Decrease of Foxp3+ Treg cell number and acquisition of effector cell phenotype during lethal infection (original) (raw)
. Author manuscript; available in PMC: 2010 Nov 20.
Abstract
Using a model of lethal oral infection with Toxoplasma gondii, we examined the fate of both induced and natural regulatory T (Treg) cells in the face of strong inflammatory responses occurring in a tolerogenic-prone environment. We found that during highly T helper 1 (Th1) cell-polarized mucosal immune responses, Treg cell numbers collapsed via multiple pathways including blockade of Treg cell induction and disruption of endogenous Treg cell homeostasis. In particular, shutdown of interleukin 2 (IL-2) in the highly Th1 cell-polarized environment triggered by infection directly contributed to Treg cell incapacity to suppress effector responses and eventually leads to immunopathogenesis. Furthermore, we found that environmental cues provided by both local dendritic cells and effector T cells can induce the expression of T-bet transcription factor and IFN-γ by Treg cells. These data reveal a mechanism for Th1 cell pathogenicity that extends beyond their proinflammatory program to limit Treg cell survival.
Introduction
Failure to properly control immune responses in the face of infections or at sites of high antigenic exposure leads to pathologic consequences. To preserve tissue integrity, complementary strategies are in place including specialized lymphocytes and antigen-presenting cell populations. Foxp3 expressing regulatory T (Treg) cells represent one of the major arms of this regulatory network by controlling both innate and adaptive immune responses (Sakaguchi et al., 2001).
In some microenvironments such as the gastrointestinal (GI) tract, in which immune reactivity against intestinal flora or dietary antigen poses a substantial risk to the host, regulatory elements are constitutively represented (Izcue et al., 2006). For instance, in addition to various populations of Treg cells, the GI tract is home to dendritic cells (DCs) displaying regulatory functions via their capacity to release cytokines or to induce Treg cells (Coombes and Powrie, 2008). We and others have shown that in the gut environment, a substantial fraction of naïve T cells can also become Foxp3+ Treg cells after oral exposure to antigen (Coombes et al., 2007; Mucida et al., 2005; Sun et al., 2007). This process is associated with the capacity of gut-associated lymphoid tissue (GALT) antigen presenting cells (APC) to generate Treg cells via a mechanism that, in addition to TGF-β, is dependent on the Vitamin A metabolite retinoic acid (RA) (Coombes et al., 2007; Denning et al., 2007; Sun et al., 2007).
Nevertheless, even in highly regulated sites, immune responses need to occur to allow proper control of microbial expansion. This implies that regulatory mechanisms have to be temporally neutralized or overcome. Several lines of evidence suggest that Treg cells themselves are subject to regulation. Such control can be direct, via triggering Toll-like receptor ligands (Wei et al., 2009), or indirect via enhanced activation of APC or effector T cells (Pasare and Medzhitov, 2003). Another means by which Treg cells could be controlled is associated with a loss of function or enhanced apoptosis (Tang et al., 2008). Recent evidence also supports the idea that Treg cells can become unstable after exposure to defined stimulatory signals or in lymphopenic environments (Degauque et al., 2008; Duarte et al., 2009; Wei et al., 2009; Xu et al., 2007). In contrast, excessive limitation of regulatory pathways can have detrimental consequences. Indeed, any failure to maintain a tight control of the equilibrium between regulation and immunity is at the core of pathologic processes from autoimmune disorders to pathogen-driven diseases. However in most circumstances and in particular during infection, critical environmental cues limiting the induction and function of Treg cells are poorly understood.
Treg cells control a large array of immune responses both in the context of highly polarized settings and in various microenvironments. This implies that maintenance of peripheral homeostasis also relies on the capacity of these cells to appropriately adapt to the environment they have to regulate. Recent evidence suggests that acquisition of additional transcription factors by Treg cells is required for proper control of defined environments. For instance, expression of the transcription factor IRF4 by Treg cells contributes to their capacity to control Th2 cells responses (Zheng et al., 2009). In contrast, a recent report demonstrated that expression of T-bet by Treg cells can favor their homing to Th1 cell environments and is required for the homeostasis and function of Treg cells during type 1 inflammation (Koch et al., 2009). How, in pathogenic situations, expression of these transcription factors could also induce the expression of an effector program by Treg cells and potentially contribute to pathogenesis has not been addressed.
One of the first models to reveal the immunologic paradox of microbial control associated with host death is murine infection with Toxoplasma gondii (T. gondii) (Gazzinelli et al., 1996). This parasite manipulates innate cells in a way that induces the development of a highly polarized Th1 cell response (Gazzinelli et al., 1994). In certain strains of mice, oral infection with T. gondii induces a severe form of intestinal inflammation referred to as the lethal ileitis model (Liesenfeld, 2002; Liesenfeld et al., 1996; Mennechet et al., 2002). When insufficiently controlled, this pathological process that is CD4+ T cell dependent leads to the death of the infected host (Liesenfeld, 2002; Mennechet et al., 2002). Using murine T. gondii infection, we explored the interplay between Treg and effector T cells in this tolerance-prone environment following challenge with a virulent pathogen. In particular, we addressed the factors controlling the induction of Treg cells and the fate of endogenous Treg cells in a situation in which regulation is clearly overwhelmed.
Using this model, we found that cues emerging from tissue resident DCs and effector T cells control the size of Treg cell populations via multiple pathways ranging from blockade of Treg cell induction to limitation of endogenous Treg cell proliferation. In particular, shut down of IL-2 by effector T cells played a major role in the pathogenesis of this infection by limiting bioavailability of Treg cell survival factors. The strong Th1 environment triggered by T. gondii infection also induced T-bet and IFN-γ expression on Treg cells. Together, our data support the idea that Th1 cells can subvert regulatory networks and become pathogenic through not only their capacity to produce inflammatory cytokines but also their reduced capacity to produce a major survival factor for Treg cells.
Results
Acute infection by T. gondii is associated with collapse of Treg cell numbers and frequencies
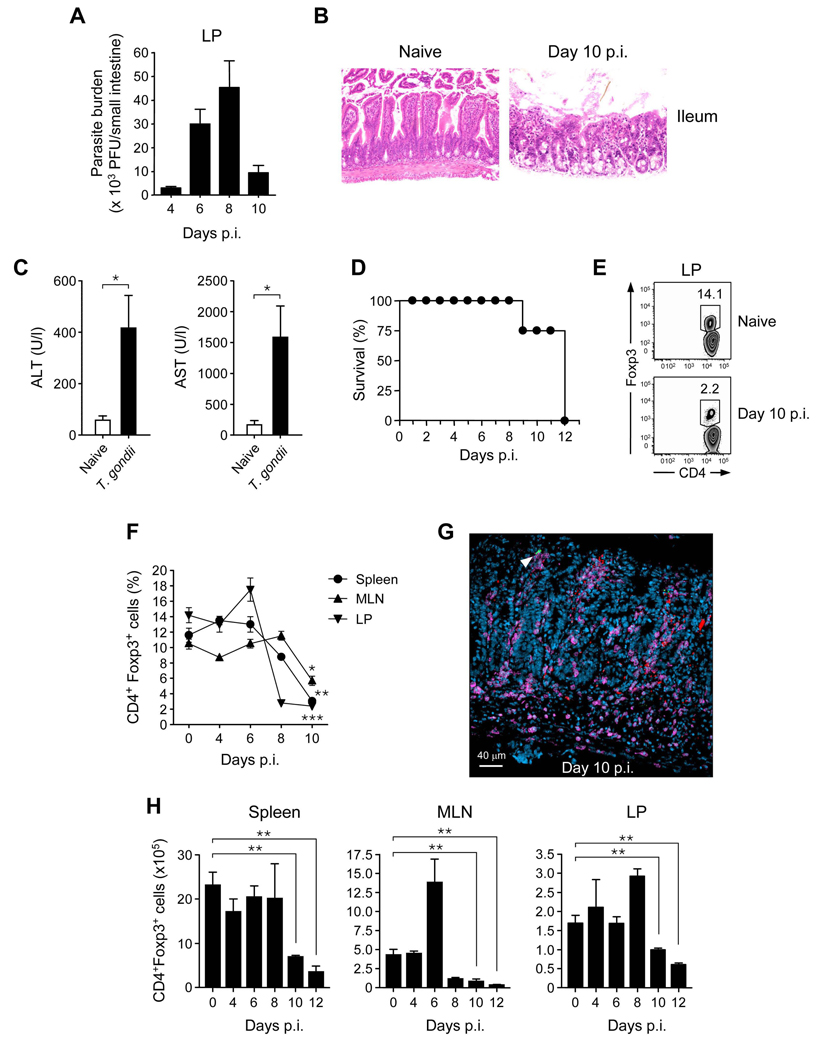
In order to evaluate the fate of Foxp3+ Treg cells in highly inflammatory settings, C57BL/6 mice were infected orally with 40 cysts of the type II T. gondii strain, ME-49. After oral infection, the parasite was primarily detected in the small intestine as well as in distal tissues (Fig 1A and data not shown). ME-49 in C57BL/6 mice induced severe small intestine inflammation with loss of epithelial architecture in the ileum and the jejunum, shortened villi, massive influx of inflammatory cells and scattered patches of necrosis, particularly in Peyer’s patches (Fig 1B, Fig S1A, (Liesenfeld, 2002; Mennechet et al., 2002)). This infection also induced severe necrosis of the liver leading to hepatic dysfunction, as indicated by increased concentrations of serum alanine and aspartate aminotransferase (Fig 1C). Although mice controled parasite expansion, they eventually succumbed to immunopathology (Fig 1A,D). At various time points after-infection, Treg cell frequencies and absolute numbers were evaluated in different tissues. Gut pathology peaked between day 8 to 10 and coincided with a strong reduction of Foxp3+ Treg cell frequencies both at the primary site (LP) and systemically (Fig 1E, F). Scattered foci of parasites were present along the small intestine, characterized by a massive influx of CD4+ T cells (Fig 1G). At these sites, Foxp3+ Treg cell were virtually absent (Fig 1G). Of note, the absolute numbers of Foxp3+T cells were also significantly reduced compared to naïve control mice (Fig 1H). In contrast, the absolute number of CD4+Foxp3− T cells was sustained in all compartments with the exception of the MLN (data not shown). Thus, after infection with a lethal dose of T. gondii, Foxp3+ Treg cell numbers and frequencies, were dramatically reduced at the site of infection and systemically.
Figure 1. Treg cell number decreases during acute toxoplasmosis.
C57/BL6 mice were infected orally with 40 cysts of ME-49. (A) Shows parasite burden in Lamina propria (LP) of small intestine at day 4, 6, 8 and 10 after infection. (B) Histological assessment of ileum from naïve or day 10 post infection (hematoxylin and eosin stain; magnification × 200). (C) Amounts of hepatic Alanine Aminotransferase (ALT) and Aspartate Aminotransferase (AST) were assessed in sera from naïve or day 9 infected mice. (D) Survival curve of infected mice, (n=6). (E) Comparative assessment of CD4+Foxp3+ Treg cell frequencies in LP compartments in naïve or day 10 infected mice. Cells were stained for TCRβ, CD4, and Foxp3 and TCR-β+CD4+-gated cells analyzed for expression of Foxp3 by flow cytometry. Numbers in quadrants refer to the percentage of CD4+ T cells expressing Foxp3. (F) Percentages of TCR-β+CD4+ cells expressing Foxp3 between day 0 and 10 after infection in spleen, MLN and LP compartments. (G) Foxp3eGFP mice were infected with 40 cysts of ME-49 expressing RFP. Day 10 after infection small intestine sections were stained for CD4 and infected sites analyzed by confocal microscopy (CD4: purple; Foxp3eGFP green; T. gondii : red). (H) Absolute numbers of TCR-β+CD4+Foxp3+ cells in various tissues between day 0 and 12 post-infection. Histograms represent the mean number of Treg cell (n=3) ± SDs. All experiments shown were performed at least 3 times, with similar results. Statistical comparisons were performed using the Student's t test (*, p < 0.05; **, p < 0.01; ***, p < 0.001).
