Pharmacology and functions of receptors for vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide: IUPHAR Review 1 (original) (raw)
Abstract
Vasoactive intestinal peptide (VIP) and pituitary adenylate cyclase-activating polypeptide (PACAP) are members of a superfamily of structurally related peptide hormones that includes glucagon, glucagon-like peptides, secretin, gastric inhibitory peptide (GIP) and growth hormone-releasing hormone (GHRH). VIP and PACAP exert their actions through three GPCRs – PAC1, VPAC1 and VPAC2– belonging to class B (also referred to as class II, or secretin receptor-like GPCRs). This family comprises receptors for all peptides structurally related to VIP and PACAP, and also receptors for parathyroid hormone, corticotropin-releasing factor, calcitonin and related peptides. PAC1 receptors are selective for PACAP, whereas VPAC1 and VPAC2 respond to both VIP and PACAP with high affinity. VIP and PACAP play diverse and important roles in the CNS, with functions in the control of circadian rhythms, learning and memory, anxiety and responses to stress and brain injury. Recent genetic studies also implicate the VPAC2 receptor in susceptibility to schizophrenia and the PAC1 receptor in post-traumatic stress disorder. In the periphery, VIP and PACAP play important roles in the control of immunity and inflammation, the control of pancreatic insulin secretion, the release of catecholamines from the adrenal medulla and as co-transmitters in autonomic and sensory neurons. This article, written by members of the International Union of Basic and Clinical Pharmacology Committee on Receptor Nomenclature and Drug Classification (NC-IUPHAR) subcommittee on receptors for VIP and PACAP, confirms the existing nomenclature for these receptors and reviews our current understanding of their structure, pharmacology and functions and their likely physiological roles in health and disease. More detailed information has been incorporated into newly revised pages in the IUPHAR database (http://www.iuphar-db.org/DATABASE/FamilyMenuForward?familyId=67).
LINKED ARTICLES
This article is part of a themed section on Secretin Family (Class B) G Protein-Coupled Receptors. To view the other articles in this section visit http://dx.doi.org/10.1111/bph.2012.166.issue-1
Keywords: accessory proteins, agonists and antagonists, class B GPCR, PAC1 receptor, PACAP, signal transduction, splice variant, VIP, VPAC1 receptor, VPAC2 receptor
Links to online information in IUPHAR-DB and the BPS Guide to Receptors and Channels
| IUPHAR database | PAC1 receptor (in GRAC) | PHV |
|---|---|---|
| 125IVIP | PAC1 receptor (in IUPHAR-DB) | Ro 25-1553 |
| 125IPACAP-27 | PAC1 receptor splice variants | Ro 25-1392 |
| Ala11,22,28VIP | PACAP-27 | VIP |
| Lys15,Arg16,Leu27VIP(1-7)/GRF(8-27)-NH2 | PACAP-38 | VPAC1 receptor (in GRAC) |
| CRF2 receptor (in GRAC) | PACAP(6-38) | VPAC1 receptor (in IUPHAR-DB) |
| CRF2 receptor (in IUPHAR-DB) | PG 97-269 | VPAC2 receptor (in GRAC) |
| M65 | PHI | VPAC2 receptor (in IUPHAR-DB) |
| maxadilan | PHM |
The endogenous peptide ligands – PACAP, VIP, PHI/PHM and PHV
Vasoactive intestinal peptide (VIP) was first isolated from porcine intestine as a 28-amino acid peptide, conserved in sequence between most mammals, capable of inducing vasodilatation in the canine femoral artery (Said and Mutt, 1970; 1972) and has subsequently been shown to have many other actions as a neuroendocrine hormone, putative neurotransmitter and cytokine. In common with the precursors of many other neuroendocrine peptides, the VIP precursor polypeptide (prepro-VIP) contains sequences encoding several additional biologically active peptides (Figure 1), including peptide histidine isoleucine [PHI; found in non-human mammals (Tatemoto and Mutt, 1981)], peptide histidine methionine [PHM; the human equivalent of PHI (Itoh et al., 1983)] and peptide histidine valine [PHV; a C-terminally extended form of PHI and PHM (Yiangou et al., 1987)]. The presence of VIP and specific VIP binding sites in defined pathways in the brain indicate that it may play an important role in CNS function (Besson et al., 1986; Martin et al., 1987). VIP is now widely accepted as a co-transmitter, with nitric oxide and carbon monoxide, of nonadrenergic, noncholinergic relaxation of both vascular and nonvascular smooth muscle (Said and Rattan, 2004) and with acetylcholine in exocrine glands (Fahrenkrug, 1993). VIP may also promote neuronal survival (Brenneman and Eiden, 1986) and regulate glycogen metabolism in the cerebral cortex (Sorg and Magistretti, 1992). VIP stimulates prolactin secretion from the pituitary (Reichlin, 1988) and catecholamine release from the adrenal medulla (Malhotra et al., 1988). In the immune system, VIP regulates T cell traffic and inhibits mitogen-activated proliferation of T cells by inhibiting IL-2 production (Ottaway, 1987). Other actions of VIP include stimulation of electrolyte secretion and protection against oxidant injury (Gozes and Brenneman, 1989; Said, 1991; 1996).
Figure 1.
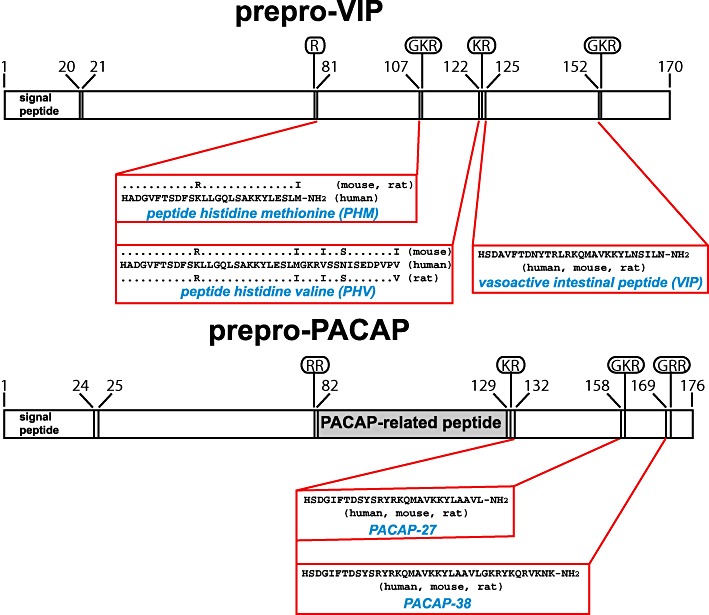
Structures of the precursors of VIP and PACAP and the biologically active peptides that they encode. Structures of the human VIP and PACAP precursors are shown, with sites of proteolytic processing (basic amino acids and glycine residues that donate the C-terminal amide groups of the mature peptides) indicated in ovals. Amino acid sequences of the human peptides and sequence variations in rat and mouse are given in single letter nomenclature. PACAP-related peptide displays sequence homology to PHM but has not been shown to be biologically active.
Pituitary adenylate cyclase-activating polypeptide (PACAP) was first identified as a 38-amino acid peptide (PACAP-38) from ovine hypothalamus on the basis of its ability to stimulate cyclic AMP production in rat anterior pituitary cells in culture (Miyata et al., 1989). Subsequently, a C-terminally truncated, 27-amino acid form of the peptide (PACAP-27) was isolated from the same source (Miyata et al., 1990). The sequences of human, mouse, rat and sheep PACAP-38 are identical (Figure 1). In the CNS, PACAP and the mRNA encoding its precursor are most abundant in the hypothalamus, with lower levels in other brain regions (Ghatei et al., 1993). PACAP is also present in peripheral tissues, such as the gastrointestinal tract, adrenal gland and testis (Ghatei et al., 1993; Arimura and Shioda, 1995). PACAP is expressed in sympathetic neurons and in the cholinergic innervation of the adrenal medulla, where it is thought to facilitate secretion of catecholamines under conditions of high stress (Przywara et al., 1996; Hamelink et al., 2002). PACAP is also thought to regulate exocrine and endocrine secretion from the pancreas (Raufman et al., 1991; Yada et al., 1994). For a recent review of the structure and functions of PACAP and its receptors, see Vaudry et al. (2009).
