Construction of folate-conjugated pRNA of bacteriophage phi29 DNA packaging motor for delivery of chimeric siRNA to nasopharyngeal carcinoma cells (original) (raw)
. Author manuscript; available in PMC: 2010 Mar 17.
Published in final edited form as: Gene Ther. 2006 May;13(10):814–820. doi: 10.1038/sj.gt.3302716
Abstract
Nasopharyngeal carcinoma is a poorly differentiated upper respiratory tract cancer that highly expresses human folate receptors (hFR). Binding of folate to hFR triggers endocytosis. The folate was conjugated into adenosine 5′-monophosphate (AMP) by 1,6-hexanediamine linkages. After reverse HPLC to reach 93% purity, the folate–AMP, which can only be used for transcription initiation but not for chain extension, was incorporated into the 5′-end of bacteriophage phi29 motor pRNA. A 16:1 ratio of folate–AMP to ATP in transcription resulted in more than 60% of the pRNA containing folate. A pRNA with a 5′-overhang is needed to enhance the accessibility of the 5′ folate for specific receptor binding. Utilizing the engineered left/right interlocking loops, polyvalent dimeric pRNA nanoparticles were constructed using RNA nanotechnology to carry folate, a detection marker, and siRNA targeting at an antiapoptosis factor. The chimeric pRNAs were processed into ds-siRNA by Dicer. Incubation of nasopharyngeal epidermal carcinoma (KB) cells with the dimer resulted in its entry into cancer cells, and the subsequent silencing of the target gene. Such a protein-free RNA nanoparticle with undetectable antigenicity has a potential for repeated long-term administration for nasopharyngeal carcinoma as the effectiveness and specificity were confirmed by ex vivo delivery in the animal trial.
Keywords: pRNA, siRNA, folate, nasopharyngeal carcinoma, gene delivery, phi29, DNA packaging motor
Introduction
siRNA1–5 and ribozyme RNA6 have been extensively utilized for post-transcriptional gene silencing in a sequence-specific manner. The delivery of siRNA has been studied by various methods, including viral vector delivery7 and lipid-encapsulated RNA injection.8 Recently, siRNAs joined to a cholesterol group have been reported to silence target gene expression in mice via intravenous injection.9 The successful application of siRNAs for the treatment of cancer and infectious diseases requires the coexistence of several features: (1) the correct folding of siRNA or ribozymes in the cell, if fused to a carrier, (2) the recognition of targeted cells, (3) the capability of entering cells owing to the size limit on membrane penetration, (4) the surviving RNase digestion within the cell, and (5) the trafficking into the appropriate cell compartment. Hence, the development of an efficient, specific and nonpathogenic system for the delivery of therapeutic RNA is highly desirable.
A 117-nt bacteriophage phi29-encoded RNA (pRNA) has been found to play a novel and essential role in DNA packaging.10 pRNA forms dimers, trimers, and, ultimately, hexamers through hand-in-hand interaction of the right and left interlocking loops.11,12,31 The structural features of pRNA, which have been studied extensively, allow for easy manipulation and permit the conversion of pRNA into a gene targeting and delivery vehicle. The pRNA molecule contains two independent folding domains with distinct functions.11,13 Replacement or insertion of nucleotides preceding residue #23 or following residue #97 does not interfere with the formation of dimers as long as the strands are paired.14 Therefore, the 5′ /3′ proximate double-stranded helical region15 of pRNA can be redesigned to carry additional sequences without altering its secondary structure or intermolecular interactions.16,17
Being a poorly differentiated carcinoma of the human upper respiratory tract, nasopharyngeal carcinoma has human folate receptors (hFR) that are highly expressed in epidermal carcinoma (KB) cells. Endocytosis of the ligand / receptor complex mediated by the binding of folate to hFR has been well studied, and macromolecules conjugated to folate have been successfully recognized by folate receptors and internalized into cells.18–20 Recently, we found that phi29 pRNA can be used as a carrier for the construction of RNA nanoparticles to deliver therapeutic RNAs such as siRNAs and / or ribozymes to specific cancer cells.12,21,22 In this report, we described the methods for the synthesis and purification of folate–adenosine 5′-monophosphate (AMP), the procedure for the construction of folate–pRNA, and the test of the purity and function of the folate-containing products. The potential of siRNA in gene therapy mediated by folate was also investigated in animal trials.