Failure to sustain Treg cell conversion after T. gondii infection
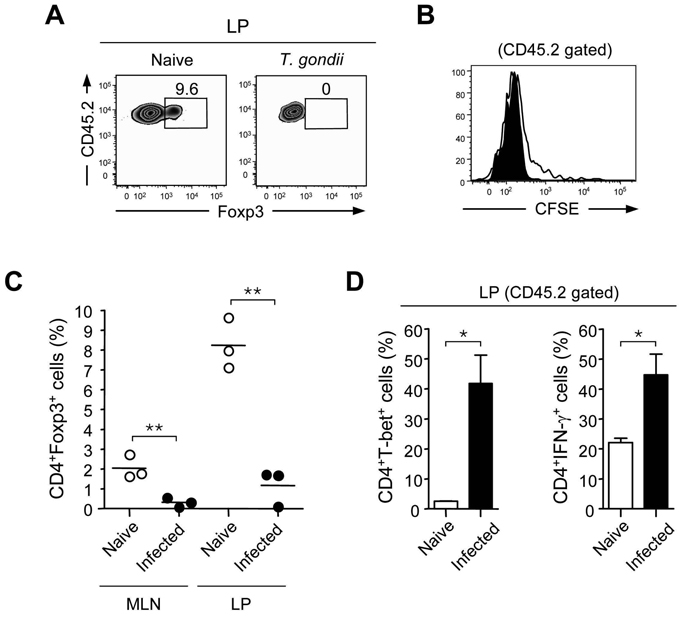
We and others demonstrated that in the GALT, oral exposure to antigen can induce conversion of naïve T cells into Treg cells (Coombes et al., 2007; Mucida et al., 2005; Sun et al., 2007). To evaluate if reduced frequencies of Treg cells during T. gondii infection could be associated with reduced Treg cell conversion, we adoptively transferred eGFP−, CD45.2+ T cells from OTII × Foxp3eGFP mice into CD45.1+ recipients that were infected or not with T. gondii. These recipient mice were then fed ovalbumin (OVA) antigen dissolved in their drinking water. After 5 days of OVA administration, CD45.2+ OVA-specific T cells had expanded and were readily detectable in the GALT, spleen, and in distal lymph nodes (LNs) of both infected and non-infected hosts (Fig 2A and data not shown). Although the frequency of transferred cells was lower in infected hosts, OVA specific cells proliferated robustly in these animals (data not shown and Fig 2B). Thus, antigen presentation was not impaired during T.gondii infection. As we previously described in naïve control mice (Sun et al., 2007), Foxp3+ OVA-specific T cells were only appreciably detected in the GALT, including the LP of naïve hosts (Fig 2C). In contrast, after infection with T. gondii, the frequency of cells expressing Foxp3 de novo was dramatically reduced. Rather, transferred CD45.2+ cells efficiently polarized toward a Th1 cell phenotype after oral feeding with OVA in the presence of T.gondii (Fig 2D).
Figure 2. Failure to sustain Treg cell conversion after T. gondii infection.
CD45.1+ mice were orally infected or not with ME-49. 4 days after infection, recipient mice received CFSE labeled CD45.2+eGFP−OT-II T cells (1.5×106) and fed with OVA antigen in drinking water for 5 days. On day 9 after infection, TCR-β+CD4+CD45.2+-gated T cells were assessed for intracellular Foxp3 expression and CFSE dilution. (A) Detection of Foxp3+ cells is illustrated for small intestinal LP in naïve or day 9 infected mice; (B) Proliferation of transferred cells from the LP was assesses by CFSE dilution (C) Percentage of CD45.2+eGFP−OT-II T cells expressing Foxp3 in LP or MLN from naïve (open circle) or day 9 (closed circle) infected mice. Each dot represents an individual mouse (n=3). (D) LP cells were briefly restimulated with PMA and ionomycin and stained for TCRβ, CD4, CD45.2, T-bet and IFN-γ. Histograms summarize the percentage of TCR-β+CD4+CD45.2+ cells expressing T-bet and IFN-γ. Error bars represent the SDs of the means of 3 individual mice. Experiment shown was performed 3 times, with similar results. (*, p < 0.05; **, p < 0.01; ***, p < 0.001).
Previous studies have demonstrated that inflammatory mediators such as IFN-γ can limit the conversion of naïve CD4+ T cells into Foxp3+T cells (Wei et al., 2007). Indeed, via their capacity to induce IFN-γ production by T cells, LpDCs purified from day 6 infected mice were significantly less efficient at inducing Foxp3+T cells compared to LpDCs from naïve mice (Fig S2). Similar data were obtained when naive LpDCs exposed to STAg (Soluble Toxoplasma Antigen) were utilized in a Treg cell conversion assay (Fig S2). Collectively, these data demonstrate that T. gondii establishes an environment that is unfavorable for the constitutive generation of Treg cells in the GI tract via manipulation of the APC status of activation and induction of effector responses.
Reduced proliferation by Foxp3+Treg cells parallels reduced IL-2 production by effector T cells
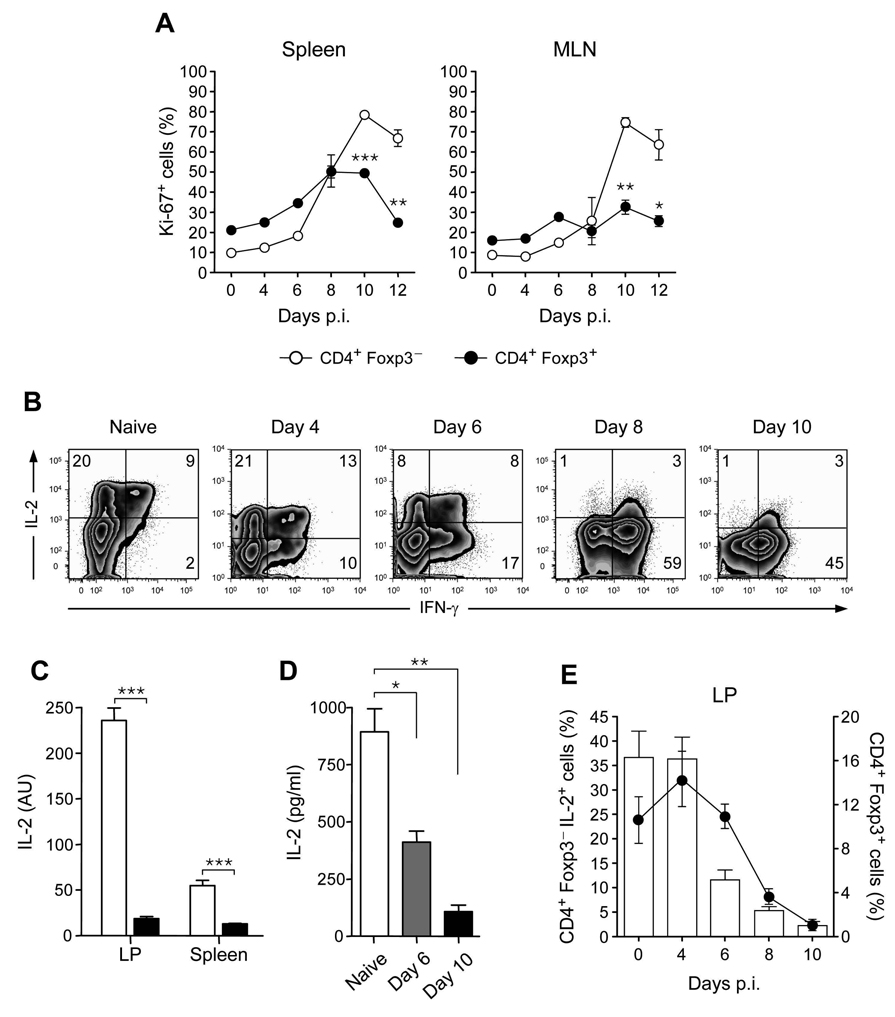
Limitation of peripheral conversion cannot solely explain the systemic reduction of Treg cells. One additional explanation could be associated with reduced proliferation or enhanced cell death of Treg compared to effector T cells. To evaluate the proliferative capacity of both populations after infection, Foxp3+T cells and Foxp3−T cells were stained for Ki-67, a cellular marker for proliferation. At day 10 post-infection, the proliferative index of Foxp3+ Treg cells was significantly reduced compared to that of Foxp3−T cells (Fig 3A). Similar data were obtained when both subsets of cells were gated on CD62L negative population (Fig S3A). We also assessed the expression of the anti-apoptotic molecule Bcl-2 on both Foxp3− and Foxp3+ cells and found that both populations downregulate Bcl-2 with the same kinetics (Fig S3B). In addition, both effector and Treg cells expressed higher amount of the apoptotic markers annexin V and caspase 3 in infected mice when compared to naïve mice (data not shown). Thus, these findings indicate that Treg cell renewal is impaired during T. gondii infection via deprivation of essential survival factors.
Figure 3. Reduced Treg cell proliferation parallels reduced IL-2 production by effector T cells during acute infection by T. gondii.
Mice were inoculated orally with 40 cysts of ME-49. Spleen and MLN cells were isolated from naïve or infected mice and stained for TCRβ, CD4, Foxp3 and Ki-67 (A) Comparative analysis of Ki-67 expression in CD4+TCR-β+Foxp3− (open circle) or CD4+TCR-β+Foxp3+ (closed circle) cells during infection by flow cytometry. Experiment shown was performed two times, with similar results (n=3). (B) At day 0, 4, 6, 8, 10 after infection, LP cells were shortly restimulated in vitro with PMA and ionomycin in the presence of Brefeldin A and stained for TCR-β, CD4, Foxp3, IL-2 and IFN-γ. Dot plots illustrate IL-2 and IFN-γ staining profiles of TCR-β+CD4+Foxp3− cells. (C) At day 9 after infection, mRNA was extracted from splenic or LP cells and quantitative RT-PCR of IL-2 mRNA was performed. Histograms show the means of 3 individuals mice +/− SDs (AU = Arbitrary Unit) (D) Spleen cells were extracted from naïve, day 6 or day 10 infected mice, stimulated with α-CD3, and IL-2 secretion evaluated by ELISA 12 hours after stimulation. (E) Histograms and dot plots represent IL-2 production by CD4+Foxp3− and Treg cell frequencies between day 0, 4, 6, 8 and 10 after infection in LP compartments. Experiments shown in panels (B), (C) and (D) were performed 3 times, with similar results (n=3; *, p < 0.05; **, p < 0.01; ***, p < 0.001).
Previous studies have demonstrated that the cytokine IL-2 plays a dominant role in promoting the expansion and survival of Treg cells (Malek and Bayer, 2004). At steady state conditions, a large fraction of CD4+ T cells from the small intestine lamina propria retained the potential to produce IL-2 (Fig 3B). However, during the course of the infection, the capacity of CD4+ T cells to produce IL-2 was dramatically reduced (29% with an MFI of 69.9 ± 1.2 for naïve mice versus 4% with an MFI of 12.3 ± 0.63 at 10 days post infection) and replaced by the ability to secrete the effector cytokine IFN-γ. At the tissue level, IL-2 mRNA was also significantly reduced in the LP and spleen (Fig 3C). To assess IL-2 protein release, splenocytes of naïve or infected mice were restimulated for 12 hours with anti-CD3. Consistent with data shown in Fig 3B, C, IL-2 release was significantly reduced in infected mice compared to naïve mice (Fig 3D). This reduced capacity to produce IL-2 paralleled Treg cell contraction (Fig 3E), supporting the idea that in the face of a strong Th1 cell response, limiting bioavailability of this cytokine may contribute to the reduction of Treg cells in the infected host.
