Synthesis of three advanced biofuels from ionic liquid-pretreated switchgrass using engineered Escherichia coli (original) (raw)
Abstract
One approach to reducing the costs of advanced biofuel production from cellulosic biomass is to engineer a single microorganism to both digest plant biomass and produce hydrocarbons that have the properties of petrochemical fuels. Such an organism would require pathways for hydrocarbon production and the capacity to secrete sufficient enzymes to efficiently hydrolyze cellulose and hemicellulose. To demonstrate how one might engineer and coordinate all of the necessary components for a biomass-degrading, hydrocarbon-producing microorganism, we engineered a microorganism naïve to both processes, Escherichia coli, to grow using both the cellulose and hemicellulose fractions of several types of plant biomass pretreated with ionic liquids. Our engineered strains express cellulase, xylanase, beta-glucosidase, and xylobiosidase enzymes under control of native E. coli promoters selected to optimize growth on model cellulosic and hemicellulosic substrates. Furthermore, our strains grow using either the cellulose or hemicellulose components of ionic liquid-pretreated biomass or on both components when combined as a coculture. Both cellulolytic and hemicellulolytic strains were further engineered with three biofuel synthesis pathways to demonstrate the production of fuel substitutes or precursors suitable for gasoline, diesel, and jet engines directly from ionic liquid-treated switchgrass without externally supplied hydrolase enzymes. This demonstration represents a major advance toward realizing a consolidated bioprocess. With improvements in both biofuel synthesis pathways and biomass digestion capabilities, our approach could provide an economical route to production of advanced biofuels.
Keywords: consolidated bioprocessing, ionic liquid pretreatment
The microbial conversion of sustainable lignocellulosic biomass into biofuels could provide a source of fully renewable transportation fuels (1). Generating these fuels from abundant feedstocks such as lignocellulose and cellulosic waste avoids many of the problems associated with current grain-based biofuels, provided the feedstock is responsibly grown and harvested (2). While early efforts toward achieving economical biofuel production have typically focused on improving yields of ethanol made from fermentation of plant sugars (3), recent advances in metabolic engineering have enabled microbial production of fuels that are compatible with existing engines and fuel distribution infrastructure (4, 5). Many of these advances have been made possible by the unparalleled genetic and metabolic tractability of the model bacterium Escherichia coli (6, 7). E. coli has been engineered to biosynthesize perhaps the most chemically diverse range of chemicals of any organism, including hydrogen (8), higher alcohols (9, 10), fatty-acid based chemicals (11), and terpenes (12, 13). Extensive knowledge of E. coli physiology will continue to aid improvements in titers beyond those achieved in proof-of-concept stages toward levels required for a commercial-scale biofuel production process.
Unfortunately, several challenges must be overcome before lignocellulose can be considered an economically competitive feedstock for biofuel production. One of the more significant challenges is the need for large quantities of glycoside hydrolase (GH) enzymes to efficiently convert lignocellulose into fermentable sugars. These enzymes are typically generated in a dedicated process that incurs substantial capital and material expense and represent the second highest contribution to raw material cost after the feedstock itself (1, 14). An alternative approach, known as consolidated bioprocessing, could potentially avoid the costs of a dedicated enzyme generation step by performing it in a combined process that includes biomass hydrolysis and fuel production (Fig. 1A) (15, 16). This can be achieved by incorporating both biomass-degrading and biofuel-producing capabilities into a single organism through genetic engineering. Several microorganisms have been engineered to ferment model cellulosic and hemicellulosic substrates directly into ethanol or other fuels (reviewed in refs. 15 and 17). For example, the yeast Saccharomyces cerevisiae (18) and the bacterium Klebsiella oxytoca (19) have been modified to convert phosphoric acid swollen cellulose (PASC) directly to ethanol without the addition of exogenous cellulase. However, PASC and similar model substrates are typically prepared using techniques that are neither suitable for actual plant biomass nor feasible on a large scale (20). Furthermore, no biofuel with the combustion properties of petrochemical fuels, which could be used directly in existing infrastructure, has been generated directly from unrefined lignocellulosic biomass.
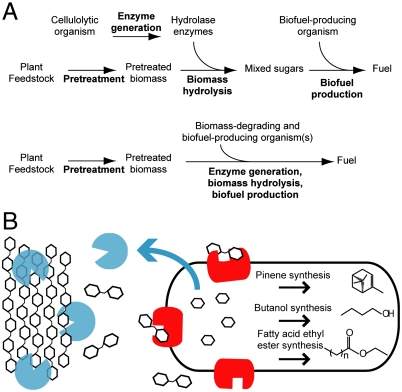
Fig. 1.
Consolidated bioprocessing of plant biomass into biofuels by E. coli. (A) Two processes for biofuel production. Typically, cellulase and hemicellulase enzymes are produced in a process step separate from biomass hydrolysis and biofuel production (top). Consolidated bioprocessing (bottom) combines enzyme generation, biomass hydrolysis, and biofuel production into a single stage. (B) Engineering E. coli for use in consolidated bioprocessing. Cellulose and hemicellulose are hydrolyzed by secreted cellulase and hemicellulose enzymes (cyan) into soluble oligosaccharides. β-glucosidase enzymes (red) further hydrolyze the oligosaccharides into monosaccharides, which are metabolized into biofuels via heterologous pathways.
