Ubiquitin and SUMO systems in the regulation of mitotic checkpoints (original) (raw)
. Author manuscript; available in PMC: 2011 Mar 21.
Published in final edited form as: Trends Biochem Sci. 2006 May 2;31(6):324–332. doi: 10.1016/j.tibs.2006.04.001
Abstract
Proteolysis mediated by the ubiquitin–proteasome system is a crucial regulatory mechanism in signal transduction cascades of temporal cellular processes such as cell division. Two principal subtypes of modular ubiquitin ligase, the anaphase-promoting complex or cyclosome (APC/C) and the Skp1/Cullin-1/F-box protein complex, have emerged as essential regulators of key events in the cell cycle. The importance of these ligases is best illustrated by their roles in the checkpoint and repair pathways or in response to multiple stresses, where they affect activation of the M-phase-promoting factor or proper formation and/or maintenance of the mitotic spindle. Recent studies have considerably improved our understanding of the function of the concerted action of the phosphorylation and ubiquitin or SUMO systems in the regulation of the stability and activity of key components of the mitotic checkpoint.
Introduction
Studying the regulation of mitotic progression is crucial for our understanding of both physiological and aberrant cell divisions. Posttranslational modifications of cell-cycle regulatory proteins have been the subject of extensive investigation. These modifications are made by a complex set of protein kinases, ubiquitin ligases and ubiquitin-like proteins (such as SUMO) that act in conjunction to control and to ensure equal and identical distribution of the genome to each daughter cell – the hallmark of mitosis. Mitotic progression requires the achievement of two general processes: activation of the M-phase-promoting factor (MPF) and formation of the dynamic mitotic spindle.
The MPF, which comprises the Cdk1/cyclin B complex, has emerged as the master regulator of mitosis through its phosphorylation of many key and accessory targets [1]. By contrast, the mitotic spindle is formed by packed chromosomes and adjacently recruited microtubules, which attach to the chromatids through centromeric kinetochores, thereby facilitating alignment of the duplicated chromosomes at the metaphase plate and preparing them for segregation [2]. Remarkably, both modules are crucial targets of the checkpoint mechanisms – namely, the surveillance responses that can be activated to avoid unfaithful or abortive cell division.
Mitotic progression is indeed impaired after cellular insults that damage chromosomal DNA and/or microtubule structures. Responses to DNA and spindle damage alter the phosphorylation, ubiquitination and/or SUMOylation of mitotic checkpoint proteins in an effort to avoid premature cytokinesis and to determine cell fate: either completion of mitosis or initiation of mitotic catastrophe and subsequent cell death.
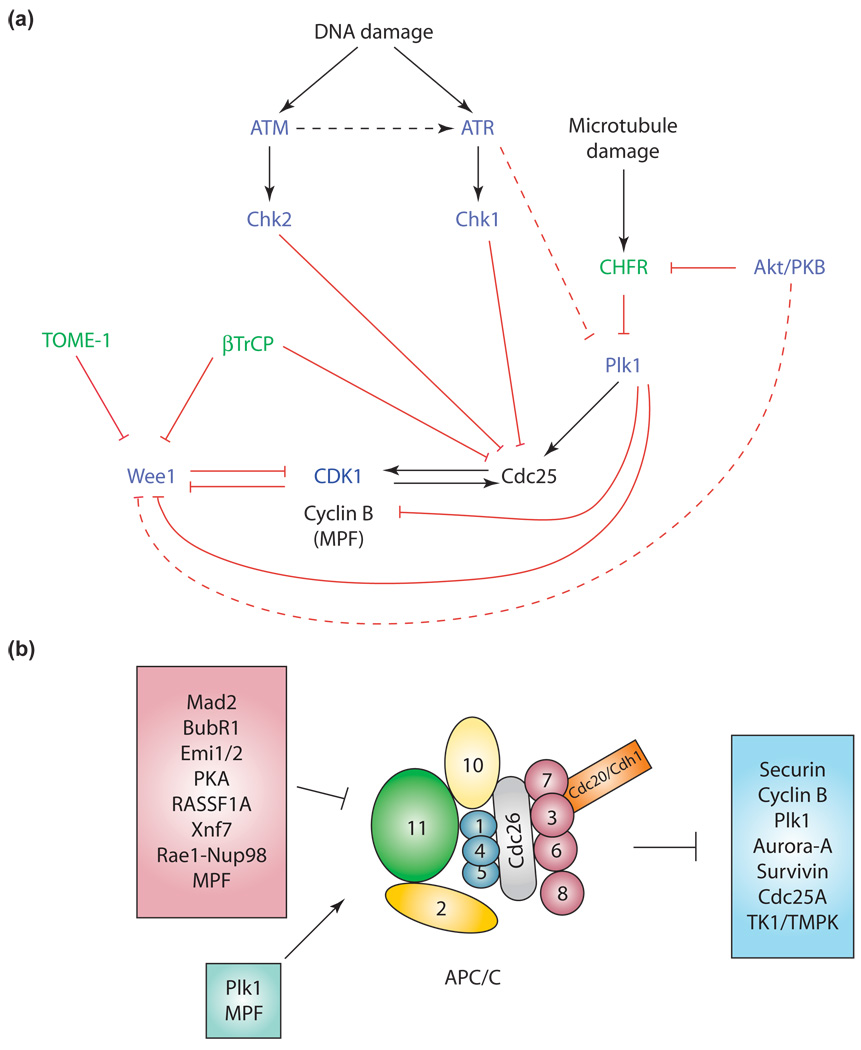
Activation of the MPF is a complex event that is regulated by inactivation of the Cdk inhibitory kinase Wee1 and concomitant activation of the Cdc25 family of phosphatases. Interestingly, Wee1 and Cdc25 have evolved as preferential targets of several checkpoint pathways and their stability is finely tuned by degradation induced by the ubiquitin–proteasome system (UPS). Another attractive ‘Achilles’ heel’ in the coordination of mitosis is the mitotic spindle. The ubiquitin ligase anaphase-promoting complex or cyclosome (APC/C) has emerged as a chief component of the spindle assembly checkpoint pathway through its regulation of key factors that timely coordinate mitosis. Finally, regulation by reversible non-canonical ubiquitination and SUMOylation also affects key components of the cell-cycle checkpoints.
Here, we discuss current understanding of the function of ubiquitin ligases and SUMO modifications in regulating the stability and activity of principal components of the spindle assembly or mitotic checkpoint, in conjunction with the role of protein kinases in this regulatory pathway.
G2/M checkpoint mechanisms: sabotaging Cdk activation
Degradation of the Cdc25 family of phosphatases
Cdk1 and Cdk2 are probably the main Cdks responsible for the general orchestration of most cell-cycle processes and the G2/M transition in particular [3]. Activation of the Cdks mainly depends both on the availability of their activating partners – the cyclins, whose levels are strongly regulated by the UPS – and on Cdc25-mediated dephosphorylation of inhibitory phosphorylation (at Thr14 and Tyr15) of Cdks (Figure 1a).
Figure 1.
The complexity of the cell-cycle checkpoint pathways. (a) Molecular circuitry showing the chief factors in cell-cycle orchestration and their positive (black lines) or negative (red lines) regulation by protein kinases (blue) and/or ubiquitin ligases (green) that are directly involved in DNA or microtubule damage responses. Broken lines indicate a possible regulatory link. Abbreviations: ATM, ataxia telangiectasia mutated; ATR, ATM and Rad3-related; CDK1, cyclin-dependent kinase 1; MPF, M-phase-promoting factor; PKB, protein kinase B. (b) Cell-cycle-related and checkpoint-related substrates of the anaphase-promoting complex or cyclosome (APC/C; blue box), together with most of its currently known activators (pale green box) and inhibitors (pink box). Of note, Emi1 and RASSF1A have been implicated in a possible early mitotic checkpoint, whereas Mad2 and BubR1 are classically involved in the spindle assembly checkpoint [74]. MPF is a classical activator of the APC/CCdc20, but it conversely inhibits the APC/CCdh1. Furthermore, Cdc20 is a substrate for the APC/CCdh1. The subunits of the APC/C are numbered according to Ref. [75]. Briefly, the APC/C contains a cullin-like protein (Apc2), a RING-H2 finger protein (Apc11) and several subunits bearing tetratricopeptide repeats (Apc3 and Apc6–8). Apc10 displays a Doc domain. The Cdc20 or Cdh1 subunits contain WD40 repeats and are the major substrate-specific APC/C activators. Proteins that putitively belong to the same family are color-matched. The numbers refer to the corresponding Apc subunit (i.e. ‘2’ refers to Apc2 and so on).