The receptors: VPAC1, VPAC2 and PAC1
Radioligand binding studies using [125I]PACAP-27 (Shivers et al., 1991) suggested the existence of two distinct receptors for PACAP in rat tissues, one with much greater affinity for PACAP than for VIP (the ‘PACAP type I receptor’) and a second with high affinity for both PACAP and VIP (the ‘PACAP type II receptor’). Subsequently, two types of high-affinity VIP (PACAP type II) receptors were identified based on the relative potencies of natural and synthetic VIP analogues. In addition to the ‘classical’ VIP receptors from intestinal cells (Laburthe et al., 1983), receptors with different pharmacology were identified in the human SUP-T1 lymphoblast cell line (Robberecht et al., 1988) and in lung cancer cell lines (Luis and Said, 1990). Subsequently, two high-affinity receptors for both VIP and PACAP (‘PACAP type II receptors’) were cloned: the VPAC1 receptor, first isolated from rat lung (Ishihara et al., 1992) and the VPAC2 receptor, first cloned from rat olfactory bulb (Lutz et al., 1993). VPAC1 and VPAC2 receptors display comparable affinity for PACAP and VIP, whereas PACAP-27 and PACAP-38 are >100-fold more potent than VIP as agonists of most isoforms of the PAC1 receptor.
The VPAC1 receptor is widely distributed in the CNS, most abundantly in the cerebral cortex and hippocampus (Ishihara et al., 1992; Usdin et al., 1994), in peripheral tissues including liver, lung and intestine (Ishihara et al., 1992; Usdin et al., 1994; Ichikawa et al., 1995; Sreedharan et al., 1995; Kaltreider et al., 1997; Reubi, 2000; Reubi et al., 2000; Harmar et al., 2004) and in T lymphocytes (Delgado et al., 1996). In the CNS, the highest concentrations of messenger RNA encoding the VPAC2 receptor are found in the thalamus and suprachiasmatic nucleus (SCN) and lower levels in the hippocampus, brainstem, spinal cord and dorsal root ganglia (Usdin et al., 1994; Sheward et al., 1995). The receptor is also present in many peripheral tissues, including smooth muscles in the cardiovascular, gastrointestinal and reproductive systems (Inagaki et al., 1994; Usdin et al., 1994; Adamou et al., 1995; Krempels et al., 1995; Wei and Mojsov, 1996; Reubi, 2000; Reubi et al., 2000; Harmar et al., 2004).
The ‘PACAP type I receptor’, which recognizes PACAP-27 and PACAP-38 with much higher potency than VIP (now referred to as PAC1) was first identified in a rat pancreatic acinar carcinoma cell line (Pisegna and Wank, 1993). mRNA encoding this receptor is expressed predominantly in the CNS, most abundantly in the olfactory bulb, thalamus, hypothalamus, the dentate gyrus of the hippocampus and in granule cells of the cerebellum (Hashimoto et al., 1996; Shioda et al., 1997). The receptor is also highly expressed in the embryonic nervous system (Sheward et al., 1998; Waschek et al., 1998; Zhou et al., 1999) and in a number of peripheral tissues, most abundantly in the adrenal medulla (Shivers et al., 1991; Spengler et al., 1993; Moller and Sundler, 1996; Reubi, 2000; Reubi et al., 2000). There is apparent heterogeneity of PAC1 receptors in tissues and cell lines, where two types of ‘PACAP type I’ pharmacology have been observed: type IA receptors, with high affinity for both PACAP-27 and PACAP-38; and type IB receptors, with high affinity for PACAP-38 but low affinity for PACAP-27 (Robberecht et al., 1991; Shivers et al., 1991). The difference between the two receptor subtypes may reflect differences in G protein coupling and second messenger mechanisms (Van Rampelbergh et al., 1996) or result from alternative splicing of PAC1 receptor mRNA.
Splice variants and accessory proteins
The diversity and functional consequences of alternative splicing events in class B GPCRs have been reviewed recently (Furness et al., 2012). Splice variants differing in amino acid sequence in the extracellular N-terminal domain or the extracellular loops may display altered ligand affinity and selectivity, whereas splice variation in the intracellular loops or the C-terminus can influence signal transduction pathways. Although there is some evidence for the existence of splice variants of the VPAC1 and VPAC2 receptors, their functional importance is not yet clear (Dickson and Finlayson, 2009). In contrast, splice variation in the PAC1 receptor is well established, complex and functionally important. Within the part of the PAC1 receptor cDNA encoding the third intracellular loop, splice variants either containing or lacking each of two alternative exons (called ‘hip’ and ‘hop’ in rodents) exist. The hop exon exists in two forms (hop1 and hop2) as the result of the existence of two alternative splice acceptor sites three nucleotides apart. Thus, six possible splice variants, which differ in their intracellular signal transduction pathways, can be generated (Spengler et al., 1993; Journot et al., 1995). Four variants of the human PAC1 receptor (null, SV-1, SV-2 and SV-3) resulting from alternative splicing of sequences equivalent to hip and hop1 have also been described (Pisegna and Wank, 1996) and were shown to differ in their ability to activate phospholipase C (PLC). In addition, splice variation in the N-terminal extracellular domain of the PAC1 receptor has been reported. Splicing out of the 4th and 5th coding exons, leading to a 21 amino acid deletion, has been reported in human and mouse (Pantaloni et al., 1996; Dautzenberg et al., 1999). Surprisingly, the human splice variant, named ‘PAC1short’ bound PACAP-27, PACAP-38 and VIP with similar high affinity and all three peptides stimulated cyclic AMP accumulation with similar potency (Dautzenberg et al., 1999). Additional N-terminal splice variants resulting from splicing out of the 3rd, 4th and 5th exons of the human gene (Dautzenberg et al., 1999) and by insertion of an additional 72 base pairs (exon 3a) encoding a sequence of 24 amino acids between coding exons 3 and 4 (Daniel et al., 2001) have also been described.
Two class B receptors – the calcitonin receptor and the calcitonin receptor-like receptor – can form heteromers with members of a family of accessory proteins called RAMPs (receptor activity-modifying proteins 1, 2 and 3) to generate multiple distinct receptor types with different specificities for endogenous peptide ligands (Barwell et al., 2012). The VPAC1 receptor (but not VPAC2 or PAC1) has been shown to be able to interact with RAMPs; in this case ligand specificity is not altered but the VPAC1 receptor-RAMP2 heteromer displays altered signal transduction specificity, with significant enhancement of agonist-mediated phosphoinositide hydrolysis with no change in cyclic AMP stimulation (Christopoulos et al., 2003).
Although there is some evidence for the presence of PHI-selective receptors in mammalian tissues and the cloning of a PHI-selective receptor from the goldfish Carassius auratus has been reported (Tse et al., 2002), there is no convincing evidence at present for a separate PHI receptor in mammals. However, it remains possible that such a receptor, either encoded by a novel gene, or resulting from alternative splicing of known genes, by interaction of known genes with accessory proteins, or through homo/hetero-oligomerization, may be discovered in the future.
Pharmacology
For recent critical reviews of the pharmacology and signalling properties of VIP and PACAP receptors, see Laburthe et al., 2007; Dickson and Finlayson, 2009. Progress in characterizing the functions of the three receptor types has been hindered by the limited number of selective drugs available (Table 1). [Ala11,22,28]VIP (Nicole et al., 2000) and [Lys15,Arg16,Leu27]VIP(1–7)/GRF(8–27)-NH2 (frequently abbreviated as [K15,R16,L27]VIP(1–7)/GRF(8–27) in the literature; Gourlet et al., 1997b) are selective agonists of the VPAC1 receptor and PG 97–269 is a selective antagonist (Gourlet et al., 1997a). Ro 25–1392 (Xia et al., 1997) is the most selective VPAC2 agonist to date. There is no highly selective VPAC2 antagonist as yet: PG99-465 (Moreno et al., 2000) has been used as a selective VPAC2 receptor antagonist in a number of physiological studies, but has been reported to be a partial agonist of VPAC2 in some functional assays (EC50= 5 nM) and act as a full agonist at VPAC1 (EC50= 8 nM) and PAC1 (EC50= 71 nM) receptors (Dickson et al., 2006). The tissue distribution of VPAC1 and VPAC2 receptors can be determined by in vitro receptor autoradiography using [125I]-VIP as the radioligand and displacement with the VPAC1 selective agonist [Lys15,Arg16,Leu27]VIP(1–7)/GRF(8–27)-NH2 and the VPAC2 selective agonist Ro 25-1553 to distinguish the two receptor types (Reubi et al., 2000; Harmar et al., 2004). [125I]-Ro 25-1553 can also be used to localize VPAC2 receptors (Vertongen et al., 1997; Reubi et al., 2000; Harmar et al., 2004).
