindle checkpoint, so we analyzed Pds1 levels in cells released from G1. However, similar to ipl1 mutants (Biggins and Murray 2001; Pinsky et al. 2006b), the ndc80-7A deg-ipl1 cells did not activate the checkpoint in response to the segregation defect (Figure 3E). Taken together, these data suggest that N-terminal Ndc80 phosphorylation contributes to both the segregation and checkpoint functions of Ipl1.
In summary, our studies show that multiple Ipl1 sites on the N-terminal domain of Ndc80 are phosphorylated when it is associated with kinetochores in vivo. Strikingly, although this phosphorylation contributes to the role of Ipl1 in both chromosome segregation and spindle checkpoint activation, our data strongly support the idea that additional key targets of Ipl1 must be phosphorylated in combination with Ndc80 to carry out the precise functions of the kinase. In the future, it will be critical to identify the complete complement of these phosphorylation events to fully understand the mechanism by which Ipl1 mediates segregation and checkpoint activation to ensure genomic stability.
Acknowledgments
We thank Min Yuan and the Institute for Systems Biology facility for proteomic analyses. This work was supported by National Institutes of Health grant GM064386 (to S.B.) and National Institute of General Medical Sciences grant (PM50 GM076547/Center for Systems Biology) (to J.A.R.). S.B. is a scholar of the Leukemia and Lymphoma Society.
References
- Biggins, S., and A. W. Murray, 2001. The budding yeast protein kinase Ipl1/Aurora allows the absence of tension to activate the spindle checkpoint. Genes Dev. 15 3118–3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggins, S., F. F. Severin, N. Bhalla, I. Sassoon, A. A. Hyman et al., 1999. The conserved protein kinase Ipl1 regulates microtubule binding to kinetochores in budding yeast. Genes Dev. 13 532–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buvelot, S., S. Y. Tatsutani, D. Vermaak and S. Biggins, 2003. The budding yeast Ipl1/Aurora protein kinase regulates mitotic spindle disassembly. J. Cell Biol. 160 329–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheeseman, I. M., S. Anderson, M. Jwa, E. M. Green, J. Kang et al., 2002. Phospho-regulation of kinetochore-microtubule attachments by the Aurora kinase Ipl1p. Cell 111 163–172. [DOI] [PubMed] [Google Scholar]
- Cheeseman, I. M., J. S. Chappie, E. M. Wilson-Kubalek and A. Desai, 2006. The conserved KMN network constitutes the core microtubule-binding site of the kinetochore. Cell 127 983–997. [DOI] [PubMed] [Google Scholar]
- Ciferri, C., S. Pasqualato, E. Screpanti, G. Varetti, S. Santaguida et al., 2008. Implications for kinetochore-microtubule attachment from the structure of an engineered Ndc80 complex. Cell 133 427–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLuca, J. G., W. E. Gall, C. Ciferri, D. Cimini, A. Musacchio et al., 2006. Kinetochore microtubule dynamics and attachment stability are regulated by Hec1. Cell 127 969–982. [DOI] [PubMed] [Google Scholar]
- Francisco, L., W. Wang and C. S. Chan, 1994. Type 1 protein phosphatase acts in opposition to Ipl1 protein kinase in regulating yeast chromosome segregation. Mol. Cell. Biol. 14 4731–4740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guimaraes, G. J., Y. Dong, B. F. McEwen and J. G. DeLuca, 2008. Kinetochore-microtubule attachment relies on the disordered N-terminal tail domain of Hec1. Curr. Biol. 18 1778–1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyt, M. A., L. Totis and B. T. Roberts, 1991. S. cerevisiae genes required for cell cycle arrest in response to loss of microtubule function. Cell 66 507–517. [DOI] [PubMed] [Google Scholar]
- Jelluma, N., A. B. Brenkman, N. J. van den Broek, C. W. Cruijsen, M. H. van Osch et al., 2008. Mps1 phosphorylates Borealin to control Aurora B activity and chromosome alignment. Cell 132 233–246. [DOI] [PubMed] [Google Scholar]
- Kemmler, S., M. Stach, M. Knapp, J. Ortiz, J. Pfannstiel et al., 2009. Mimicking Ndc80 phosphorylation triggers spindle assembly checkpoint signalling. EMBO J. 28 1099–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, B., A. I. Nesvizhskii, P. G. Rani, S. Hahn, R. Aebersold et al., 2007. The transcription elongation factor TFIIS is a component of RNA polymerase II preinitiation complexes. Proc. Natl. Acad. Sci. USA 104 16068–16073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, R., and A. W. Murray, 1991. Feedback control of mitosis in budding yeast. Cell 66 519–531. [DOI] [PubMed] [Google Scholar]
- Miller, S. A., M. L. Johnson and P. T. Stukenberg, 2008. Kinetochore attachments require an interaction between unstructured tails on microtubules and Ndc80(Hec1). Curr. Biol. 18 1785–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng, T. M., W. G. Waples, B. D. Lavoie and S. Biggins, 2009. Pericentromeric sister chromatid cohesion promotes kinetochore biorientation. Mol. Biol. Cell. 20 3818–3827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinsky, B. A., C. V. Kotwaliwale, S. Y. Tatsutani, C. A. Breed and S. Biggins, 2006. a Glc7/protein phosphatase-1 regulatory subunits can oppose the Ipl1/Aurora protein kinase by redistributing Glc7. Mol. Cell. Biol. 26 2648–2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinsky, B. A., C. Kung, K. M. Shokat and S. Biggins, 2006. b The Ipl1-Aurora protein kinase activates the spindle checkpoint by creating unattached kinetochores. Nat. Cell Biol. 8 78–83. [DOI] [PubMed] [Google Scholar]
- Straight, A. F., A. S. Belmont, C. C. Robinett and A. W. Murray, 1996. GFP tagging of budding yeast chromosomes reveals that protein-protein interactions can mediate sister chromatid cohesion. Curr. Biol. 6 1599–1608. [DOI] [PubMed] [Google Scholar]
- Tanaka, T. U., N. Rachidi, C. Janke, G. Pereira, M. Galova et al., 2002. Evidence that the Ipl1-Sli15 (Aurora kinase-INCENP) complex promotes chromosome bi-orientation by altering kinetochore-spindle pole connections. Cell 108 317–329. [DOI] [PubMed] [Google Scholar]
- Wei, R. R., J. Al-Bassam and S. C. Harrison, 2007. The Ndc80/HEC1 complex is a contact point for kinetochore-microtubule attachment. Nat. Struct. Mol. Biol. 14 54–59. [DOI] [PubMed] [Google Scholar]