The Cdc25 family of phosphatases has at least three members: Cdc25A, Cdc25B and Cdc25C. These proteins have been shown to be tightly regulated during the cell cycle by both phosphorylation and the UPS [4]. Mammalian Cdc25A, an intrinsically unstable protein, is specifically stabilized during mitosis by phosphorylation by the Cdk1/cyclin B1 complex as part of a positive feedback loop that ensures maximal activation of the MPF and entry into mitosis [5]. The labile state of Cdc25A throughout most phases of the cell cycle has been attributed to at least two different ubiquitin ligases that function in cell-cycle control: the APC/CCdh1 ubiquitin ligase complex, which is responsible for degradation of Cdc25A at late mitosis exit and early G1 through recognition of a KEN-box motif located at the N terminus of the protein; and an Skp1/Cullin-1/F-box protein (SCF) complex containing βTrCP1 or βTrCP2 (SCFβTrCP1/2), which is required for degradation of Cdc25A during the S and G2 phases [6–8].
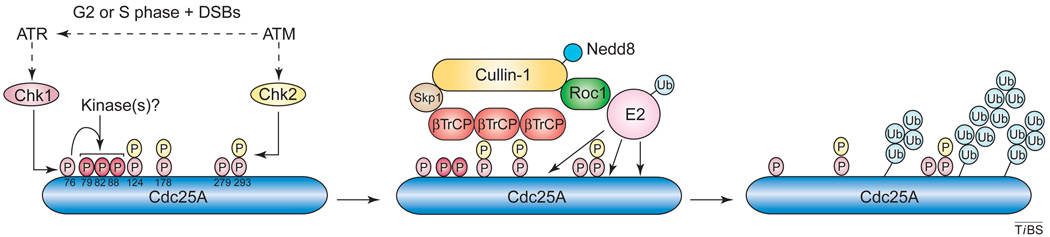
Degradation of Cdc25A mediated by the SCFβTrCP1/2 complex involves phosphorylation by the ATR–Chk1 kinase module, coupled with the protein claspin and the complex Rad9–Rad1–Hus1, which probably act as DNA damage sensors. This proteolytic mechanism represents an integral part of the physiological progression of S phase. DNA damage, however, further activates and switches this pathway from a constitutively operating surveillance mode (which is compatible with DNA replication and/or mitotic entry) into an emergency checkpoint response (which blocks cell-cycle progression) [9]. Chk1 phosphorylates Cdc25 directly at several residues (reviewed in Ref. [10]), although how this complex phosphorylation facilitates recognition of Cdc25A by SCFβTrCP1/2 remains to be clarified (Box 1).
Box 1. Phosphorylation-dependent association of Cdc25A with βTrCP.
Cdc25A is a key target of the G1, S/M, intra-S and G2 (or G2/M) checkpoints [9]. After cells are exposed to DNA-damaging agents (e.g. etoposide, X-ray or UV irradiation) or the generation of stalled replication forks (e.g. by hydroxyurea), Cdc25A is heavily phosphorylated on Ser76 (by Chk1) and on Ser124, Ser178 and Ser293 (by Chk1 and Chk2); other sites that are phosphorylated by as yet unidentified kinases include Ser79, Ser82 and probably Ser88 [76] (Figure I).
Some phosphorylation events (e.g. on Ser124) could create a degenerate docking site for βTrCP1 or βTrCP2 (βTrCP1/2) that encompasses the natural negative charges of Asp125 and Asp130. In addition, it has been proposed that the sequence DDGFVD (residues 215–220), described as the ‘DDG motif’, could comprise a constitutively active or primed interaction motif for βTrCP1/2 in which Asp216 and Asp220 would provide the negative charges recognized by the F-box protein [12]. Furthermore, some of the phospho-acceptor sites (Ser212, Thr208 and Thr210) that are proximal to the DDG motif might strengthen the interaction between βTrCP1/2 and Cdc25A.
A phosphodegron centered on Ser82 was initially proposed as the main recognition motif for βTrCP1/2. A priming phosphorylation event on Ser76 that could facilitate phosphorylation around the local ubiquitin ligase recognition motif (which is embedded in a PEST sequence) has been also suggested [77]. Together, these modifications enhance recognition of hyperphosphorylated Cdc25A by the SCFβTrCP1/2 complex. Although the role of such massive phosphorylation remains unclear, it might (i) increase ubiquitination kinetics owing to possible structural changes that ensure more efficient recognition by the E3 enzyme, that bring putative lysine acceptors into close proximity of the E2 enzyme or that induce a more efficient multimeric architectural arrangement for the ubiquitin transfer; (ii) recruit other, unidentified proteins for a more processive and efficient reaction; (iii) facilitate the interaction of several βTrCP1/2 molecules with the substrate.
It is also plausible that the presence of several (at least two or three) βTrCP-binding sites in Cdc25A makes it possible to recruit different βTrCP molecules to each site. If so, then there must be some sort of oligomerization mechanism for βTrCP. Indeed, the βTrCP1 and βTrCP2 proteins are able to homo- and heterodimerize [78]. The sequential binding of multiple ubiquitin ligases to Cdc25A could function (i) to induce structural changes required for efficient ubiquitination or for multi-polyubiquitination or (ii) as a mechanism to ‘seek and find’ the most advantageous lysine acceptor capable of inducing proteasomal targeting.
Regarding the other members of the Cdc25 family, it has been reported that mammalian Cdc25C is also degraded when cells are treated with arsenite (which induces a G2/M arrest). Degradation of Cdc25C under these conditions requires an intact KEN box, which suggests that the APC/C is involved [11]. Cdc25B proteins also seem to be subject to a basal degradation that is mediated via a DDG motif, coupled with some negatively charged residues in its vicinity, and the SCFβTrCP1/2 complex [12].
Degradation of the Cdk inhibitor Wee1
Inhibitory phosphorylation of mammalian Cdk1 and Cdk2 is mediated by the Wee1 or Myt1 family of protein kinases. Initially, the activity of Wee1 was reported to be regulated mainly by cell-cycle-dependent phosphorylation [13]. Subsequently, it has been shown that Wee1 protein levels are also regulated by the UPS [14].
In Xenopus egg extracts, induction of S-phase arrest by aphidicolin (an inhibitor of DNA polymerase that triggers a replication checkpoint) induces stabilization of Wee1 [14]. In this system, the E2 enzyme Cdc34 (which assembles into an SCF-type complex) is part of the machinery involved in Wee1 degradation after the completion of S phase.
In fission yeast, a Wee1-related kinase called Mik1 was found to be stabilized during the S-phase checkpoint arrest [15]; Mik1 has been subsequently shown to be essential for both the G2/M DNA damage and replication checkpoints [16]. In budding yeast, a unique Wee1-related kinase termed Swe1 is also regulated by proteolysis and is specifically stabilized at the so-called ‘morphogenesis checkpoint’ (which responds to insults that perturb bud formation; reviewed in Ref. [17]). Degradation of Swe1 has been documented to be triggered by direct phosphorylation by the Cdc28p/cyclin (MPF) complex and to be regulated by relocalization of the kinase from the nucleus to the mother–bud neck and probably by interaction with proteins such as Hsl7p or Hsl1p.
