FlhA provides the adaptor for coordinated delivery of late flagella building blocks to the type III secretion system (original) (raw)
Abstract
Flagella are the bacterial organelles of motility and can play important roles in pathogenesis. Flagella biosynthesis requires the coordinated export of huge protein amounts from the cytosol to the nascent flagellar structure at the cell surface and employs a type III secretion system (T3SS). Here we show that the integral membrane protein FlhA from the gram-positive bacterium Bacillus subtilis acts as an adaptor for late export substrates at the T3SS. The major filament protein (flagellin) and the filament-cap protein (FliD) bind to the FlhA cytoplasmic domain (FlhA-C) only in complex with their cognate chaperones (FliS and FliT). To understand the molecular details of these interactions we determined the FlhA-C crystal structure at 2.3 Å resolution. FlhA-C consists of an N-terminal linker region, three subdomains with a novel fold, and a disordered region essential for the adaptor function. We show that the export protein FliJ associates with the linker region and modulates the binding properties of FlhA-C. While the interaction of FliD/FliT is enhanced, flagellin/FliS is not affected. FliJ also keeps FliT associated with FlhA-C and excess of FliT inhibits binding of FliD/FliT, suggesting that empty FliT chaperones stay associated with FliJ after export of FliD. Taken together, these results allow to propose a model that explains how the T3SS may switch from the stoichiometric export of FliD to the high-throughput secretion of flagellin.
Keywords: chaperone, flagella biosynthesis, motility, protein transport
Flagella represent one of nature’s largest molecular machines and are the bacterial organelles of locomotion (reviewed in refs. 1–3). In many pathogenic bacteria, they also act as virulence factors, because motility is needed to reach the primary site of infection, and thereby establish the first step of a bacterial infection (4, 5). A flagellum consists of the cytosolic C-ring, the membrane-embedded basal body and the exterior hook, hook-filament junction, and filament structures. The flagellar filament is a long, tubular structure and consists of about 20,000 subunits of the protein flagellin. The assembly of the filament is strictly sequential: At first the pentameric FliD cap must be formed to promote flagellin self-assembly at the distal end of the growing filament (6). Flagellar proteins (e.g., flagellin and FliD) are synthesized in the cytosol, transferred into the central channel of the growing flagellar structure, and travel as monomers to the nascent end of the flagellum where they assemble (7). Translocation into the central channel is achieved by a flagellum-specific type 3 secretion system (T3SS) in the cytoplasmic membrane and driven by the proton-motive force (8–10). To prevent futile self-assembly/aggregation within the cytosol, specialized chaperones (FliS for flagellin and FliT for FliD) prevent premature interactions of flagellar components by binding to their C-terminal, amphipathic oligomerization domains (11–13). They also seem to be involved in the entry of subunits into the T3SS (14–16), but the molecular details are not known.
T3SSs are used for the export of flagella building blocks (17) as well as for the transmission of bacterial virulence effectors into host cells (18). The inner membrane part of the T3SS is formed by six integral membrane proteins (FlhA, FlhB, FliP, FliQ, FliR, FliO) and is thought to reside within the MS-ring (19, 20). In the cytosol three proteins (FliI, FliH, and FliJ) are functionally associated to the T3SS. The FliI ATPase (21) together with its negative regulator FliH (22) seems to regulate initial entry and sorting of export substrates into the T3SS (14, 23). The membrane-associated protein FliJ is essential for the export of flagella building blocks (20). It binds to empty chaperones of the hook-filament junction and of filament-cap substructures but not of the filament (24). This led to the proposal that FliJ selectively recycles empty chaperones during flagella assembly (24, 25).
The integral transmembrane protein FlhA (InvA, YscV in pathogen related T3SSs) is the largest component of the T3SS (26) and belongs to the “flagellum/hypersensitive response/invasion” (FHIPEP) family of proteins (27). It consists of an N-terminal transmembrane (TM) domain with eight predicted TM α-helices and a large cytosolic domain at its C terminus (FlhA-C) (Fig. 1A) (28). Disruption of the FlhA gene in gram-positive or gram-negative bacteria leads to nonmotile cells, which lack flagella and are unable to export flagellar proteins (20, 29). Although FlhA has been implicated in substrate translocation (30, 31), and even though interactions of its cytoplasmic domain with all components of the T3SS were reported (28, 30, 32–34), its precise function is still unknown. Our biochemical and structural analyses establish FlhA-C as an adaptor that receives the late flagella building blocks FliD and Flagellin only when bound to their cognate chaperones FliT and FliS, respectively.