Results
Characterization of folate–AMP
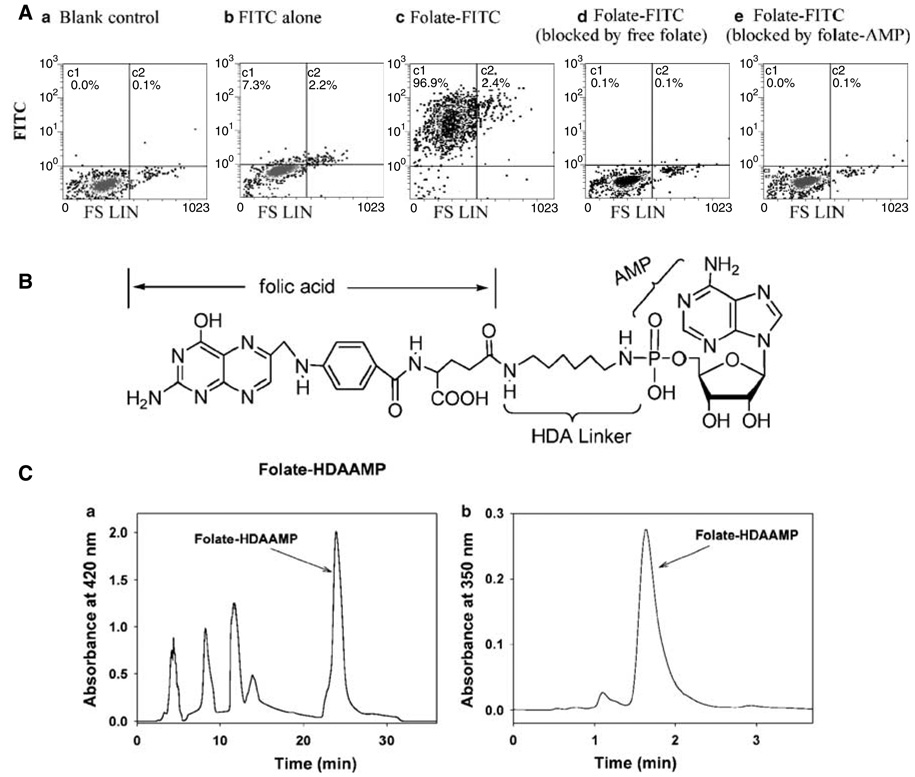
A folate–AMP complex (Figure 1) was synthesized by conjugating folic acid with AMP through the linker molecule 1,6-hexanediamine (HDA) by established chemistry.23 The complex was purified by semipreparative reverse phase HPLC (Figure 1C). The purity of the folate–AMP complex was determined by both reverse phase HPLC and thin-layer chromatography. The compound, exhibiting 93% purity (Figure 1C), was used for the synthesis of folate–pRNA as discussed below.
Figure 1.
Structure, purification, and characterization of folate–AMP. (A). The binding of folate–AMP to the folate receptor shown by a competition assay. KB cells were left without treatment (a) or incubated with folate-FITC (c). Also shows the binding of FITC without folate labeling (b). The numbers shown in the upper quadrants represent the percentage of fluorescence-positive cells. The binding of folate-FITC to KB cells was blocked with free folate (d) or folate–AMP (e). (B) Structure of folate–AMP. (C-a) Purification of folate–AMP by reverse phase HPLC under the following conditions: Column: Delta Pak C18 7.8 × 300 mm, preequilibrated with 20 mm phosphate, pH 7.0; flow rate at 6 ml/min. After sample loading, the eluting solvent was changed manually according to the following order: from 100% 20 mm phosphate to 100% water at 8 min, to 10% MeOH / 90% water at 19 min, and finally to 15% MeOH / 90% water at 20 min. The 22–31 min folate–AMP fraction was collected and lyophilized. (C-b) Purity analysis of folate–HDA–AMP by HPLC. Econosphere C18, 4.6 × 50 mm, 20% MeOH / 80% 20 mm phosphate, pH 7.0, flow rate = 1 ml / min. The sample contains 96% folate–HDA–AMP.
To determine whether folate–AMP is able to bind to the folate receptor on the cell surface, the capability of folate–AMP to compete with folate-FITC for binding to human nasopharyngeal carcinoma KB cells was assessed by flow cytometry. Ninety-seven percent of KB cells, which are folate-receptor positive, exhibited strong binding by folate–FITC (Figure 1A). However, only 0.1% of cells were detected to contain folate-FITC when KB cells were preincubated with folate–AMP, which served as a competitor with folate-FITC (100 nm) for folate receptor binding (Figure 1A). Similar blockage was observed when free folate was used. These results indicate that the folate moiety incorporated into AMP retains a high binding capacity for the folate receptor. FITC dye (100 mm) was also included as a negative control to exclude the nonspecific binding between FITC and KB cells.
Synthesis of folate pRNA
Previously, specific 5′-end modifications of pRNA had been achieved through the use of T7 RNA polymerase with GMPS for transcription initiation.24 The main limitation in such an application is that only RNA starting with a ‘G’ can be applied.25 Currently, through the use of the T7 class II promoter,26,27 adenosine can also serve in the initiation of transcription, and so we have been able to incorporate AMP derivatives into the 5′-end of the pRNA or circular permutated pRNA (cpRNA) in one-step labeling. AMP derivatives such as folate–AMP can only be used for initiation but not for chain extension, thus ensuring that labeling occurs only at the 5′-end.