Table 1.
Useful pharmacological tools for the characterization of VIP and PACAP receptors
| Receptor | VPAC1 | VPAC2 | PAC1 |
|---|---|---|---|
| Endogenous ligands | VIP (8.5–9.8) | VIP (7.8–8.8) | VIP (6.0–6.3) |
| PACAP-27 (8.9) | PACAP-27 (7.6–8.0) | PACAP-27 (8.5) | |
| PACAP-38 (8.2) | PACAP-38 (pEC50= 7.7–9.3) | PACAP-38 (8.8–9.0) | |
| PHI (pIC50= 6.0) | PHI (pIC50= 7.5) | Values for the ‘PAC1short’ splice variant (Dautzenberg et al., 1999) are as follows: | |
| PHM (5.7) | PHV (pIC50= 8.8) | ||
| PHV (pIC50= 5.5) | |||
| VIP (8.4) | |||
| PACAP-27 (8.5) | |||
| PACAP-38 (8.8) | |||
| Selective agonists | [Ala11,22,28]VIP (8.1: Nicole et al., 2000) | Ro 25-1553 (8.0: Gourlet et al., 1997b,c) | Maxadilan (pEC50 = 9.2: Moro and Lerner, 1997) |
| [Lys15,Arg16,Leu27]VIP(1–7)/GRF(8–27)-NH2 (pIC50= 7.7–9.0: Gourlet et al., 1997b) | Ro 25-1392 (8.0: Xia et al., 1997) | ||
| Selective antagonists | PG97-269 (pIC50= 7.1–8.7: Gourlet et al., 1997a) | – | Max.d.4 (Tatsuno et al., 2001) |
| M65 (pIC50= 6.6–6.8: Uchida et al., 1998) | |||
| Radioligands | [125I]-VIP, [125I]-PACAP-27, [125I]-Ro 25-1553 | [125I]-VIP, [125I]-PACAP-27 | [125I]-PACAP-27 |
The most selective agonist of PAC1 receptors is maxadilan, a peptide isolated from the salivary glands of sand flies (Lutzomyia longipalpis), which has no sequence homology to VIP or PACAP (Moro and Lerner, 1997). Max.d.4 (maxadilan Δ24–42) and M65 (maxadilan Δ25–41; Uchida et al., 1998) are synthetic variants of maxadilan, which display activity as PAC1 antagonists but the use of these peptides has been limited due to problems with their availability. Finally, it is important to note that although PACAP(6–38) has been used as a PAC1 receptor antagonist in many studies, it also exhibits high potency at VPAC2 receptors (Dickinson et al., 1997).
Receptor structure and signal transduction mechanisms
For all class B receptors, the large N-terminal ectodomain plays a crucial role in ligand recognition, prompting structural studies of this domain (Laburthe and Couvineau, 2002; Laburthe et al., 2007; Couvineau and Laburthe, 2012a, b). As initially described for the mouse CRF2 receptor (Grace et al., 2004), the structure comprises a crucial sushi domain characterized by two antiparallel β sheets and stabilized by three disulphide bonds and a salt bridge sandwiched between aromatic rings of two tryptophan residues. Structures of the ectodomains of PAC1 receptor (Sun et al., 2007; Kumar et al., 2011) and VPAC2 receptors (PDB ID: 2X57) have been determined by X-ray or NMR and a structural model of the VPAC1 receptor obtained by homology modelling associated with photoaffinity experiments (Tan et al., 2006). Photoaffinity labelling has also demonstrated that the ectodomain of the PAC1 receptor is the major binding site for PACAP (Dejda et al., 2011). The data are consistent with the two-site model for peptide binding to class B GPCRs (Figure 2), in which the C-terminal and central α-helical parts of the peptide hormone interact with the sushi domain in the N-terminal ectodomain (N-ted) ultimately positioning the N-terminus of the peptide to contact the transmembrane region resulting in receptor activation (Laburthe and Couvineau, 2002; Laburthe et al., 2007; Bourgault et al., 2009; Couvineau and Laburthe, 2012b). This latter contact region remains elusive since no structure for a full-length class B GPCR has been determined yet. However, the presence of a helix N-capping motif in cognate peptide ligands of all class B receptors, including VIP and PACAP, supports that the folded backbone conformation of a N-cap is formed upon receptor binding and constitutes a key element underlying class B GPCR activation (Neumann et al., 2008).
Figure 2.
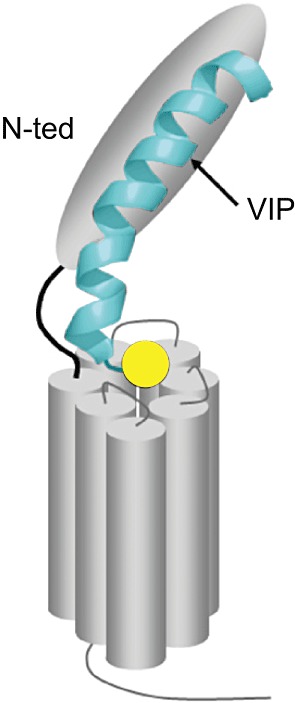
Schematic model of VPAC1 receptor activation. The receptor N-ted traps the central and C-terminal parts (6–28) of VIP (shown in blue) and positions the N-terminal part (1–5) of VIP (yellow circle) in the receptor core for activation (adapted from Laburthe et al., 2007).
Functions of VIP and PACAP receptors in the CNS
The widespread distribution of VIP and PACAP and their receptors in the brain and periphery has led to many hypotheses concerning the physiological functions of these receptors. However, the availability of mutant mice lacking VIP (Colwell et al., 2003), PACAP (Kawaguchi et al., 2003; Colwell et al., 2004), the VPAC2 receptor (Asnicar et al., 2002; Harmar et al., 2002), the VPAC1 receptor (Fabricius et al., 2011) and the PAC1 receptor (Hannibal et al., 2001; Jongsma et al., 2001; Otto et al., 2001b) has permitted experimental validation of a number of physiological functions for these receptors.
Both VIP and PACAP play roles in the control of circadian rhythms in the brain's ‘master clock’ in the SCN of the hypothalamus. Light entrains the SCN clock through a population of retinal ganglion cells that project to the SCN via the retinohypothalamic tract and contain both glutamate and PACAP. Studies of knockout mice lacking the PAC1 receptor or its ligand PACAP (Hannibal et al., 2001; 2008; Kawaguchi et al., 2003; Colwell et al., 2004) show that PACAP plays a role in modulating the light-induced resetting of the behavioural rhythm and light-induced clock gene expression and physiology in the SCN. In contrast, VIP is synthesized in a population of SCN neurones, many of which are thought to receive a direct retinal innervation, and acts on VPAC2 receptors, which are expressed throughout the SCN. Studies of knockout mice lacking the VPAC2 receptor indicate that this receptor is necessary for the generation of normal circadian rhythms of electrical activity, clock gene expression, physiology and behaviour (Harmar et al., 2002; Cutler et al., 2003; Hughes et al., 2004; Aton et al., 2005; Bechtold et al., 2008; Hannibal et al., 2011). VIP-deficient mice also display a severely disrupted circadian phenotype, sharing many common features with that of VPAC2 receptor null mice (Colwell et al., 2003; Aton et al., 2005).
Studies on PAC1 receptor knockout mice point to a role for presynaptic PAC1-mediated signalling at the mossy fibre synapse in long-term potentiation (LTP) and hippocampus-dependent associative learning (Otto et al., 2001a; Matsuyama et al., 2003). The PAC1 receptor is also expressed in brain areas implicated in the emotional control of behaviour, such as the amygdala, bed nucleus of the stria terminalis (BNST), hypothalamus, locus coeruleus and periaqueductal grey. Consistent with this, PACAP and PAC1 receptors are up-regulated in the BNST following chronic stress, and heightened BNST PACAP signalling produces anxiogenic behavioural responses (Hammack et al., 2010). PACAP and PAC1 receptor null mice demonstrate reduced anxiety behaviour and mice with a ubiquitous but not with a forebrain-specific deletion of the PAC1 receptor exhibited elevated locomotor activity with strongly reduced anxiety-like behaviour (Otto et al., 2001a; Matsuyama et al., 2003). Furthermore, the glucocorticoid response in PACAP null animals is altered after emotional stressors (Stroth and Eiden, 2010; Tsukiyama et al., 2011). PAC1 receptor signalling in the CNS also alters feeding behaviour (Hawke et al., 2009; Mounien et al., 2009); PAC1 signalling decreases food intake and promotes anorexic-like responses, which may be related to enhanced anxiety. An intronic single nucleotide polymorphism in a putative oestrogen response element within the PAC1 receptor gene (ADCYAP1R1) has been associated with post-traumatic stress disorder in the female population (Ressler et al., 2011), consistent with evidence that stress and oestrogen regulate the expression of the gene in animal models.