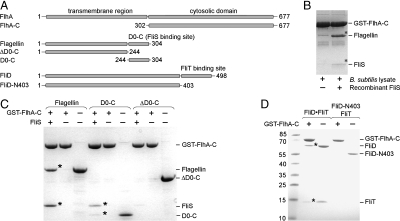
Fig. 1.
FlhA-C interacts with late flagellar substrate-chaperone complexes. (A) Scheme of FlhA, flagellin, and FliD constructs used in this study. The numbers correspond to amino acid positions. The FliS and FliT binding sites at flagellin and FliD, respectively, are indicated. (B) Binding of native flagellin to immobilized GST-FlhA-C in the absence (lane 1) and presence of recombinant FliS (lane 2). Flagellin and FliS, which were confirmed by mass spectrometry, are indicated by asterisks. (C) Binding of recombinantly expressed and purified flagellin variants to GST-tagged FlhA-C in the presence or absence of FliS. Lanes 3, 6, and 9 show the quality of the employed flagellin variants. Binding of flagellin/FliS and D0-C/FliS to FlhA-C is indicated by asterisks. (D) Binding of recombinantly expressed and purified FliD variants to GST-tagged FlhA-C in the presence or absence of FliT. Lanes 3 and 5 show the size and quality of the employed FliD variants. Binding of FliD/FliT to FlhA-C is indicated by asterisks. B_–_D show Coomassie-stained SDS/PAGE.
Results and Discussion
FlhA-C Interacts with Late Flagella Substrate-Chaperone Complexes.
To specify the role of FlhA-C in the T3SS of flagella, we performed Glutathione-S-transferase (GST) pulldown assays in Bacillus subtilis cell lysates. FlhA-C interacts with the major filament protein flagellin (Fig. 1B). Addition of the flagellin-specific chaperone FliS (11) to the cell lysates enhanced the interaction of FlhA-C with flagellin. This indicates that the flagellin/FliS complex binds to FlhA-C and that FliS was probably limiting in the cell lysate. Next we performed the same assay with recombinantly expressed and purified flagellin and FliS. The same results as before were obtained, indicating that no other cytosolic factors are involved in the binding of flagellin/FliS to FlhA-C (Fig. 1C). In the absence of FliS binding of flagellin to FlhA-C was not observed (Fig. 1C). A flagellin variant lacking the FliS binding site (35, 36) (ΔD0-C, amino acid residues 1–244, Fig. 1A) was unable to interact with FlhA-C even in the presence of FliS (Fig. 1C). This experiment also revealed that empty FliS does not bind to FlhA-C. A complex of FliS and its binding domain in flagellin (D0-C, residues 244–304, Fig. 1A) was sufficient for binding to FlhA-C (Fig. 1C). Taken together we show that the flagellin/FliS complex binds to FlhA-C but not the individual proteins.
To investigate whether other late substrate-chaperone complexes also bind to FlhA-C, the filament-capping protein FliD and its chaperone FliT were chosen. Like flagellin/FliS, purified FliD and FliT form a heterodimer (Fig. 1A and Fig. S1), which binds to FlhA-C (Fig. 1D). A FliD variant lacking the C-terminal FliT binding site (FliD-N403, Fig. 1A) was unable to associate with FlhA-C in the presence or absence of FliT (Fig. 1D). FliT alone did not bind to FlhA-C (Fig. 1D). Taken together, targeting of late flagella building blocks (FliD, flagellin) to FlhA-C follows a general mechanism and relies on the recognition of the secretion substrate by its cognate chaperone. Thereby, FlhA receives flagella building blocks in a state competent for translocation.
The Crystal Structure of FlhA-C Shows Three Subdomains with a Novel Fold.
