Cell death throes (original) (raw)
Living cells come equipped with a highly efficient death machinery that is readily activated when they reach their duly allotted life span or are deemed to be ineffectual, redundant, or damaged. The existence of a genetic death program was first inferred from the ordered morphologic changes revealed by electron micrographs of dying cells from organisms as diverse as amphibians and humans (1). The cell’s death throes are evidenced by compaction of the chromatin, which collapses against the nuclear envelope. The cell then shrinks, undergoing vigorous membrane blebbing—a dance of death—before finally breaking up into multiple membrane-enclosed vesicles (apoptotic bodies) that are engulfed by neighboring cells. Death is fast, 30–60 min, and clean—there is no leakage of intracellular contents, and inflammatory responses therefore are avoided.
Mysterious for decades, the biochemical pathways underlying this death program now are rapidly coming into focus. Key conjectures made from cancer and developmental genetics are being confirmed by structural studies and by gene targeting in mice. Surprisingly, at least one cardinal feature of cell death—DNA cleavage—appears to be dispensable, as reported in this issue of the Proceedings by Zhang et al. (2).
Degradation of DNA was in fact the biochemical marker of apoptosis first recognized. Scission occurs at internucleosomal sites and the resulting characteristic DNA “ladder” became the hallmark of apoptosis (3, 4). Over the years several nucleases have been indicted as candidate effectors, but no consensus emerged as to which, if any, was the culprit. Although DNA cleavage initially was regarded as the crucial event initiating apoptosis, it now is recognized to be a downstream consequence (see below).
The central engine of apoptosis is in fact a family of highly conserved proteases. The founder is Ced-3, the product of one of three genes (ced-3, _ced-4, and egl-1) essential for developmental cell deaths in_Caenorhabditis elegans (5, 6). It is homologous to a family of mammalian proteases with the unusual specificity of cleaving after aspartic acid (7–10). Like Ced-3, many of these mammalian caspases play a vital role in apoptosis, although some instead regulate inflammation.
To preclude accidental cell suicide, caspases are synthesized as zymogens having little catalytic activity. They are activated by proteolytic scissions that release the N-terminal pro-domain and a large (≈p20) and small (≈p10) subunit. Importantly, the cleavage sites themselves are caspase consensus sites, implying that activation is accomplished by autocatalysis, by previously activated caspases, or by other enzymes of similar specificity (only one such is known, granzyme B, which is introduced into target cells by killer T cells). The picture that emerges, therefore, is that apoptosis is set in train by a caspase cascade.
How do the caspases choreograph the dance of death? Individual caspases have different recognition sites, which include at least three amino acids preceding the aspartic acid (11), but only a relatively restricted set of proteins is cleaved. In addition to the other caspases, some 40 or so potential victims have been identified (10), but the relevance of many to the apoptotic phenotype has yet to be established.
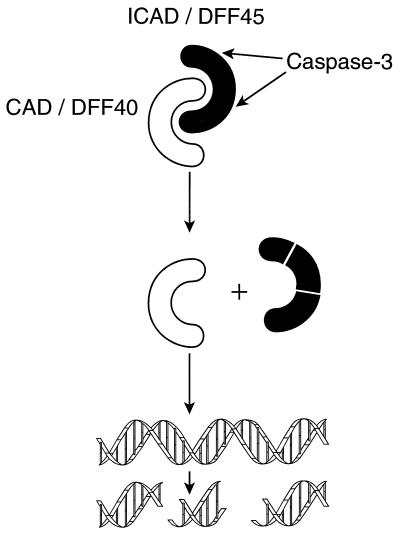
One of the clearest targets is an inhibitor of the nuclease responsible for chromatin cleavage. By using an assay in which nuclei were incubated with cytoplasmic extracts of Hela cells plus active recombinant caspase, Wang and his colleagues (12) purified a DNA fragmentation factor (DFF) activated by caspase-3. A similar complex was isolated by Nagata’s group (13) from mouse cells. Healthy cells contain a heterodimer comprised of a 40-kDa nuclease (DFF40 or CAD, caspase activated DNase) complexed to a 45-kDa inhibitor (DFF45 or ICAD, inhibitor of CAD). Caspase-3 activates the nuclease by cleaving the inhibitor at two sites strongly conserved between the mouse and human proteins (Fig. 1) (12–15).