A cellulolytic strain of E. coli capable of growth on plant biomass would be a first step toward producing many varieties of advanced biofuels at lowered cost. One obstacle to engineering E. coli for consumption of lignocellulose is the organism’s inferior capacity for protein export, which renders it unable to secrete cellulases in quantities required for industrial-scale lignocellulose hydrolysis. Various techniques, developed over decades of research, can be applied to generate secreted yields from E. coli of 0.5–0.8 g protein/L (21). Unfortunately, these concentrations are still too low for an industrial process, which are most efficient around levels of 20 mg cellulase/g solids and 200 g/L solids loading (22) [although recent work (23) has demonstrated that removal of soluble hydrolase inhibitors may substantially reduce the enzyme loading required]. To further engineer a cellulolytic E. coli strain for use in consolidated bioprocessing, biofuel production pathways must also be introduced and expressed at levels that yield high titers while not overburdening the cell. The integration of engineered cellulolytic capabilities together with pathways for advanced biofuel production into a single organism may present an insurmountable metabolic burden for E. coli, or indeed any microbe, without appropriate regulation.
We engineered E. coli to convert plant biomass into three advanced biofuels without the addition of exogenous GH enzymes (Fig. 1B). The carefully regulated expression of heterologous GH enzymes made suitable for export by E. coli allows rapid and efficient growth on model cellulosic and hemicellulosic substrates, as well as on the cellulose and hemicellulose components of raw plant biomass pretreated with ionic liquids (IL). IL pretreatment of plant biomass is a promising approach for enabling efficient biomass conversion (20). While the price of IL is currently a substantial barrier to commercialization, recent work has identified performance targets that could eventually enable adoption of this highly effective pretreatment technology (24). Unlike other pretreatment techniques, dissolution of plant biomass in IL nearly eliminates cellulose crystallinity and significantly decreases lignin content, thereby significantly decreasing the enzyme load required for hydrolysis (25). Our E. coli is capable of growing on the cellulose and hemicellulose fractions of several types of IL-pretreated plant biomass, even with low yields of secreted protein (< 0.1 mg enzymes/g solids). Furthermore, we show that cellulolytic and hemicellulolytic capabilities can be expressed with any of three distinct biofuel synthesis pathways in the same organism. By using cocultures of fuel-producing cellulolytic and hemicellulolytic strains, we demonstrate the production of fuel substitutes or precursors suitable for three engine types (gasoline, diesel, jet) directly from both the cellulose and hemicellulose components of IL-treated switchgrass. This represents a major advance toward combining the extensive biosynthetic capabilities of E. coli with lignocellulose utilization, while avoiding a dedicated process for enzyme generation, a substantial cost barrier to advanced biofuel production. Our results are a proof-of-concept that provides the foundation to further developments in both E. coli engineering and IL pretreatment that could eventually realize the cost savings achievable by consolidated bioprocessing. The modifications described here could likely be transplanted into other industrial microorganisms.
Results
The first step of lignocellulose metabolism is hydrolysis of cellulose and hemicellulose by secreted cellulase and hemicellulase enzymes, respectively (Fig. 1B). We found previously that the Clostridium stercorarium endoxylanase Xyn10B can be produced extracellularly by E. coli when fused with the protein OsmY (11), a fusion shown to enable protein export (26). To find a cellulase exportable by E. coli, we expressed a library of 10 family 5 endocellulases as fusions with OsmY (Table S1). Expression of two of the OsmY-cellulase fusions generated endocellulase activity in the growth medium (Fig. 2A and Fig. S1), with the Cel enzyme from Bacillus sp. D04 (cellulase #7) (27) demonstrating the highest activity. Both Cel and Xyn10B demonstrated activity against IL-treated switchgrass, indicating that enzymes expressed extracellularly by E. coli could potentially reduce or eliminate the need for exogenously added cellulolytic enzymes. Extracellular OsmY-Cel released glucose equivalent to 5% of the cellulose, producing cellotriose and cellobiose, while OsmY-Xyn10B hydrolyzed 11% of the xylan, mostly into xylotriose and xylobiose (Fig. S2). The combined biomass hydrolysis yield represents 8% of the total sugars available in the IL-treated switchgrass (28).
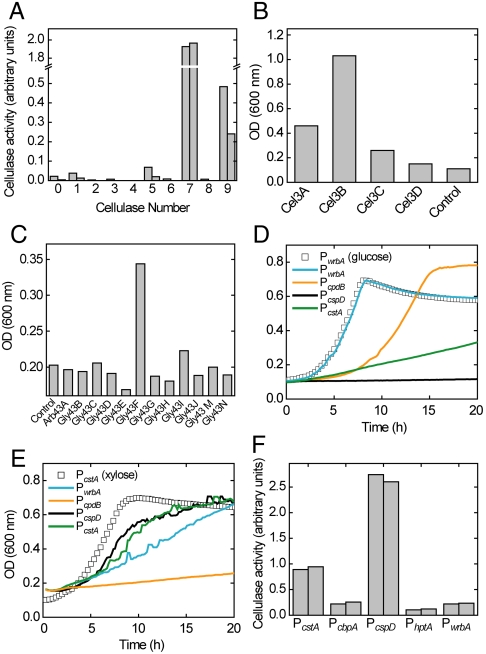
Fig. 2.
Assembling biological parts required for lignocellulose hydrolysis and consumption by E. coli. (A) Secretion of cellulases. Cellulases were expressed as fusions with the OsmY protein, and extracellular cellulase activity measured using an azo-CMC assay. Cellulase identities that correspond to the numbers used can be found in Table S1. Two measurements from each cellulase are shown. (B) Growth after 18 h in M9/0.2% cellobiose medium of E. coli expressing four β-glucosidases from Cellvibrio japonicus under control of the lacUV5 promoter. (C) Growth of E. coli in MOPS-M9/0.2% xylodextrins after 15 h, enabled by expression of xylobiosidases. (D) Growth curves on MOPS-M9/0.5% cellobiose medium when expressing β-glucosidase Cel3A under control of E. coli promoters. A growth curve on glucose is shown for comparison. (E) Growth curves on enzymatically hydrolyzed xylan of E. coli expressing the xylobiosidase Gly43F under control of E. coli promoters, with a growth curve on xylose for comparison. Each curve is an average of two separate experiments. For growth curves on glucose and xylose, half of the data points are omitted for clarity. (F) Extracellular endocellulase activity levels of cellulase #7 (Cel from _Bacillus sp._D04) when expressed under the control of several native E. coli promoters after 20 h of growth in LB medium. Measurements from biological duplicates are shown.