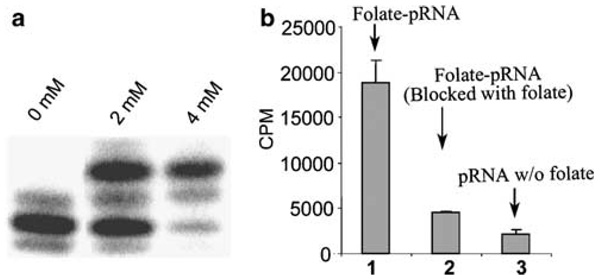
In vitro transcription of pRNA was performed in the presence of both folate–AMP and ATP, together with CTP, UTP and GTP. Different molar ratios of AMP:ATP were examined in transcription reactions. Our results show that 16:1 is the best ratio of folate–AMP to ATP in considering both total yield of the RNA production and the percentage of pRNA carrying the folate labeling. The RNA labeled with folate had a slower migration rate compared to nonlabeled RNA on denaturant urea / PAGE (Figure 2a). Results indicate that under the optimal transcription conditions, more than 60% of the pRNA contains a folate moiety, as estimated from the gel (Figure 2a).
Figure 2.
5′ labeling of pRNA by folate and binding of folate–pRNA to KB cells. (a) folate–AMP was included in the in vitro transcription together with nonmodified NTPs. Trace amounts of [α32P]ATP were included to indicate the position of RNA. Only one major band was detected on the PAGE / urea gel when folate–AMP was skipped. About 50–60% of the RNA shifted to the upper position when 2 mm and 4 mm folate–AMP were utilized. (b) Binding of 3H-labeled folate–RNA to KB cells.
Binding of folate–pRNA to nasopharyngeal carcinoma cells
The size of a motor pRNA monomer was determined to be around 11 nm.28 The binding of this nanometer-scale particle with folate labeling was examined. A truncated pRNA (7–106) with a 5′-overhang was constructed to enhance the accessibility of the 5′ folate for receptor binding (Figure 2b). [3H]UTP was included in the transcription reaction to uniformly label the RNA. [3H]folate-RNA exhibited strong binding to KB cells compared to the RNA without folate (Figure 2b). As the binding was blocked by free folate, the specificity in binding mediated by the folate receptor was demonstrated. The recessive blunt or overhanging of the 3′-end noticeably reduced the binding efficiency of the folate–pRNA to the receptor.
Binding of nanoparticles containing both folate–pRNA and siRNA chimera to nasopharyngeal carcinoma cells
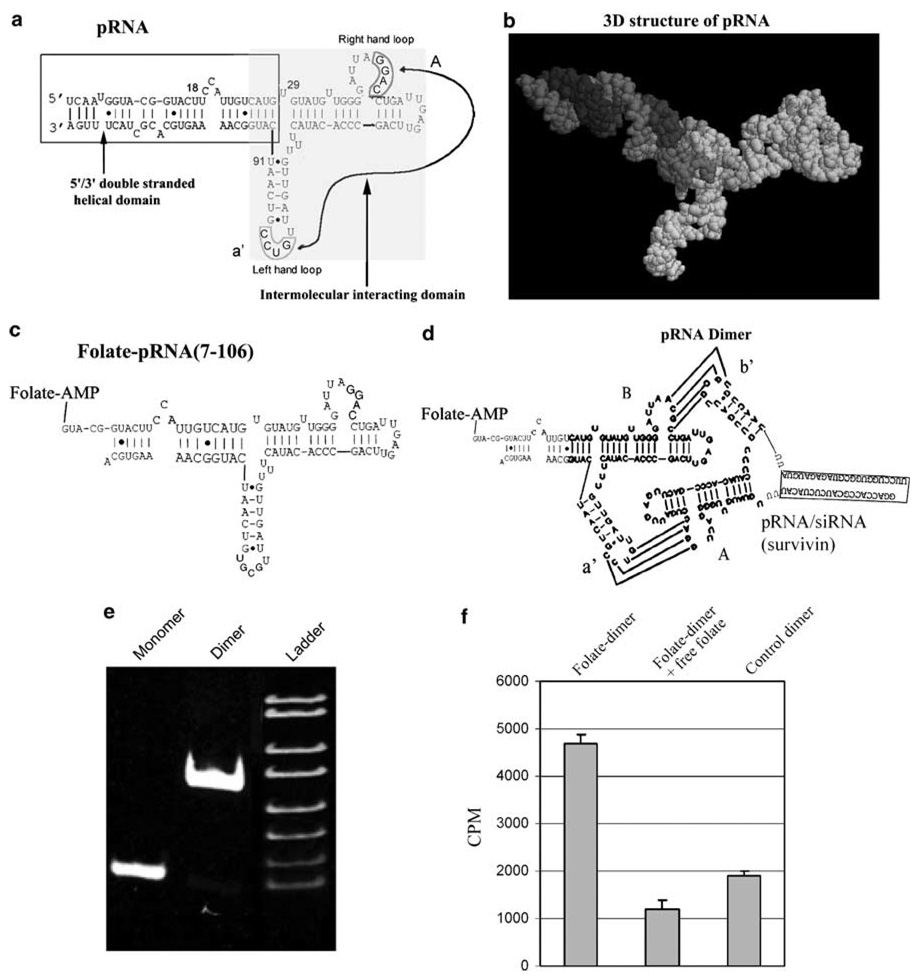
Phi29 pRNA contains two interlocking loops that can be manipulated to produce desired stable dimers approximately 20 nm in size.28,29 For example, pRNA (A-b′) contains a right-hand loop A (5′ G45G46A47C48) and a left-hand loop b′ (3′ U85G84C83G82), which together can pair with the left-hand loop a′ (3′ C85C84U83G82) and the right-hand loop B (5′ A45C46G47C48) of pRNA (B-a′), respectively (Figure 3). A chimeric pRNA / siRNA monomer was constructed by replacing the double-stranded helical region of pRNA with siRNA sequences without affecting the gene silencing function and the pRNA secondary structure.21,22 The deliverable folate containing nanoparticles was conjugated by mixing equal molar amounts of folate–pRNA (B-a′) with a chimeric pRNA / siRNA (A-b′) via the interaction of the interlocking loops (Figure 3). The formation of the dimer was demonstrated by native-PAGE (Figure 3), cryo-AFM, and ultracentrifugation (data not shown). To assess the binding capacity of such a pRNA dimer to a folate receptor, an RNA complex composed of [3H](A-b′) pRNA and unlabeled folate-(B-a′) pRNA was incubated with KB cells. The folate-labeled RNA dimer showed much stronger binding compared to the control RNA dimer without folate labeling (Figure 3). The binding specificity was demonstrated by blockage with free folate.