More recently, an F-box protein called TOME-1 has been shown to be responsible for degradation of Wee1 phosphorylated at Ser38 (Ser85 in human Wee1A) before the ‘G2/M’ transition in the Xenopus system [18]. Interestingly, TOME-1 itself is degraded during G1 by the APC/CCdh1 complex. Surprisingly, although phosphorylation and degradation of Wee1 in frog egg extracts require the presence of a nucleus, TOME-1 seems likely to be a cytosolic protein. Conversely, other studies have found that the human phosphorylated protein Wee1A is not recognized by TOME-1 but instead by the SCFβTrCP1/2 complex [19].
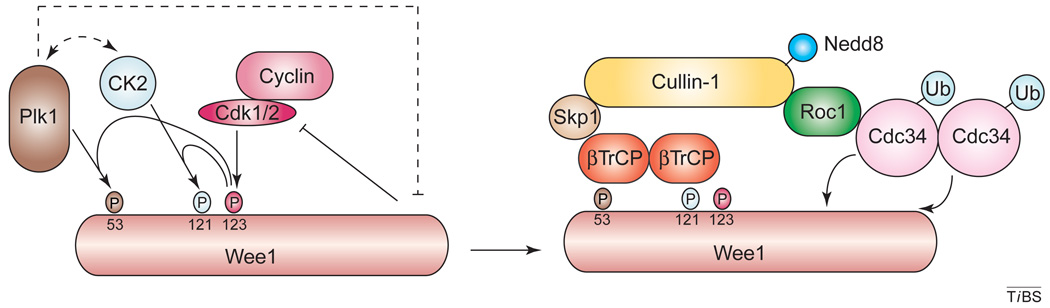
It was initially shown that Ser53 and Ser123, which are phosphorylated by Plk1 and Cdk1, respectively, act as possible ‘phosphodegrons’ for degradation of Wee1A at the onset of mitosis [19]. It turns out that degradation of Wee1A, similar to degradation of Cdc25A, depends on phosphorylation at multiple sites. For Wee1A, Cdk1/2-mediated phosphorylation at Ser123 functions as a double-priming phosphorylation event for subsequent phosphorylation at residues Ser53 (by Plk1) and Ser121 (by Casein kinase 2) [20]. It seems, then, that several molecules of βTrCP can bind to different phosphodegrons in Wee1A (Box 2).
Box 2. Different phosphodegrons in Wee1A might serve βTrCP.
The first degron in Wee1A is formed by phosphorylated Ser53 and Glu57, which are present in a degenerate and non-canonical DpSAFQE motif (residues 52–57) as opposed to the classical DSGΦXS site; a second degron is formed by Glu117 (or even probably Glu116) and phosphorylated Ser121 in a non-canonical and extended EEGFGpSSpS motif (residues 116–123). It seems that phosphorylation of Ser123 alone could help to establish some accessory interactions with charged amino acids present in the WD40 repeats of βTrCP. A third degron could be formed by the Ser85 residue (equivalent to Ser38 in the Xenopus Wee1A protein) in the sequence GpSPGELEED (residues 84–92) (Figure I). It is not yet clear whether this last degron is sufficient for recognition by TOME-1 or βTrCP proteins; however, studying this putative degron (and the kinase responsible for phosphorylation at Ser85) could clarify the differences observed between the human and Xenopus systems.
It remains to be determined whether all of these sites constitute optimal or suboptimal sites for βTrCP. It is conceivable that several βTrCP molecules might bind Wee1 and that the number of bound molecules (monomers, dimers or even oligomers) will determine the ubiquitination of Wee1A and the rate or the timing of Wee1A degradation. In this context, a recent study in the Xenopus system showed that Ser38 (Ser85 in human Wee1A), Thr53 (not conserved) and Ser62 (Ser121) are required for Wee1A degradation in interphase egg extracts, whereas Thr104 (Thr190) and Thr150 (Thr239) are necessary for degradation of Wee1A at M phase [79]. In human Wee1A, Thr239 is close to another cryptic putative recognition site for βTrCP: DSLLLHSSG (residues 241–249).
The priming phosphorylation event mediated by Cdk1 during the G2/M transition seems to be conserved in budding yeast, where Swe1 is apparently phosphorylated first by the Cdc28–Clb2 complex (probably in the nucleus) and then at its septin ring in a process involving Cla4 (known as PAK in mammals) and the scaffolding activities of Hsl1 (which tethers Hsl7) and Hsl7 itself. Swe1 is translocated, together with the Plk1 ortholog Cdc5 (this translocation also depends in part on Cdc28 and on Swe1 itself), to the bud neck, where Cdc5 hyperphosphorylates the primed phosphorylated Swe1, thereby inducing its degradation [80]. The ubiquitin ligase or ligases responsible for the ubiquitination of Swe1 after its heavy phosphorylation remain unknown.
As mentioned earlier, Wee1 is stabilized during DNA replication checkpoints. Although little is known about the cell-cycle regulation (during normal progression and checkpoint establishment) of βTrCP, which has emerged as a key factor in the control of two of the main Cdk regulators (Cdc25 and Wee1), it is plausible that the stabilization of Wee1 during stalled replication is due to inhibition of the Cdk itself (either through direct events or through indirect feedback loop processes) and/or Plk1. Indeed, it has been demonstrated that Plk1 is a selected target of the DNA damage checkpoint [21]. Notably, Plk1-mediated induced degradation of Wee1 has been also implicated in the process that occurs after DNA damage to ensure mitotic re-entry during mitosis in cells recovering from DNA-damage-induced arrest [22].
Finally, it has been reported that Chk1-catalyzed phosphorylation of Xenopus Wee1 at Ser549 (Ser642 in human Wee1A) helps to activate Wee1 after checkpoint induction [23,24]. It is likely that this phosphorylation event also contributes to the stabilization of Wee1.
Regulation of Plk1 by CHFR in the DNA damage checkpoint
Plks contribute to entry into mitosis and bipolar spindle formation, but also regulate mitotic exit and have been implicated in the temporal and spatial coordination of cytokinesis. Furthermore, it has been established that several DNA-damaging agents (such as adriamycin, X-ray or UV irradiation) inactivate Plk1 in a manner dependent on ATM or ATR kinase. Thus, Plk1 seems to be an important target of the DNA damage checkpoint, facilitating cell-cycle arrest at several points during G2 and mitosis [21]. Experiments in budding yeast have also shown that the Plk ortholog Cdc5 is controlled by the DNA damage checkpoint [25].