The soluble oligosaccharides that are produced by enzymatic hydrolysis of cellulose and xylan (cellodextrins and xylodextrins, respectively) cannot be metabolized by E. coli MG1655. To further hydrolyze cellodextrins into glucose, we screened four β-glucosidases cloned from Cellvibrio japonicus, a Gram-negative cellulolytic bacterium, and determined if their expression in E. coli could permit growth on cellobiose (Fig. 1B) (29, 30). E. coli grew best on cellobiose when expressing either cel3A or cel3B (Fig. 2B). To enable growth of E. coli on xylodextrins, the oligosaccharide products of xylan hydrolysis, we screened 12 xylobiosidase genes from C. japonicus (29). Expression of gly43F enabled growth on enzymatically hydrolyzed beechwood xylan (Fig. 2C).
The need for exogenously added chemicals to activate expression of biomass-consumption pathways might require extensive optimization of both the timing of induction and the induction strength, complicating engineering of biofuel generation from biomass. Therefore, we used native E. coli promoters to control expression of the selected β-glucosidase and xylobiosidase genes. This places expression of the biomass-consumption pathways under control of environmentally responsive promoters and avoids the costs of expensive chemical inducers for activation of the biomass-consumption pathways. We sought to achieve growth rates of oligosaccharide-utilizing E. coli that matched rates observed on the corresponding monosaccharide. Reasoning that expression of biomass-consumption pathways should be limited to periods when E. coli is starved of carbon (for instance, when the cells are freshly inoculated into biomass-containing medium from a glucose-based seed culture), we screened several promoters that have been shown to increase in transcriptional activity prior to stationary phase (31) or known to be activated by the gene regulator CRP (32). We expressed cel3A and cel3B using several native E. coli promoters to determine which promoter-enzyme combination would permit the fastest growth on cellobiose. Remarkably, a strain expressing cel3A under the control of the wrbA promoter (P wrbA) grew on cellobiose as fast as on glucose (Fig. 2D and Fig. S3). We screened the same set of promoters to optimize expression of gly43F as determined by growth on xylodextrins. We found that expression of gly43F using the promoters P cstA or P cspD enabled a growth rate on xylodextrins nearly as high as on xylose (Fig. 2E). Surprisingly, the use of native promoters to drive expression of appropriate β-glucosidase and xylobiosidase genes enables E. coli to grow on oligosaccharides at a rate limited only by the consumption rate of the monosaccharides and perhaps as fast as native cellulolytic organisms.
To express the complete biomass conversion pathways under native promoters rather than the chemically inducible promoter used to screen the cellulase library, we placed expression of the osmY-cel fusion under control of the members of our promoter library to determine which promoter generated the maximum extracellular cellulase yield. Expression of osmY-cel using the promoter for the cspD gene (P cspD) resulted in the highest cellulase activity of the promoters tested (Fig. 2F). We combined P _cspD_-_osmY_-cel with P _wrbA_-cel_3_A into a single plasmid designated pCellulose (Fig. 3A) to enable growth on cellulose. E. coli bearing pCellulose grew on the model substrate PASC as the sole carbon source (Fig. 3B), though growth on cellobiose was slowed relative to plasmids bearing P _wrbA_-cel_3_A alone (Fig. S4). In the same manner, we combined P _cspD_-_osmY_-xyn_10_B with P _cstA_-gly_43_F into a single plasmid, designated pXylan (Fig. 3A). Impressively, E. coli bearing pXylan grew on beechwood xylan, a model hemicellulosic substrate, at nearly the limit set by the consumption rate of xylose (Fig. 3C).
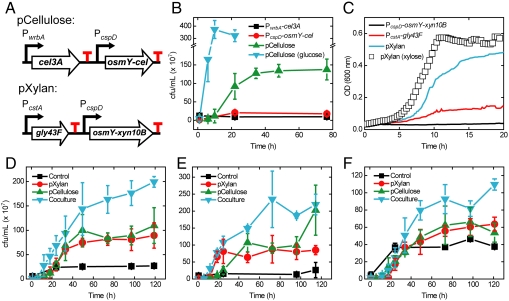
Fig. 3.
Engineered E. coli grows on model cellulosic substrates and IL-treated plant biomass. (A) Gene schematics for the pCellulose and pXylan plasmids, designed to enable E. coli to metabolize cellulose and xylan, respectively. (B) Growth on phosphoric acid swollen cellulose (PASC) monitored by serial dilution, plating, and colony counting. Cells expressing either Cel3A or OsmY-Cel alone, or containing the pCellulose plasmid, were grown in MOPS-M9/0.7% PASC. Growth of the pCellulose-bearing strain in MOPS-M9/0.4% glucose is shown for comparison. (C) Growth of strains expressing either Gly43F or OsmY-XynB alone, or bearing pXylan in MOPS-M9/0.5% beechwood xylan. Growth of pXylan-bearing strain in 0.5% xylose is shown for comparison. Each curve is an average of three separate growth experiments. (D_–_F) Growth on the cellulose and hemicellulose fractions of IL-treated switchgrass, eucalyptus, and yard waste, respectively. Error bars represent standard deviation of biological triplicates, except for yard waste control strain (biological duplicates).