Figure 3.
Sketch of the sequence and structure of pRNA chimeras. (a) Phi29 pRNA sequence and secondary structure. The right- and left-hand loops are circled, the double-helical domain is framed, and the intermolecular interacting domain is shaded. The curved line points to the two interacting loops. (b) 3D structure of pRNA monomer. (c) Illustration of the construction of folate-labeled pRNA (7–106) with truncated sequences. (d) Folate-labeled chimeric pRNA dimers harboring siRNA against survivin. (e) The high-efficiency formation of pRNA dimer was demonstrated by native-PAGE. (f) Specific binding of folate–pRNA dimer to KB cells. Cells were incubated with RNA dimer composed of folate–pRNA (A-b′) and [3H]-pRNA (B-a′) in the presence (center column) or absence (left column) of free folate. The right column is the [3H]-dimer without folate labeling as a negative control.
Entry of nanoparticles containing both folic-pRNA and siRNA chimera to nasopharyngeal carcinoma cells
To determine whether the folate moiety on the chimeric pRNA dimer could mediate the entry of the complex into KB cells, a chimeric siRNA complex was constructed for specific gene silencing. RNA dimer (1.75 µm), containing both folate and siRNA against firefly luciferase, was incubated with, rather than transfected into, KB cells. The expression level of firefly luciferase in cells treated with folate–RNA dimer decreased to 30% of cells without RNA treatment. In contrast, cells treated with a control folate-free RNA dimer retained 85% of luciferase gene expression. These results suggest that specific knockdown of the firefly luciferase gene was achieved by folate receptor-mediated internalization of chimeric pRNA dimer in the absence of transfection reagents.
Processing of the pRNA chimera and the complex of dimer pRNA chimera into siRNA by Dicer
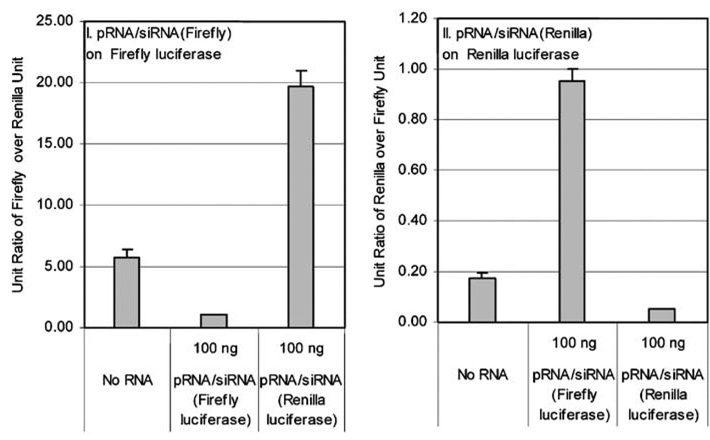
Our previous work has shown that exogenous sequences added to the 5′ / 3′-end of pRNA, such as siRNA or hammerhead ribozyme, retained biological function in cells.16,21,22 In this study, a chimeric pRNA / siRNA targeting firefly or renilla luciferase was constructed, and the silencing efficiency was tested by transient transfection. The chimeric siRNA construct suppressed its target gene specifically and efficiently as demonstrated by a Dual reporter assay, in which the expression levels of two different luciferases were measured in the presence of a chimeric pRNA harboring the siRNA targeting one of the luciferases. The nontargeted luciferase served as the internal control. No silencing of the luciferase gene occurred when mutation was introduced into the siRNA of the pRNA complex (Figure 4).
Figure 4.
Specific knockdown of gene expression by transfection of pRNA / siRNA. Dual luciferase assay showing the specific silencing of the gene for firefly luciferase (left) and renilla luciferase (right) by pRNA / siRNA (Firefly) and pRNA / siRNA (Renilla), respectively, by transient transfection.