There is clear evidence that PACAP exerts neurotrophic activities during development and may prevent brain damage provoked by various types of injury. PACAP and its receptors are expressed actively in the CNS during development (Basille et al., 1993; 1994). In particular, high concentrations of PAC1 receptors are found in the external granule cell layer of the rodent cerebellum during the first two postnatal weeks (Zhou et al., 1999; Basille et al., 2000), a period of intense multiplication and migration of granule cells. Treatment of cultured granule cells with PACAP enhances cell survival and stimulates neurite outgrowth (Cavallaro et al., 1996; Gonzalez et al., 1997; Kienlen Campard et al., 1997). The neurotrophic effect of PACAP is mediated through two distinct mechanisms, that is, activation of the adenylyl cyclase and PLC pathways leads to inhibition of caspase-3 activity and promotion of cell survival (Vaudry et al., 2000), whereas activation of the adenylyl cyclase and MAPK pathways regulates gene expression and causes differentiation of granule neurons (Villalba et al., 1997; Vaudry et al., 1998; 1999). Injection of PACAP at the surface of the cerebellum of rat pups augments the number of migrating granule cells and increases the thickness of the internal granule cell layer (Vaudry et al., 1999), suggesting that PACAP is a potent inhibitor of apoptosis in the cerebellum during the development. In adult animals, PACAP reduces the severity of injury in models of focal cerebral (Ohtaki et al., 2006) and retinal (Szabadfi et al., 2012) ischaemia. In vitro PACAP also exerts a neuroprotective effect on cerebellar neurons against apoptotic cell death induced by ethanol (Vaudry et al., 2002b), cisplatin (Aubert et al., 2008), ceramides (Vaudry et al., 2003) and oxidative stress (Vaudry et al., 2002a).
VIP is also thought to play a role in neurodevelopment and in neuroprotection following injury to the CNS. For example, VIP has been shown to be protective against excitotoxin-induced white matter lesions in neonatal mice (Gressens et al., 1997; 1999; Rangon et al., 2005), probably acting through VPAC2 receptors. VPAC2 receptors have also been implicated in the control of astrocyte proliferation (Zupan et al., 1998). VPAC2 receptors have also been implicated in the VIP-induced expression of the neuroprotective protein activity-dependent neuroprotective protein (ADNP) in astrocytes (Zusev and Gozes, 2004) and NAP (davunetide), an active fragment of ADNP, is in clinical development for the treatment of neurodegenerative disorders (Gozes, 2011). In studies of post-natal hippocampus in vitro, VPAC2 receptor activation was found to expand the pool of neural stem/progenitor cells by preventing either a neuronal or glial fate choice and by supporting their survival, whereas selective VPAC1 receptor activation promoted a neurogenic granule cell fate (Zaben et al., 2009). Two recent publications from independent groups have found associations between copy number variation in the gene encoding the VPAC2 receptor and susceptibility to schizophrenia (Levinson et al., 2011; Vacic et al., 2011). These findings have generated some excitement in the field because they may imply that the VPAC2 receptor is a potential target for the development of new antipsychotic drugs (Piggins, 2011).
Functions of VIP and PACAP receptors in the immune system
VIP and PACAP play important roles in the control of immunity and inflammation. PAC1 receptor mRNA is constitutively expressed in macrophages and monocytes. PACAP, acting through the PAC1 receptor appears to be protective against endotoxin-induced septic shock, acting at least in part by attenuating lipopolysaccharide-induced production of proinflammatory IL-6 (Martinez et al., 2002). VIP has potent effects in the immune system, influencing T cell differentiation and migration and modulating the production of cytokines by the two subsets of mouse helper T cells: T helper 1 (Th1) cells, which mediate classical delayed-type cellular immunity and T helper 2 (Th2) cells, which mediate hypersensitivity reactions, such as allergy. VPAC1 receptors are highly expressed constitutively on T cells, especially Th cells, whereas VPAC2 receptors are expressed marginally or not at all by unstimulated Th cells but are up-regulated to high levels by Th cell stimulation. Studies on VPAC2 receptor knockout mice (Goetzl et al., 2001; Voice et al., 2002) and on transgenic mice overexpressing the VPAC2 receptor in CD4 T cells (Voice et al., 2001; 2002; 2003) suggest that the receptor regulates the balance between Th1 and Th2 by stimulating production of more Th2-type cytokines, due to expansion of the Th2-type subset. PACAP knockout mice exhibited the predicted hyperinflammatory response in the experimental autoimmune encephalomyelitis (EAE) model of multiple sclerosis, with an enhanced Th1/Th2 cytokine profile, but also with a reduced expansion of regulatory T cells (Tan et al., 2009). VIP-deficient mice, on the other hand, exhibited a paradoxical resistance to EAE, with a failed entry of inflammatory cells into the CNS parenchyma (Abad et al., 2010), pointing to a critical role for VIP in T cell trafficking. VIP receptor agonists and antagonists may have therapeutic potential in the treatment of inflammatory and autoimmune diseases such as Crohn's disease (Abad et al., 2003), rheumatoid arthritis (Juarranz et al., 2004) and multiple sclerosis (Gonzalez-Rey et al., 2006).
Functions in the gastrointestinal tract
PACAP and VIP and their receptors are expressed widely in the gastrointestinal tract. In the gastric mucosa, PACAP-containing enteric nerve fibres have been described, which are co-localized with PAC1 receptors (Miampamba et al., 2002). PACAP appears to have diverse functions in the stomach that depend on whether the cell target expresses either PAC1 or VPAC1 receptors. PAC1 is expressed on gastric enterochromaffin-like (ECL) cells and involved in the regulation of gastric acid secretion, whereas VPAC1 is expressed by the somatostatin containing gastric D cells and thought to inhibit gastric acid secretion. In the rat stomach, PACAP released by enteric neurons innervating the mucosa appears to mediate the nocturnal increase in gastric acid secretion (Zeng et al., 1999). VIP knockout mice exhibit a reduction in gastrointestinal motility similar to that observed in patients with Hirschsprung's disease (Lelievre et al., 2007). These observations suggest that PACAP, VIP and their receptors appear to play an important role in the regulation of gastrointestinal function and underscore the importance of further studies in this area.
Relevance to cancer
The best-known association of VIP with cancer is the ‘watery diarrhoea syndrome’ (also known as ‘pancreatic cholera’ or Verner Morrison syndrome) caused by the ectopic secretion of VIP by some tumours (VIPomas), usually of non-β pancreatic islet cell origin (Modlin et al., 1978). The symptoms of this condition are thought to result from the action of VIP on VPAC1 receptors in the intestinal mucosa to stimulate chloride secretion and water movement into the intestinal lumen, an effect mimicked by selective VPAC1 receptor agonists in animal models (Tsutsumi et al., 2002).
Most of the commonly occurring human tumours express VPAC1 receptors (Reubi et al., 2000). VPAC2 expressing tumours are much rarer: they include a high proportion of gastrointestinal stromal tumours (Reubi et al., 2004) and also leiomyomata (benign smooth muscle neoplasms, e.g. uterine fibroids). Consistent with the role of PACAP in the CNS and the sympathoadrenal system, PAC1 receptors are often expressed in tumours of neuroectodermal origin (e.g. neuroblastoma, glioma, phaeochromocytoma and pituitary adenoma; Robberecht et al., 1993; 1994; Vertongen et al., 1996) as well as in endometrial carcinoma (Reubi et al., 2000). The use of radioactive ligands of VIP and PACAP receptors for imaging or therapy of tumours has met with limited success, perhaps due to difficulties in achieving selectivity for tumour tissue over normal tissues and in identifying analogues with appropriate pharmacokinetic properties. Numerous cell lines derived from tumours have been found to express receptors for VIP and PACAP (see http://www.tumor-gene.org/GPCR/gpcr.html) and have provided useful tools for the study of receptor function. For example, the first report of a receptor with VPAC2 pharmacology used SUP-T1 lymphoblasts (Robberecht et al., 1988) and PC12 phaeochromocytoma cells have been used extensively to study the effects of PACAP on cell survival, cell proliferation, neurite outgrowth and the underlying signalling pathways (Vaudry et al., 2009).