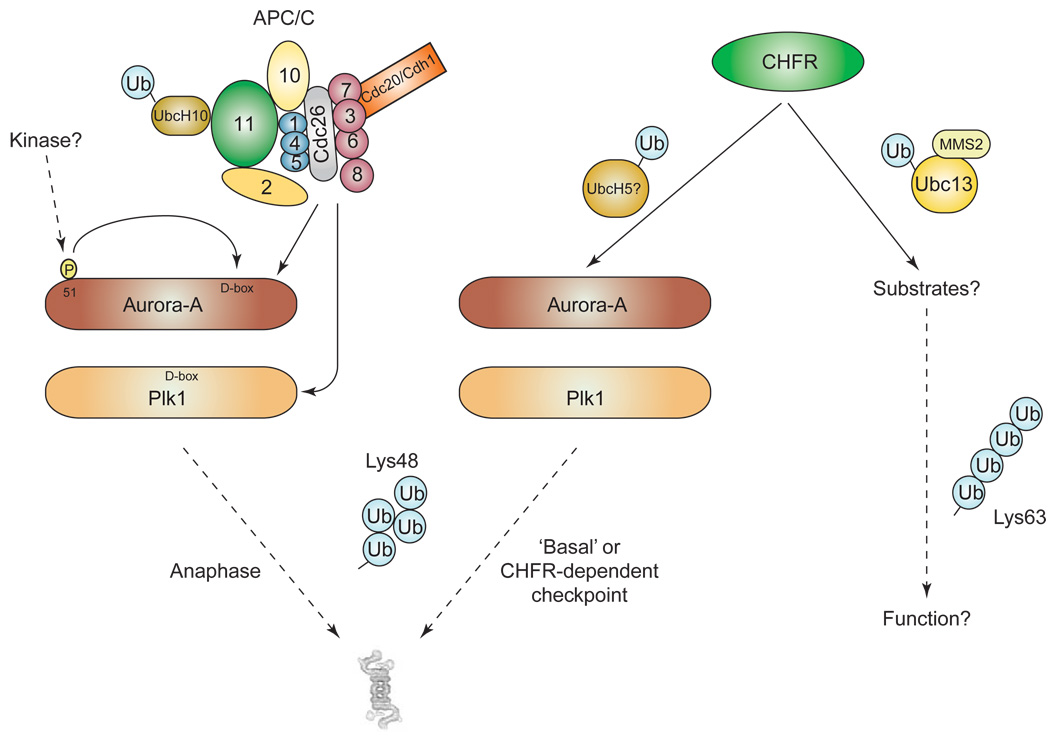
The product of the CHFR (checkpoint with forkhead associated and ring-finger) gene was initially found to be essential for the cellular checkpoint response that delays entry into mitosis in the presence of stresses that disrupt microtubule assembly and/or dynamics [26]. Subsequently, it has been shown that CHFR in fact functions as a RING-finger-type ubiquitin ligase for Plk1 in the Xenopus system [27]. Ubiquitination of Plk1 by CHFR delays activation of Cdk1, leading in turn to a delay in mitosis. It was initially thought that CHFR-mediated ubiquitination of Plk1 induces its degradation by the proteasome. Recent studies suggest, however, that the physiological E2 enzyme that is used by CHFR is the Ubc13–MMS2 complex [28], which generates non-canonical polyubiquitin chains with a Lys63 linkage – a topology that is not associated with degradation [29]. Accordingly, further studies are required to clarify how Plk1 is readily degraded by a classical UPS pathway involving CHFR.
A detailed analysis of cells in which cell-cycle progression was delayed in a CHFR-dependent manner found that CHFR is involved in a unique checkpoint response that arrests the cell cycle in early prophase (after chromosomal condensation but before mitosis commitment; also called ‘antephase’), the final target of which seems to be the Cdk1/cyclin B complex [30,31]. This checkpoint is primarily mediated by the p38 stress kinases and requires ubiquitination but not proteasomal activity (consistent with Lys63 topology) [31]. By contrast, Chfr knockout mouse embryonic fibroblasts have been clearly shown to have enhanced amounts of both the Plk1 and Aurora-A (but not Aurora-B) protein kinases [32], strongly suggesting that CHFR has a role in the basal stability of these proteins and probably also during CHFR-dependent cell-cycle arrest. Aurora-A and Plk1 are also degraded during the physiological cell cycle via ubiquitination by the APC/CCdh1 ubiquitin ligase. Degradation of Aurora-A occurs in a D-box-, A-box- and phosphorylation-dependent manner [33,34], whereas that of Plk1 requires a D-box [35] (Figure 2).
Figure 2.
Two different ubiquitin ligases control the stability of the Aurora-A and Polo-like kinases. In normally dividing cells, CHFR is inactivated during progression of the cell cycle (probably by phosphorylation mediated by Akt/protein kinase B at the G2/M transition; not shown). Under these conditions, Aurora-A and Plk1 are degraded during anaphase by a mechanism that involves APC/C-mediated ubiquitination and, at least for Aurora-A, a distributed degron. On microtubule disassembly or altered microtubule dynamics, a checkpoint response that depends on CHFR activation is initiated. The C-terminal cysteine-rich region of CHFR then recognizes the N terminus of Aurora-A, inducing its polyubiquitination and its subsequent proteasomal degradation. Note that this mechanism should also act under normal conditions that affect the basal stability of both Aurora-A and Plk1. CHFR might also be able to generate ubiquitin chains with a Lys63 topology, but the putative substrates for this alternative pathway remain unknown. Notably, CHFR- and APC/C-mediated ubiquitination of Aurora-A and/or Plk1 could target the same lysine residue. A possible interplay between the CHFR and APC/C pathways is thus conceivable, especially at the level of Aurora-A because both ubiquitin ligases require the intact N terminus of this kinase.
Concerning the effect of DNA damage on these components, inactivation of CHFR by Akt/PKB-mediated phosphorylation has been correlated with the promotion of mitosis after DNA damage [36]. Furthermore, activation of Aurora-A at the G2/M transition is inhibited by DNA damage, and overexpression of Aurora-A induces abrogation of the DNA-damage-induced G2 checkpoint [37]. Moreover, in response to DNA damage such as X-ray irradiation, the APCCdh1 complex is activated. The importance of Cdh1 in the DNA damage response is illustrated by Cdh1-depleted chicken DT40 cells, which fail to maintain DNA-damage-induced G2 arrest. Activation of APCCdh1 has a crucial role in both p27/Kip1-dependent G1 arrest and in DNA-damage-induced G2 arrest [38]. Finally, in budding yeast, the Aurora–Ipl1 protein kinase has been recently found to be responsible for activating the spindle assembly checkpoint in response to tension defects by creating unattached kinetochores [39].
The spindle assembly or mitotic checkpoint
The spindle assembly checkpoint delays the onset of anaphase if chromosomes fail to align completely at the metaphase plate during mitosis. Alterations in this checkpoint pathway have important implications for genomic instability.
The spindle assembly checkpoint is mediated by inhibition of the APC/C ubiquitin ligase complex (Figure 1b). Components of this checkpoint include members of the Bub (Bub1–Bub3) and Mad (Mad1, Mad2 and BubR1/Mad3) families, the Mps1 kinase, the kinesin-like CENP-E protein and the dynein-interacting proteins Zw10 and Rod [40]. Inhibition of the APC/C is thought to be mediated by Mad2 and BubR1, which inactivate the Cdc20 protein – a diffusible and activating factor of the APC/C that is required for degradation of securin (also known as Pds1) and cyclin B during the metaphase to anaphase transition [41].
Moreover, recent studies have also implicated the chromosomal passenger complex (CPC), which comprises at least survivin, INCENP, Dasra-B (also known as borealin) and the Aurora-B kinase, as a factor that can sense the lack of tension between chromosomes and microtubules. Therefore, the CPC also has an auxiliary, but important, role as a putative regulator of the spindle assembly checkpoint [42]. The CPC, and survivin in particular, has been shown to undergo changes in its localization during mitosis: initially, survivin associates with inner centromeres from G2 until the metaphase to anaphase transition; subsequently, it translocates to the spindle midzone, where it participates in the control of cell cleavage [43].
Ubiquitination cycle of survivin
Recently, a regulatory cascade involving the ubiquitination of survivin has been shown to control distribution of this protein during mitosis [44]. Localization of survivin (and thus Aurora-B) at centromeres and its local dynamic behavior are achieved via its polyubiquitination through a Lys63-linked chain. The length of this chain is regulated positively and negatively by, respectively, a complex containing p97 (a member of the AAA + ATPases), Npl4 (a zinc-finger protein) and Ufd1 (a protein with a ubiquitin-interacting motif), and the protein human fat facet in mouse (hFAM).
Whereas the p97–NP14–Ufd1 complex has been associated with an E4-type activity through the recruitment of putative E3 enzymes [45], hFAM is a member of the large family of deubiquitinating enzymes or ‘DUBs’ [46]. It seems that the precise control of the length of Lys63 polyubiquitination on survivin determines the duration of its localization at centromeres, thereby contributing to proper chromosomal attachment to the spindle and subsequent segregation during anaphase [44].