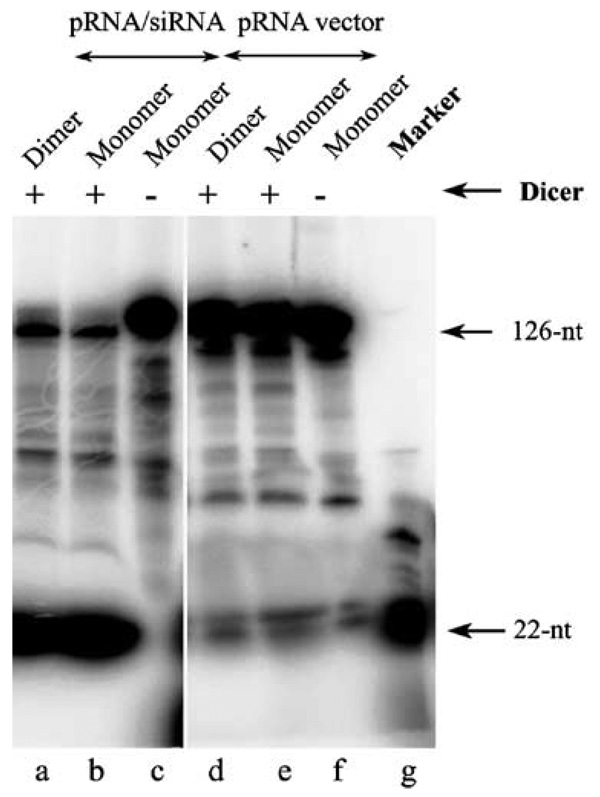
To determine whether the knockdown effects shown above were siRNA-specific, chimeric pRNA / siRNA monomers or dimers were treated with cell lysate or recombinant purified Dicer (Figure 5), which is known for its unique function in processing long double-stranded RNA into 22-bp siRNA.30 Incubation of the 5′-[γ32P]-pRNA / siRNA complex with cell lysate resulted in the processing of the chimeric RNA complex into a 29-base double-stranded siRNA (not shown). Such processing is expected as we intentionally introduced two uridines at the three-way junction to increase the free energy for folding of the junction area into a single-stranded loop. Incubation of the pRNA / siRNA complex, harboring a 29-base double-stranded siRNA, with purified Dicer resulted in the processing of the chimeric RNA complex into a 22-base double-stranded siRNA (Figure 5), as revealed by denaturant urea PAGE. These results suggest that the chimeric pRNA / siRNA complex specifically cleaved to release the functional double-stranded siRNA at the 5′ /3′-ends, and that the function of gene silencing resulted from the siRNA after the RNA complex was delivered into the cells. The dimer formation of chimeric pRNA / siRNA did not interfere with the processing and release of functional siRNA.
Figure 5.
Processing of chimeric pRNA / siRNA complex by recombinant Dicer into 22-bp siRNA. The chimeric pRNA / siRNA (lane a–c) or pRNA vector (lane e–f) with 5′-end [γ32P] was incubated with purified recombinant Dicer for 30 min and 2 h, respectively, and then separated on denaturant PAGE / urea gel. 22-nt RNA was used as the marker. The processing of dimeric RNA (lane a and d) was also examined.
Animal trials demonstrate specific suppression of tumorigenicity of cancer cells by ex vivo delivery of chimeric siRNA against survivin
Animal trials were conducted to test the specificity by ex vivo delivery using a dimer containing both pRNA (B-a′) / folate and pRNA (A-b′) / siRNA (survivin). The potential of this RNA dimer to suppress tumor formation was tested in athymic nude mice. KB cells were incubated with various dimeric RNA samples before being introduced into the nude mice by axilla injection. Four out of the eight mice receiving cells alone developed tumors within 3 weeks; six out of the eight mice receiving dimer (pRNA (B-a′) + pRNA / siRNA (survivin) (A-b′)) without folate labeling developed tumors, and seven out of the eight mice receiving dimer (pRNA (B-a′) + pRNA / mutant siRNA (A-b′)) developed tumors. Each group of these mice exhibited an average tumor size in excess of 100 mm3 within 41 days of injection. In contrast, only one of the eight mice receiving cells pretreated with dimers with pRNA (A-b′) / folate and pRNA (B-a′) / siRNA (survivin) developed a tumor. This single mouse produced a plaque within a week, much earlier than the mice in any of the other groups, and therefore, given these special circumstances, it was treated as an outlier. The inhibition of tumor formation is specific as the control dimer RNA without folate conjugation used in control mouse groups did not affect tumor development.
Discussion
The goal of this work was to construct folate-conjugated phi29 pRNA for the delivery of chimeric siRNA to nasopharyngeal carcinoma cells via folate receptor. Folate labeling was achieved by utilizing folate–AMP as an initiator of RNA transcription with a T7II promoter, although folate–AMP might have some inhibitory effects on transcription yields when used at high concentrations. Phage phi29 pRNA was used as a vector to carry siRNA sequences. In addition, both breast cancer cells and ovary cancer cells were specifically stained by folate-FITC, indicating that this delivery method can also apply to at least two additional kinds of cancer cells.