Acquisition of effector phenotype by Treg cell during T. gondii infection
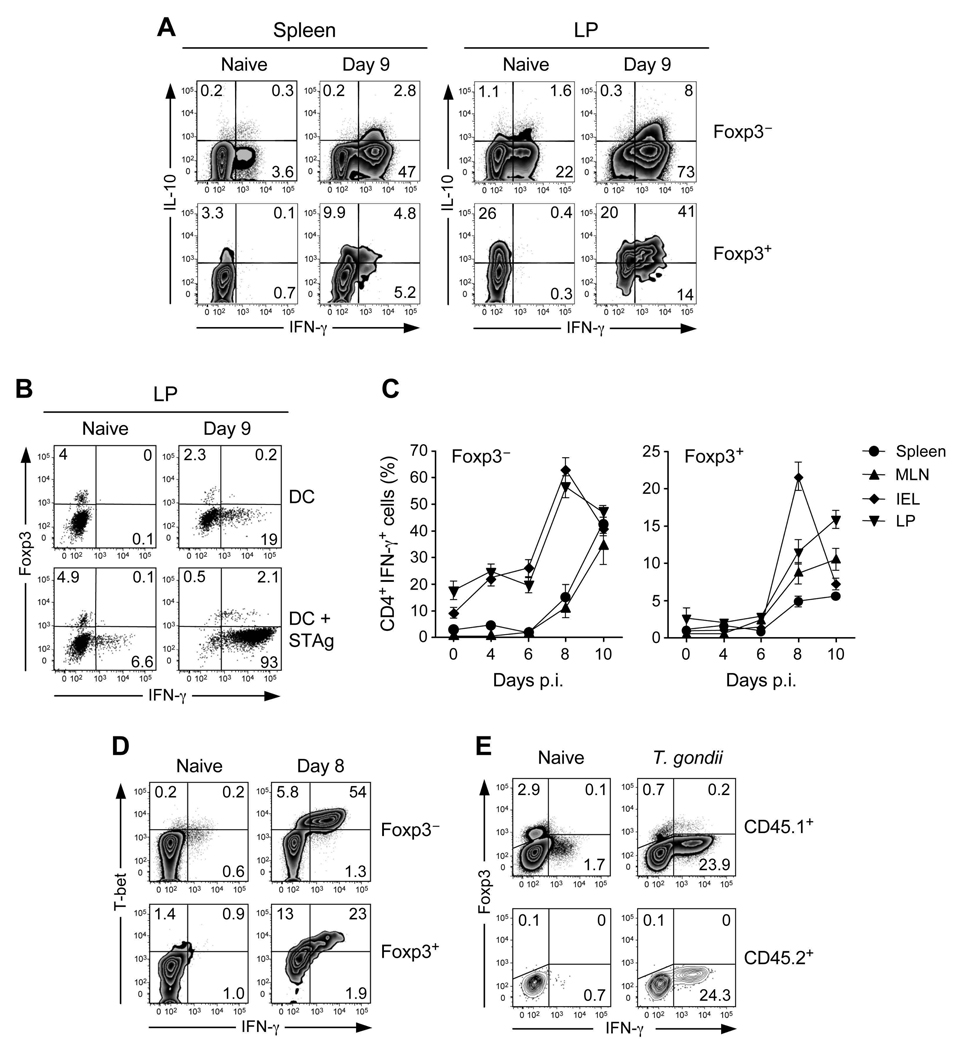
IL-10 was previously shown to play a dominant role in the control of gut inflammation and to limit immunopathological processes induced by T. gondii infection (Gazzinelli et al., 1996; Suzuki et al., 2000; Wilson et al., 2005). We therefore assessed whether the remaining Treg cells during the inflammatory process had an altered capacity to produce IL-10. As previously shown, under steady state conditions, a substantial proportion of LP Treg cells can produce IL-10 in the GI tract (Uhlig et al., 2006) (Fig 4A). At day 9 after-infection, a sizable fraction of Foxp3− T cells acquired the capacity to produce both IL-10 and IFN-γ, consistent with previous observations showing that cells with this phenotype are induced during T. gondii infection (Jankovic et al., 2007). At this time point, IL-10 production by Treg cells in the small intestine increased and was acquired in the spleen (Fig 4A). Thus, although the number of Treg cells was dramatically reduced after T. gondii infection, their capacity to produced IL-10 was not impaired.
Figure 4. Acquisition of effector phenotype by Treg cell during T. gondii infection.
CD4+TCR-β+ cells from the spleen, MLN, LP and IEL compartments were analyzed for expression of Foxp3, IL-10 and IFN-γ by flow cytometry 8 days after oral infection with 40 ME-49 cysts. (A) Dot plots illustrate IL-10 and IFN-γ intracellular staining profiles of Foxp3− or Foxp3+ cells from naïve or day 8 infected spleen and LP compartments. Numbers in quadrants refer to the percentage of each subset. (B) CD4+TCRβ+ cells from naïve or day 9 infected LP compartment were incubated with splenic DC in presence of STAg for 15 hours and Brefeldin A. Dot plots illustrate Foxp3 and IFN-γ intracellular staining profiles of TCR-β+CD4+ gated cells. (C) Percentages of Foxp3− or Foxp3+ CD4+TCR-β+ cells producing IFN-γ during the infection in spleen, MLN, LP and IEL compartments (cells were restimulted as in A) (D) T-bet and IFN-γ expression were analyzed by flow cytometry in naïve or day 8 infected MLN compartment on CD4+TCR-β+Foxp3− and CD4+TCR-β+Foxp3+ cells. (D) CD45.2+CD4+eGFP− T cells (2×106) isolated from Foxp3eGFP mice were transferred into CD45.1+ recipient mice. At day 8 after infection, CD4+TCR-β+CD45.1+ or CD45.2+ cells from the spleen were analyzed by flow cytometry for Foxp3 and IFN-γ expression. The results shown are representative of 3 panels C) independent experiments. Each dot plot represents one mouse and error bars represent the SD of the means of 3 individual mice.
In spite of their ability to produce IL-10, Foxp3+T cells also acquired the capacity to express the effector cytokine IFN-γ, albeit to a lower level than effector T cells (Fig 4A). After exposure of purified CD4+T cells to DCs loaded with STAg, more than 80% of Foxp3+ T cells produced IFN-γ (Fig 4B). The expression of this cytokine paralleled that of effector T cells with the highest proportion of IFN-γ expressing Foxp3+ cells in the GALT (Fig 4C). A recent report showed that Treg cells can express T-bet during Th1 settings (Koch et al., 2009). To address if T-bet could control IFN-γ production by Foxp3+ cells, we evaluated the expression of this transcription factor by Foxp3+ cells. We observed that a high fraction of Treg cells also expressed T-bet during T. gondii infection with the highest proportion found in the spleen and LP (Fig S4A). All IFN-γ expressing Foxp3+T cells also expressed T-bet (Fig 4D). To assess if IFN-γ production by Treg cells was compatible with effector function, purified Foxp3+T cells from T.gondii infected mice were incubated with macrophages previously infected with T.gondii. In vitro, Foxp3+T cells from infected mice displayed effector function based on their capacity to limit parasite expansion and induce NO by infected macrophages (Fig S5). We next assessed if this phenotype could interfere with their suppressive capacity. We found that, in vitro, Treg cells from infected mice were as efficient as Treg cells from naïve mice to suppress proliferation of responder T cells from infected mice (Fig S4B). In these suppression assays, the production of IFN-γ by effector T cells was reduced although not fully suppressed in the presence of Treg cells from infected mice (Fig S4C).
Although we had previously shown that Treg cell conversion was impaired in the context of T. gondii infection (Fig 2), we still needed to address the possibility that Foxp3+ cells producing IFN-γ could arise from effector T cells. To this end, we transferred highly purified Foxp3− T cells into infected recipient mice expressing the congenic marker CD45.1. At day 8 after-transfer, transferred cells expressing CD45.2 expressed comparable amount of IFN-γ to their endogenous counterpart but did not acquire Foxp3 (Fig 4E). Conversely, when highly purified Treg cells were transferred into congenic recipients, these cells acquired the capacity to express T-bet and IFN-γ (data not shown). Together, these data reveal that during T. gondii infection, Foxp3+ Treg cells acquire Th1 cells properties, namely expression of T-bet and IFN-γ.
DCs from infected mice induce T-bet expression and IFN-γ production on Treg cells
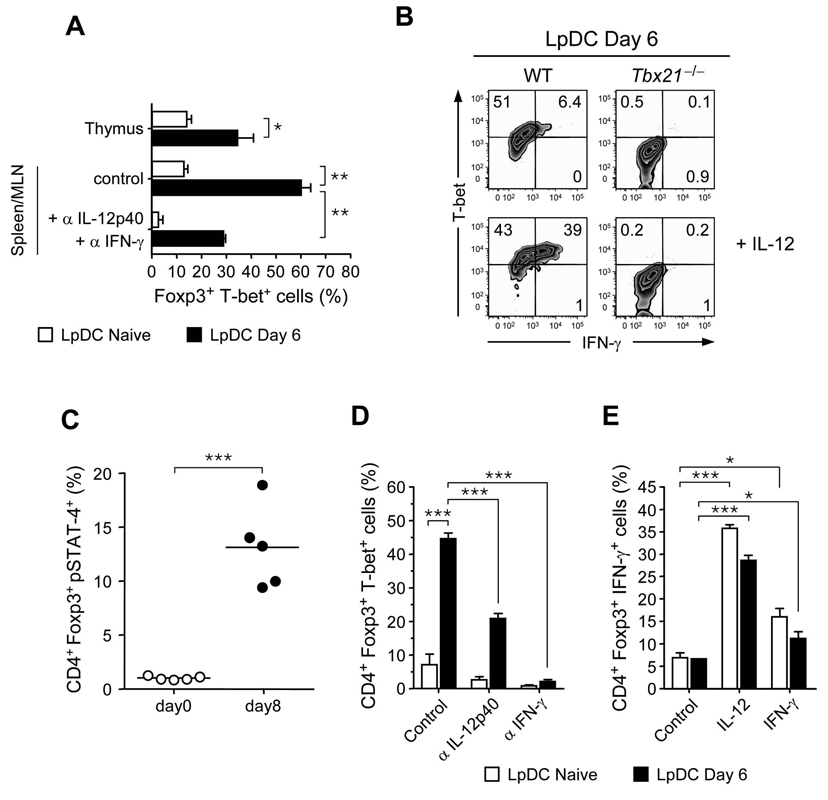
We next assessed the cues that induce T-bet and IFN-γ expression by Foxp3+ Treg cells. LpDCs purified from either naive or infected mice were incubated with highly purified Foxp3+ T cells from peripheral lymphoid organs and expression of T-bet was evaluated after TCR stimulation. Previous reports demonstrated that T. gondii can induce the production of a large array of inflammatory cytokines by splenic DCs (Gazzinelli et al., 1994; Reis e Sousa et al., 1999; Sher et al., 1998). Similarly, we found that in contrast to LpDCs purified from naïve mice, LpDCs purified from infected mice expressed high amount of message for p35 and p28, subunits of the IL-12 and IL-27 cytokines, respectively (Fig S6). Previous reports have suggested that induced Treg cells may be less stable than naturally occurring Treg cells (Floess et al., 2007). To obtain a pure population of natural Treg cells and to minimize contamination by peripherally induced Treg cells, Foxp3+T cells were purified from the thymus and expression of T-bet was compared to Treg cells from the spleen and MLN. After 5 days in culture, a small fraction of Foxp3+Treg acquired T-bet expression when exposed to LpDCs from naïve mice (Fig 5A). In contrast, after exposure of Treg cells to LpDCs from infected mice, a substantial fraction of Treg cells had acquired T-bet expression regardless of their origin (Fig 5A). Acquisition of T-bet by Treg cells was partially blocked by incubation with α-IFN-γ and α-IL-12 antibodies (Fig 5A).