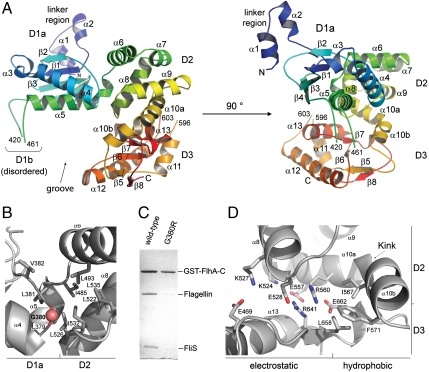
In order to understand how FlhA-C receives substrate-chaperone complexes we determined its crystal structure at 2.3 Å resolution (Table S1, Fig. S2). The structure with the overall dimensions of 45 Å × 55 Å × 50 Å comprises amino acid residues 318–677 (with only two disordered regions: residues 420–461 and 596–603). FlhA-C consists of three subdomains, named D1, D2, and D3 that form a groove (Fig. 2A Left and Fig. S3). D1, D2, and D3 have novel folds as determined by the DALI server (37). D1 can be divided into two parts: a well-folded D1a part (residues 340–484) and a disordered D1b part (residues 420–461) that is inserted into D1a just before helix α5. The N terminus of D1 is connected to the FlhA transmembrane domain by a linker region (residues 318–342) that contains two helices (Figs. 2A Right and 1A). D1a consists of two α-helices that pack against a four-stranded, mixed β-sheet. D1b is only poorly ordered in the electron density map and seems therefore highly flexible and/or unstructured (see Table S2). D2 (residues 485–571) consists of five α-helices (α6–α10) and interacts with D1a by a predominantly hydrophobic interface with a total buried surface area of ∼1350 _Å_2 (Fig. 2B). Disruption of this interface by mutation of the conserved Gly380 impairs the function of FlhA in vivo (38) and in vitro (Fig. 2C). Thus, the hydrophobic D1a-D2 contact seems to be essential for the binding of substrate-chaperone complexes to FlhA-C. D3 (residues 578–677) consists of a four-stranded β-sheet (β5–β8), which is surrounded by three α-helices (α11–α13). Helices α11 and α12 are connected by a partially disordered loop (amino acids 596–603). D2 and D3 interact by a bipartite network of electrostatic and hydrophobic contacts with a total buried surface area of ∼1,500 _Å_2 (Fig. 2D). Helix α10 with a kink of about 80° at leucine 563 is part of the D2-D3 interface. Two conserved glutamate residues in the interface are essential for flagellar export in Salmonella typhimurium (Glu547 and Glu676, Fig. S4) (38). Analysis of the corresponding mutants of B. subtilis FlhA-C (Glu528 and Glu662) in our in vitro assay was hindered, because both mutant proteins tend to degrade during expression and purification. This implies that the D2-D3 interface is crucial for the stability of FlhA-C. Taken together, the arrangement of the D1, D2 and D3 domains of FlhA-C and the specific interdomain interactions observed in the crystal structure are essential for the correct function of FlhA.
Fig. 2.
Crystal structure of FlhA-C. (A) Cartoon representation of the crystal structure of B. subtilis FlhA-C. The domains D1a, D1b, D2, and D3 are indicated and rainbow colored from the N terminus to the C terminus. The secondary structure elements are labeled with α and β for α-helix and β-strand, respectively. (B) Close-up of the D1a/D2 interface. The majority of hydrophobic residues are provided by helices α6, α8, and α5 of D2 and D1a, respectively. The conserved glycine (Gly380) is indicated by a red ball. In S. typhimurium, replacement of the corresponding Gly399 by arginine abolishes the secretion of flagellar proteins at nonpermissive temperature (38). (C) The Gly380Arg variant of B. subtilis FlhA-C does not bind the flagellin/FliS complex in our in vitro assay. Replacement of Gly380 (and Gly399 in S. typhimurium) by arginine most likely destroys the hydrophobic interface between D1a and D2 which is conserved also in S. typhimurium. The figure shows a Coomassie-stained SDS/PAGE. (D) Close-up of the D2/D3 interface. D2 and D3 interact via an extended, bipartite interface of electrostatic and hydrophobic interactions. A central part of the interface is formed by the kinked helix α10. The residues forming the interface are conserved among FlhA proteins of different bacterial species (Fig. S3).
D1b Is Essential for Binding of Late Substrate-Chaperone Complexes to FlhA-C.