The pRNA / siRNA was processed by Dicer, and released double-stranded siRNA duplex, which led to specific suppression of gene expression. A stable pRNA dimer was generated by mixing two pRNAs, one of which carried folate labeling, whereas the other carried siRNA sequences. When this RNA complex was mixed with KB cells in the absence of transfection reactions, the folate moiety was shown to (1) mediate the binding of dimeric complex and (2) mediate the knockdown of targeted luciferase gene expression. Furthermore, the suppression of tumor growth was achieved in mouse trials by incubating the folate–siRNA complex against the survivin gene, which plays an important role in tumor development.
Phi29 pRNA forms dimers as a result of the interaction of interlocking loops of each pRNA. In the future, chimeric trimers or even hexamers will be assembled by manipulating the sequence of interlocking loops. The polyvalent nature of pRNA will facilitate carrying multiple components with various functions including cell recognition, detection, endosome escape and gene suppression. Nucleotide derivatives will be utilized to produce stable RNase-resistant RNA to improve the silencing efficiency.9 This polyvalent RNA complex could also be potentially useful in treating chronic viral infectious diseases caused by HIV or HBV by targeting the specific virus-glycoproteins present on the infected cell surface.
One advantage of this strategy is that gene silencing can be achieved simply by mixing an RNA complex with cancer cells, without the aid of transfection reagents derived from cationic lipids or CaCl2. More importantly, as cancer cells express a variety of signature receptors at different stages of development, some endocytable receptors could be used as carriers to mediate the entry of therapeutic reagents labeled with the receptor ligand. Another advantage in using RNA as a delivery vehicle is the ability to avoid the problem of immune response and the rejection of protein vectors after repeated long-term drug administration.
Materials and methods
Preparation of RNA
RNA preparation and the characterization of dimer were described in our previous publications.13,31 Briefly, RNAs were prepared by in vitro transcription using T7-MegaShortscript Kit purchased from Ambion. DNA templates with T7 polymerase were used in the presence of 7.5 mm ATP, 7.5 mm GTP, 7.5 mm UTP, and 7.5 mm CTP. 1 µl [α-32P]ATP or [3H]UTP was included for radioactive labeling of RNA. Transcription products were purified by 8% PAGE / urea and eluted with 0.5 m sodium acetate, 0.1 mm EDTA, and 0.1% SDS. RNAs were ethanol precipitated and resuspended in depc-treated water. To label the 5′-end of RNA with folate, both 4 mm folate–AMP and 0.25 mm ATP were included in a transcription reaction, together with 1 mm UTP, CTP, and GTP. The solution also contains 40 mm Tris (pH 8.0), 6 mm MgCl2, 2mm spermidine, 0.01% Triton X-100, 5 mm DTT, 0.2 µm DNA templates, and 5 U / µl T7 RNA polymerase (Promega).32
Synthesis and purification of folate–AMP
The conjugation of folate with AMP was achieved by introducing a folate moiety to AMP through the linker molecule HDA, based on similar conjugation chemistry as published.32 5′ Folate–AMP was then purified to 93% purity by semipreparative reverse phase HPLC. After lyophilization, the compound was dissolved in water. Its concentration was determined by absorbance at 350 nm, with a molar extinction coefficient of ε 350 = 8000 m−1 cm−1. The folate–AMP was then used directly for the preparation of folate-conjugated RNA under the T7 ϕ2.5 promoter under the published conditions.
Cell culture
KB cells were maintained in a folate-free RPMI1640 medium (Gibco) supplemented with 10% FBS and penicillin / streptomycin in a 5% CO2 incubator. The serum provided a normal complement of endogenous folate for cell growth.
Flow cytometry analysis
KB cells (4 × 105) were seeded into a six-well plate and grown for 24 h. After being rinsed twice with PBS, the cells were incubated with 100 nm folate-FITC for 20 min at room temperature, with or without the presence of a blocking reagent. Free folate or folate–AMP (166 µm) was included as blocking reagents. Cells were then washed and harvested in PBS and analyzed by flow cytometry.
Binding of folate RNA to KB cells
One micromolar RNA was added to a suspension of 107 cells in 0.5 ml of medium in the presence of 10 mm Mg2+ and incubated at 37°C for 30 min. Cells were then washed twice with RPMI1640 medium, and the radio-activity of cells was measured by a liquid scintillation counter.
Dual-luciferase assays
Gene silencing assay by transfection was performed by cotransfecting various chimeric siRNA into mouse fibroblast PA317-PAR cells with both pGL3 plasmid encoding firefly luciferase and pRL-TK plasmid encoding Renilla luciferase. Both luciferase activities were measured by Dual-Luciferase Reporter Assay System (Promega).
For the incubation assay, the folate-dimer was prepared by mixing folate–pRNA (7–106) B-a′ and pRNA / siRNA (Firefly luciferase) A-b′ with 10 mm Mg2+. KB cells were seeded in a six-well plate in a folate-free medium. After being washed by PBS-supplied MgCl2, the premixed dimer RNA (1.75 µm) was then added to cells and incubated for 3 h at 37°C. RNase inhibitor SUPERRNaseIN (1 U / µl) (Ambion) was added into the binding buffer. After incubation, free RNA was washed off, and pGL3 and pRL-TK plasmids were introduced into cells using Lipofectamine 2000 (Promega). Luciferase activities were measured the next day.