Figure 5. DCs from infected mice imprint T-bet expression and IFN-γ production on both endogenous and induced Treg cells.
(A) CD4+eGFP+ cells isolated from peripheral lymphoid tissue (spleen, MLN) or the thymus of Foxp3eGFP mice were cultivated with purified LpDC from naïve or day 6 infected mice in presence the of IL-2 and α-CD3. Isotype controls or αIL-12p40 and αIFN-γ were added at the beginning of cocultures of peripheral Treg cell and LpDCs. After 5 days of culture, cells were stained for TCR-β, CD4, Foxp3 and T-bet and analyzed by flow cytometry. Histogram represents the mean of triplicate wells ± SDs. (B) Spleen and MLN CD4+CD45RBloCD25hi cells isolated from WT or Tbx21−/− mice were cultivated with purified LpDCs from day 6 infected mice in presence of IL-2 and α-CD3, with or without addition of IL-12. After 5 days of culture, cells were shortly restimulated in vitro by PMA and ionomycin in the presence of Brefeldin A and stained for TCR-β, CD4 and Foxp3. Dot plots are representative staining for T-bet and IFN-γ on TCR-β+CD4+Foxp3+ cells. Experiments shown in panel A and B were performed 3 times, with similar results. (C) At day 8 after oral infection with 40 ME-49 cysts, MLN cells were briefly restimulated in vitro with IL-12, stained for TCR-β, CD4, Foxp3 and pSTAT-4, and analyzed by flow cytometry. Graphs show the percentage of TCR-β+CD4+Foxp3+ expressing pSTAT-4 from naïve (open circle) or day 8 infected (closed circle). Each dot represents one mouse. (D) and (E) CD4+CD25−CD44loFoxp3− T cells isolated from Foxp3eGFP were cultured with exogenous TGF-β and α-CD3 in presence of LpDC purified from naïve or day 6 infected mice with indicated blocking mAb (αIL-12p40, αIFN-γ) or isotype control or with indicated cytokines (IL-12p70, IFN-γ). After 5 days of culture, cells were shortly restimulated in vitro and T-bet (D) or IFN-γ (E) expression was analyzed on TCR-β+CD4+Foxp3+ cells by flow cytometry. Histograms show summary of results obtained for triplicate wells ± SDs. Experiments shown in panel C, D and E were performed two times, with similar results (*, p < 0.05; **, p < 0.01; ***, p < 0.001).
After incubation with LpDCs from infected mice, only a small fraction of Treg cells produced IFN-γ and addition of exogenous IL-12 was required to induce substantial amounts of IFN-γ, suggesting that Treg cells have acquired the capacity to respond to IL-12 (Fig 5B). Indeed, exposure of Treg cells from T. gondii infected mice to IL-12 induced significantly more Stat4 phosphorylation, a hallmark of IL-12 signaling (Jacobson et al., 1996), compared to Treg cells from naïve mice (Fig 5C). IFN-γ production by Treg cells was strictly dependent on T-bet expression as Tbx21−/− Treg cells fail to produce this cytokine even in the presence of high amount of IL-12 (Fig 5B).
Although Treg cell conversion was strongly inhibited during T. gondii infection, we wanted to evaluate if the induction of a Th1 cell phenotype could also be observed on induced Treg cells. LpDCs from infected mice were less efficient at inducing Foxp3 expression in vitro when compared to LpDCs from naïve mice (Fig S2). However unlike LpDCs from naïve mice, LpDCs from infected mice promoted T-bet expression by Foxp3+ Treg cells (Fig 5D). Neutralizing IFN-γ abolished T-bet expression whereas blocking IL-12 only had a partial effect, suggesting that T-bet expression is more dependent on IFN-γ than IL-12 signaling (Fig 5D). In contrast, addition of IL-12 dramatically enhanced expression of IFN-γ by Treg cells in the presence of LpDCs from naïve or infected mice whereas IFN-γ had only a modest effect (Fig 5E).
Thus, for both naturally occurring and induced Treg cells, T-bet is readily induced by LpDCs from infected mice. In both cases, expression is dependent on IFN-γ but acquisition of IFN-γ by Treg cells is only observed in the presence of high amounts of IL-12.
Enhancement of Treg cell survival and stability protects mice from pathology
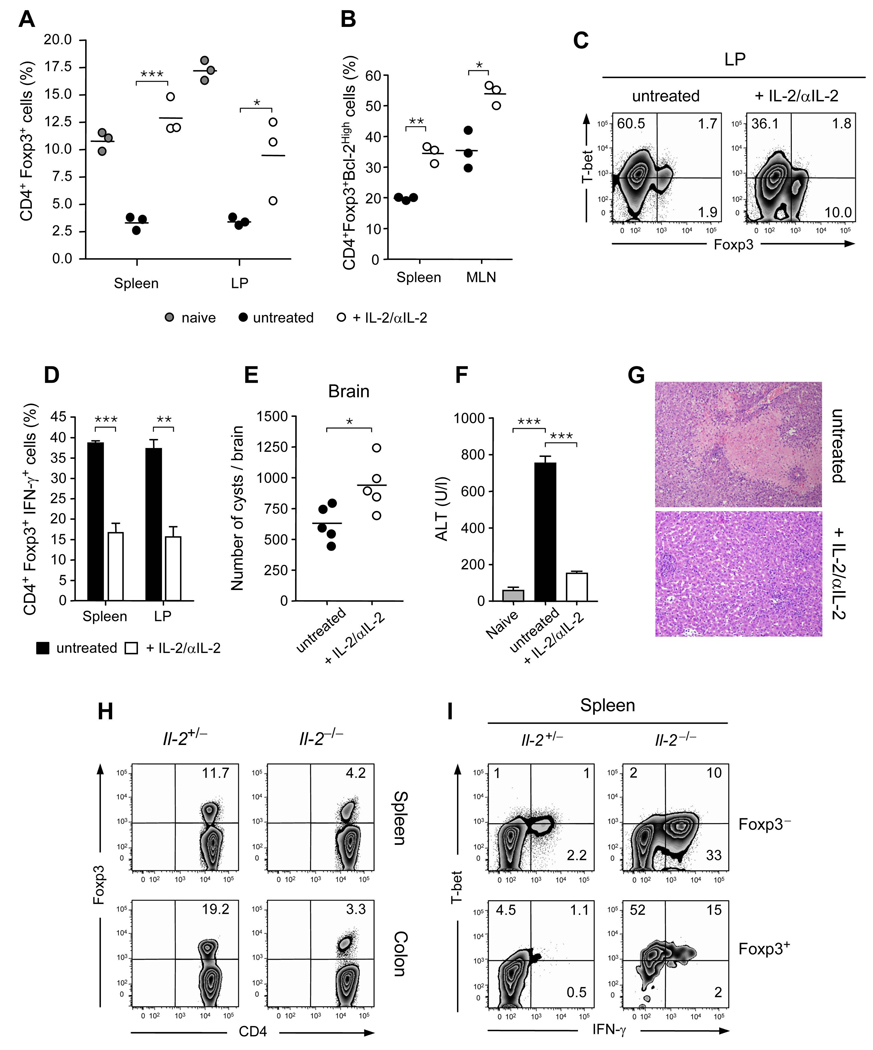
Recent studies have shown that co-administration of IL-2 and α-IL-2 antibody in the form of a complex increases the biological activity of this cytokine. Some of these complexes can specifically amplify Treg cell populations in vivo (Boyman et al., 2006). We utilized this approach to evaluate how IL-2 availability could contribute to Treg cell contraction and T. gondii pathogenesis. Mice infected with T. gondii were treated with IL-2-anti-IL-2 complexes and immune responses and pathology were evaluated at day 9 after-infection. Strikingly, treatment with IL-2 complexes limited the crash of Treg cells in all tissues (Fig 6A). Such treatment also significantly increased the expression of the anti-apototic Bcl-2 molecule in these cells (Fig 6B). Enhanced Treg cell frequency and survival was associated with reduced T-bet and IFN-γ expression by Treg cells in all compartments analyzed (Fig 6 C, D). To confirm that IL-2 treatment acted on the endogenous Treg cell compartment, highly purified Treg cells were transferred into congenic hosts that were infected with T. gondii and treated with IL-2 complexes. Treatment with IL-2 complexes prevented the expression of IFN-γ by transferred Treg cells (Fig S7A). Enhanced Treg cell frequencies were also associated with reduced effector responses in most compartments (data not shown) and as a consequence, enhanced brain cyst numbers (Fig 6E). Importantly, infected mice treated with IL-2-anti-IL-2 complexes exhibited significantly reduced morbidity compared to control mice with reduced inflammation of the small intestine (Fig S7B) and reduced necrosis of the liver with restoration of liver enzymes to control levels (Fig 6F, G).
Figure 6. Enhancement of Treg cell survival and stability protects mice from lethal outcome.
Mice inoculated orally with 40 ME-49 cysts were treated with IL-2-anti-IL-2 complexes or not for 5 consecutive days. At day 9 after infection, TCR-β+CD4+cells were analyzed for expression of Foxp3, Bcl2 and T-bet by flow cytometry. (A) Treg cell percentages in spleen and LP compartments from naïve (grey circle), infected and untreated (closed circle) or infected and treated (open circle) mice. Each dot represents an individual mouse. (B) Percentages of Treg cell expressing Bcl-2 in spleen or MLN compartments. (C) Representative staining for Foxp3 and T-bet expression by TCR-β+CD4+ gated LP cells. (D) Histograms summarize the percentage of TCR-β+CD4+Foxp3+ cells producing IFN-γ in the spleen and LP compartments from day 9 infected mice treated or not with IL-2-anti IL-2 complexes. Error bars represent the SDs of the means of 3 individual mice. (E) Mice were inoculated orally with 5 ME-49 cysts and the number of cysts was counted in the brain at 40 days after infection. Each dot represents an individual mouse (n=5). (F) The amount of hepatic Alanine Aminotransferase (ALT) was assessed in the serum at day 9 after infection (n=4). (G) Histological assessment of liver. (I) Comparative assessment of CD4+Foxp3+ Treg cells in spleen and colon compartments in age-matched Il2+/− or Il2−/− naïve mice. (H) Dot plots illustrate the T-bet and IFN-γ expression of TCR-β+CD4+ Foxp3+ or Foxp3− cells in the spleen of naïve Il2+/− or Il2−/− mice. The results shown are representative 3 independent experiments with similar results (*, p < 0.05; **, p < 0.01; ***, p < 0.001).
To further probe the potential link between IL-2 starvation and the acquisition of an effector phenotype by Treg cells, we evaluated the phenotype of Treg cells in the complete absence of IL-2. In the absence of infection, mice deficient in IL-2 spontaneously develop an autoimmune inflammatory disease associated with a dramatic reduction in peripheral Treg cell frequencies (Fig 6H), (Kramer et al., 1995). Foxp3+ Treg cells purified from _il2_−/− mice sustained suppressive function in vitro (data not shown). Concomitant with development of the inflammatory disease, and in contrast with il-2+/− littermate controls, Foxp3+ T cells from il-2−/− mice expressed high amount of T-bet in all the tissues analyzed (Fig 6I and data not shown). Furthermore, a significant proportion of Treg cells expressed IFN-γ in tissues undergoing severe inflammation such as the colon and the spleen (Fig 6I and data not shown). Thus, the development of inflammatory disease in IL-2 deficient mice is not only associated with reduced Treg cell frequencies but also can be correlated to the acquisition of a Th1 cell phenotype by Treg cells. Altogether, these results support the concept that in T. gondii infection, IL-2 withdrawal during the Th1 cell response contributes to Treg cell collapse and directly or indirectly to the acquisition of a Th1 cell phenotype by Treg cells.