Figure 1.
Regulation of DNA fragmentation during apoptosis. ICAD/DFF45 binds tightly to the nuclease CAD/DFF40 and inhibits it. When caspase-3 is activated, it cleaves ICAD/DFF45, thereby releasing CAD/DFF40 to cleave DNA.
Intriguingly, CAD/DFF40 synthesized in the absence of ICAD/DFF45 is inactive, suggesting that ICAD acts as a chaperone for correct folding and that the complex is formed cotranslationally (13, 14). This double safety catch ensures that cells are protected even if CAD/DFF40 production exceeds that of ICAD/DFF45. It now appears that the complex is nuclear in intact cells, and the observation that CAD nuclease activity is stimulated by high mobility group protein and histone 1, but not core histones, provides a rationale for internucleosomal DNA degradation (14).
To clarify further the role of DFF in chromosomal fragmentation, Zhang_et al._ (2) have generated mice with a nonfunctional ICAD/DFF45 gene. Surprisingly, homozygous mutants were born in normal Mendelian ratios and were healthy and fertile, with no obvious abnormalities. As expected, no DFF activity could be detected in lysates of tissues from these mice. Moreover, splenocytes and thymocytes exposed for 10 hr to cytotoxic agents such as etoposide and staurosporine exhibited no DNA fragmentation, although some became apparent by 24 hr. Chromatin condensation in the mutant cells was severely impaired.
These experiments suggest that ICAD/DFF45 is the principal regulator of DNA fragmentation during apoptosis and that the cleavage initiates chromatin condensation. Further work is needed to determine the basis for the low level of DNA degradation achievable in its absence. Perhaps CAD/DFF40 synthesized in the absence of its chaperone possesses some residual nuclease activity. Alternatively, there may a redundant, but slower-acting, mechanism for DNA degradation.
Surprisingly, DNA degradation may not be the essential coup de grace sometimes imagined. All major organs of the ICAD/DFF45 mutant mice appeared normal, even lympho-myeloid tissues, which are known to undergo substantial apoptosis during development and differentiation. Furthermore, apart from a small increase in circulating granulocytes, there was no significant change in either the total numbers or relative proportions of the major hematopoietic cell types. The apparently normal phenotype suggests that DNA degradation is dispensable during apoptosis of mammalian cells. This inference had been made earlier from mutant cell lines that remained susceptible to apoptosis even though unable to undergo genome digestion (16, 17) and from the observation that enucleated cells exposed to cytotoxic agents undergo many of the key morphological features of apoptosis (18, 19). A similar conclusion had been reached from_nuc-1_ (nuclease abnormal) mutants of C. elegans, in which cells die and are engulfed normally even though their DNA remains undegraded (20). Thus DNA degradation appears to be an expendable luxury, although it presumably facilitates efficient disposal of the cellular corpse and would deter any inadvertent gene transfer to the phagocytosing cells (17) or autoimmunity provoked by anti-DNA antibodies.
Caspase-3 may be the sole caspase controlling ICAD/DFF. In MCF-7 breast carcinoma cells, which lack caspase-3 owing to an inactivating mutation, tumor necrosis factor, and staurosporine provoke apoptosis without DNA fragmentation (or shrinkage and blebbing), and these features can be restored by transfection of the cells with a functional caspase-3 gene (21). Furthermore, on exposure to cytotoxic stimuli, a variety of cell types derived from_caspase-3_ −/− mice fail to undergo chromatin condensation or DNA degradation, though the cells do die with other hallmarks of apoptosis (22).
What in turn controls caspase-3? Evidence is accumulating that effector caspases such as caspase-3, -6, and -7 are activated by a distinct group of activator caspases whose activity is, in turn, triggered by apoptotic signals that promote their aggregation and autocatalysis via adaptor and cofactor proteins (10, 23–27).