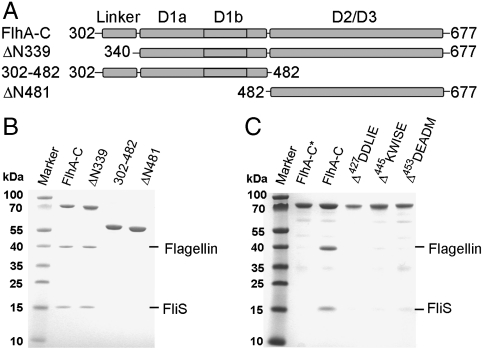
Guided by the X-ray structure we tested whether the linker/D1 (residues 302–482) or D2/D3 (ΔN481, residues 482–677) regions alone would be sufficient to bind flagellin/FliS or FliD/FliT (Fig. 3A). None of the two FlhA-C variants was able to bind late substrate-chaperone complexes showing that all three FlhA-C subdomains are required (Fig. 3B and Fig. S5). Only the linker region of FlhA-C (ΔN339, residues 302–339) is dispensable for FlhA-C interaction with flagellin/FliS (Fig. 3B). In S. typhimurium FlhA, two temperature-sensitive mutants (Leu449Arg and Gly451Asp), which are unable to export flagellar proteins at restrictive temperature (20, 38), localize within D1b (Fig. 2A Left and Fig. S3). We hypothesized that D1b might participate in binding substrate-chaperone complexes to FlhA-C and generated different FlhA-C variants with five amino acid deletions in D1b. While all deletion variants behaved like the full-length FlhA-C in purification, indicating proper folding, none of them was able to bind flagellin/FliS (Fig. 3C) or FliD/FliT (Fig. S5) in our in vitro assay. Therefore, D1b is essential for the interaction of late substrate-chaperone complexes with FlhA-C.
Fig. 3.
Functional analysis of FlhA-C. (A) Scheme of FlhA-C variants used in this study. The numbers correspond to amino acid positions. (B) Analysis of association of FlhA-C variants with flagellin/FliS. FlhA-C and FlhA-ΔN339 are able to bind flagellin/FliS. FlhA-302-482 and ΔN481, which represent the D1 and D2/D3, respectively, are unable to bind flagellin/FliS. (C) Binding studies of flagellin/FliS to “five amino acid” deletion variants in D1b of FlhA-C. All mutants failed to bind flagellin/FliS. The asterisk indicates the negative control: GST-FlhA-C incubated with flagellin in the absence of FliS. B and C show Coomassie-stained SDS/PAGE.
FliJ Enhances FliD/FliT Binding to FlhA-C.
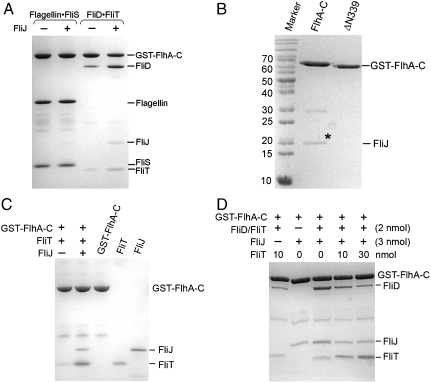
We noticed that the binding efficiencies of the two substrate-chaperone complexes to FlhA-C differ significantly. While flagellin/FliS binds almost stoichiometrically to FlhA-C, FliD/FliT binds substoichiometrically (Fig. 4A, compare lanes 1 and 3). Therefore, the interaction with of FliD/FliT with FlhA-C might require an additional factor. In S. typhimurium an interaction between FlhA-C and FliJ was described (30, 32, 39). We confirm this interaction for the B. subtilis homologues (Fig. 4B). Deletion of the linker region (ΔN339) abolishes the interaction (Fig. 4B), indicating that the linker region is required for binding FliJ to FlhA-C. We then tested whether FliJ influences the ability of FlhA-C to interact with flagellin/FliS or FliD/FliT. The presence of FliJ did not influence the binding of flagellin/FliS (Fig. 4A, lane 2), but a significant increase in FliD/FliT binding was observed (Fig. 4A, Lane 4). Therefore, FliJ selectively enhances association of FliD/FliT with FlhA-C.