These findings provide mechanistic insight into the prevention of cell proliferation and the induction of apoptosis in cells where survivin expression has been inhibited [47] and into the observed sensitization of p53-mutanted lung cancer cells to adriamycin after survivin depletion [48]. Survivin has been also implicated in the sensitization of cells to DNA-damage-induced apoptosis caused by PS-341 (bortezomib), a clinically approved proteasomal inhibitor for the treatment of multiple myelomas, in a p53- and 14–3–3-dependent manner [49].
Degradation of Aurora-B
The Aurora-B kinase is degraded by the APC/CCdh1 after metaphase during normal progression of the cell cycle [50]. Aurora-B seems to be able to interact with both Cdc20 and Cdh1, and probably also directly with core subunits of the APC/C such as Cdc27 [51].
Aurora-B physically and specifically associates with the BRCA-1 C-terminal (BRCT) domain of PARP-1, resulting in its poly(ADP)ribosylation in response to DNA damage and consequential inhibition of its kinase activity [52]. Such inhibition is expected to contribute to the physiological response to DNA damage.
Although it has been reported that degradation of Aurora-B depends on a KEN box and an atypical A-box (as suggested for Aurora-A) that does not contain a phosphorylatable residue [51], another study has implicated a D-box in Aurora-B degradation [53]. It has not been determined whether degradation of either Aurora-A or Aurora-B is involved in any particular checkpoint signaling, but the implied role of these kinases in generating a robust response of the spindle assembly checkpoint as a result of the loss of spindle tension makes them attractive (direct or indirect) targets of this pathway. Moreover, there seems to be a clear connection among the spindle assembly checkpoint, the generation of chromosome instability and subsequent aneuploidy (which is commonly seen in cancer), and the regulation of cell-death pathways [54] – events in which the Aurora family of kinases has been implicated to a considerable extent [55].
Degradation of Pds1/securin
The protein Pds1/securin has been shown to be a direct target of the DNA damage response pathway in budding yeast [25]. Pds1/securin acts as an inhibitor of the protease separase that triggers chromosome segregation at the onset of anaphase by cleaving cohesin, the chromosomal protein complex responsible for sister chromatid cohesion. Pds1/securin is degraded at the metaphase to anaphase transition by the APC/C to enable activation of separase.
Pds1/securin is phosphorylated by Chk1, a modification that blocks its ubiquitination by the APC/CCdc20, whereas Chk2 inhibits the interaction between Pds1/securin and Cdc20. Both events lead to stabilization of Pds1/securin and blockade of the metaphase to anaphase transition. Pds1/securin has been proposed to be involved in recovery after repair of the DNA damage, leading to its dephosphorylation [56]. The possibility that the Pds1/securin–separase–cohesin pathway assists DNA repair by removing local cohesin in interphase fission yeast cells, has been also suggested [57].
Regulation of the APC/C
The APC/C is probably the key target of the mitotic spindle assembly checkpoint through its inhibition via Mad2 and BubR1 proteins (Figure 1b). There has been considerable progress in the identification of several other inhibitors of the APC/C such as Emi1, Xrp1 (also known as Emi2), RASSF1A, Xnf7 (a ubiquitin ligase), the Rae1–Nup98 complex, protein kinase A (PKA) and Mnd2 (a specific inhibitor of the meiotic APC/CAma1 identified in yeast).
In particular, the Emi1 protein is expected to block the APC/C during S phase, the beginning of prophase and prometaphase. Emi1 is degraded by the SCFβTrCP1/2 complex through a classical βTrCP-recognition phosphodegron motif [58]. Direct phosphorylation of Emi1 by the Cdk1/cyclin B complex and Plk1 has been associated with Emi1 degradation [59,60]. The current data suggest that massive phosphorylation of Emi1 by Cdk1 (as described earlier for Cdc25A by Chk1) could create a phosphorylation cascade (involving Plk1) that would ultimately result in phosphorylation of the classical βTrCP recognition motif (and perhaps other cryptic motifs), thereby inducing the interaction between Plk1 and one or several molecules of βTrCP [59,60].
In budding yeast, inhibition of the APC/C by PKA has been clearly linked to the DNA damage response. PKA is a crucial component of the nutrient sensing pathway and some stress-activated pathways. DNA damage activates PKA (through activation of Mec1), which in turn directly phosphorylates the APC/C regulatory activating protein Cdc20. This event blocks degradation of Clb2 (a B-type cyclin) and Pds1/securin, inducing the cell-cycle arrest required for launching the DNA repair machinery [61,62].
In addition to its role in the mitotic checkpoint, the APC/CCdh1 has been found to regulate human thymidine kinase 1 (hTK1) throughout the cell cycle (with very low levels at G1 phase owing to its degradation starting at the end of mitosis). Thymidine kinase enzymes are essential for synthesis of a dNTP pool (from the salvage pathway) required for DNA replication. Degradation of TK1 is dependent on the presence of an intact KEN box in its C terminus [63]. Another target of the APC/C has been subsequently found in the same pathway: namely, the thymidylate kinase (TMPK). This kinase seems to be a target of both of the APC/C activators, Cdc20 and Cdh1, via an essential D-box motif and probably an accessory KEN-box. More importantly, overexpression of non-degradable mutants of TK1 and TMPK in cells disrupts the dNTP pool by creating a surplus that eventually leads to growth retardation and an increased rate of gene mutation and genomic instability [64].
Role of SUMOylation in mitosis and its associated checkpoints
The protein Ubc9 is the classical E2 enzyme associated with the SUMO conjugation process. In budding and fission yeasts, cells that are mutant in Ubc9 or in SUMO itself show defects in progression through the G2/M transition, in addition to defects in chromosome integrity, telomere length, kinetochore function, chromosome segregation, proper checkpoint responses to DNA damage and survival mechanisms under stress conditions. SUMO isopeptidases (enzymes that counteract the SUMO modification) such as Ulp1 and Ulp2 are also required for, and are regulated by, progression of the cell cycle in budding yeast. In addition, yeast mutants deficient in Ulp2 are hypersensitive to several DNA-damaging agents [65].
Recently, the SUMO E3 ligase Pli1p has been found to be necessary for centromere and telomere maintenance in fission yeast and, interestingly, Pli1p mutants are highly sensitive to microtubule-destabilizing drugs [66]. In accordance with these observations, SUMOylated proteins are upregulated under oxidative and ethanol stress. It has been also suggested that, at least in budding yeast, the SUMO system is also required for the APC/C-mediated degradation of cyclin B and Pds1/securin that is controlled by the spindle assembly checkpoint [67].
In vertebrates, cells derived from Ubc9 knockout mice show major deficiencies in chromosome condensation and segregation, along with severely altered nuclear morphology [68]. The first substrate found to be modified by SUMO was the RanGAP1 protein – the activating factor for the small GTPase Ran that is involved in nuclear and spindle formation and in nucleo-cytoplasmic transport. It has been demonstrated that RanBP2 (also known as Nup358), which is a component of the nuclear pore complex that can function as a SUMO E3 ligase, interacts with SUMOylated RanGAP1, and that during mitosis both proteins are targeted to the mitotic spindle and kinetochores, where they are required to ensure proper microtubule–kinetochore attachment [69].
Similar to the ubiquitination cycle of survivin described earlier, at least two different proteins have been reported to be subject, during the metaphase to anaphase progression, to a SUMOylation cycle that might be related to proper coordination of the sequential mitotic events and eventually to checkpoint mechanisms. The first is the Pds5 protein, which has been implicated in sister chromatid cohesion maintenance in a way that might be partially independent of the cohesin complex [70]. SUMOylation of Pds5 is regulated by the cell cycle (it peaks during mitosis), is antagonized by the SUMO isopeptidase Smt4 (also known as Ulp2) and seems to be necessary to promote dissolution of cohesion. The second protein is the topoisomerase II enzyme that undergoes mitotic modification via SUMO-2/3 conjugation mediated by the Uba2–Aos1 (E1), Ubc9 (E2) and PIASy (E3) cascade [71]. Interestingly, SUMOylation of topoisomerase II can be reverted by Ulp2. This modification is maximal in metaphase and is followed by rapid deconjugation during anaphase. It has been suggested that the pool of SUMO-modified topoisomerase II might be mobilized from mitotic chromatin in a manner that is important for sister chromatid segregation and/or the remodeling of mitotic chromosomes.