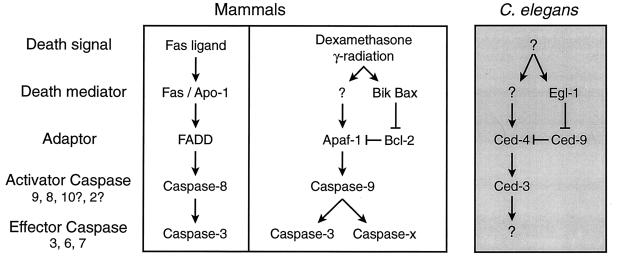
Two principal caspase pathways have emerged in mammalian cells (Fig.2). One involves ligand-induced oligomerization of plasma membrane-bound “death receptors,” such as Fas/Apo-1 and other members of the tumor necrosis factor receptor family. This pathway activates caspase-8 via the adaptor protein FADD (Fas-associated death domain; also called Mort-1) (28). A range of other death signals, such as dexamethasone and γ radiation, instead activate a second pathway, involving caspase-9, cytochrome_c,_ and Apaf-1 (29), a mammalian homolog of the adaptor protein encoded by the C. elegans death gene_ced-4_ (26, 29).
Figure 2.
Pathways to cell death in C. elegans and mammals (see text).
The apoptosis antagonist Bcl-2 fails to block Fas/Apo-1-induced death, at least in lymphocytes, so the caspase-8-dependent death pathway triggered by death receptors appears to bypass the step controlled by Bcl-2 and its homologs (30–32). In contrast, the mammalian Apaf-1-caspase-9 pathway and the analogous Ced-4-Ced-3 pathway in C. elegans are held in check by Bcl-2 and Ced-9, respectively (Fig. 2) (33–38). Proapoptotic cousins of Bcl-2 such as Bax, or more distant relatives such as Bik, may act by heterodimerizing with Bcl-2, thereby releasing Apaf-1 for its death duties. Likewise, Egl-1 interacts with Ced-9, releasing Ced-4 to activate Ced-3.
Gene targeting strongly supports the existence of the two mammalian pathways outlined in Fig. 2. Thus fibroblasts derived from_caspase-8_ −/− mice were resistant to tumor necrosis factor and to agonistic antibodies reactive with the death receptors Fas/Apo-1 and DR3, but remained sensitive to a range of other agents such as UV radiation, etoposide, and staurosporine (39), and the converse held for caspase-9 −/− fibroblasts (40). Moreover, caspase-9 −/− thymocytes were refractory to dexamethasone and γ radiation but remained sensitive to Fas-induced death (40, 41). There appear to be at least two classes of caspase-9-dependent pathways. In embryonic stem (ES) cells and neuronal progenitor cells, caspase-9-dependent death appears to require activation of caspase-3, because both caspase-9 −/− and_caspase-3_ −/− ES cells resist UV radiation-induced apoptosis, and the two types of mutant mice exhibit similar defects in brain development. The caspase-9-dependent death of thymocytes, however, also can use other effector caspases, because_caspase-9_ −/− but not caspase-3 −/− thymocytes are resistant to dexamethasone and γ radiation.
Although the outlines of the death circuitry in cells are rapidly emerging, our current models undoubtedly are simplistic. It seems highly likely that there are additional pathways, driven by other activator caspases and their corresponding adaptors—neither of the above pathways accounts for the culling of autoreactive thymocytes, for example. Significant differences probably will emerge in the circuitry of various cell types and there may be cross-talk between pathways as well as feedback loops to amplify or dampen signals. Much remains to be established, particularly about how Bcl-2-related proteins regulate the caspase cascade. Further gene targeting currently on line and crosses of mutant mice hold great promise for unraveling the cellular secrets of life and death.
Footnotes
A commentary on this article begins on page 12480.