We next attempted to grow E. coli bearing either pXylan or pCellulose on plant biomass treated with the ionic liquid 1-ethyl-3-methylimidazolium acetate [C2mim][OAc]. We inoculated E. coli MG1655 strains bearing pXylan, pCellulose, or a control plasmid into minimal medium containing 2.6% w/vol IL-treated switchgrass as the sole carbon source, without adding exogenous enzymes. The strains containing pXylan and pCellulose grew well, indicating that both the cellulose and hemicellulose components of the pretreated switchgrass can be used as carbon sources (Fig. 3D, red and green curves). The control strain showed minimal growth (Fig. 3D, black curve), indicating that most of the growth observed by the pCellulose and pXylan strains is enabled via enzymatic hydrolysis of cellulose and xylan, rather than any monosaccharides present in the switchgrass or released by pretreatment. The monocultures continued to produce up to 0.5 mg/L xylanase and cellulase enzymes during growth (Fig. S5). We also observed leakage of other cellular proteins into the growth medium, which may be a consequence of expressing fusions with a periplasmic protein (33) (Fig. S5). When the strains were combined and grown on switchgrass as a coculture, the cells grew to a cell density approximately equal to the sum of the individual monocultures (Fig. 3D, cyan curve), demonstrating growth on both fractions of switchgrass in one medium.
We tested growth on IL-pretreated Eucalyptus globulus to determine if IL pretreatment could render a range of lignocellulose types digestible by our engineered E. coli. Both pXylan and pCellulose monocultures and a coculture of the two strains grew well in minimal medium containing 4.0% w/vol IL-treated eucalyptus (Fig. 3E). Finally, we tested growth on IL-treated yard waste, a feedstock that could avoid the costs of growing dedicated energy crops while decreasing landfill usage (34). Once again, both monocultures and coculture grew in minimal medium containing 2.6% w/vol yard waste (Fig. 3F).
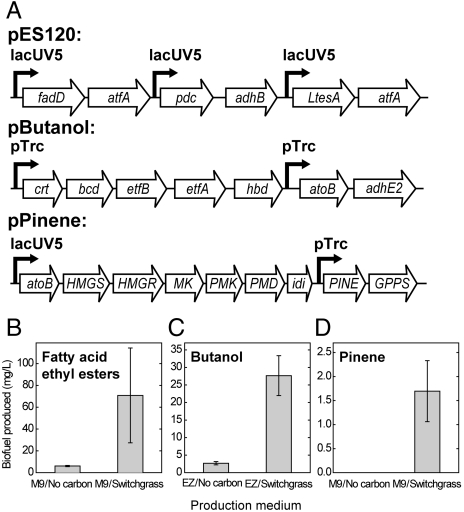
To demonstrate production of advanced biofuels from plant biomass without the use of exogenously added GH enzymes, we next engineered the biomass-consuming E. coli strains to generate three advanced biofuels directly from IL-treated switchgrass. We chose pathways that produce alcohols, linear hydrocarbons, or branched-chain hydrocarbons to test the integration of our biomass-consumption pathways with the extensive biosynthesis capabilities of E. coli. Biodiesel, typically made from plant oils that have been chemically esterified with methanol or ethanol, can also be made by E. coli in vivo in the form of fatty-acid ethyl esters (FAEE) (11). We encoded a six-gene FAEE production pathway on a single plasmid (pES120, Fig. 4A) and introduced the construct into a strain of E. coli MG1655 lacking the acyl-CoA dehydrogenase gene fadE. We found that this strain generates 405 ± 27 mg/L from MOPS-M9/1% glucose (10 g/L) (or 0.04 g FAEE/g glucose, 12% of the theoretical yield of 0.33 FAEE/g glucose) (11), and 0.022 g FAEE/g xylose from 10 g/L xylose. E. coli MG1655 Δ_fadE_ pES120 bearing pXylan or pCellulose produced FAEE from xylan or cellobiose, respectively (Fig. S6_A_), indicating that both strains are capable of FAEE production from their substrates. In order to produce FAEE from plant biomass, a coculture of both strains was grown in minimal medium containing 5.5% w/vol IL-treated switchgrass. The coculture produced 71 ± 43 mg/L of FAEE, well above the no-carbon control (6.1 ± 0.5 mg/L, Fig. 4B) and the noncellulolytic E. coli control (4 ± 3 mg/L), indicating production of FAEE directly from pretreated switchgrass. This corresponds to 80% of the estimated yield obtainable with this pathway from the amount of sugars anticipated to be released from 5.5% switchgrass by the Cel and Xyn10B enzymes (0.14% glucose and 0.14% xylose).
Fig. 4.
Conversion of IL-treated switchgrass into advanced biofuels. (A) Gene schematics of plasmids encoding biofuel production pathways demonstrated in this work. Gene names listed in Table S2. Production of fatty-acid ethyl esters (B), butanol (C), and pinene (D) from IL-treated switchgrass by cocultures of cellulose- and xylan-consuming E. coli. Error bars represent standard deviation of biological triplicates.
Butanol has been proposed as a gasoline replacement because it is fully compatible with existing internal combustion engines. Based in part on previous work (9), we constructed a heterologous butanol pathway encoded on a single plasmid (pButanol, Fig. 4A) and inserted into an E. coli DH1 strain lacking the alcohol dehydrogenase gene, adhE. When bearing either pXylan or pCellulose, E. coli DH1 Δ_adhE_ pButanol produced butanol from either xylan or cellobiose, respectively (Fig. S6_B_). A coculture of both strains yielded 28 ± 5 mg/L butanol from defined rich medium containing 3.3% w/vol IL-treated switchgrass as the main carbon source (Fig. 4C). A control strain lacking pXylan or pCellulose produced 8 ± 2 mg/L butanol from pretreated switchgrass.