Chimeric pRNA/siRNA processing
The purified recombinant Dicer was purchased from Gene Therapy Systems.
In vivo animal studies
Five-week-old male athymic nude mice (Harlan Sprague Dawley) were housed in a pathogen-free environment. Animals were randomly assigned to experimental groups (n = 8). Cells were maintained in an antibiotic-free medium for 1 week before injection. On the day of the injection, cells were rinsed with PBS twice and incubated with the dimeric pRNA complex at 37°C for 3 h before being collected by trypsin digestion. Cells (2.5 × 105) in 0.1 ml of media were used for the injection of each mouse. Cells were inoculated subcutaneously into the right axilla of the forelimb. Once xenografts were visible, their size was determined two times per week by externally measuring tumors in two dimensions. Volume was calculated by the following equation: V = (L × _W_2) × 0.5, where L is the length and W is the width of the xenograft.
Acknowledgements
This work was supported mainly by NIH grant R01-EB03730 (Nanoscience and Nanotechnology in Biology and Medicine Program to PG), and partially by NIH grant R01-GM59944 (Institute of General Medicine to PG). We thank Taejin Lee for preparing the data of Figure 4, Philip Low for providing materials and insightful discussions, Paul Robinson for the cytometry assays, Sulma Mohammed for the animal trials, and Jeremy Hall for manuscript preparation.
References
- 1.Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411:494–498. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- 2.Ryther RC, Flynt AS, Phillips JA, III, Patton JG. siRNA therapeutics: big potential from small RNAs. Gene Therapy. 2005;12:5–11. doi: 10.1038/sj.gt.3302356. [DOI] [PubMed] [Google Scholar]
- 3.Li H, Li WX, Ding SW. Induction and suppression of RNA silencing by an animal virus. Science. 2002;296:1319–1321. doi: 10.1126/science.1070948. [DOI] [PubMed] [Google Scholar]
- 4.Brummelkamp TR, Bernards R, Agami R. A system for stable expression of short interfering RNAs in mammalian cells. Science. 2002;296:550–553. doi: 10.1126/science.1068999. [DOI] [PubMed] [Google Scholar]
- 5.Carmichael GG. Medicine: silencing viruses with RNA. Nature. 2002;418:379–380. doi: 10.1038/418379a. [DOI] [PubMed] [Google Scholar]
- 6.Rossi JJ. Ribozyme therapy for HIV infection. Adv Drug Deliv Rev. 2000;44:71–78. doi: 10.1016/s0169-409x(00)00085-5. [DOI] [PubMed] [Google Scholar]
- 7.Devroe E, Silver PA. Therapeutic potential of retroviral RNAi vectors. Expert Opin Biol Ther. 2004;4:319–327. doi: 10.1517/14712598.4.3.319. [DOI] [PubMed] [Google Scholar]
- 8.Morrissey DV, Lockridge JA, Shaw L, Blanchard K, Jensen K, Breen W, et al. Potent and persistent in vivo anti-HBV activity of chemically modified siRNAs. Nat Biotechnol. 2005;23:1002–1007. doi: 10.1038/nbt1122. [DOI] [PubMed] [Google Scholar]
- 9.Soutschek J, Akinc A, Bramlage B, Charisse K, Constien R, Donoghue M, et al. Therapeutic silencing of an endogenous gene by systemic administration of modified siRNAs. Nature. 2004;432:173–178. doi: 10.1038/nature03121. [DOI] [PubMed] [Google Scholar]
- 10.Guo P, Erickson S, Anderson D. A small viral RNA is required for in vitro packaging of bacteriophage ϕ29 DNA. Science. 1987;236:690–694. doi: 10.1126/science.3107124. [DOI] [PubMed] [Google Scholar]
- 11.Guo P. Structure and function of phi29 hexameric RNA that drive viral DNA packaging motor: review. Prog Nucl Acid Res Mole Biol. 2002;72:415–472. doi: 10.1016/s0079-6603(02)72076-x. [DOI] [PubMed] [Google Scholar]
- 12.Shu D, Moll D, Deng Z, Mao C, Guo P. Bottom-up assembly of RNA arrays and superstructures as potential parts in nanotechnology. Nano Lett. 2004;4:1717–1724. doi: 10.1021/nl0494497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang CL, Lee C-S, Guo P. The proximate 5′ and 3′ ends of the 120-base viral RNA (pRNA) are crucial for the packaging of bacteriophage ϕ29 DNA. Virology. 1994;201:77–85. doi: 10.1006/viro.1994.1267. [DOI] [PubMed] [Google Scholar]