Fig. 4.
FliJ selectively enhances interaction of FliD/FliT with FlhA-C. (A) Binding of flagellin/FliS and FliD/FliT to FlhA-C in the absence and the presence of FliJ. FliJ does not influence binding of flagellin/FliS to FlhA-C (lanes 1 and 2). The presence of FliJ significantly increases binding of FliD/FliT to FlhA-C (lanes 3 and 4). (B) Association of FliJ and FlhA-C requires the linker region that connects that the transmembrane domain of FlhA with its cytoplasmic domain. FliJ is indicated by an asterisk. FlhA-ΔN339, which lacks the linker region, is unable to bind FliJ. (C) FliT binds to FliJ in the context of FlhA-C. Lanes 3–5 show the quality of FlhA-C, FliT, and FliJ, respectively. (D) FliT and FliD/FliT compete for a common binding site at FliJ bound to FlhA-C. FliT does not influence FliD/FliT binding to FlhA-C in the absence of FliJ (lane 1). In the presence of FliJ, increasing amounts of FliT lead to a decreased binding of FliD/FliT (lanes 3–5). A_–_D show Coomassie-stained SDS/PAGE.
FliT and FliD/FliT Compete for a Common Binding Site at FliJ.
Isolated FliJ was previously reported to bind FliT, but not FliS or substrate/chaperone complexes (24). We now show that FliJ binds FliT also when associated with FlhA-C (Fig. 4C). To address a possible competition between FliT and FliD/FliT for FliJ at FlhA-C, increasing amounts of FliT were added to the in vitro assay. FliT decreased the interaction of FliD/FliT with FlhA-C in the presence of FliJ (Fig. 4D) indicating that FliT and FliD/FliT compete for a common binding site at FliJ.
A Model for Coordinated Substrate-Chaperone Binding to FlhA-C.
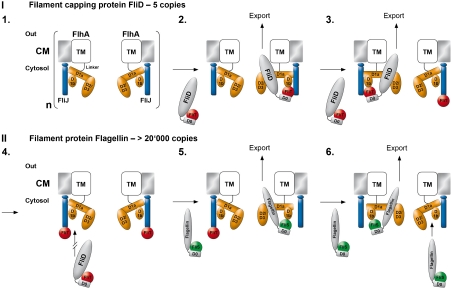
After export of FliD and flagellin subunits by the T3SS machinery, these proteins travel through a central channel formed by the already established hook and hook-filament structures. FliD stably assembles into a pentameric cap structure at the nascent end of the growing flagellum, which is essential for the subsequent polymerization of flagellin into the filament (6, 40). Mutant strains of S. typhimurium with a nonfunctional FliD fail to assemble the filament, leading to the diffusion of flagellin monomers into the culture medium (reviewed in ref. 7). Once the FliD pentamer is completed, it cannot accept another monomer in the cap. Current electron-microscopic data on the FliD cap of the filament (41) suggest that accessory FliD monomers would presumably block the central channel and, therefore, subsequent filament assembly. In this respect a weak association of FliD/FliT with FlhA-C in the absence of FliJ might be necessary to prevent uncontrolled FliD binding and secretion. In order to assure the precise stoichiometry of the cap structure it would be advantageous if the T3SS restricted the number of secreted FliD molecules to five. Based on our data, it is tempting to propose the following working model (Fig. 5): FliJ modifies the binding abilities of FlhA-C or provides an additional binding site for the FliD/FliT complex in close proximity to the T3SS pore (step 1–2). After the secretion of FliD, empty FliT chaperones stay associated with FlhA via FliJ (step 3). Assuming that FlhA is an oligomer (1, 19), the stoichiometry of FlhA could define the number of binding sites (step 4). Thereby, the number of secreted FliD molecules would be simply restricted by the number of FliT molecules that stay associated with FliJ. Because efficient binding of FliD/FliT to FlhA-C requires a vacant FliJ, while that of Flagellin/FliS does not, the occupation of FliJ might switch the T3SS to the high-throughput secretion of the filament component flagellin (> 20,000 copies). Reduction in complexity elegantly ensures filament assembly with a minimal regulatory effort and low error probability. However, this model needs to be further validated by the determination of the stoichiometry of the integral membrane protein FlhA. The export of defined protein copy numbers for establishing extracellular superstructures is a unique problem in biology (e.g., the bacterial injectosome) (18), and we imagine that a similar mechanism might be employed.