Finally, we constructed a metabolic pathway to produce the monoterpene pinene, an immediate chemical precursor to a potential jet fuel (35), directly from switchgrass. The pinene synthesis pathway was encoded on a single plasmid (pPinene, Fig. 4A) and introduced into E. coli MG1655. We combined pXylan and pCellulose into separate strains of E. coli MG1655 pPinene and confirmed that each strain is capable of producing pinene from either xylan or cellobiose, respectively (Fig. S6_C_). We inoculated the strains as a coculture into MOPS-M9 medium containing either 3.9% IL-treated switchgrass or no carbon source. The pinene pathway yielded 1.7 ± 0.6 mg/L pinene from pretreated switchgrass (Fig. 4D). No pinene was produced from a culture grown in MOPS-M9 medium without a carbon source or from switchgrass medium inoculated with a strain lacking pXylan or pCellulose.
Discussion
We have demonstrated the engineering of E. coli to produce three advanced biofuels suitable for existing fuel infrastructure directly from lignocellulosic plant biomass without using externally supplied GH enzymes. While our engineering was greatly facilitated by the tractability of E. coli, the approach we have described here could be readily adapted for other microorganisms for use in a consolidated bioprocess to generate advanced biofuels from biomass. IL pretreatment using [C2mim][OAc] rendered three types of lignocellulose suitable for use by our strains as sole carbon sources, indicating that our system is likely applicable to lignocellulose feedstocks that are ecologically and economically appropriate to grow and harvest anywhere in the world. Overall, our results illustrate that the wide portfolio of compounds that can be synthesized by E. coli, or any other microorganism, can be produced directly from any IL-pretreated plant feedstock.
In order to make our E. coli strains suitable for use in an industrial bioprocess, both biofuel-producing and biomass-degrading capabilities require significant improvements. For instance, an optimal strain capable of producing FAEE at theoretical yield (0.33 g FAEE/ 1 g glucose) and achieving complete hydrolysis of IL-treated switchgrass, of which 78% is cellulose and xylan by weight (28), would obtain 0.26 g FAEE/ 1 g of IL-treated switchgrass, far higher than what could be achieved here. This requires an eightfold improvement in biofuel yield from glucose over what the current FAEE production pathway can achieve. More relevant to the engineered cellulolytic capabilities described in this work, the cellulose and hemicellulose fractions (95% and 89%, respectively) not digested by the enzymes we use here must be saccharified by the E. coli strain to achieve high biofuel yields or even to reach cell densities typical of an industrial fermentation process. A wide variety of enzymes found to have activity against IL-treated plant biomass was recently found in a cow rumen metagenome (36), and these enzymes could be screened to find either replacements for or supplements to the hydrolysis activity of Cel and Xyn10B enzymes. Furthermore, protein export pathways that do not compromise the cell membrane (as our OsmY fusions may be doing) should be used instead to avoid compromising cellular fitness and biofuel yields. Along with chromosomal integration of the biomass-consumption pathways (as opposed to encoding the pathways on plasmids), these steps should also improve the genetic stability of our modifications as well as their suitability for industrial-scale fermentations. In parallel with optimizations of the E. coli strain, the IL pretreatment could be modified to render the lignocellulose completely susceptible to hydrolysis by the GH enzymes we used here. For instance, acid catalysis during IL pretreatment of cellulose has been shown to dramatically increase the extent of subsequent enzymatic hydrolysis (37). These improvements will be required to fully realize the cost savings of a consolidated bioprocess that could provide a versatile platform for producing any advanced biofuel from any plant biomass at economical yields.
Materials and Methods
Selection, Optimization, and Screening of Cellulase Genes.
The set of cellulases was chosen to maximize diversity within family 5 endocellulases. First, the CAZy database was used to collate all known family 5 enzymes (38). At the time this work was done, there were 689 such enzymes in the database. The enzymes were aligned using Muscle (39) and then 10 were selected to maximize diversity using HyperTree (40). The 10 genes were then optimized for expression in E. coli using GeneDesigner and synthesized by DNA 2.0 (41). Two cultures each of E. coli DH10B cells bearing pGB012 plasmids encoding each individual OsmY-cellulase fusion were grown overnight in LB medium supplemented with 100 μg/mL carbenicillin and inoculated 1/100 into fresh LB. Cultures bearing pGB012 were used as a cellulase-free control. Cultures were grown at 37 °C to an optical density at 600 nm (OD600) of 0.4 and induced by addition of IPTG to 200 μM, and expression proceeded at 37 °C for 20 h. As described in SI Text, 200 μL of the supernatant was assayed for endocellulase activity.
Measurement of Native Promoter-Driven Cellulase Secretion.
E. coli MG1655 bearing plasmids with osmY-cel under control of several E. coli promoters was grown in LB medium (100 μg/mL carbenicillin) for 20 h before endocellulase activity present in the supernatant was measured.
Beta-Glucosidase Screening and Native Promoter Selection.
E. coli BL21 bearing beta-glucosidase genes was grown overnight in LB medium with 100 μg/mL carbenicillin, transferred 1/100 into M9/0.2% cellobiose medium with 100 μg/mL carbenicillin, and allowed to grow for 18 h at 37 °C before OD measurements were taken. A cell line bearing a plasmid with a beta-xylosidase was used as a control. For Cel3B-native promoter screening, plasmids bearing Cel3B under control of several E. coli promoters were introduced into E. coli BL21 cells, and transformants were grown in LB medium with 100 μg/mL carbenicillin overnight and inoculated 1/25 into a 96-well plate with 200 μL of M9/0.2% cellobiose medium or M9/0.2% glucose medium with 200 μg/mL carbenicillin. Growth was monitored with a microplate incubator and reader (TECAN). For Cel3A-native promoter screening, plasmids bearing Cel3A under control of one of several promoters were introduced into MG1655 cells, and overnight cultures were inoculated 1/40 into 800 μL of MOPS-M9/0.5% cellobiose or MOPS-M9/0.5% dextrose with 100 μg/mL carbenicillin in a 24-well plate. Growth was monitored with a microplate incubator and reader (TECAN).