References
- Kerr J F R, Wyllie A H, Currie A R. Br J Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang J, Liu X, Scherer D C, van Kaer L, Wang X, Xu M. Proc Natl Acad Sci USA. 1998;95:12480–12485. doi: 10.1073/pnas.95.21.12480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Skalka M, Matyásová J, Cejková M. FEBS Lett. 1976;72:271–274. doi: 10.1016/0014-5793(76)80984-2. [DOI] [PubMed] [Google Scholar]
- 4.Wyllie A H. Nature (London) 1980;284:555–556. doi: 10.1038/284555a0. [DOI] [PubMed] [Google Scholar]
- 5.Ellis H M, Horvitz H R. Cell. 1986;44:817–829. doi: 10.1016/0092-8674(86)90004-8. [DOI] [PubMed] [Google Scholar]
- 6.Conradt B, Horvitz H R. Cell. 1998;93:519–529. doi: 10.1016/s0092-8674(00)81182-4. [DOI] [PubMed] [Google Scholar]
- 7.Yuan J, Shaham S, Ledoux S, Ellis H M, Horvitz H R. Cell. 1993;75:641–652. doi: 10.1016/0092-8674(93)90485-9. [DOI] [PubMed] [Google Scholar]
- 8.Nicholson D W, Thornberry N A. Trends Biochem Sci. 1997;22:299–306. doi: 10.1016/s0968-0004(97)01085-2. [DOI] [PubMed] [Google Scholar]
- 9.Cohen G M. Biochem J. 1997;326:1–16. doi: 10.1042/bj3260001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thornberry N A, Lazebnik Y. Science. 1998;281:1312–1316. doi: 10.1126/science.281.5381.1312. [DOI] [PubMed] [Google Scholar]
- 11.Thornberry N A, Rano T A, Peterson E P, Rasper D M, Timkey T, Garcia-Calvo M, Houtzager V M, Nordstrom P A, Roy S, Vaillancourt J P, et al. J Biol Chem. 1997;272:17907–17911. doi: 10.1074/jbc.272.29.17907. [DOI] [PubMed] [Google Scholar]
- 12.Liu X, Zou H, Slaughter C, Wang X. Cell. 1997;89:175–184. doi: 10.1016/s0092-8674(00)80197-x. [DOI] [PubMed] [Google Scholar]
- 13.Enari M, Sakahira H, Yokoyama H, Okawa K, Iwamatsu A, Nagata S. Nature (London) 1998;391:43–50. doi: 10.1038/34112. [DOI] [PubMed] [Google Scholar]
- 14.Liu X, Li P, Widlak P, Zou H, Luo X, Garrard W T, Wang X. Proc Natl Acad Sci USA. 1998;95:8461–8466. doi: 10.1073/pnas.95.15.8461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sakahira H, Enari M, Nagata S. Nature (London) 1998;391:96–99. doi: 10.1038/34214. [DOI] [PubMed] [Google Scholar]
- 16.Ucker D S, Obermiller P S, Eckhart W, Apgar J R, Berger N A, Meyers J. Mol Cell Biol. 1992;12:3060–3069. doi: 10.1128/mcb.12.7.3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peitsch M C, Mannherz H C, Tschopp J. Trends Cell Biol. 1994;4:37–41. doi: 10.1016/0962-8924(94)90002-7. [DOI] [PubMed] [Google Scholar]
- 18.Jacobson M D, Burne J F, Raff M C. EMBO J. 1994;13:1899–1910. doi: 10.1002/j.1460-2075.1994.tb06459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schulze-Osthoff K, Walczak H, Dröge W, Krammer P H. J Cell Biol. 1994;127:15–20. doi: 10.1083/jcb.127.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hedgecock E M, Sulston J E, Thomson J N. Science. 1983;220:1277–1279. doi: 10.1126/science.6857247. [DOI] [PubMed] [Google Scholar]
- 21.Jänicke R U, Sprengart M L, Wati M R, Porter A G. J Biol Chem. 1998;273:9357–9360. doi: 10.1074/jbc.273.16.9357. [DOI] [PubMed] [Google Scholar]