Other functions in the periphery
VIP and PACAP, acting through PAC1 and VPAC2 receptors on pancreatic β-cells, have been implicated in the control of pancreatic insulin secretion. PAC1 receptor-deficient mice display impaired insulinotropic response to glucose, reduced glucose tolerance and impaired glucagon response to insulin-induced hypoglycaemia (Jamen et al., 2000; Persson and Ahren, 2002) and overexpression of PACAP in mouse pancreatic β-cells has been reported to enhance insulin secretion and ameliorate streptozotocin-induced diabetes (Yamamoto et al., 2003) and to inhibit hyperinsulinemia and islet hyperplasia in agouti yellow mice (Tomimoto et al., 2004). VPAC2 receptor null mice have been reported to be able to maintain a normal response to glucose challenge with lower levels of insulin than wild-type mice, suggesting a significant increase in insulin sensitivity in the knockout mice (Asnicar et al., 2002). A selective peptide agonist of the VPAC2 receptor stimulated glucose-dependent insulin secretion in isolated rat and human pancreatic islets, increased insulin synthesis in purified rat islets and caused a dose-dependent increase in plasma insulin levels in fasted rats, suggesting that VPAC2 receptor agonists may be a useful therapy for the treatment of type 2 diabetes (Tsutsumi et al., 2002).
Consistent with the expression of PAC1 and VPAC1 receptors in adrenomedullar cells (Usdin et al., 1994; Moller and Sundler, 1996; Yon et al., 1998; Shioda et al., 2000), PACAP and VIP are potent activators of catecholamine release in vitro (Cheung and Holzwarth, 1986; Watanabe et al., 1992) and in vivo (Lamouche et al., 1999). Interestingly, PACAP strongly increases VIP mRNA expression in bovine chromaffin cells (Lee et al., 1999). Intravenous administration of PACAP enhances the secretion of corticosteroids in dog (Kawai et al., 1994) and calf (Edwards and Jones, 1994). In human, PACAP-induced stimulation of cortisol secretion from adrenal slices is suppressed by the β-adrenoceptor antagonists (Neri et al., 1996; Breault et al., 2000) suggesting that the effect of PACAP on corticosteroid secretion is mediated through its stimulatory action on catecholamine release.
The presence of PACAP in primary sensory neurones and the PAC1 receptor in the dorsal horn of the spinal cord (Jongsma et al., 2000) suggest a role for the PAC1 receptor in pain responses. PAC1 receptor knockout mice displayed impaired nociceptive responses to chemical, thermal and mechanical stimuli (Jongsma et al., 2001) and PACAP-deficient mice also displayed abnormal pain responses (Mabuchi et al., 2004).
Although most studies of PAC1 receptor knockout mice have found these animals to be superficially normal and viable, it has been reported that when crossed onto a C57BL/6 background, almost all PAC1 receptor knockout mice developed pulmonary hypertension and right heart failure after birth, suggesting an important role for PAC1-mediated signalling for the maintenance of normal pulmonary vascular tone during early postnatal life (Otto et al., 2004).
Perspectives
VIP and PACAP play diverse and important roles in the CNS, with functions in the control of circadian rhythms, learning and memory, anxiety and responses to stress and brain injury. The development of drugs acting on these receptors may lead to new treatments for sleep disorders, stroke, neurodegenerative disorders and age-related memory impairment. Genetic studies also implicate the VPAC2 receptor as a potential target for the development of new antipsychotic drugs and suggest involvement of the PAC1 receptor in post-traumatic stress disorder. The role of VIP and PACAP in the gastrointestinal tract suggest that these hormones and their receptors would provide a therapeutic target for the management of gastric acid secretory disorders and disorders affecting gastrointestinal motility such as functional bowel syndromes. The involvement of VIP and PACAP in immune function suggests potential applications in the treatment of diseases such as Crohn's disease, rheumatoid arthritis and multiple sclerosis. Other peripheral functions of VIP and PACAP indicate potential importance in diabetes, pain and hypertension. The major impediment to translational research on VIP and PACAP is that all of the currently useful pharmacological tools are peptides. The first small molecule antagonists of PAC1 receptors (Beebe et al., 2008) and human VPAC2 receptors (Chu et al., 2010) have been described recently and may herald important breakthroughs in understanding the therapeutic opportunities offered by these receptors.
Acknowledgments
AJH is supported by IUPHAR, the British Pharmacological Society and the British Heart Foundation Centre of Research Excellence Award (RE/08/001).
Glossary
BNST
bed nucleus of the stria terminalis
EAE
experimental autoimmune encephalomyelitis
ECL
enterochromaffin-like
GHRH
growth hormone-releasing hormone
GIP
gastric inhibitory peptide
LTP
long-term potentiation
NC-IUPHAR
International Union of Basic and Clinical Pharmacology Committee on Receptor Nomenclature and Drug Classification
N-ted
N-terminal ectodomain
PACAP
pituitary adenylate cyclase-activating polypeptide
PHI
peptide histidine isoleucine
PHM
peptide histidine methionine
PHV
peptide histidine valine
RAMP
receptor activity-modifying protein
SCN
suprachiasmatic nuclei
Th1
T helper 1
Th2
T helper 2
VIP
vasoactive intestinal peptide
Conflicts of interest
Prof. Illana Gozes serves as a Director, Chief Scientific Officer at Allon Therapeutics Inc. clinically developing davunetide (NAP).
References
- Abad C, Martinez C, Juarranz MG, Arranz A, Leceta J, Delgado M, et al. Therapeutic effects of vasoactive intestinal peptide in the trinitrobenzene sulfonic acid mice model of Crohn's disease. Gastroenterology. 2003;124:961–971. doi: 10.1053/gast.2003.50141. [DOI] [PubMed] [Google Scholar]
- Abad C, Tan YV, Lopez R, Nobuta H, Dong H, Phan P, et al. Vasoactive intestinal peptide loss leads to impaired CNS parenchymal T-cell infiltration and resistance to experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A. 2010;107:19555–19560. doi: 10.1073/pnas.1007622107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamou JE, Aiyar N, Van Horn S, Elshourbagy NA. Cloning and functional characterization of the human vasoactive intestinal peptide (VIP)-2 receptor. Biochem Biophys Res Commun. 1995;209:385–392. doi: 10.1006/bbrc.1995.1515. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 5th Edition. Br J Pharmacol. 2011;164(Suppl. 1):S1–S324. doi: 10.1111/j.1476-5381.2011.01649_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arimura A, Shioda S. Pituitary adenylate cyclase activating polypeptide (PACAP) and its receptors: neuroendocrine and endocrine interaction. Front Neuroendocrinol. 1995;16:53–88. doi: 10.1006/frne.1995.1003. [DOI] [PubMed] [Google Scholar]
- Asnicar MA, Koster A, Heiman ML, Tinsley F, Smith DP, Galbreath E, et al. Vasoactive intestinal polypeptide/pituitary adenylate cyclase-activating peptide receptor 2 deficiency in mice results in growth retardation and increased basal metabolic rate. Endocrinology. 2002;143:3994–4006. doi: 10.1210/en.2002-220354. [DOI] [PubMed] [Google Scholar]