- 14.Chen C, Zhang C, Guo P. Sequence requirement for hand-in-hand interaction in formation of pRNA dimers and hexamers to gear phi29 DNA translocation motor. RNA. 1999;5:805–818. doi: 10.1017/s1355838299990350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang CL, Tellinghuisen T, Guo P. Confirmation of the helical structure of the 5′ / 3′ termini of the essential DNA packaging pRNA of phage ϕ29. RNA. 1995;1:1041–1050. [PMC free article] [PubMed] [Google Scholar]
- 16.Hoeprich S, ZHou Q, Guo S, Qi G, Wang Y, Guo P. Bacterial virus phi29 pRNA as a hammerhead ribozyme escort to destroy hepatitis B virus. Gene Therapy. 2003;10:1258–1267. doi: 10.1038/sj.gt.3302002. [DOI] [PubMed] [Google Scholar]
- 17.Shu D, Huang L, Hoeprich S, Guo P. Construction of phi29 DNA-packaging RNA (pRNA) monomers, dimers and trimers with variable sizes and shapes as potential parts for nanodevices. J Nanosci and Nanotech (JNN) 2003;3:295–302. doi: 10.1166/jnn.2003.160. [DOI] [PubMed] [Google Scholar]
- 18.Lee RJ, Low PS. Delivery of liposomes into cultured KB cells via folate receptor-mediated endocytosis. J Biol Chem. 1994;269:3198–3204. [PubMed] [Google Scholar]
- 19.Mathias CJ, Wang S, Lee RJ, Waters DJ, Low PS, Green MA. Tumor-selective radiopharmaceutical targeting via receptor-mediated endocytosis of gallium-67-deferoxamine-folate. J Nucl Med. 1996;37:1003–1008. [PubMed] [Google Scholar]
- 20.Benns JM, Maheshwari A, Furgeson DY, Mahato RI, Kim SW. Folate-PEG-folate-graft-polyethylenimine-based gene delivery. J Drug Target. 2001;9:123–139. doi: 10.3109/10611860108997923. [DOI] [PubMed] [Google Scholar]
- 21.Guo S, Tschammer N, Mohammed S, Guo P. Specific delivery of therapeutic RNAs to cancer cells via the dimerization mechanism of phi29 motor pRNA. Human Gene Ther. 2005;16:1097–1109. doi: 10.1089/hum.2005.16.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khaled A, Guo S, Li F, Guo P. Controllable self-assembly of nanoparticles for specific delivery of multiple therapeutic molecules to cancer cells using RNA nanotechnology. Nano Lett. 2005;5:1797–1808. doi: 10.1021/nl051264s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang F, Bugg CW, Yarus M. RNA-Catalyzed CoA, NAD, and FAD synthesis from phosphopantetheine, NMN, and FMN. Biochemistry. 2000;39:15548–15555. doi: 10.1021/bi002061f. [DOI] [PubMed] [Google Scholar]
- 24.Garver K, Guo P. Boundary of pRNA functional domains and minimum pRNA sequence requirement for specific connector binding and DNA packaging of phage phi29. RNA. 1997;3:1068–1079. [PMC free article] [PubMed] [Google Scholar]
- 25.Seelig B, Jaschke A. Ternary conjugates of guanosine monophosphate as initiator nucleotides for the enzymatic synthesis of 5′-modified RNAs1. Bioconjug Chem. 1999;10:371–378. doi: 10.1021/bc980085h. [DOI] [PubMed] [Google Scholar]
- 26.Huang F. Efficient incorporation of CoA, NAD and FAD into RNA by in vitro transcription. Nucleic Acids Res. 2003;31:e8. doi: 10.1093/nar/gng008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pan T, Gutell RR, Uhlenbeck OC. Folding of circularly permuted transfer RNAs. Science. 1991;254:1361–1364. doi: 10.1126/science.1720569. [DOI] [PubMed] [Google Scholar]
- 28.Hoeprich S, Guo P. Computer modeling of three-dimensional structure of DNA-packaging RNA(pRNA) monomer, dimer, and hexamer of Phi29 DNA packaging motor. J Biol Chem. 2002;277:20794–20803. doi: 10.1074/jbc.M112061200. [DOI] [PubMed] [Google Scholar]
- 29.Chen C, Sheng S, Shao Z, Guo P. A dimer as a building block in assembling RNA. A hexamer that gears bacterial virus phi29 DNA-translocating machinery. J Biol Chem. 2000;275(23):17510–17516. doi: 10.1074/jbc.M909662199. [DOI] [PubMed] [Google Scholar]
- 30.Carmell MA, Hannon GJ. RNase III enzymes and the initiation of gene silencing. Nat Struct Mol Biol. 2004;11:214–218. doi: 10.1038/nsmb729. [DOI] [PubMed] [Google Scholar]
- 31.Guo P, Zhang C, Chen C, Trottier M, Garver K. Inter-RNA interaction of phage phi29 pRNA to form a hexameric complex for viral DNA transportation. Mol Cell. 1998;2:149–155. doi: 10.1016/s1097-2765(00)80124-0. [DOI] [PubMed] [Google Scholar]
- 32.Huang F, Wang G, Coleman T, Li N. Synthesis of adenosine derivatives as transcription initiators and preparation of 5′ fluorescein- and biotin-labeled RNA through one-step in vitro transcription. RNA. 2003;9:1562–1570. doi: 10.1261/rna.5106403. [DOI] [PMC free article] [PubMed] [Google Scholar]