Treg cell number collapse correlates with parasite pathogenesis
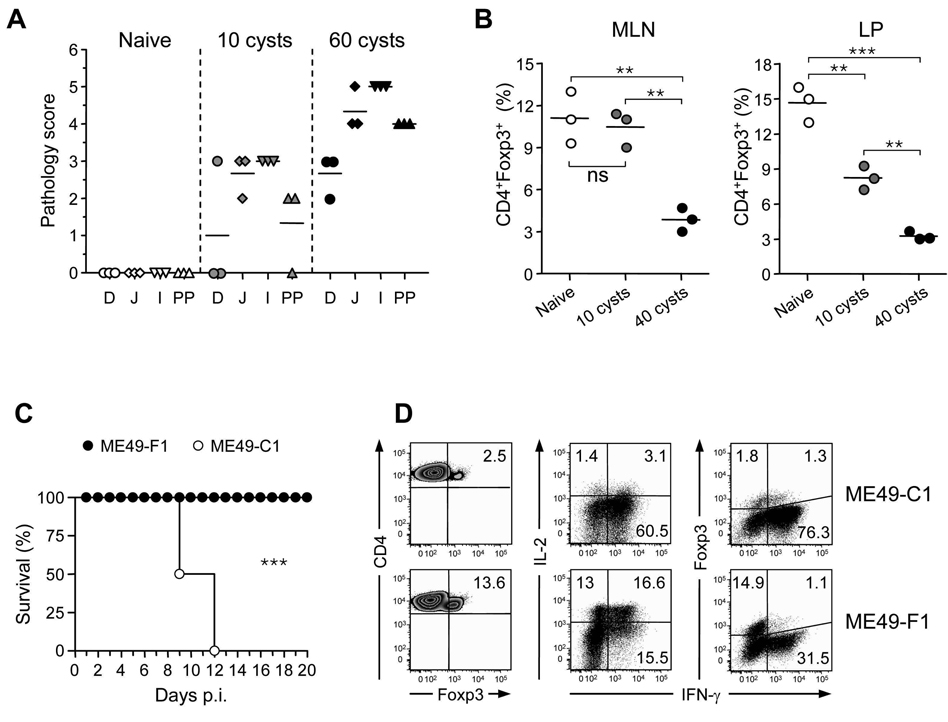
We postulated that Treg cell number collapse and acquisition of effector phenotype during T. gondii infection should correlate with pathogenicity. The capacity of T. gondii to cause disease can be associated with the strain of mice, the dose of parasite, or the type or clone of parasite used. When C57BL/6 mice were infected with a lower dose of parasite that allows host survival and only induces marginal pathology (Fig 7A), Treg cell were maintained at a higher frequency than in the lethal dose (Fig 7B). We next infected mice with clones derived from ME-49 that display distinct virulence. At 40 cysts, clone C1 induced high amount of gut and systemic pathology leading to the death of the infected host (Fig 7C and data not shown). In contrast, clone F1 induced only marginal pathology and allows for host survival at the same dose.
Figure 7. Treg cell collapse correlates with parasite virulence.
(A) Mice were inoculated orally with 10 or 60 ME-49 cysts. At day 9 after infection, pathology in the small intestine duodenum (D), jejunum (J), ileum (I) and Peyer’s patches (PP) was compared (n=3). (B) Mice were inoculated orally with 10 or 40 cysts of ME-49. Comparative assessment of CD4+Foxp3+ Treg cells frequencies in MLN and LP compartments in naïve or day 9 infected C57BL/6 mice. Cells were stained for TCR-β, CD4, and Foxp3. TCR-β+CD4+-gated cells were analyzed for Foxp3 expression by flow cytometry. Each dot represents one mouse (open circle: naïve, grey circle: 10 cysts, closed circle: 40 cysts). The results shown are representative of two (panel A) or three (panel B) independent experiments with similar results. Statistical comparisons were performed using the Student's t test (*, p < 0.05; **, p < 0.01; ***, p < 0.001). Mice were inoculated orally with 40 cysts of ME-49 clone F1 or clone C1. (C) The survival curves of infected mice were compared (n=5; ***, p < 0.001). (D) At day 9 after infection, LP cells were analyzed by flow cytometry for TCR-β, CD4 and Foxp3 expression or for TCR-β, CD4, IL-2, IFN-γ, and Foxp3 expression after in vitro restimulation with PMA and ionomycin in the presence of Brefeldin A. Each dot plot represents one mouse among 3 used per condition. Numbers in quadrants represent the percentage of each subset. The results shown in panel C and D are representative of two independent experiments.
As previously shown for ME-49, clone C1 induced Treg cell number collapse and a dramatic reduction of IL-2 production by effector T cells in the GALT and systemically (Fig 7D and data not shown). Furthermore, after C1 infection, a substantial fraction of Treg cells expressed IFN-γ. In contrast, after infection with clone F1, Treg cell populations were maintained and this correlated with sustained production of IL-2 by effector T cells (Fig 7D). In addition, Treg cells expressed only marginal amounts of IFN-γ compared to infection with the more virulent C1 clone (7% of Treg cells versus 42% with C1 infection, Fig 7D). Similar data were obtained under conditions associated with lower level of pathogenicity such as in BALB/c mice (Fig S8). Thus, our data suggest that Treg cell number collapse and acquisition of IFN-γ only occur in highly pathogenic situations.
Discussion
Here, we showed that a highly Th1 cell type-polarized mucosal immune response can induce the breakdown of the Treg cell population. Such a decline occurred via multiple mechanisms, including blockade of Treg cell induction and a dramatic reduction of the endogenous Treg cell population. In particular, our data suggest that shutdown of IL-2 in highly Th1 cell type-polarized environment contributes to the incapacity of Treg cells to parallel effector responses and consequently leads to immunopathogenesis. In addition, we found that environmental cues provided by both local DCs and effector T cells can confer T-bet expression in Treg cells, which leads to IFN-γ production by these cells. Thus, this study provides an evaluation of mechanisms that negatively control Treg cells during an infectious disease and demonstrate its importance in pathogenicity.
During T.gondii infection, protective lymphocytes concomitantly drive the immunopathologic process (Liesenfeld et al., 1996). In a non-lethal model of T.gondii after intraperitoneal infection, IL-27 as well as IL-10 producing Th1 cells limit excessive effector responses (Gazzinelli et al., 1996; Jankovic et al., 2007; Villarino et al., 2003). Given Treg cells ability to dampen excessive effector responses, these cells should also limit responses to this pathogen and preserve tissue integrity. Indeed, selective depletion of Foxp3+ cells using mice expressing a diphtheria toxin (DT) receptor under the control of the foxp3 gene locus (Lahl et al., 2007) further enhanced effector T cell responses during non lethal T.gondii infection (data not shown). Conversely, preventing this numerical collapse through treatment by increasing the amount of IL-2 resulted in marked reductions of both morbidity and immunopathology. Altogether, our results support the idea that Treg cells play a major role in preservation of host tissue integrity during T. gondii infection.
We and others have shown that the gut-associated lymphoid tissue is a preferential site for driving peripheral induction of Foxp3+ Treg cells (Coombes et al., 2007; Mucida et al., 2005; Mucida et al., 2007; Sun et al., 2007). Our data demonstrate that this process is dramatically inhibited during T. gondii infection. Several reports suggest that presence of Th1 effector cytokines or high level of costimulation—both events that characterize T. gondii infection (Gaddi and Yap, 2007)—had an antagonistic effect on Treg cell conversion (Benson et al., 2007; Bettelli et al., 2006; Kretschmer et al., 2005; O'Malley et al., 2009; Veldhoen et al., 2006; Wang et al., 2008; Wei et al., 2007). We recently showed that gut DCs no longer induce Treg cells when stimulated with bacterial DNA (Hall et al., 2008). Similarly, gut DCs exposed to Toxoplasma antigen are impaired in their capacity to induce Treg cells. Thus, tolerogenic DCs lose their capacity to induce Treg cells when activated by defined commensal products or pathogens in favor of the induction of effector responses. Upon challenge with T. gondii, gut-resident APCs are likely to be replaced by inflammatory cells that have not been conditioned by the gut environment (Dunay et al., 2008). Previous reports support the idea that restricted or defective Treg cell conversion can enhance immunopathology in the gut or lung (Curotto de Lafaille et al., 2008; Izcue et al., 2008). The relative contribution of impaired Treg cell conversion to the pathology induced by T. gondii remains difficult to evaluate but is likely to play a role in the overall decrease of Treg cells during infection. Our findings also raise the possibility that exposure to antigen at a time of acute infection may impair the acquisition of tolerance against innocuous antigens that could, in turn, further contribute to the pathologic process.
Recent findings suggest that acquisition of transcription factors in addition to Foxp3 can confer unique functional characteristics to Treg cells (Zheng et al., 2009). For instance, their expression of T-bet was recently shown to favor their capacity to control Th1 responses (Koch et al., 2009). In the context of T.gondii infection, Treg cells express high levels of the Th1 transcription factor T-bet. When isolated from the primary site of T. gondii infection, lamina propria DCs can readily induce T-bet expression by Treg cells via, in part, their capacity to induce IFN-γ by T cells. As LpDCs gain the capacity to produce IL-12 in this environment, we found that T-bet expression was associated with acquisition of responsiveness to IL-12 via enhanced Stat4 phosphorylation. Other factors—such as IL-27 that we found highly expressed in LpDCs from infected mice—are also likely to contribute to this imprinting. Based on recent findings that T-bet favors Treg cell tropism to sites of Mycobacterium tuberculosis infection, we could speculate that under non-lethal T.gondii infection, T-bet may confer an advantage to Treg cells. Indeed, we found that expression of T-bet did not interfere with the capacity of Treg cells to suppress proliferation of effector T cells in vitro. Furthermore, T-bet expression correlated with expression of CXCR3 (data not shown).
Our data reveal that in highly virulent settings, Treg cells do not accumulate preferentially at site of infection. Further, T-bet expression led to IFN-γ production, a cytokine responsible for both effector and pathogenic responses during T. gondii infection. Recent evidence shows that lymphocytes maintain a certain degree of plasticity with respect to their capacity to produce cytokines (Lee et al., 2009; Wei et al., 2009). Cells expressing both Foxp3 and IL-17 can be found in mucosal tissue or in vitro cultures (Lee et al., 2009; Xu et al., 2007; Yang et al., 2008). Genome wide mapping of H3K4me3 and H3K27me3 performed in Treg cells revealed markers of both repression and induction at the tbx21 locus. On the other hand, Ifng locus did not shown any sign of induction or repression (Wei et al., 2009) suggesting that it is poised for transcriptional activation. A previous report demonstrated that regulatory T cells expressing both Foxp3 and T-bet were capable to control airway hyperactivity (Stock et al., 2004). A role for IFN-γ in mediating Treg cell function has been reported in a model of graft transplants (Sawitzki et al., 2005) and recent evidence demonstrates that, in vitro, Treg cells can produce this cytokine (Wei et al., 2009); however, the capacity of Treg cells to express IFN-γ under physiologic settings, and in particular during infection, remained unclear. Our data support the idea that such an effect may be associated with, or arise as a consequence of pathology. Indeed, we only detected IFN-γ production by Treg cells in situations leading to death of the infected host. This would suggest that in the presence of high amount of inflammatory mediators, T-bet expression may reach a threshold that superimposes an effector program on Treg cells.