- Aton SJ, Colwell CS, Harmar AJ, Waschek J, Herzog ED. Vasoactive intestinal polypeptide mediates circadian rhythmicity and synchrony in mammalian clock neurons. Nat Neurosci. 2005;8:476–483. doi: 10.1038/nn1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubert N, Vaudry D, Falluel-Morel A, Desfeux A, Fisch C, Ancian P, et al. PACAP prevents toxicity induced by cisplatin in rat and primate neurons but not in proliferating ovary cells: involvement of the mitochondrial apoptotic pathway. Neurobiol Dis. 2008;32:66–80. doi: 10.1016/j.nbd.2008.06.014. [DOI] [PubMed] [Google Scholar]
- Barwell J, Gingell JJ, Watkins HA, Archbold JK, Poyner DR, Hay DL. Calcitonin and calcitonin receptor-like receptors: common themes with family B GPCRs? Br J Pharmacol. 2012;166:51–65. doi: 10.1111/j.1476-5381.2011.01525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basille M, Gonzalez BJ, Leroux P, Jeandel L, Fournier A, Vaudry H. Localization and characterization of PACAP receptors in the rat cerebellum during development: evidence for a stimulatory effect of PACAP on immature cerebellar granule cells. Neuroscience. 1993;57:329–338. doi: 10.1016/0306-4522(93)90066-o. [DOI] [PubMed] [Google Scholar]
- Basille M, Gonzalez BJ, Fournier A, Vaudry H. Ontogeny of pituitary adenylate cyclase-activating polypeptide (PACAP) receptors in the rat cerebellum: a quantitative autoradiographic study. Brain Res Dev Brain Res. 1994;82:81–89. doi: 10.1016/0165-3806(94)90150-3. [DOI] [PubMed] [Google Scholar]
- Basille M, Vaudry D, Coulouarn Y, Jegou S, Lihrmann I, Fournier A, et al. Comparative distribution of pituitary adenylate cyclase-activating polypeptide (PACAP) binding sites and PACAP receptor mRNAs in the rat brain during development. J Comp Neurol. 2000;425:495–509. [PubMed] [Google Scholar]
- Bechtold DA, Brown TM, Luckman SM, Piggins HD. Metabolic rhythm abnormalities in mice lacking VIP-VPAC2 signaling. Am J Physiol Regul Integr Comp Physiol. 2008;294:R344–R351. doi: 10.1152/ajpregu.00667.2007. [DOI] [PubMed] [Google Scholar]
- Beebe X, Darczak D, Davis-Taber RA, Uchic ME, Scott VE, Jarvis MF, et al. Discovery and SAR of hydrazide antagonists of the pituitary adenylate cyclase-activating polypeptide (PACAP) receptor type 1 (PAC1-R) Bioorg Med Chem Lett. 2008;18:2162–2166. doi: 10.1016/j.bmcl.2008.01.052. [DOI] [PubMed] [Google Scholar]
- Besson J, Sarrieau A, Vial M, Marie JC, Rosselin G, Rostene W. Characterization and autoradiographic distribution of vasoactive intestinal peptide binding sites in the rat central nervous system. Brain Res. 1986;398:329–336. doi: 10.1016/0006-8993(86)91493-9. [DOI] [PubMed] [Google Scholar]
- Bourgault S, Vaudry D, Segalas-Milazzo I, Guilhaudis L, Couvineau A, Laburthe M, et al. Molecular and conformational determinants of pituitary adenylate cyclase-activating polypeptide (PACAP) for activation of the PAC1 receptor. J Med Chem. 2009;52:3308–3316. doi: 10.1021/jm900291j. [DOI] [PubMed] [Google Scholar]
- Breault L, Yon L, Montero M, Chouinard L, Contesse V, Delarue C, et al. Occurrence and effect of PACAP in the human fetal adrenal gland. Ann N Y Acad Sci. 2000;921:429–433. doi: 10.1111/j.1749-6632.2000.tb07010.x. [DOI] [PubMed] [Google Scholar]
- Brenneman DE, Eiden LE. Vasoactive intestinal peptide and electrical activity influence neuronal survival. Proc Natl Acad Sci U S A. 1986;83:1159–1162. doi: 10.1073/pnas.83.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavallaro S, Copani A, D'Agata V, Musco S, Petralia S, Ventra C, et al. Pituitary adenylate cyclase activating polypeptide prevents apoptosis in cultured cerebellar granule neurons. Mol Pharmacol. 1996;50:60–66. [PubMed] [Google Scholar]
- Cheung CY, Holzwarth MA. Fetal adrenal VIP: distribution and effect on medullary catecholamine secretion. Peptides. 1986;7:413–418. doi: 10.1016/0196-9781(86)90007-0. [DOI] [PubMed] [Google Scholar]
- Christopoulos A, Christopoulos G, Morfis M, Udawela M, Laburthe M, Couvineau A, et al. Novel receptor partners and function of receptor activity-modifying proteins. J Biol Chem. 2003;278:3293–3297. doi: 10.1074/jbc.C200629200. [DOI] [PubMed] [Google Scholar]
- Chu A, Caldwell JS, Chen YA. Identification and characterization of a small molecule antagonist of human VPAC(2) receptor. Mol Pharmacol. 2010;77:95–101. doi: 10.1124/mol.109.060137. [DOI] [PubMed] [Google Scholar]
- Colwell CS, Michel S, Itri J, Rodriguez W, Tam J, Lelievre V, et al. Disrupted circadian rhythms in VIP- and PHI-deficient mice. Am J Physiol Regul Integr Comp Physiol. 2003;285:R939–R949. doi: 10.1152/ajpregu.00200.2003. [DOI] [PubMed] [Google Scholar]
- Colwell CS, Michel S, Itri J, Rodriguez W, Tam J, Lelievre V, et al. Selective deficits in the circadian light response in mice lacking PACAP. Am J Physiol Regul Integr Comp Physiol. 2004;287:R1194–R1201. doi: 10.1152/ajpregu.00268.2004. [DOI] [PubMed] [Google Scholar]
- Couvineau A, Laburthe M. VPAC receptors: structure, molecular pharmacology and interaction with accessory proteins. Br J Pharmacol. 2012a;166:42–50. doi: 10.1111/j.1476-5381.2011.01676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couvineau A, Laburthe M. The family B1 GPCR: structural aspects and interaction with accessory proteins. Curr Drug Targets. 2012b;13:103–115. doi: 10.2174/138945012798868434. [DOI] [PubMed] [Google Scholar]
- Cutler DJ, Haraura M, Reed HE, Shen S, Sheward WJ, Morrison CF, et al. The mouse VPAC2 receptor confers suprachiasmatic nuclei cellular rhythmicity and responsiveness to vasoactive intestinal polypeptide in vitro. Eur J Neurosci. 2003;17:197–204. doi: 10.1046/j.1460-9568.2003.02425.x. [DOI] [PubMed] [Google Scholar]
- Daniel PB, Kieffer TJ, Leech CA, Habener JF. Novel alternatively spliced exon in the extracellular ligand-binding domain of the pituitary adenylate cyclase-activating polypeptide (PACAP) type 1 receptor (PAC1R) selectively increases ligand affinity and alters signal transduction coupling during spermatogenesis. J Biol Chem. 2001;276:12938–12944. doi: 10.1074/jbc.M009941200. [DOI] [PubMed] [Google Scholar]
- Dautzenberg FM, Mevenkamp G, Wille S, Hauger RL. N-terminal splice variants of the type I PACAP receptor: isolation, characterization and ligand binding/selectivity determinants. J Neuroendocrinol. 1999;11:941–949. doi: 10.1046/j.1365-2826.1999.00411.x. [DOI] [PubMed] [Google Scholar]
- Dejda A, Bourgault S, Doan ND, Letourneau M, Couvineau A, Vaudry H, et al. Identification by photoaffinity labeling of the extracellular N-terminal domain of PAC1 receptor as the major binding site for PACAP. Biochimie. 2011;93:669–677. doi: 10.1016/j.biochi.2010.12.010. [DOI] [PubMed] [Google Scholar]
- Delgado M, Martinez C, Johnson MC, Gomariz RP, Ganea D. Differential expression of vasoactive intestinal peptide receptors 1 and 2 (VIP-R1 and VIP-R2) mRNA in murine lymphocytes. J Neuroimmunol. 1996;68:27–38. doi: 10.1016/0165-5728(96)00063-x. [DOI] [PubMed] [Google Scholar]
- Dickinson T, Fleetwood-Walker SM, Mitchell R, Lutz EM. Evidence for roles of vasoactive intestinal polypeptide (VIP) and pituitary adenylate cyclase activating polypeptide (PACAP) receptors in modulating the responses of rat dorsal horn neurons to sensory inputs. Neuropeptides. 1997;31:175–185. doi: 10.1016/s0143-4179(97)90087-1. [DOI] [PubMed] [Google Scholar]