Beta-Xylosidase Screening and Native Promoter Selection.
E. coli DH10B carrying beta-xylosidase genes under control of P cspD was grown overnight in LB medium with 100 μg/mL carbenicillin. The cultures did not grow at similar rates, likely due to the expression of proteins at toxic levels. Cultures were inoculated into MOPS-M9/0.2% xylan with 0.5 μg/mL thiamin and 100 μg/mL carbenicillin, into which sterile LB containing secreted OsmY-Xyn10B had been added (1/10 volume) to hydrolyze the xylan into xylodextrins. Growth was monitored on a 96-well plate with a microplate reader (TECAN). For Gly43F-native promoter screening, plasmids carrying gly43F under control of several E. coli promoters were introduced into E. coli MG1655 cells. Cells were grown overnight in LB medium with 100 μg/mL carbenicillin and inoculated 1/40 into 800 μL of MOPS-M9/0.5% beechwood xylan or xylose with 100 μg/mL carbenicillin supplemented with 5% of sterile LB containing secreted OsmY-Xyn10B on a 24-well plate. Growth was monitored with a microplate reader.
Growth Measurement on Beechwood Xylan.
Biological triplicates of E. coli MG1655 cells carrying either pXylan, pP _cstA_-gly_43_F/p_15_A, or pP _cspD_-_osmY_-xyn_10_B/_SC_101∗∗ was inoculated into LB with 100 μg/mL carbenicillin and grown at 37 °C for 16 h. Overnight cultures were inoculated 1/20 into 800 μL MOPS-M9/0.5% xylan or 0.5% xylose medium with 100 μg/mL carbenicillin and grown with shaking in a microplate reader (TECAN) at 37 °C. Curves shown are averages of the triplicates, and median-averaged over a five-point window.
Growth Curves on Biomass and PASC.
For biomass medium, IL-treated biomass (prepared as described in SI Text) was washed with water to remove any growth inhibitors present (such as residual IL). A full description of washing procedure can be found in SI Text. We used 10 mL of MOPS-M9 with biomass and 100 μg/mL carbenicillin as growth medium. For growth on PASC, triplicates of E. coli MG1655 bearing plasmids pCellulose, pP _wrbA_-cel_3_A/p_15_A, or p(P _cspD_-_RBS_3-_osmY_-cel/_SC_101∗∗) were grown overnight in LB with 100 μg/mL carbenicillin. For growth on plant biomass, E. coli MG1655 bearing either plasmid pXylan, pCellulose, or a control plasmid were grown for 18 h at 37 °C in LB medium containing 100 μg/mL carbenicillin. For growth of monocultures on biomass, the biomass medium was inoculated 1/20 (0.5 mL) with either the control, pXylan, or pCellulose cultures. For growth of pXylan/pCellulose cocultures, the biomass medium was inoculated with 0.25 mL pXylan and pCellulose cultures. All growth curves were performed with biological triplicates (three different colonies), with the exception of the yard waste control culture, which was performed in duplicate. Growth was measured by serially diluting a sample 10-6 (2 μL in 200 μL three times) in sterile phosphate-buffered saline, and 100 μL of the 10-6 dilution was spread on an LB-agar plate. Colonies were counted the next day.
Conversion of Switchgrass to FAEE.
Three aliquots of 5 mL MOPS-M9 medium containing either 5.5% sterilized washed switchgrass or no carbon source were prepared and inoculated 1/20 with cultures of E. coli MG1655 Δ_fadE_ pES120 with either pXylan or pCellulose (or 1/10 with control culture) grown for 24 h in LB medium. Cultures were grown at 37 °C for 92 h, at which point FAEE production was induced by addition of 50 μM IPTG. The production cultures were left at room temperature for 4 h after induction and returned to 37 °C for 96 h of production time. Free fatty-acid ethyl esters, and free fatty acids, were measured largely as described in ref. 11.
Conversion of Switchgrass to Butanol.
Twelve cultures of 5-mL EZ-Rich medium (Teknova) were prepared as described by the manufacturer except without glucose. Six of the cultures contained 3.3% (w/vol) washed, IL-treated switchgrass. E. coli DH1 Δ_adhE_ pButanol carrying pCelluose or pXylan was grown in LB medium for 38 or 25 h, respectively, at 37 °C. Biomass and null media were inoculated with 0.25 mL of each culture (0.5 mL total inoculum size) and cultures moved to 37 °C for 6.5 h, after which 2 mL of EZ-Rich salts (to final concentration, including original formulation, of 2X) was added to the cultures. Cultures were grown at 30 °C and induced after 30 min by addition of 200 μM IPTG, sealed with parafilm (creating a microaerobic environment), and returned to 30 °C for 96 h. Butanol was extracted and quantified as described in SI Text.
Conversion of Switchgrass to Pinene.
Twelve aliquots of 5 mL MOPS-M9 medium, six of which contained 3.9% (w/vol) washed switchgrass, were prepared. Three overnight cultures each of E. coli MG1655/pPinene carrying a control plasmid, pXylan or pCellulose were grown for 24 h in LB medium. 0.5 mL of control culture, or 0.25 mL of both pXylan and pCellulose, was added to the switchgrass and null media and the cultures grown at 37 °C. After 22 h, pinene production was induced by addition of 200 μM IPTG, 0.55 mL dodecane was added to trap the pinene, and the cultures were incubated to 30 °C for 72 h. Extraction and quantification of pinene was performed as described in SI Text.