Fig. 5.
Model for the delivery of late flagella building blocks to FlhA-C. The transmembrane (TM) protein FlhA resides in the cytosolic membrane (CM). Its cytoplasmic part (orange) consists of three main domains (D1, D2, and D3). The color code is: FliJ (blue), FliD/FliT complex (gray/red), Flagellin/FliS complex (gray/green). The flexible nature of D1b is indicated by a dashed line. The oligomeric nature of FlhA is indicated by a bracket, and “n” refers to the still unknown stoichiometry.
Methods Summary
A detailed description of the applied methods can be found in SI Methods. Shortly, all genes were amplified by polymerase chain reaction (PCR) from the B. subtilis genome and cloned into pET24d (Novagen), pET16b (Novagen) or pGAT2 (EMBL). Proteins were expressed in Escherichia coli BL21(DE3) and purified by Ni-ion affinity and size exclusion chromatography. Crystallization, structure determination, and crystallographic statistics are in SI Methods and Table S1. The GST-pulldown assay is described in detail in SI Methods. The biophysical properties of FlhA-C variants were analyzed by static light scattering (Minidawn Tristar, Wyatt) and refractive index measurements (ΔN1000, Dr. Bures).
Supplementary Material
Supporting Information
Acknowledgments.
We thank Astrid Hendricks for excellent technical assistance, Jürgen Kopp from our Crystallization Platform (BZH/Cluster of Excellence:CellNetworks) for excellent support, and Dieter Kressler for stimulating discussion. We thank Hans Helferdorfer (Die Graphiker, Heidelberg) for support in preparing the cartoon. Data collection was performed at the European Synchrotron Radiation Facility, Grenoble. This work was supported by a collaborative research grant from the Deutsche Forschungsgemeinschaft (SFB 638) and by the interdisciplinary PhD program “Molecular Machines: Mechanisms and Functional Interconnections” of the Land Baden-Württemberg.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
Data deposition: The atomic coordinates for the structure have been deposited in the Protein Data Bank, www.pdb.org (PDB ID code 3MIX).
References
- 1.Macnab RM. How bacteria assemble flagella. Annu Rev Microbiol. 2003;57:77–100. doi: 10.1146/annurev.micro.57.030502.090832. [DOI] [PubMed] [Google Scholar]
- 2.Chevance FF, Hughes KT. Coordinating assembly of a bacterial macromolecular machine. Nat Rev Microbiol. 2008;6:455–465. doi: 10.1038/nrmicro1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McCarter LL. Regulation of flagella. Curr Opin Microbiol. 2006;9:180–186. doi: 10.1016/j.mib.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 4.Ottemann KM, Miller JF. Roles for motility in bacterial-host interactions. Mol Microbiol. 1997;24:1109–1117. doi: 10.1046/j.1365-2958.1997.4281787.x. [DOI] [PubMed] [Google Scholar]
- 5.Moens S, Vanderleyden J. Functions of bacterial flagella. Crit Rev Microbiol. 1996;22:67–100. doi: 10.3109/10408419609106456. [DOI] [PubMed] [Google Scholar]
- 6.Ikeda T, Asakura S, Kamiya R. “Cap” on the tip of Salmonella flagella. J Mol Biol. 1985;184:735–737. doi: 10.1016/0022-2836(85)90317-1. [DOI] [PubMed] [Google Scholar]
- 7.Minamino T, Namba K. Self-assembly and type III protein export of the bacterial flagellum. J Mol Microb Biotech. 2004;7:5–17. doi: 10.1159/000077865. [DOI] [PubMed] [Google Scholar]
- 8.Minamino T, Namba K. Distinct roles of the FliI ATPase and proton motive force in bacterial flagellar protein export. Nature. 2008;451:485–488. doi: 10.1038/nature06449. [DOI] [PubMed] [Google Scholar]
- 9.Paul K, Erhardt M, Hirano T, Blair DF, Hughes KT. Energy source of flagellar type III secretion. Nature. 2008;451:489–492. doi: 10.1038/nature06497. [DOI] [PubMed] [Google Scholar]