Given their high degree of self-reactivity, it is plausible that Treg cells can contribute to tissue damage or lose suppressive capacity if armed with effector cytokines (Hsieh et al., 2006). Indeed, a recent report highlighted that Foxp3 instability and acquisition of IFN-γ can favor the development of autoimmune diabetes (Zhou et al., 2009). However, the fate of bonafide Treg cells was not evaluated in this study. Here we demonstrate that Treg cells from T.gondii infected mice can produce IFN-γ and exert effector function as illustrated by their capacity to limit parasite growth. However based on the small frequency of remaining Treg cells in this particular setting, the overall contribution of these cells to the pathogenesis of the infection remains difficult to assess.
Our data compellingly suggest that one trigger of immunopathology during Th1 polarized responses may be the limitation of IL-2 availability. Over the past few years, a strong body of literature suggests that a primary function of IL-2 is to promote the expansion and survival of Treg cells (Malek and Bayer, 2004). Indeed, previous work showed that the number of Treg cells can be indexed to the number of IL-2-producing effector cells (Almeida et al., 2006). Intriguingly, IL-2 production declines upon differentiation into effector T cells. Accordingly, we found that following oral infection with T. gondii, the emergence of a Th1 response was associated with a sytemic reduction of IL-2 production by CD4+ T cells. During T. gondii infection, IL-27 and IL-12 were shown to synergize to limit IL-2 production (Villarino et al., 2007). A link between defective IL-2 production and Treg cell dysfunction has been previously proposed as one of the mechanisms associated with break down of tolerance in nonobese diabetic mice (Tang et al., 2008). In our study, we found that following infection, Treg cells expressed lower levels of Bcl-2 and increased levels of apoptotic markers compared to Treg cells from naïve mice suggesting that deregulation of Treg cell frequencies is a consequence of impaired Treg cell turnover. Because our data support the idea that cytokine reduction contributes to Treg cell collapse, such effect is likely to occur regardless to Treg cell specificity. A role for IL-2 is further supported by our observation that treatment of mice with an IL-2-anti-IL-2 complex (Boyman et al., 2006) can restore Treg cell proliferation, frequencies and survival following infection. Of note, in this particular setting we cannot fully exclude the possibility that IL-2 directly increases Treg cell expansion (Webster et al., 2009) rather than restoring IL-2 levels in infected mice. Nevertheless, the observation that Treg cells from IL-2 deficient mice recapitulate the phenotype in _T. gondii_-infected mice supports the idea that IL-2 withdrawal plays a major role in the alterations observed during infection. It is tempting to speculate that under most circumstances, Treg cells would be the primary bystander targets of IL-2 withdrawal during a Th1 response, thus providing an opportunity for effector responses to control infection.
Our work offers new insights into microbial pathogenesis associated with the subversion of regulatory pathways by effector responses. Our data demonstrating that Treg cell acquire effector functions are highly relevant in the context of therapeutic approaches aimed at inducing or transferring Treg cells. during the onset of autoimmune diseases. Furthermore, understanding how pathogenic microbes manipulate niches to undermine regulatory responses provides potential therapeutic strategies to control host homeostasis at large.
Material and Methods
Mice
C57BL/6 (WT) and B6.SJL mice were purchased from Taconic Farms and Jackson Laboratory or bred in house. Tbx21−/− and Il-2−/− mice were purchased from Jackson Laboratory. Foxp3eGFP reporter mice (Foxp3eGFP) and DEREG mice were obtained from Dr. M. Oukka (Bettelli et al., 2006) and Dr. T. Sparwasser (Lahl et al., 2007), respectively. We generated OT-IIxFoxp3eGFP mice. All mice were bred and maintained under pathogen-free conditions at an American Association for the Accreditation of Laboratory Animal Care-accredited animal facility at the NIAID and housed in accordance with the procedures outlined in the Guide for the Care and Use of Laboratory Animals under an animal study proposal approved by the NIAID Animal Care and Use Committee. Mice between 8 and 12 weeks of age were used. Mice were gender, vendor and age-matched for each experiment.
Parasite and infection protocol
ME-49 type II strain (ATCC # 50840) (American Type Culture Collection, Manassas, VA, USA) of T. gondii was used for production of tissue cysts in C57BL/6 mice. Tissue cysts used in experiments were obtained from mice that were inoculated 1–3 months previously with five cysts by gavage. Animals were sacrificed, the brains were removed and homogenized in 1 ml of phosphate buffer saline (PBS), pH 7.2, and tissue cysts were counted on the basis of 3 or more aliquots of 20 µL. For challenge studies, 8- to 12-week-old mice were used for all studies. Mice were infected by oral route. Parental ME-49 was electroporated with RFP and selected for red fluorescence. Two clones (ME-49 C1 and ME-49 F1) were established that possessed different degrees of virulence when passed through mice. STAg was prepared as previously described (Grunvald et al., 1996).
Quantitation of parasite tissue loads
Human fibroblast (Hs27; ATCC # CRL-1634) cultures were used for parasite burden quantification as previously described (Pfefferkorn and Pfefferkorn, 1976; Roos et al., 1994). Tissue single cell suspensions (104 to 106 cells) were added to the fibroblast monolayer cultures and titrated by plaque formation. The results of these titrations are reported in Plaque Forming Units (PFU).
In Vitro Assessment of Tachyzoite Killing
Macrophages harvested from naïve mice by injecting cold RPMI into the peritoneal cavity were plated in flat-bottom 96-well plates (3×105 per well) in complete medium and incubated 2 hours at 37°C. 5×104 ME-49-RFP tachyzoites were then added for 2 hours. After washing the infected monolayer, 105 CD4+GFP+ or GFP− cells purified from Foxp3eGFP mice orally infected with ME-49 were added in each well. 2 days later parasite growth was measured by fluorescence and NO2 − levels were measured in culture supernatants by the Griess assay as an indicator of reactive nitrogen intermediates.
Phenotypic analysis
Cells from spleen, MLN, LP and IEL were prepared as previously described (Sun et al., 2007). Single-cell suspensions were incubated with anti-FcγIII/II receptor antibody (93) and stained with fluorochrome-conjugated antibodies against CD4 (RM4–5), CD8α (53-6.7), CD25 (PC61.5), CD62L (MEL-14), TCR-β chain (H57–597), CD45.1 (A20), CD45.2 (104), in PBS containing or not 1% FBS for 20 min on ice and then washed. 7-amino-actinomycin D (7-AAD, eBioscience) or LIVE/DEAD Fixable Blue Dead cell stain kit (invitrogen) was used to exclude dead cells. For Foxp3 staining, cells were subsequently stained using the Foxp3 staining set (eBioscience) according to the manufacturer's protocol. Nuclear Ki-67 and Bcl-2 staining were performed using anti-Ki-67 (B56) and anti-Bcl-2 (3F11) from BD Pharmingen. For T-bet staining anti-mouse/human T-bet (eBio4B10) from eBioscience was used. Cell acquisition was performed on an LSRII machine using FACSDiVa software (BD Biosciences). For each sample, at least 300,000 events were collected. Data were analyzed using FlowJo software (TreeStar).
In vitro restimulation and intracellular cytokine detection
RPMI 1640 supplemented with 10% FBS, penicillin, streptomycin, gentamicin, HEPES, glutamine, nonessential amino acids, and 50 mM of β-mercaptoethanol was used for in vitro restimulation. For basal cytokine detection, spleen, MLN, LP and IEL single cell suspensions were cultured in triplicate at 1×106 cells/ml in a 96-well U-bottom plate and stimulated with 50 ng/ml PMA (Sigma) and 5 µg/ml ionomycin (Sigma), in the presence of brefeldin A (GolgiPlug, BD Biosciences). After 4 hrs, samples were stained for dead cells as described previously, washed twice with PBS and fixed using 2% paraformaldehyde (Electron Microscopy Sciences) solution. Cells were then stained with fluorochrome-conjugated antibodies against TCR-β chain (H57–597), CD4 (RM4–5), CD8α (53-6.7), IFN-γ (XMG1.2), IL-10 (JES5-16E3), IL-2 (JES6-1A12), Foxp3 (FJK-16s), T-bet (eBio4B10) or isotype controls: rat IgG1 (eBRG1), rat IgG2a (eBR2a), rat IgG2b (eB149/10H5), mouse IgG1 (clone P3) in the presence of anti-FcγIII/II receptor for 60 min in FACS buffer containing 0.6% saponin. All antibodies were purchased from eBioscience or BD Biosciences. In some experiments, 2.5×105 LP TCR-β+CD4+ cells from naïve or day 9 mice were incubated with 5×104 cells MACS-sorted splenic dendritic cells in complete medium in a round-bottom 96-well plate in presence of STAg (5 µg/ml) for 15 hours. Brefeldin A was added for the last 6 hours and cells were stained for TCR-β, CD4, Foxp3 and IFN-γ as previously described.
Lamina propria DCs (LpDCs) purification
LpDCs were purified from naïve or T. gondii infected small intestine. After LP digests were passed through 70- and 40-µm cell strainers, cells were resuspended in 1.077 g/cm3 iso-osmotic NycoPrep medium (Accurate Chemical & Scientific Corp.), and overlayed with RPMI 1640. The low-density fraction was collected after centrifugation at 1,650 g for 15 min. Cells were washed and incubated with a mixture of mAb containing anti-FcγIII/II receptor, 7-AAD viability staining solution, α-CD11c (HL-3), α-MHCII (AF6-120.1), as well as the non-DC components α-DX5 (DX5), α-NK1.1 (PK136), and α-B220 (RA3-6B2) (all from eBioscience). DCs were defined as CD11c+MHCII+ cells and non-DCs were excluded when sorted by flow cytometry on a FACSAria. CD11c+MHCII+ cells were > 90% and used for in vitro conversion assays or co-cultured with purified Treg cells.
T cell purification
Single cell suspensions of spleen and MLN extracted from naïve or day 8 infected Foxp3eGFP, Tbx21−/− and C57BL/6 WT mice were enriched for CD4+ T cells by negative selection using an autoMACs (Miltenyi Biotec). The enriched fraction was further labeled with fluorescent dye-conjugated mAb, including CD4 (RM4–5), CD25 (7D4), CD44 (IM7), CD45RB (16A) (all from eBioscience) and sorted by flow cytometry on a FACSVantage or FACSAria (BD Biosciences). Purified CD4+CD25−CD44loeGFP− T cells (< 0.5 % Foxp3+) were used for in vitro conversion assays. Purified CD4+eGFP+ or CD4+CD25+CD45RBlow were used for Treg cell-DC incubation. In proliferation assays, purified CD4+eGFP− T cells and CD4+eGFP+ T cells were used.
In vitro conversion assay
Conversion protocol was performed as previously described (Bettelli et al., 2006) with T cells and LpDCs obtained as described above. LpDCs were purified from naïve or day 6 infected mice. In brief, FACS purified LpDCs and CD4+ T cells were cocultured at a 1:10 ratio (1×105 CD4+ T cells) in complete medium (RPMI-1640 containing 10% FBS, 50 mM of β-mercaptoethanol and antibiotics) and Treg cell polarizing conditions (soluble α-CD3 mAb (1 µg/ml) (BD Bioscience) and human rTGF-β (0.6 ng/ml) (R&D)). 5 ng/ml of IL-2 was supplemented in cocultures every 2 days. STAg (5 µg/ml) and various combinations of cytokines (10 ng/ml; IL-12, IFN-γ from R&D) or mAb (10 µg/ml; α-IL12/23p40 (C17.8), α-IFN-γ (XMG1.2 or 11B11) or isotype controls α-IgG2a (R35–95), α-IgG1κ (R3–34) from BD Biosciences) were added at the start of cocultures in Treg cells polarizing conditions. On day 5, cells were stained with the viability marker 7-AAD (eBioscience) or with LIVE/DEAD kit (Invitrogen), αCD4, αCD25, α-Foxp3 and/or α-T-bet and analyzed on an LSRII.