The topoisomerase I and II enzymes are also rapidly SUMOylated in response to inhibition of their activity by poisons such as camptothecin. This SUMOylation event seems to amplify the formation of cleavable complexes (the covalent topoisomerase–DNA intermediates) and might constitute a mechanism involved in DNA repair. Remarkably, degradation of topoisomerase I and II by the UPS has been also reported in cells treated with topoisomerase inhibitors. Degradation of topoisomerase is dependent on an intact SUMO system and has been associated with the resistance to camptothecin shown by several tumor cells (see Ref. [72] and references therein).
The topoisomerase-binding protein Topors is also SUMOylated. It has been proposed that Topors might act as both a ubiquitin and a SUMO ligase for the p53 tumor suppressor. Finally, topoisomerase IIβ-binding protein 1 (TopBP1) has been shown to be degraded through hHYD-mediated ubiquitination. X-ray irradiation blocks degradation of TopBP1, which is accompanied by its phosphorylation and its colocalization with γH2AX nuclear foci in DNA breaks [73]. Of note, the deubiquitinating enzyme Doa4 protects cells from camptothecin-induced cell death.
Concluding remarks
The prevailing theme in our present understanding of checkpoint control is the multiplicity of enzymes involved in regulating each of the mitotic checkpoints. Multiple kinases and phospho-acceptor sites (‘distributed degrons’) modulate the checkpoint control proteins to enable access to the ubiquitin ligase. The possibility that a priming phosphorylation event is required to achieve a functional modification (i.e. recognition of the substrate) is becoming apparent for a growing number of proteins. Further complexity is demonstrated by the realization that more than one molecule of ligase might be required to target a substrate, and that different members of the ubiquitin ligase family might encounter cooperative or regulatory directed association.
The notion that not all modified proteins are destined for degradation owing to non-canonical ubiquitination seems to apply to several key regulatory proteins. These proteins are modified by a different set of ubiquitin-conjugating enzymes, and the underlying mechanism that enables the association and activity of one type of E2 enzyme versus another remains largely unknown, although a dependency on posttranslational modifications seems highly feasible. Non-canonical ubiquitination is expected to result in endless topologies of polyubiquitin chains, and we expect that each one will possess a distinct functional significance (e.g. localization or assembly into different complexes mediated by specific adaptors).
If we add to these sources of complexity the role of ubiquitin-like proteins in the regulatory modules and the reversible nature of the modifications, it is clear that we are only at the early stages of understanding the mitotic checkpoint circuits.
Figure I.
Sequential model of the interplay between the phosphorylation and ubiquitination cascades that function in Cdc25A degradation. Cdc25A (dark blue) is massively phosphorylated by Chk1 (pink), Chk2 (yellow) and other unidentified kinases in response to DNA double strand breaks (DSBs) that occur during either the G2 or S phase. Hyperphosphorylated Cdc25A is then recognized by a putative oligomeric βTrCP complex as part of a modular SCF-type ubiquitin ligase that probably induces multi-polyubiquitination of the phosphatase.
Figure I.
Model of the regulation of Wee1 degradation by phosphorylation and subsequent ubiquitination. The protein kinase Wee1 (light brown) is first phosphorylated by a Cdk–cyclin complex (red and pink). This initial event triggers phosphorylation of Wee1 by Plk1 (brown) and Casein kinase 2 (CK2; pale blue). Hyperphosphorylated Wee1 is then recognized and ubiquitinated by a SCFCdc34–βTrCP complex, in which both the Cdc34 and βTrCP subunits might be present as oligomers.
Acknowledgements
We thank J. Chen for critically reading the manuscript. A grant from the National Cancer Institute (CA078419 to Z.R.) is gratefully acknowledged.
References
- 1.Doree M, Hunt T. From Cdc2 to Cdk1: when did the cell cycle kinase join its cyclin partner? J. Cell Sci. 2002;115:2461–2464. doi: 10.1242/jcs.115.12.2461. [DOI] [PubMed] [Google Scholar]
- 2.Gadde S, Heald R. Mechanisms and molecules of the mitotic spindle. Curr. Biol. 2004;14:R797–R805. doi: 10.1016/j.cub.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 3.Malumbres M, Barbacid M. Mammalian cyclin-dependent kinases. Trends Biochem. Sci. 2005;30:630–641. doi: 10.1016/j.tibs.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 4.Boutros R, et al. The when and wheres of CDC25 phosphatases. Curr. Opin. Cell Biol. 2006;18:185–191. doi: 10.1016/j.ceb.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 5.Mailand N, et al. Regulation of G2/M events by Cdc25A through phosphorylation-dependent modulation of its stability. EMBO J. 2002;21:5911–5920. doi: 10.1093/emboj/cdf567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Donzelli M, et al. Dual mode of degradation of Cdc25 A phosphatase. EMBO J. 2002;21:4875–4884. doi: 10.1093/emboj/cdf491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Busino L, et al. Degradation of Cdc25A by βTrCP during S phase and in response to DNA damage. Nature. 2003;426:87–91. doi: 10.1038/nature02082. [DOI] [PubMed] [Google Scholar]
- 8.Jin J, et al. SCFβTRCP links Chk1 signaling to degradation of the Cdc25A protein phosphatase. Genes Dev. 2003;17:3062–3074. doi: 10.1101/gad.1157503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bartek J, et al. Checking on DNA damage in S phase. Nat. Rev. Mol. Cell Biol. 2004;5:792–804. doi: 10.1038/nrm1493. [DOI] [PubMed] [Google Scholar]
- 10.Busino L, et al. Cdc25A phosphatase: combinatorial phosphorylation, ubiquitylation and proteolysis. Oncogene. 2004;23:2050–2056. doi: 10.1038/sj.onc.1207394. [DOI] [PubMed] [Google Scholar]
- 11.Chen F, et al. Arsenite-induced Cdc25C degradation is through the KEN-box and ubiquitin-proteasome pathway. Proc. Natl. Acad. Sci. U. S. A. 2002;99:1990–1995. doi: 10.1073/pnas.032428899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kanemori Y, et al. β-TrCP recognizes a previously undescribed nonphosphorylated destruction motif in Cdc25A and Cdc25B phosphatases. Proc. Natl. Acad. Sci. U. S. A. 2005;102:6279–6284. doi: 10.1073/pnas.0501873102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kellogg DR. Wee1-dependent mechanisms required for coordination of cell growth and cell division. J. Cell Sci. 2003;116:4883–4890. doi: 10.1242/jcs.00908. [DOI] [PubMed] [Google Scholar]
- 14.Michael WM, Newport J. Coupling of mitosis to the completion of S phase through Cdc34-mediated degradation of Wee1. Science. 1998;282:1886–1889. doi: 10.1126/science.282.5395.1886. [DOI] [PubMed] [Google Scholar]
- 15.Boddy MN, et al. Replication checkpoint enforced by kinases Cds1 and Chk1. Science. 1998;280:909–912. doi: 10.1126/science.280.5365.909. [DOI] [PubMed] [Google Scholar]