- 22.Woo M, Hakem R, Soengas M S, Duncan G S, Shahinian A, Kagi D, Hakem A, McCurrach M, Khoo W, Kaufman S A, et al. Genes Dev. 1998;12:806–819. doi: 10.1101/gad.12.6.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boldin M P, Goncharov T M, Goltsev Y V, Wallach D. Cell. 1996;85:803–815. doi: 10.1016/s0092-8674(00)81265-9. [DOI] [PubMed] [Google Scholar]
- 24.Muzio M, Chinnaiyan A M, Kischkel F C, O’Rourke K, Shevchenko A, Ni J, Scaffidi C, Bretz J D, Zhang M, Gentz R, et al. Cell. 1996;85:817–827. doi: 10.1016/s0092-8674(00)81266-0. [DOI] [PubMed] [Google Scholar]
- 25.Muzio M, Stockwell B R, Stennicke H R, Salvesen G S, Dixit V M. J Biol Chem. 1998;273:2926–2930. doi: 10.1074/jbc.273.5.2926. [DOI] [PubMed] [Google Scholar]
- 26.Li P, Nijhawan D, Budihardjo I, Srinivasula S M, Ahmad M, Alnemri E S, Wang X. Cell. 1997;91:479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 27.Srinivasula S M, Ahmad M, Fernandes-Alnemri T, Alnemri E S. Mol Cell. 1998;1:949–957. doi: 10.1016/s1097-2765(00)80095-7. [DOI] [PubMed] [Google Scholar]
- 28.Ashkenazi A, Dixit V M. Science. 1998;281:1305–1308. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- 29.Zou H, Henzel W J, Liu X, Lutschg A, Wang X. Cell. 1997;90:405–413. doi: 10.1016/s0092-8674(00)80501-2. [DOI] [PubMed] [Google Scholar]
- 30.Strasser A, Harris A W, Huang D C S, Krammer P H, Cory S. EMBO J. 1995;14:6136–6147. doi: 10.1002/j.1460-2075.1995.tb00304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith K G C, Strasser A, Vaux D L. EMBO J. 1996;15:5167–5176. [PMC free article] [PubMed] [Google Scholar]
- 32.Newton K, Harris A W, Bath M L, Smith K G C, Strasser A. EMBO J. 1998;17:706–718. doi: 10.1093/emboj/17.3.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Adams J M, Cory S. Science. 1998;281:1322–1326. doi: 10.1126/science.281.5381.1322. [DOI] [PubMed] [Google Scholar]
- 34.Chinnaiyan A M, O’Rourke K, Lane B R, Dixit V M. Science. 1997;275:1122–1126. doi: 10.1126/science.275.5303.1122. [DOI] [PubMed] [Google Scholar]
- 35.Chaudhary D, O’Rourke K, Chinnaiyan A M, Dixit V M. J Biol Chem. 1998;273:17708–17712. doi: 10.1074/jbc.273.28.17708. [DOI] [PubMed] [Google Scholar]
- 36.Spector M S, Desnoyers S, Hoeppner D J, Hengartner M O. Nature (London) 1997;385:653–656. doi: 10.1038/385653a0. [DOI] [PubMed] [Google Scholar]
- 37.Wu D, Wallen H D, Nuñez G. Science. 1997;275:1126–1129. doi: 10.1126/science.275.5303.1126. [DOI] [PubMed] [Google Scholar]
- 38.Seshagiri S, Miller L K. Curr Biol. 1997;7:455–460. doi: 10.1016/s0960-9822(06)00216-8. [DOI] [PubMed] [Google Scholar]
- 39.Varfolomeev E E, Schuchmann M, Luria V, Chiannilkulchai N, Beckmann J S, Mett I L, Rebrikov D, Brodianski V M, Kemper O C, Kollet O, et al. Immunity. 1998;9:267–276. doi: 10.1016/s1074-7613(00)80609-3. [DOI] [PubMed] [Google Scholar]
- 40.Hakem R, Hakem A, Duncan G S, Henderson J T, Woo M, Soengas M S, Elia A, de la Pompa J L, Kagi D, Khoo W, et al. Cell. 1998;94:339–352. doi: 10.1016/s0092-8674(00)81477-4. [DOI] [PubMed] [Google Scholar]
- 41.Kuida K, Haydar T F, Kuan C-Y, Gu Y, Taya C, Karasuyama H, Su M S-S, Rakic P, Flavell R A. Cell. 1998;94:325–337. doi: 10.1016/s0092-8674(00)81476-2. [DOI] [PubMed] [Google Scholar]