- 10.Galan JE. Energizing type III secretion machines: What is the fuel? Nat Struct Mol Biol. 2008;15:127–128. doi: 10.1038/nsmb0208-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Auvray F, Thomas J, Fraser GM, Hughes C. Flagellin polymerisation control by a cytosolic export chaperone. J Mol Biol. 2001;308:221–229. doi: 10.1006/jmbi.2001.4597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bennett JC, Thomas J, Fraser GM, Hughes C. Substrate complexes and domain organization of the Salmonella flagellar export chaperones FlgN and FliT. Mol Microbiol. 2001;39:781–791. doi: 10.1046/j.1365-2958.2001.02268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fraser GM, Bennett JC, Hughes C. Substrate-specific binding of hook-associated proteins by FlgN and FliT, putative chaperones for flagellum assembly. Mol Microbiol. 1999;32:569–580. doi: 10.1046/j.1365-2958.1999.01372.x. [DOI] [PubMed] [Google Scholar]
- 14.Thomas J, Stafford GP, Hughes C. Docking of cytosolic chaperone-substrate complexes at the membrane ATPase during flagellar type III protein export. Proc Natl Acad Sci USA. 2004;101:3945–3950. doi: 10.1073/pnas.0307223101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Akeda Y, Galan JE. Chaperone release and unfolding of substrates in type III secretion. Nature. 2005;437:911–915. doi: 10.1038/nature03992. [DOI] [PubMed] [Google Scholar]
- 16.Gauthier A, Finlay BB. Translocated intimin receptor and its chaperone interact with ATPase of the type III secretion apparatus of enteropathogenic Escherichia coli. J Bacteriol. 2003;185:6747–6755. doi: 10.1128/JB.185.23.6747-6755.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Macnab RM. Type III flagellar protein export and flagellar assembly. Biochim Biophys Acta. 2004;1694:207–217. doi: 10.1016/j.bbamcr.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 18.Moraes TF, Spreter T, Strynadka NC. Piecing together the type III injectisome of bacterial pathogens. Curr Opin Struct Biol. 2008;18:258–266. doi: 10.1016/j.sbi.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 19.Fan F, Ohnishi K, Francis NR, Macnab RM. The FliP and FliR proteins of Salmonella typhimurium, putative components of the type III flagellar export apparatus, are located in the flagellar basal body. Mol Microbiol. 1997;26:1035–1046. doi: 10.1046/j.1365-2958.1997.6412010.x. [DOI] [PubMed] [Google Scholar]
- 20.Minamino T, Macnab RM. Components of the Salmonella flagellar export apparatus and classification of export substrates. J Bacteriol. 1999;181:1388–1394. doi: 10.1128/jb.181.5.1388-1394.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fan F, Macnab RM. Enzymatic characterization of FliI. An ATPase involved in flagellar assembly in Salmonella typhimurium. J Biol Chem. 1996;271:31981–31988. doi: 10.1074/jbc.271.50.31981. [DOI] [PubMed] [Google Scholar]
- 22.Minamino T, MacNab RM. FliH, a soluble component of the type III flagellar export apparatus of Salmonella, forms a complex with FliI and inhibits its ATPase activity. Mol Microbiol. 2000;37:1494–1503. doi: 10.1046/j.1365-2958.2000.02106.x. [DOI] [PubMed] [Google Scholar]
- 23.Stafford GP, et al. Sorting of early and late flagellar subunits after docking at the membrane ATPase of the type III export pathway. J Mol Biol. 2007;374:877–882. doi: 10.1016/j.jmb.2007.09.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Evans LD, Stafford GP, Ahmed S, Fraser GM, Hughes C. An escort mechanism for cycling of export chaperones during flagellum assembly. Proc Natl Acad Sci USA. 2006;103:17474–17479. doi: 10.1073/pnas.0605197103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Evans LD, Hughes C. Selective binding of virulence type III export chaperones by FliJ escort orthologues InvI and YscO. FEMS Microbiol Lett. 2009;293:292–297. doi: 10.1111/j.1574-6968.2009.01535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kihara M, Minamino T, Yamaguchi S, Macnab RM. Intergenic suppression between the flagellar MS ring protein FliF of Salmonella and FlhA, a membrane component of its export apparatus. J Bacteriol. 