- 16.Rhind N, Russell P. Roles of the mitotic inhibitors Wee1 and Mik1 in the G2 DNA damage and replication checkpoints. Mol. Cell. Biol. 2001;21:1499–1508. doi: 10.1128/MCB.21.5.1499-1508.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee KS, et al. Monitoring the cell cycle by multi-kinase-dependent regulation of Swe1/Wee1 in budding yeast. Cell Cycle. 2005;4:1346–1349. doi: 10.4161/cc.4.10.2049. [DOI] [PubMed] [Google Scholar]
- 18.Ayad NG, et al. Tome-1, a trigger of mitotic entry, is degraded during G1 via the APC. Cell. 2003;113:101–113. doi: 10.1016/s0092-8674(03)00232-0. [DOI] [PubMed] [Google Scholar]
- 19.Watanabe N, et al. M-phase kinases induce phospho-dependent ubiquitination of somatic Wee1 by SCFβTrCP. Proc. Natl. Acad. Sci. U. S. A. 2004;101:4419–4424. doi: 10.1073/pnas.0307700101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Watanabe N, et al. Cyclin-dependent kinase (CDK) phosphorylation destabilizes somatic Wee1 via multiple pathways. Proc. Natl. Acad. Sci. U. S. A. 2005;102:11663–11668. doi: 10.1073/pnas.0500410102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smits VA, et al. Polo-like kinase-1 is a target of the DNA damage checkpoint. Nat. Cell Biol. 2000;2:672–676. doi: 10.1038/35023629. [DOI] [PubMed] [Google Scholar]
- 22.van Vugt MA, et al. Polo-like kinase-1 controls recovery from a G2 DNA damage-induced arrest in mammalian cells. Mol. Cell. 2004;15:799–811. doi: 10.1016/j.molcel.2004.07.015. [DOI] [PubMed] [Google Scholar]
- 23.Lee J, et al. Positive regulation of Wee1 by Chk1 and 14-3-3 protein. Mol. Biol. Cell. 2001;12:551–563. doi: 10.1091/mbc.12.3.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stanford JS, Ruderman JV. Changes in regulatory phosphorylation of Cdc25C Ser287 and Wee1 Ser549 during normal cell cycle progression and checkpoint arrests. Mol. Biol. Cell. 2005;16:5749–5760. doi: 10.1091/mbc.E05-06-0541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sanchez Y, et al. Control of the DNA damage checkpoint by Chk1 and Rad53 protein kinases through distinct mechanisms. Science. 1999;286:1166–1171. doi: 10.1126/science.286.5442.1166. [DOI] [PubMed] [Google Scholar]
- 26.Scolnick DM, Halazonetis TD. Chfr defines a mitotic stress checkpoint that delays entry into metaphase. Nature. 2000;406:430–435. doi: 10.1038/35019108. [DOI] [PubMed] [Google Scholar]
- 27.Kang D, et al. The checkpoint protein Chfr is a ligase that ubiquitinates Plk1 and inhibits Cdc2 at the G2 to M transition. J. Cell Biol. 2002;156:249–259. doi: 10.1083/jcb.200108016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bothos J, et al. The Chfr mitotic checkpoint protein functions with Ubc13-Mms2 to form Lys63-linked polyubiquitin chains. Oncogene. 2003;22:7101–7107. doi: 10.1038/sj.onc.1206831. [DOI] [PubMed] [Google Scholar]
- 29.Andersen PL, et al. Distinct regulation of Ubc13 functions by the two ubiquitin-conjugating enzyme variants Mms2 and Uev1A. J. Cell Biol. 2005;170:745–755. doi: 10.1083/jcb.200502113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Summers MK, et al. The CHFR mitotic checkpoint protein delays cell cycle progression by excluding Cyclin B1 from the nucleus. Oncogene. 2005;24:2589–2598. doi: 10.1038/sj.onc.1208428. [DOI] [PubMed] [Google Scholar]
- 31.Matsusaka T, Pines J. Chfr acts with the p38 stress kinases to block entry to mitosis in mammalian cells. J. Cell Biol. 2004;166:507–516. doi: 10.1083/jcb.200401139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu X, et al. Chfr is required for tumor suppression and Aurora A regulation. Nat. Genet. 2005;37:401–406. doi: 10.1038/ng1538. [DOI] [PubMed] [Google Scholar]
- 33.Castro A, et al. The D-Box-activating domain (DAD) is a new proteolysis signal that stimulates the silent D-Box sequence of Aurora-A. EMBO Rep. 2002;3:1209–1214. doi: 10.1093/embo-reports/kvf241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Littlepage LE, Ruderman JV. Identification of a new APC/C recognition domain, the A box, which is required for the Cdh1-dependent destruction of the kinase Aurora-A during mitotic exit. Genes Dev. 2002;16:2274–2285. doi: 10.1101/gad.1007302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lindon C, Pines J. Ordered proteolysis in anaphase inactivates Plk1 to contribute to proper mitotic exit in human cells. J. Cell Biol. 2004;164:233–241. doi: 10.1083/jcb.200309035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shtivelman E. Promotion of mitosis by activated protein kinase B after DNA damage involves polo-like kinase 1 and checkpoint protein CHFR. Mol. Cancer Res. 2003;1:959–969. [PubMed] [Google Scholar]
- 37.Marumoto T, et al. Roles of aurora-A kinase in mitotic entry and G2 checkpoint in mammalian cells. Genes Cells. 2002;7:1173–1182. doi: 10.1046/j.1365-2443.2002.00592.x. [DOI] [PubMed] [Google Scholar]
- 38.Sudo T, et al. Activation of Cdh1-dependent APC is required for G1 cell cycle arrest and DNA damage-induced G2 checkpoint in vertebrate cells. EMBO J. 2001;20:6499–6508. doi: 10.1093/emboj/20.22.6499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pinsky BA, et al. The Ipl1-Aurora protein kinase activates the spindle checkpoint by creating unattached kinetochores. Nat. Cell Biol. 2006;8:78–83. doi: 10.1038/ncb1341. [DOI] [PubMed] [Google Scholar]
- 40.Kops GJ, et al. On the road to cancer: aneuploidy and the mitotic checkpoint. Nat. Rev. Cancer. 2005;5:773–785. doi: 10.1038/nrc1714. [DOI] [PubMed] [Google Scholar]
- 41.Nasmyth K. How do so few control so many? Cell. 2005;120:739–746. doi: 10.1016/j.cell.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 42.Lens SM, Medema RH. The survivin/Aurora B complex: its role in coordinating tension and attachment. Cell Cycle. 2003;2:507–510. doi: 10.4161/cc.2.6.559. [DOI] [PubMed] [Google Scholar]
- 43.Vagnarelli P, Earnshaw WC. Chromosomal passengers: the four-dimensional regulation of mitotic events. Chromosoma. 2004;113:211–222. doi: 10.1007/s00412-004-0307-3. [DOI] [PubMed] [Google Scholar]
- 44.Vong QP, et al. Chromosome alignment and segregation regulated by ubiquitination of survivin. Science. 2005;310:1499–1504. doi: 10.1126/science.1120160. [DOI] [PubMed] [Google Scholar]
- 45.Hoppe T. Multiubiquitylation by E4 enzymes: ‘one size’ doesn’t fit all. Trends Biochem. Sci. 2005;30:183–187. doi: 10.1016/j.tibs.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 46.Nijman SM, et al. A genomic and functional inventory of deubiquitinating enzymes. Cell. 2005;123:773–786. doi: 10.1016/j.cell.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 47.Ambrosini G, et al. Induction of apoptosis and inhibition of cell proliferation by survivin gene targeting. J. Biol. Chem. 1998;273:11177–11182. doi: 10.1074/jbc.273.18.11177. [DOI] [PubMed] [Google Scholar]
- 48.Yonesaka K, et al. Small interfering RNA targeting survivin sensitizes lung cancer cell with mutant p53 to adriamycin. Int. J. Cancer. 2006;118:812–820. doi: 10.1002/ijc.21350. [DOI] [PubMed] [Google Scholar]
- 49.Vaziri SA, et al. Sensitization of DNA damage-induced apoptosis by the proteasome inhibitor PS-341 is p53 dependent and involves target proteins 14-3-3σ and survivin. Mol. Cancer Ther. 2005;4:1880–1890. doi: 10.1158/1535-7163.MCT-05-0222. [DOI] [PubMed] [Google Scholar]