- Dickson L, Finlayson K. VPAC and PAC receptors: from ligands to function. Pharmacol Ther. 2009;121:294–316. doi: 10.1016/j.pharmthera.2008.11.006. [DOI] [PubMed] [Google Scholar]
- Dickson L, Aramori I, McCulloch J, Sharkey J, Finlayson K. A systematic comparison of intracellular cyclic AMP and calcium signalling highlights complexities in human VPAC/PAC receptor pharmacology. Neuropharmacology. 2006;51:1086–1098. doi: 10.1016/j.neuropharm.2006.07.017. [DOI] [PubMed] [Google Scholar]
- Edwards AV, Jones CT. Adrenal responses to the peptide PACAP in conscious functionally hypophysectomized calves. Am J Physiol. 1994;266:E870–E876. doi: 10.1152/ajpendo.1994.266.6.E870. [DOI] [PubMed] [Google Scholar]
- Fabricius D, Karacay B, Shutt D, Leverich W, Schafer B, Takle E, et al. Characterization of intestinal and pancreatic dysfunction in VPAC1-null mutant mouse. Pancreas. 2011;40:861–871. doi: 10.1097/MPA.0b013e318214c783. [DOI] [PubMed] [Google Scholar]
- Fahrenkrug J. Transmitter role of vasoactive intestinal peptide. Pharmacol Toxicol. 1993;72:354–363. doi: 10.1111/j.1600-0773.1993.tb01344.x. [DOI] [PubMed] [Google Scholar]
- Furness SGB, Wootten D, Christopoulos A, Sexton PM. Consequences of splice variation on Secretin family G protein-coupled receptor function. Br J Pharmacol. 2012;166:98–109. doi: 10.1111/j.1476-5381.2011.01571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghatei MA, Takahashi K, Suzuki Y, Gardiner J, Jones PM, Bloom SR. Distribution, molecular characterization of pituitary adenylate cyclase-activating polypeptide and its precursor encoding messenger RNA in human and rat tissues. J Endocrinol. 1993;136:159–166. doi: 10.1677/joe.0.1360159. [DOI] [PubMed] [Google Scholar]
- Goetzl EJ, Voice JK, Shen S, Dorsam G, Kong Y, West KM, et al. Enhanced delayed-type hypersensitivity and diminished immediate-type hypersensitivity in mice lacking the inducible VPAC2 receptor for vasoactive intestinal peptide. Proc Natl Acad Sci U S A. 2001;98:13854–13859. doi: 10.1073/pnas.241503798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez BJ, Basille M, Vaudry D, Fournier A, Vaudry H. Pituitary adenylate cyclase-activating polypeptide promotes cell survival and neurite outgrowth in rat cerebellar neuroblasts. Neuroscience. 1997;78:419–430. doi: 10.1016/s0306-4522(96)00617-3. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Rey E, Fernandez-Martin A, Chorny A, Martin J, Pozo D, Ganea D, et al. Therapeutic effect of vasoactive intestinal peptide on experimental autoimmune encephalomyelitis: down-regulation of inflammatory and autoimmune responses. Am J Pathol. 2006;168:1179–1188. doi: 10.2353/ajpath.2006.051081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourlet P, De Neef P, Cnudde J, Waelbroeck M, Robberecht P. In vitro properties of a high affinity selective antagonist of the VIP1 receptor. Peptides. 1997a;18:1555–1560. doi: 10.1016/s0196-9781(97)00230-1. [DOI] [PubMed] [Google Scholar]
- Gourlet P, Vandermeers A, Vertongen P, Rathe J, De Neef P, Cnudde J, et al. Development of high affinity selective VIP1 receptor agonists. Peptides. 1997b;18:1539–1545. doi: 10.1016/s0196-9781(97)00228-3. [DOI] [PubMed] [Google Scholar]
- Gourlet P, Vertongen P, Vandermeers A, Vandermeers-Piret MC, Rathe J, De Neef P, et al. The long-acting vasoactive intestinal polypeptide agonist RO 25-1553 is highly selective of the VIP2 receptor subclass. Peptides. 1997c;18:403–408. doi: 10.1016/s0196-9781(96)00322-1. [DOI] [PubMed] [Google Scholar]
- Gozes I. NAP (davunetide) provides functional and structural neuroprotection. Curr Pharm Des. 2011;17:1040–1044. doi: 10.2174/138161211795589373. [DOI] [PubMed] [Google Scholar]
- Gozes I, Brenneman DE. VIP: molecular biology and neurobiological function. Mol Neurobiol. 1989;3:201–236. doi: 10.1007/BF02740606. [DOI] [PubMed] [Google Scholar]
- Grace CR, Perrin MH, DiGruccio MR, Miller CL, Rivier JE, Vale WW, et al. NMR structure and peptide hormone binding site of the first extracellular domain of a type B1 G protein-coupled receptor. Proc Natl Acad Sci U S A. 2004;101:12836–12841. doi: 10.1073/pnas.0404702101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gressens P, Marret S, Hill JM, Brenneman DE, Gozes I, Fridkin M, et al. Vasoactive intestinal peptide prevents excitotoxic cell death in the murine developing brain. J Clin Invest. 1997;100:390–397. doi: 10.1172/JCI119545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gressens P, Besse L, Robberecht P, Gozes I, Fridkin M, Evrard P. Neuroprotection of the developing brain by systemic administration of vasoactive intestinal peptide derivatives. J Pharmacol Exp Ther. 1999;288:1207–1213. [PubMed] [Google Scholar]
- Hamelink C, Tjurmina O, Damadzic R, Young WS, Weihe E, Lee HW, et al. Pituitary adenylate cyclase-activating polypeptide is a sympathoadrenal neurotransmitter involved in catecholamine regulation and glucohomeostasis. Proc Natl Acad Sci U S A. 2002;99:461–466. doi: 10.1073/pnas.012608999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammack SE, Roman CW, Lezak KR, Kocho-Shellenberg M, Grimmig B, Falls WA, et al. Roles for pituitary adenylate cyclase-activating peptide (PACAP) expression and signaling in the bed nucleus of the stria terminalis (BNST) in mediating the behavioral consequences of chronic stress. J Mol Neurosci. 2010;42:327–340. doi: 10.1007/s12031-010-9364-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannibal J, Jamen F, Nielsen HS, Journot L, Brabet P, Fahrenkrug J. Dissociation between light-induced phase shift of the circadian rhythm and clock gene expression in mice lacking the pituitary adenylate cyclase activating polypeptide type 1 receptor. J Neurosci. 2001;21:4883–4890. doi: 10.1523/JNEUROSCI.21-13-04883.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannibal J, Brabet P, Fahrenkrug J. Mice lacking the PACAP type I receptor have impaired photic entrainment and negative masking. Am J Physiol Regul Integr Comp Physiol. 2008;295:R2050–R2058. doi: 10.1152/ajpregu.90563.2008. [DOI] [PubMed] [Google Scholar]
- Hannibal J, Hsiung HM, Fahrenkrug J. Temporal phasing of locomotor activity, heart rate rhythmicity, and core body temperature is disrupted in VIP receptor 2-deficient mice. Am J Physiol Regul Integr Comp Physiol. 2011;300:R519–R530. doi: 10.1152/ajpregu.00599.2010. [DOI] [PubMed] [Google Scholar]
- Harmar AJ, Marston HM, Shen S, Spratt C, West KM, Sheward WJ, et al. The VPAC2 receptor is essential for circadian function in the mouse suprachiasmatic nuclei. Cell. 2002;109:497–508. doi: 10.1016/s0092-8674(02)00736-5. [DOI] [PubMed] [Google Scholar]
- Harmar AJ, Sheward WJ, Morrison CF, Waser B, Gugger M, Reubi JC. Distribution of the VPAC2 receptor in peripheral tissues of the mouse. Endocrinology. 2004;145:1203–1210. doi: 10.1210/en.2003-1058. [DOI] [PubMed] [Google Scholar]