2001;183:1655–1662. doi: 10.1128/JB.183.5.1655-1662.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hueck CJ. Type III protein secretion systems in bacterial pathogens of animals and plants. Microbiol Mol Biol Rev. 1998;62:379–433. doi: 10.1128/mmbr.62.2.379-433.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McMurry JL, Van Arnam JS, Kihara M, Macnab RM. Analysis of the cytoplasmic domains of Salmonella FlhA and interactions with components of the flagellar export machinery. J Bacteriol. 2004;186:7586–7592. doi: 10.1128/JB.186.22.7586-7592.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carpenter PB, Ordal GW. Bacillus subtilis FlhA: A flagellar protein related to a new family of signal-transducing receptors. Mol Microbiol. 1993;7:735–743. doi: 10.1111/j.1365-2958.1993.tb01164.x. [DOI] [PubMed] [Google Scholar]
- 30.Minamino T, MacNab RM. Interactions among components of the Salmonella flagellar export apparatus and its substrates. Mol Microbiol. 2000;35:1052–1064. doi: 10.1046/j.1365-2958.2000.01771.x. [DOI] [PubMed] [Google Scholar]
- 31.Ghelardi E, et al. Requirement of flhA for swarming differentiation, flagellin export, and secretion of virulence-associated proteins in Bacillus thuringiensis. J Bacteriol. 2002;184:6424–6433. doi: 10.1128/JB.184.23.6424-6433.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fraser GM, Gonzalez-Pedrajo B, Tame JR, Macnab RM. Interactions of FliJ with the Salmonella type III flagellar export apparatus. J Bacteriol. 2003;185:5546–5554. doi: 10.1128/JB.185.18.5546-5554.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Minamino T, et al. Role of the C-terminal cytoplasmic domain of FlhA in bacterial flagellar type III protein export. J Bacteriol. doi: 10.1128/JB.01328-09. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rust M, et al. The Helicobacter pylori anti-sigma factor FlgM is predominantly cytoplasmic and cooperates with the flagellar basal body protein FlhA. J Bacteriol. 2009;191:4824–4834. doi: 10.1128/JB.00018-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Evdokimov AG, et al. Similar modes of polypeptide recognition by export chaperones in flagellar biosynthesis and type III secretion. Nat Struct Biol. 2003;10:789–793. doi: 10.1038/nsb982. [DOI] [PubMed] [Google Scholar]
- 36.Ozin AJ, Claret L, Auvray F, Hughes C. The FliS chaperone selectively binds the disordered flagellin C-terminal D0 domain central to polymerisation. FEMS Microbiol Lett. 2003;219:219–224. doi: 10.1016/S0378-1097(02)01208-9. [DOI] [PubMed] [Google Scholar]
- 37.Holm L, Sander C. Dali: A network tool for protein structure comparison. Trends Biochem Sci. 1995;20:478–480. doi: 10.1016/s0968-0004(00)89105-7. [DOI] [PubMed] [Google Scholar]
- 38.Saijo-Hamano Y, Minamino T, Macnab RM, Namba K. Structural and functional analysis of the C-terminal cytoplasmic domain of FlhA, an integral membrane component of the type III flagellar protein export apparatus in Salmonella. J Mol Biol. 2004;343:457–466. doi: 10.1016/j.jmb.2004.08.067. [DOI] [PubMed] [Google Scholar]
- 39.Gonzalez-Pedrajo B, Fraser GM, Minamino T, Macnab RM. Molecular dissection of Salmonella FliH, a regulator of the ATPase FliI and the type III flagellar protein export pathway. Mol Microbiol. 2002;45:967–982. doi: 10.1046/j.1365-2958.2002.03047.x. [DOI] [PubMed] [Google Scholar]
- 40.Homma M, Iino T. Locations of hook-associated proteins in flagellar structures of Salmonella typhimurium. J Bacteriol. 1985;162:183–189. doi: 10.1128/jb.162.1.183-189.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yonekura K, et al. The bacterial flagellar cap as the rotary promoter of flagellin self-assembly. Science. 2000;290:2148–2152. doi: 10.1126/science.290.5499.2148. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information