- 50.Pines J. Mitosis: a matter of getting rid of the right protein at the right time. Trends Cell Biol. 2005 doi: 10.1016/j.tcb.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 51.Nguyen HG, et al. Mechanism of Aurora-B degradation and its dependency on intact KEN and A-boxes: identification of an aneuploidy-promoting property. Mol. Cell. Biol. 2005;25:4977–4992. doi: 10.1128/MCB.25.12.4977-4992.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Monaco L, et al. Inhibition of Aurora-B kinase activity by poly(ADP-ribosyl)ation in response to DNA damage. Proc. Natl. Acad. Sci. U. S. A. 2005;102:14244–14248. doi: 10.1073/pnas.0506252102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stewart S, Fang G. Destruction box-dependent degradation of aurora B is mediated by the anaphase-promoting complex/cyclosome and Cdh1. Cancer Res. 2005;65:8730–8735. doi: 10.1158/0008-5472.CAN-05-1500. [DOI] [PubMed] [Google Scholar]
- 54.Weaver BA, Cleveland DW. Decoding the links between mitosis, cancer, and chemotherapy: the mitotic checkpoint, adaptation, and cell death. Cancer Cell. 2005;8:7–12. doi: 10.1016/j.ccr.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 55.Giet R, et al. Aurora kinases, aneuploidy and cancer, a coincidence or a real link? Trends Cell Biol. 2005;15:241–250. doi: 10.1016/j.tcb.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 56.Agarwal R, et al. Two distinct pathways for inhibiting Pds1 ubiquitination in response to DNA damage. J. Biol. Chem. 2003;278:45027–45033. doi: 10.1074/jbc.M306783200. [DOI] [PubMed] [Google Scholar]
- 57.Nagao K, et al. Separase-mediated cleavage of cohesin at interphase is required for DNA repair. Nature. 2004;430:1044–1048. doi: 10.1038/nature02803. [DOI] [PubMed] [Google Scholar]
- 58.Margottin-Goguet F, et al. Prophase destruction of Emi1 by the SCFβTrCP/Slimb ubiquitin ligase activates the anaphase promoting complex to allow progression beyond prometaphase. Dev. Cell. 2003;4:813–826. doi: 10.1016/s1534-5807(03)00153-9. [DOI] [PubMed] [Google Scholar]
- 59.Moshe Y, et al. Role of Polo-like kinase in the degradation of early mitotic inhibitor 1, a regulator of the anaphase promoting complex/cyclosome. Proc. Natl. Acad. Sci. U. S. A. 2004;101:7937–7942. doi: 10.1073/pnas.0402442101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hansen DV, et al. Plk1 regulates activation of the anaphase promoting complex by phosphorylating and triggering SCFβTrCP-dependent destruction of the APC inhibitor Emi1. Mol. Biol. Cell. 2004;15:5623–5634. doi: 10.1091/mbc.E04-07-0598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Searle JS, Sanchez Y. Stopped for repairs: a new role for nutrient sensing pathways? Cell Cycle. 2004;3:865–868. [PubMed] [Google Scholar]
- 62.Searle JS, et al. The DNA damage checkpoint and PKA pathways converge on APC substrates and Cdc20 to regulate mitotic progression. Nat. Cell Biol. 2004;6:138–145. doi: 10.1038/ncb1092. [DOI] [PubMed] [Google Scholar]
- 63.Ke PY, Chang ZF. Mitotic degradation of human thymidine kinase 1 is dependent on the anaphase-promoting complex/cyclosome-CDH1-mediated pathway. Mol. Cell. Biol. 2004;24:514–526. doi: 10.1128/MCB.24.2.514-526.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ke PY, et al. Control of dTTP pool size by anaphase promoting complex/cyclosome is essential for the maintenance of genetic stability. Genes Dev. 2005;19:1920–1933. doi: 10.1101/gad.1322905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li SJ, Hochstrasser M. The yeast ULP2 (SMT4) gene encodes a novel protease specific for the ubiquitin-like Smt3 protein. Mol. Cell. Biol. 2000;20:2367–2377. doi: 10.1128/mcb.20.7.2367-2377.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xhemalce B, et al. Role of the fission yeast SUMO E3 ligase Pli1p in centromere and telomere maintenance. EMBO J. 2004;23:3844–3853. doi: 10.1038/sj.emboj.7600394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dieckhoff P, et al. Smt3/SUMO and Ubc9 are required for efficient APC/C-mediated proteolysis in budding yeast. Mol. Microbiol. 2004;51:1375–1387. doi: 10.1046/j.1365-2958.2003.03910.x. [DOI] [PubMed] [Google Scholar]
- 68.Nacerddine K, et al. The SUMO pathway is essential for nuclear integrity and chromosome segregation in mice. Dev. Cell. 2005;9:769–779. doi: 10.1016/j.devcel.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 69.Joseph J, et al. The RanGAP1-RanBP2 complex is essential for microtubule-kinetochore interactions in vivo. Curr. Biol. 2004;14:611–617. doi: 10.1016/j.cub.2004.03.031. [DOI] [PubMed] [Google Scholar]
- 70.Stead K, et al. Pds5p regulates the maintenance of sister chromatid cohesion and is sumoylated to promote the dissolution of cohesion. J. Cell Biol. 2003;163:729–741. doi: 10.1083/jcb.200305080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Azuma Y, et al. PIASy mediates SUMO-2 conjugation of Topoisomerase-II on mitotic chromosomes. EMBO J. 2005;24:2172–2182. doi: 10.1038/sj.emboj.7600700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jacquiau HR, et al. Defects in SUMO (small ubiquitin-related modifier) conjugation and deconjugation alter cell sensitivity to DNA topoisomerase I-induced DNA damage. J. Biol. Chem. 2005;280:23566–23575. doi: 10.1074/jbc.M500947200. [DOI] [PubMed] [Google Scholar]
- 73.Honda Y, et al. Cooperation of HECT-domain ubiquitin ligase hHYD and DNA topoisomerase II-binding protein for DNA damage response. J. Biol. Chem. 2002;277:3599–3605. doi: 10.1074/jbc.M104347200. [DOI] [PubMed] [Google Scholar]
- 74.Jackson PK. Linking tumor suppression, DNA damage and the anaphase-promoting complex. Trends Cell Biol. 2004;14:331–334. doi: 10.1016/j.tcb.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 75.Castro A, et al. The anaphase-promoting complex: a key factor in the regulation of cell cycle. Oncogene. 2005;24:314–325. doi: 10.1038/sj.onc.1207973. [DOI] [PubMed] [Google Scholar]
- 76.Sorensen CS, et al. Chk1 regulates the S phase checkpoint by coupling the physiological turnover and ionizing radiation-induced accelerated proteolysis of Cdc25A. Cancer Cell. 2003;3:247–258. doi: 10.1016/s1535-6108(03)00048-5. [DOI] [PubMed] [Google Scholar]
- 77.Donzelli M, et al. Hierarchical order of phosphorylation events commits Cdc25A to βTrCP-dependent degradation. Cell Cycle. 2004;3:469–471. [PubMed] [Google Scholar]
- 78.Suzuki H, et al. Homodimer of two F-box proteins βTrCP1 or βTrCP2 binds to IκBα for signal-dependent ubiquitination. J. Biol. Chem. 2000;275:2877–2884. doi: 10.1074/jbc.275.4.2877. [DOI] [PubMed] [Google Scholar]
- 79.Kim SY, et al. Multisite M-phase phosphorylation of Xenopus Wee1A. Mol. Cell. Biol. 2005;25:10580–10590. doi: 10.1128/MCB.25.23.10580-10590.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Asano S, et al. Concerted mechanism of Swe1/Wee1 regulation by multiple kinases in budding yeast. EMBO J. 2005;24:2194–2204. doi: 10.1038/sj.emboj.7600683. [DOI] [PMC free article] [PubMed] [Google Scholar]