Suppression assay
CD4+eGFP− T cells were labeled with 1.25 µM CFSE (Invitrogen) in HBSS (Mediatech Inc.) for 15 min at 37 C. Cells were washed twice in media containing 10 % FBS. 5×104 CFSE labeled CD4+eGFP− T cells were cultured per well of a 96-well U-bottom plate with 0.5 µg/ml of α-CD3 mAb (BD Bioscience) and in presence of 105 irradiated T-depleted spleen cell feeders and decreasing amounts of CD4+eGFP+ cells (at indicated ratio) during 3 days.
Treg cell stimulation by DCs
5×104 CD4+CD25+CD45RBlow or CD4+eGFP+ T cells were cocultured in each well with LpDCs purified from naïve or day 6 infected mice (ratio 1:1) in complete medium with soluble α-CD3 mAb (1 µg/ml) (BD Bioscience) and 5 ng/ml of IL-2. STAg (5 µg/ml) and various combinations of cytokines or mAb including, IL-12, IFN-γ, α-IL12/23p40, α-IFN-γ or isotype controls were added at the start of coculture as described above. After a 5-day culture at 37°C, 5% CO2, Foxp3, T-bet and cytokine staining was performed as previously described.
Oral antigen administration
Mice were infected orally or not with 40 ME49 cysts. Foxp3− T lymphocytes from the secondary lymph nodes and spleen of OT-IIxFoxp3eGFP mice (CD45.2) were purified by cell-sorting, stained with CFSE and adoptively transferred into naïve or infected (day 4 post-infection) B6.SJL recipient mice (CD45.1). Each mouse received 1.5×106 cells and then 1.5 % OVA solution dissolved in drinking water (grade V; Sigma-Aldrich) for 5 consecutive days. On day 9, MLN and LP were collected from B6.SJL hosts and Foxp3 expression was assessed in transferred cells. LN and LP single cell suspensions were prepared as described above.
Pathology assessment
Mice were euthanized 9 or 10 days post oral infection with 40 cysts of ME-49. Their small intestines were removed and immediately fixed in a solution containing 10% formalin. Paraffin embedded sections were cut at 0.5 µm and stained with hematoxylin and eosin. The entire length of the small intestine (Duodenum, Jejunum, Ileum and Peyers Patch) was examined histologically. Inflammation was scored on the following modified scale (Asseman et al., 1999) of 0–5: 0 – within normal limits; 1 – minimal inflammatory leukocyte infiltrates multifocally or locally; 2 – mild inflammatory leukocytic and granulocytic infiltrates within the lamina propria; 3 – mild to moderate inflammatory infiltrates in the lamina propria and submucosa, alterations in intestinal crypts, local to diffuse thickening of the mucosal epithelium; 4 – moderate to marked inflammatory infiltrates of primarily viable and degenerate neutrophils, eosinophils, lymphocytes, plasma cells admixed with cellular debris within the lamina propria and submucosa with separation or loss of crypts; 5 – marked to severe inflammation diffusely with altered or complete loss of normal histologic structures and abundant inflammatory infiltrates that may extend transmurally (with peritonitis). Sections of livers were evaluated for the numbers of inflammatory loci and necrosis. Liver alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels were measured in serum samples, using commercially available kits (Boehringer Mannheim).
Treatment with IL-2/anti-IL-2 complexes
Mice were inoculated orally with ME49 cysts and IL-2 + αIL2 complexes were administered or not intraperitoneally for 5 consecutive days. The complex was formed by incubating 1.5 µg recombinant mouse IL-2 and 15 µg of Functional Grade Purified anti-mouse IL-2 (Clone: JES6-1A12, eBioscience) for 5 min at room temperature.
Quantitative RT-PCR
Splenic and LP total cells or DCs were purified from mice at 6 or 9 days post-infection. RNA were then extracted with RNAeasy columns (Qiagen) and analyzed by quantitative RT-PCR according to the manufacturer’s instructions using primers for murine IL-2, IL-12p40, IL-12p35, EBI3, IL-27p28 (Qiagen).
pSTAT-4 detection
5×105 MLN cells from ME-49 infected mice were stimulated in vitro with 10 ng/ml of recombinant mouse IL-12 (R&D) during 20 min. Cells were then fixed for 20 minutes using 2% paraformaldehyde solution, permeabilized with 90% methanol solution for 30 min and stained in PBS with antibodies against TCR-β, CD4, Foxp3 and pSTAT-4 (38/p-Stat4, BD Bioscience) or mouse α–IgG2bκ isotype control.
Confocal analysis
Small intestines for confocal imaging were processed by the “swiss roll” method. Briefly, the small intestines were removed, cut open longitudinally, and washed in PBS. They were then sectioned into 3 pieces of equal length and rolled around a small wooden dowel with the villi facing outward. After fixation overnight in 4% formaldehyde, the intestines were dehydrated in 30% sucrose for at least 24 hours. The rolls were then frozen in Tissue Freezing Medium (Electron Microscopy Sciences) and sectioned at 15 µm. Sections were mounted on Superfrost Plus slides (Electron Microscopy Sciences) and stained with CD4 Alexa Fluor 700 (eBioscience) and Hoechst 33342 (Invitrogen) followed by mounting with Prolong Gold (Invitrogen). Images were taken on a Leica SP5 microscope and image processing was performed using Imaris software (Bitplane).
Statistical analysis
Groups were compared with Prism software (GraphPad) using the unpaired or paired Student’s t test.
Supplementary Material
01
Acknowledgements
This work was supported by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health. We would like to thank the students of the Biology of Parasitism Course (Woods hole MBL 2008–2009) for contributing to some of the experiments. We thank Dr. Fernando Torres for help with pathological evaluation of samples. We thank Dr. Kevin Holmes and the NIAID sorting facility and Kim Beacht for technical assistance. We thank Aishling O’Hara (University of Pennsylvania, Immunology Graduate Program) for help with the parasite and Dr George Yap for helpful suggestions. We thank Drs. F.A. Sher and D. Jankovic for critical reading of the manuscript.
Abbreviations used
BMDC
BM-derived DC
GALT
gut-associated lymphoid tissue
GI
gastrointestinal tract
gfDNA
gut flora DNA
IBD
inflammatory bowel disease
IEL
intraepithelial lymphocyte
inf. DC
infected DC
Lp
lamina propria
LpDC
lamina propria DC
MLN
mesenteric lymph node
PP
Peyer’s patch
TLR
Toll-like receptor
Teff cell
effector T cell
Treg cell
regulatory T cell
SpDC
spleen DC
RA
retinoic acid
LPS
Lipopolysaccharide
T. gondii
Toxoplasma gondii
IL
interleukin
IFN-γ
interferon gamma
STAg
soluble toxoplasma antigen
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Almeida AR, Zaragoza B, Freitas AA. Indexation as a novel mechanism of lymphocyte homeostasis: the number of CD4+CD25+ regulatory T cells is indexed to the number of IL-2-producing cells. J Immunol. 2006;177:192–200. doi: 10.4049/jimmunol.177.1.192. [DOI] [PubMed] [Google Scholar]
- Asseman C, Mauze S, Leach MW, Coffman RL, Powrie F. An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J Exp Med. 1999;190:995–1004. doi: 10.1084/jem.190.7.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson MJ, Pino-Lagos K, Rosemblatt M, Noelle RJ. All-trans retinoic acid mediates enhanced T reg cell growth, differentiation, and gut homing in the face of high levels of co-stimulation. J Exp Med. 2007;204:1765–1774. doi: 10.1084/jem.20070719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- Boyman O, Surh CD, Sprent J. Potential use of IL-2/anti-IL-2 antibody immune complexes for the treatment of cancer and autoimmune disease. Expert Opin Biol Ther. 2006;6:1323–1331. doi: 10.1517/14712598.6.12.1323. [DOI] [PubMed] [Google Scholar]
- Coombes JL, Powrie F. Dendritic cells in intestinal immune regulation. Nat Rev Immunol. 2008;8:435–446. doi: 10.1038/nri2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, Hall J, Sun CM, Belkaid Y, Powrie F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-{beta}- and retinoic acid-dependent mechanism. J Exp Med. 2007 doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curotto de Lafaille MA, Kutchukhidze N, Shen S, Ding Y, Yee H, Lafaille JJ. Adaptive Foxp3+ regulatory T cell-dependent and -independent control of allergic inflammation. Immunity. 2008;29:114–126. doi: 10.1016/j.immuni.2008.05.010. [DOI] [PubMed] [Google Scholar]
- Degauque N, Mariat C, Kenny J, Zhang D, Gao W, Vu MD, Alexopoulos S, Oukka M, Umetsu DT, DeKruyff RH, et al. Immunostimulatory Tim-1-specific antibody deprograms Tregs and prevents transplant tolerance in mice. J Clin Invest. 2008;118:735–741. doi: 10.1172/JCI32562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denning TL, Wang YC, Patel SR, Williams IR, Pulendran B. Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17-producing T cell responses. Nat Immunol. 2007;8:1086–1094. doi: 10.1038/ni1511. [DOI] [PubMed] [Google Scholar]
- Duarte JH, Zelenay S, Bergman ML, Martins AC, Demengeot J. Natural Treg cells spontaneously differentiate into pathogenic helper cells in lymphopenic conditions. Eur J Immunol. 2009;39:948–955. doi: 10.1002/eji.200839196. [DOI] [PubMed] [Google Scholar]
- Dunay IR, Damatta RA, Fux B, Presti R, Greco S, Colonna M, Sibley LD. Gr1(+) inflammatory monocytes are required for mucosal resistance to the pathogen Toxoplasma gondii. Immunity. 2008;29:306–317. doi: 10.1016/j.immuni.2008.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floess S, Freyer J, Siewert C, Baron U, Olek S, Polansky J, Schlawe K, Chang HD, Bopp T, Schmitt E, et al. Epigenetic control of the foxp3 locus in regulatory T cells. PLoS Biol. 2007;5:e38. doi: 10.1371/journal.pbio.0050038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaddi PJ, Yap GS. Cytokine regulation of immunopathology in toxoplasmosis. Immunol Cell Biol. 2007;85:155–159. doi: 10.1038/sj.icb.7100038. [DOI] [PubMed] [Google Scholar]
- Gazzinelli RT, Wysocka M, Hayashi S, Denkers EY, Hieny S, Caspar P, Trinchieri G, Sher A. Parasite-induced IL-12 stimulates early IFN-gamma synthesis and resistance during acute infection with Toxoplasma gondii. J Immunol. 1994;153:2533–2543. [PubMed] [Google Scholar]
- Gazzinelli RT, Wysocka M, Hieny S, Scharton-Kersten T, Cheever A, Kuhn R, Muller W, Trinchieri G, Sher A. In the absence of endogenous IL-10, mice acutely infected with Toxoplasma gondii succumb to a lethal immune response dependent on CD4+ T cells and accompanied by overproduction of IL-12, IFN-gamma and TNF-alpha. J Immunol. 1996;157:798–805. [PubMed] [Google Scholar]
- Grunvald E, Chiaramonte M, Hieny S, Wysocka M, Trinchieri G, Vogel SN, Gazzinelli RT, Sher A. Biochemical characterization and protein kinase C dependency of monokine-inducing activities of Toxoplasma gondii. Infect Immun. 1996;64:2010–2018. doi: 10.1128/iai.64.6.2010-2018.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall JA, Bouladoux N, Sun CM, Wohlfert EA, Blank RB, Zhu Q, Grigg ME, Berzofsky JA, Belkaid Y. Commensal DNA Limits Regulatory T Cell Conversion and Is a Natural Adjuvant of Intestinal Immune Responses. Immunity. 2008 doi: 10.1016/j.immuni.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh CS, Zheng Y, Liang Y, Fontenot JD, Rudensky AY. An intersection between the self-reactive regulatory and nonregulatory T cell receptor repertoires. Nat Immunol. 2006;7:401–410. doi: 10.1038/ni1318. [DOI] [PubMed] [Google Scholar]