Supplementary Material
Supporting Information
Acknowledgments.
We thank our colleagues at Joint BioEnergy Institute and S. del Cardayre and B. da Costa at LS9 for assistance with all aspects of this project. E. Luning and J. Gin helped with primer design, M. Ouellet assisted with GC-MS measurements, and H. Burd assisted with HPLC analysis. We thank Dr. Ken Vogel at the United States Department of Agriculture, Lincoln, NE, for supplying switchgrass and Arborgen for supplying Eucalyptus globulus. This work was supported by the University of California Discovery Grant program and LS9; the Joint BioEnergy Institute (www.jbei.org) supported by the U.S. Department of Energy, Office of Science, Office of Biological and Environmental Research, through contract DE-AC02-05CH11231 between Lawrence Berkeley National Laboratory and the U.S. Department of Energy; and the Synthetic Biology Engineering Research Center. C.V. and D.T.E. were supported by National Science Foundation Grants BES-0547637 and SynBERC, National Institutes of Health Grant AI67699, Amryis Biotechnologies, DNA 2.0, and UC-Discovery Grant bio05-10556.
Footnotes
Conflict of interest statement: J.K. and C.V. have financial interests in Amyris, and J.K. has a financial interest in LS9 and Lygos.
This article is a PNAS Direct Submission.
References
- 1.National Research Council (U.S.) Liquid Transportation Fuels from Coal and Biomass:Technological Status, Costs, and Environmental Impacts. Washington: Natl Academies Press; 2009. Panel on alternative liquid transportation fuels; p. 370. [Google Scholar]
- 2.Tilman D, et al. Beneficial biofuels—the food, energy, and environment trilemma. Science. 2009;325:270–271. doi: 10.1126/science.1177970. [DOI] [PubMed] [Google Scholar]
- 3.Ingram LO, et al. Enteric bacterial catalysts for fuel ethanol production. Biotechnol Prog. 1999;15:855–866. doi: 10.1021/bp9901062. [DOI] [PubMed] [Google Scholar]
- 4.Fortman JL, et al. Biofuel alternatives to ethanol: pumping the microbial well. Trends Biotechnol. 2008;26:375–381. doi: 10.1016/j.tibtech.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 5.Keasling JD. Manufacturing molecules through metabolic engineering. Science. 2010;330:1355–1358. doi: 10.1126/science.1193990. [DOI] [PubMed] [Google Scholar]
- 6.Atsumi S, Liao JC. Metabolic engineering for advanced biofuels production from Escherichia coli. Curr Opin Biotechnol. 2008;19:414–419. doi: 10.1016/j.copbio.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clomburg JM, Gonzalez R. Biofuel production in Escherichia coli: The role of metabolic engineering and synthetic biology. Appl Microbiol Biotechnol. 2010;86:419–434. doi: 10.1007/s00253-010-2446-1. [DOI] [PubMed] [Google Scholar]
- 8.Maeda T, Sanchez-Torres V, Wood TK. Enhanced hydrogen production from glucose by metabolically engineered Escherichia coli. Appl Microbiol Biotechnol. 2007;77:879–890. doi: 10.1007/s00253-007-1217-0. [DOI] [PubMed] [Google Scholar]
- 9.Atsumi S, et al. Metabolic engineering of Escherichia coli for 1-butanol production. Metab Eng. 2008;10:305–311. doi: 10.1016/j.ymben.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 10.Atsumi S, Hanai T, Liao JC. Non-fermentative pathways for synthesis of branched-chain higher alcohols as biofuels. Nature. 2008;451:86–89. doi: 10.1038/nature06450. [DOI] [PubMed] [Google Scholar]
- 11.Steen EJ, et al. Microbial production of fatty-acid-derived fuels and chemicals from plant biomass. Nature. 2010;463:559–562. doi: 10.1038/nature08721. [DOI] [PubMed] [Google Scholar]
- 12.Peralta-Yahya PP, et al. Identification and microbial production of a terpene-based advanced biofuel. Nat Commun. 2011;2:483. doi: 10.1038/ncomms1494. 10.1038/ncomms1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang C, et al. Farnesol production from Escherichia coli by harnessing the exogenous mevalonate pathway. Biotechnol Bioeng. 2010;107:421–429. doi: 10.1002/bit.22831. [DOI] [PubMed] [Google Scholar]
- 14.Blanch HW, Klein-Marcuschamer D, Oleskowicz-Popiel P, Simmons BA. Technoeconomic analysis of biofuels: A wiki-based platform for lignocellulosic biorefineries. Biomass Bioenergy. 2010;34:1914–1921. [Google Scholar]
- 15.Lynd LR, van Zyl WH, McBride JE, Laser M. Consolidated bioprocessing of cellulosic biomass: An update. Curr Opin Biotechnol. 2005;16:577–583. doi: 10.1016/j.copbio.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 16.Lynd LR, Weimer PJ, van Zyl WH, Pretorius IS. Microbial cellulose utilization: Fundamentals and biotechnology. Microbiol Mol Biol Rev. 2002;66:506–577. doi: 10.1128/MMBR.66.3.506-577.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.la Grange DC, den Haan R, van Zyl WH. Engineering cellulolytic ability into bioprocessing organisms. Appl Microbiol Biotechnol. 2010;87:1195–1208. doi: 10.1007/s00253-010-2660-x. [DOI] [PubMed] [Google Scholar]
- 18.Den Haan R, Rose SH, Lynd LR, van Zyl WH. Hydrolysis and fermentation of amorphous cellulose by recombinant Saccharomyces cerevisiae. Metab Eng. 2007;9:87–94. doi: 10.1016/j.ymben.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 19.Zhou SD, Ingram LO. Simultaneous saccharification and fermentation of amorphous cellulose to ethanol by recombinant Klebsiella oxytoca SZ21 without supplemental cellulase. Biotechnol Lett. 2001;23:1455–1462. [Google Scholar]
- 20.Alvira P, Tomas-Pejo E, Ballesteros M, Negro MJ. Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: A review. Bioresour Technol. 2010;101:4851–4861. doi: 10.1016/j.biortech.2009.11.093. [DOI] [PubMed] [Google Scholar]
- 21.Georgiou G, Segatori L. Preparative expression of secreted proteins in bacteria: Status report and future prospects. Curr Opin Biotechnol. 2005;16:538–545. doi: 10.1016/j.copbio.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 22.Stickel JJ, Roche CM, Dibble CJ, Knutsen JS, Liberatore MW. Particle concentration and yield stress of biomass slurries during enzymatic hydrolysis at high-solids loadings. Biotechnol Bioeng. 2009;104:290–300. doi: 10.1002/bit.22381. [DOI] [PubMed] [Google Scholar]
- 23.Ladisch MR, Kim Y, Ximenes E, Mosier NS. Soluble inhibitors/deactivators of cellulase enzymes from lignocellulosic biomass. Enzyme Microb Technol. 2011;48:408–415. doi: 10.1016/j.enzmictec.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 24.Klein-Marcuschamer D, Simmons BA, Blanch HW. Techno-economic analysis of a lignocellulosic ethanol biorefinery with ionic liquid pre-treatment. Biofuel Bioprod Bior. 2011;5:562–569. [Google Scholar]
- 25.Li CL, et al. Comparison of dilute acid and ionic liquid pretreatment of switchgrass: Biomass recalcitrance, delignification and enzymatic saccharification. Bioresour Technol. 2010;101:4900–4906. doi: 10.1016/j.biortech.2009.10.066. [DOI] [PubMed] [Google Scholar]
- 26.Qian ZG, Xia XX, Choi JH, Lee SY. Proteome-based identification of fusion partner for high-level extracellular production of recombinant proteins in Escherichia coli. Biotechnol Bioeng. 2008;101:587–601. doi: 10.1002/bit.21898. [DOI] [PubMed] [Google Scholar]
- 27.Han SJ, Yoo YJ, Kang HS. Characterization of a bifunctional cellulase and its structural gene—the Cel gene of Bacillus Sp D04 has exoglucanase and endoglucanase activity. J Biol Chem. 1995;270:26012–26019. doi: 10.1074/jbc.270.43.26012. [DOI] [PubMed] [Google Scholar]
- 28.Singh S, et al. Monitoring and analyzing process streams towards understanding ionic liquid pretreatment of switchgrass (Panicum virgatum L.) Bioenergy Research. 2010;3:134–145. [Google Scholar]
- 29.Deboy RT, et al. Insights into plant cell wall degradation from the genome sequence of the soil bacterium Cellvibrio japonicus. J Bacteriol. 2008;190:5455–5463. doi: 10.1128/JB.01701-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rixon JE, et al. Characterization of the gene celd and its encoded product 1,4-beta-D-glucan glucohydrolase-D from Pseudomonas-fluorescens subsp cellulosa. Biochem J. 1992;285:947–955. doi: 10.1042/bj2850947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zaslaver A, et al. A comprehensive library of fluorescent transcriptional reporters for Escherichia coli. Nat Methods. 2006;3:623–628. doi: 10.1038/nmeth895. [DOI] [PubMed] [Google Scholar]
- 32.Keseler IM, et al. EcoCyc: A comprehensive view of Escherichia coli biology. Nucleic Acids Res. 2009;37:D464–D470. doi: 10.1093/nar/gkn751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Georgiou G, Shuler ML, Wilson DB. Release of periplasmic enzymes and other physiological-effects of beta-lactamase overproduction in Escherichia-coli. Biotechnol Bioeng. 1988;32:741–748. doi: 10.1002/bit.260320603. [DOI] [PubMed] [Google Scholar]
- 34.Antizar-Ladislao B, Turrion-Gomez JL. Second-generation biofuels and local bioenergy systems. Biofuels Bioprod Bior. 2008;2:455–469. [Google Scholar]
- 35.Harvey BG, Wright ME, Quintana RL. High-density renewable fuels based on the selective dimerization of pinenes. Energy Fuels. 2010;24:267–273. [Google Scholar]
- 36.Hess M, et al. Metagenomic discovery of biomass-degrading genes and genomes from cow rumen. Science. 2011;331:463–467. doi: 10.1126/science.1200387. [DOI] [PubMed] [Google Scholar]
- 37.Rinaldi R, Engel P, Buchs J, Spiess AC, Schuth F. An integrated catalytic approach to fermentable sugars from cellulose. Chemsuschem. 2010;3:1151–1153. doi: 10.1002/cssc.201000153. [DOI] [PubMed] [Google Scholar]
- 38.Cantarel BL, et al. The Carbohydrate-Active EnZymes database (CAZy): An expert resource for glycogenomics. Nucleic Acids Res. 2009;37:D233–D238. doi: 10.1093/nar/gkn663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Edgar RC. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bingham J, Sudarsanam S. Visualizing large hierarchical clusters in hyperbolic space. Bioinformatics. 2000;16:660–661. doi: 10.1093/bioinformatics/16.7.660. [DOI] [PubMed] [Google Scholar]
- 41.Villalobos A, Ness JE, Gustafsson C, Minshull J, Govindarajan S. Gene Designer: a synthetic biology tool for constructing artificial DNA segments. BMC Bioinformatics. 2006;7:285. doi: 10.1186/1471-2105-7-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information