- Izcue A, Coombes JL, Powrie F. Regulatory T cells suppress systemic and mucosal immune activation to control intestinal inflammation. Immunol Rev. 2006;212:256–271. doi: 10.1111/j.0105-2896.2006.00423.x. [DOI] [PubMed] [Google Scholar]
- Izcue A, Hue S, Buonocore S, Arancibia-Carcamo CV, Ahern PP, Iwakura Y, Maloy KJ, Powrie F. Interleukin-23 restrains regulatory T cell activity to drive T cell-dependent colitis. Immunity. 2008;28:559–570. doi: 10.1016/j.immuni.2008.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson NG, Szabo SJ, Guler ML, Gorham JD, Murphy KM. Regulation of interleukin-12 signalling during T helper phenotype development. Adv Exp Med Biol. 1996;409:61–73. doi: 10.1007/978-1-4615-5855-2_9. [DOI] [PubMed] [Google Scholar]
- Jankovic D, Kullberg MC, Feng CG, Goldszmid RS, Collazo CM, Wilson M, Wynn TA, Kamanaka M, Flavell RA, Sher A. Conventional T-bet(+)Foxp3(−) Th1 cells are the major source of host-protective regulatory IL-10 during intracellular protozoan infection. J Exp Med. 2007;204:273–283. doi: 10.1084/jem.20062175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch MA, Tucker-Heard G, Perdue NR, Killebrew JR, Urdahl KB, Campbell DJ. The transcription factor T-bet controls regulatory T cell homeostasis and function during type 1 inflammation. Nature Immunology. 2009;10:1–7. doi: 10.1038/ni.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer S, Schimpl A, Hunig T. Immunopathology of interleukin (IL) 2-deficient mice: thymus dependence and suppression by thymus-dependent cells with an intact IL-2 gene. J Exp Med. 1995;182:1769–1776. doi: 10.1084/jem.182.6.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretschmer K, Apostolou I, Hawiger D, Khazaie K, Nussenzweig MC, von Boehmer H. Inducing and expanding regulatory T cell populations by foreign antigen. Nat Immunol. 2005;6:1219–1227. doi: 10.1038/ni1265. [DOI] [PubMed] [Google Scholar]
- Lahl K, Loddenkemper C, Drouin C, Freyer J, Arnason J, Eberl G, Hamann A, Wagner H, Huehn J, Sparwasser T. Selective depletion of Foxp3+ regulatory T cells induces a scurfy-like disease. J Exp Med. 2007;204:57–63. doi: 10.1084/jem.20061852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YK, Turner H, Maynard CL, Oliver JR, Chen D, Elson CO, Weaver CT. Late developmental plasticity in the T helper 17 lineage. Immunity. 2009;30:92–107. doi: 10.1016/j.immuni.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liesenfeld O. Oral infection of C57BL/6 mice with Toxoplasma gondii: a new model of inflammatory bowel disease? J Infect Dis. 2002;185 Suppl 1:S96–S101. doi: 10.1086/338006. [DOI] [PubMed] [Google Scholar]
- Liesenfeld O, Kosek J, Remington JS, Suzuki Y. Association of CD4+ T cell-dependent, interferon-gamma-mediated necrosis of the small intestine with genetic susceptibility of mice to peroral infection with Toxoplasma gondii. J Exp Med. 1996;184:597–607. doi: 10.1084/jem.184.2.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malek TR, Bayer AL. Tolerance, not immunity, crucially depends on IL-2. Nat Rev Immunol. 2004;4:665–674. doi: 10.1038/nri1435. [DOI] [PubMed] [Google Scholar]
- Mennechet FJ, Kasper LH, Rachinel N, Li W, Vandewalle A, Buzoni-Gatel D. Lamina propria CD4+ T lymphocytes synergize with murine intestinal epithelial cells to enhance proinflammatory response against an intracellular pathogen. J Immunol. 2002;168:2988–2996. doi: 10.4049/jimmunol.168.6.2988. [DOI] [PubMed] [Google Scholar]
- Mucida D, Kutchukhidze N, Erazo A, Russo M, Lafaille JJ, Curotto de Lafaille MA. Oral tolerance in the absence of naturally occurring Tregs. J Clin Invest. 2005;115:1923–1933. doi: 10.1172/JCI24487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M, Cheroutre H. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256–260. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- O'Malley JT, Sehra S, Thieu VT, Yu Q, Chang HC, Stritesky GL, Nguyen ET, Mathur AN, Levy DE, Kaplan MH. Signal transducer and activator of transcription 4 limits the development of adaptive regulatory T cells. Immunology. 2009;127:587–595. doi: 10.1111/j.1365-2567.2008.03037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science. 2003;299:1033–1036. doi: 10.1126/science.1078231. [comment]. [DOI] [PubMed] [Google Scholar]
- Pfefferkorn ER, Pfefferkorn LC. Toxoplasma gondii: isolation and preliminary characterization of temperature-sensitive mutants. Exp Parasitol. 1976;39:365–376. doi: 10.1016/0014-4894(76)90040-0. [DOI] [PubMed] [Google Scholar]
- Reis e Sousa C, Yap G, Schulz O, Rogers N, Schito M, Aliberti J, Hieny S, Sher A. Paralysis of dendritic cell IL-12 production by microbial products prevents infection-induced immunopathology. Immunity. 1999;11:637–647. doi: 10.1016/s1074-7613(00)80138-7. [DOI] [PubMed] [Google Scholar]
- Roos DS, Donald RG, Morrissette NS, Moulton AL. Molecular tools for genetic dissection of the protozoan parasite Toxoplasma gondii. Methods Cell Biol. 1994;45:27–63. doi: 10.1016/s0091-679x(08)61845-2. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S, Sakaguchi N, Shimizu J, Yamazaki S, Sakihama T, Itoh M, Kuniyasu Y, Nomura T, Toda M, Takahashi T. Immunologic tolerance maintained by CD25+ CD4+ regulatory T cells: their common role in controlling autoimmunity, tumor immunity, and transplantation tolerance. Immunol Rev. 2001;182:18–32. doi: 10.1034/j.1600-065x.2001.1820102.x. [DOI] [PubMed] [Google Scholar]
- Sawitzki B, Kingsley CI, Oliveira V, Karim M, Herber M, Wood KJ. IFN-gamma production by alloantigen-reactive regulatory T cells is important for their regulatory function in vivo. J Exp Med. 2005;201:1925–1935. doi: 10.1084/jem.20050419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sher A, Hieny S, Charest H, Scharton-Kersten T, Collazo C, Germain RN, Reis e Sousa C. The role of dendritic cells in the initiation of host resistance to Toxoplasma gondii. Adv Exp Med Biol. 1998;452:103–110. doi: 10.1007/978-1-4615-5355-7_12. [DOI] [PubMed] [Google Scholar]
- Stock P, Akbari O, Berry G, Freeman GJ, Dekruyff RH, Umetsu DT. Induction of T helper type 1-like regulatory cells that express Foxp3 and protect against airway hyper-reactivity. Nat Immunol. 2004;5:1149–1156. doi: 10.1038/ni1122. [DOI] [PubMed] [Google Scholar]
- Sun CM, Hall JA, Blank RB, Bouladoux N, Oukka M, Mora JR, Belkaid Y. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med. 2007 doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki Y, Sher A, Yap G, Park D, Neyer LE, Liesenfeld O, Fort M, Kang H, Gufwoli E. IL-10 is required for prevention of necrosis in the small intestine and mortality in both genetically resistant BALB/c and susceptible C57BL/6 mice following peroral infection with Toxoplasma gondii. J Immunol. 2000;164:5375–5382. doi: 10.4049/jimmunol.164.10.5375. [DOI] [PubMed] [Google Scholar]
- Tang Q, Adams JY, Penaranda C, Melli K, Piaggio E, Sgouroudis E, Piccirillo CA, Salomon BL, Bluestone JA. Central role of defective interleukin-2 production in the triggering of islet autoimmune destruction. Immunity. 2008;28:687–697. doi: 10.1016/j.immuni.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlig HH, Coombes J, Mottet C, Izcue A, Thompson C, Fanger A, Tannapfel A, Fontenot JD, Ramsdell F, Powrie F. Characterization of Foxp3+CD4+CD25+ and IL-10-secreting CD4+CD25+ T cells during cure of colitis. J Immunol. 2006;177:5852–5860. doi: 10.4049/jimmunol.177.9.5852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Villarino A, Hibbert L, Lieberman L, Wilson E, Mak T, Yoshida H, Kastelein RA, Saris C, Hunter CA. The IL-27R (WSX-1) is required to suppress T cell hyperactivity during infection. Immunity. 2003;19:645–655. doi: 10.1016/s1074-7613(03)00300-5. [DOI] [PubMed] [Google Scholar]
- Villarino AV, Tato CM, Stumhofer JS, Yao Z, Cui YK, Hennighausen L, O'Shea JJ, Hunter CA. Helper T cell IL-2 production is limited by negative feedback and STAT-dependent cytokine signals. J Exp Med. 2007;204:65–71. doi: 10.1084/jem.20061198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Pino-Lagos K, de Vries VC, Guleria I, Sayegh MH, Noelle RJ. Programmed death 1 ligand signaling regulates the generation of adaptive Foxp3+CD4+ regulatory T cells. Proc Natl Acad Sci U S A. 2008 doi: 10.1073/pnas.0710441105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster KE, Walters S, Kohler RE, Mrkvan T, Boyman O, Surh CD, Grey ST, Sprent J. In vivo expansion of T reg cells with IL-2-mAb complexes: induction of resistance to EAE and long-term acceptance of islet allografts without immunosuppression. J Exp Med. 2009;206:751–760. doi: 10.1084/jem.20082824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei G, Wei L, Zhu J, Zang C, Hu-Li J, Yao Z, Cui K, Kanno Y, Roh TY, Watford WT, et al. Global mapping of H3K4me3 and H3K27me3 reveals specificity and plasticity in lineage fate determination of differentiating CD4+ T cells. Immunity. 2009;30:155–167. doi: 10.1016/j.immuni.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J, Duramad O, Perng OA, Reiner SL, Liu YJ, Qin FX. Antagonistic nature of T helper 1/2 developmental programs in opposing peripheral induction of Foxp3+ regulatory T cells. Proc Natl Acad Sci U S A. 2007;104:18169–18174. doi: 10.1073/pnas.0703642104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson EH, Wille-Reece U, Dzierszinski F, Hunter CA. A critical role for IL-10 in limiting inflammation during toxoplasmic encephalitis. J Neuroimmunol. 2005;165:63–74. doi: 10.1016/j.jneuroim.2005.04.018. [DOI] [PubMed] [Google Scholar]
- Xu L, Kitani A, Fuss I, Strober W. Cutting edge: regulatory T cells induce CD4+CD25-Foxp3- T cells or are self-induced to become Th17 cells in the absence of exogenous TGF-beta. J Immunol. 2007;178:6725–6729. doi: 10.4049/jimmunol.178.11.6725. [DOI] [PubMed] [Google Scholar]
- Yang XO, Pappu BP, Nurieva R, Akimzhanov A, Kang HS, Chung Y, Ma L, Shah B, Panopoulos AD, Schluns KS, et al. T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR alpha and ROR gamma. Immunity. 2008;28:29–39. doi: 10.1016/j.immuni.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Chaudhry A, Kas A, Deroos P, Kim JM, Chu TT, Corcoran L, Treuting P, Klein U, Rudensky AY. Regulatory T-cell suppressor program co-opts transcription factor IRF4 to control T(H)2 responses. Nature. 2009 doi: 10.1038/nature07674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Bailey-Bucktrout SL, Jeker LT, Penaranda C, Martinez-Llordella M, Ashby M, Nakayama M, Rosenthal W, Bluestone JA. Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat Immunol. 2009 doi: 10.1038/ni.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
01