- Hashimoto H, Nogi H, Mori K, Ohishi H, Shigemoto R, Yamamoto K, et al. Distribution of the mRNA for a pituitary adenylate cyclase-activating polypeptide receptor in the rat brain: an in situ hybridization study. J Comp Neurol. 1996;371:567–577. doi: 10.1002/(SICI)1096-9861(19960805)371:4<567::AID-CNE6>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Hawke Z, Ivanov TR, Bechtold DA, Dhillon H, Lowell BB, Luckman SM. PACAP neurons in the hypothalamic ventromedial nucleus are targets of central leptin signaling. J Neurosci. 2009;29:14828–14835. doi: 10.1523/JNEUROSCI.1526-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes AT, Fahey B, Cutler DJ, Coogan AN, Piggins HD. Aberrant gating of photic input to the suprachiasmatic circadian pacemaker of mice lacking the VPAC2 receptor. J Neurosci. 2004;24:3522–3526. doi: 10.1523/JNEUROSCI.5345-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichikawa S, Sreedharan SP, Owen RL, Goetzl EJ. Immunochemical localization of type I VIP receptor and NK-1-type substance P receptor in rat lung. Am J Physiol. 1995;268:L584–L588. doi: 10.1152/ajplung.1995.268.4.L584. [DOI] [PubMed] [Google Scholar]
- Inagaki N, Yoshida H, Mizuta M, Mizuno N, Fujii Y, Gonoi T, et al. Cloning and functional characterization of a third pituitary adenylate cyclase-activating polypeptide receptor subtype expressed in insulin-secreting cells. Proc Natl Acad Sci U S A. 1994;91:2679–2683. doi: 10.1073/pnas.91.7.2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara T, Shigemoto R, Mori K, Takahashi K, Nagata S. Functional expression and tissue distribution of a novel receptor for vasoactive intestinal polypeptide. Neuron. 1992;8:811–819. doi: 10.1016/0896-6273(92)90101-i. [DOI] [PubMed] [Google Scholar]
- Itoh N, Obata K, Yanaihara N, Okamoto H. Human preprovasoactive intestinal polypeptide contains a novel PHI-27-like peptide, PHM-27. Nature. 1983;304:547–549. doi: 10.1038/304547a0. [DOI] [PubMed] [Google Scholar]
- Jamen F, Persson K, Bertrand G, Rodriguez-Henche N, Puech R, Bockaert J, et al. PAC1 receptor-deficient mice display impaired insulinotropic response to glucose and reduced glucose tolerance. J Clin Invest. 2000;105:1307–1315. doi: 10.1172/JCI9387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jongsma H, Danielsen N, Sundler F, Kanje M. Alteration of PACAP distribution and PACAP receptor binding in the rat sensory nervous system following sciatic nerve transection. Brain Res. 2000;853:186–196. doi: 10.1016/s0006-8993(99)02233-7. [DOI] [PubMed] [Google Scholar]
- Jongsma H, Pettersson LM, Zhang Y, Reimer MK, Kanje M, Waldenstrom A, et al. Markedly reduced chronic nociceptive response in mice lacking the PAC1 receptor. Neuroreport. 2001;12:2215–2219. doi: 10.1097/00001756-200107200-00034. [DOI] [PubMed] [Google Scholar]
- Journot L, Waeber C, Pantaloni C, Holsboer F, Seeburg PH, Bockaert J, et al. Differential signal transduction by six splice variants of the pituitary adenylate cyclase-activating peptide (PACAP) receptor. Biochem Soc Trans. 1995;23:133–137. doi: 10.1042/bst0230133. [DOI] [PubMed] [Google Scholar]
- Juarranz MG, Santiago B, Torroba M, Gutierrez-Canas I, Palao G, Galindo M, et al. Vasoactive intestinal peptide modulates proinflammatory mediator synthesis in osteoarthritic and rheumatoid synovial cells. Rheumatology (Oxford) 2004;43:416–422. doi: 10.1093/rheumatology/keh061. [DOI] [PubMed] [Google Scholar]
- Kaltreider HB, Ichikawa S, Byrd PK, Ingram DA, Kishiyama JL, Sreedharan SP, et al. Upregulation of neuropeptides and neuropeptide receptors in a murine model of immune inflammation in lung parenchyma. Am J Respir Cell Mol Biol. 1997;16:133–144. doi: 10.1165/ajrcmb.16.2.9032120. [DOI] [PubMed] [Google Scholar]
- Kawaguchi C, Tanaka K, Isojima Y, Shintani N, Hashimoto H, Baba A, et al. Changes in light-induced phase shift of circadian rhythm in mice lacking PACAP. Biochem Biophys Res Commun. 2003;310:169–175. doi: 10.1016/j.bbrc.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Kawai K, Yokota C, Ohashi S, Isobe K, Suzuki S, Nakai T, et al. Pituitary adenylate cyclase-activating polypeptide: effects on pancreatic-adrenal hormone secretion and glucose-lipid metabolism in normal conscious dogs. Metabolism. 1994;43:739–744. doi: 10.1016/0026-0495(94)90124-4. [DOI] [PubMed] [Google Scholar]
- Kienlen Campard P, Crochemore C, Rene F, Monnier D, Koch B, Loeffler JP. PACAP type I receptor activation promotes cerebellar neuron survival through the cAMP/PKA signaling pathway. DNA Cell Biol. 1997;16:323–333. doi: 10.1089/dna.1997.16.323. [DOI] [PubMed] [Google Scholar]
- Krempels K, Usdin TB, Harta G, Mezey E. PACAP acts through VIP type 2 receptors in the rat testis. Neuropeptides. 1995;29:315–320. doi: 10.1016/0143-4179(95)90001-2. [DOI] [PubMed] [Google Scholar]
- Kumar S, Pioszak A, Zhang C, Swaminathan K, Xu HE. Crystal structure of the PAC1R extracellular domain unifies a consensus fold for hormone recognition by class B G-protein coupled receptors. PLoS ONE. 2011;6:e19682. doi: 10.1371/journal.pone.0019682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laburthe M, Couvineau A. Molecular pharmacology and structure of VPAC Receptors for VIP and PACAP. Regul Pept. 2002;108:165–173. doi: 10.1016/s0167-0115(02)00099-x. [DOI] [PubMed] [Google Scholar]
- Laburthe M, Amiranoff B, Boige N, Rouyer-Fessard C, Tatemoto K, Moroder L. Interaction of GRF with VIP receptors and stimulation of adenylate cyclase in rat and human intestinal epithelial membranes. Comparison with PHI and secretin. FEBS Lett. 1983;159:89–92. doi: 10.1016/0014-5793(83)80422-0. [DOI] [PubMed] [Google Scholar]
- Laburthe M, Couvineau A, Tan V. Class II G protein-coupled receptors for VIP and PACAP: structure, models of activation and pharmacology. Peptides. 2007;28:1631–1639. doi: 10.1016/j.peptides.2007.04.026. [DOI] [PubMed] [Google Scholar]
- Lamouche S, Martineau D, Yamaguchi N. Modulation of adrenal catecholamine release by PACAP in vivo. Am J Physiol. 1999;276:R162–R170. doi: 10.1152/ajpregu.1999.276.1.R162. [DOI] [PubMed] [Google Scholar]
- Lee HW, Hahm SH, Hsu CM, Eiden LE. Pituitary adenylate cyclase-activating polypeptide regulation of vasoactive intestinal polypeptide transcription requires Ca2+ influx and activation of the serine/threonine phosphatase calcineurin. J Neurochem. 1999;73:1769–1772. doi: 10.1046/j.1471-4159.1999.731769.x. [DOI] [PubMed] [Google Scholar]
- Lelievre V, Favrais G, Abad C, Adle-Biassette H, Lu Y, Germano PM, et al. Gastrointestinal dysfunction in mice with a targeted mutation in the gene encoding vasoactive intestinal polypeptide: a model for the study of intestinal ileus and Hirschsprung's disease. Peptides. 2007;28:1688–1699. doi: 10.1016/j.peptides.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinson DF, Duan J, Oh S, Wang K, Sanders AR, Shi J, et al. Copy number variants in schizophrenia: confirmation of five previous findings and new evidence for 3q29 microdeletions and VIPR2 duplications. Am J Psychiatry. 2011;168:302–316. doi: 10.1176/appi.ajp.2010.10060876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luis J, Said SI. Characterization of VIP- and helodermin-preferring receptors on human small cell lung carcinoma cell lines. Peptides. 1990;11:1239–1244. doi: 10.1016/0196-9781(90)90158-2. [DOI] [PubMed] [Google Scholar]
- Lutz EM, Sheward WJ, West KM, Morrow JA, Fink G, Harmar AJ. The VIP2 receptor: molecular characterisation of a cDNA encoding a novel receptor for vasoactive intestinal peptide. FEBS Lett. 1993;334:3–8. doi: 10.1016/0014-5793(93)81668-p. [DOI] [PubMed] [Google Scholar]
- Mabuchi T, Shintani N, Matsumura S, Okuda-Ashitaka E, Hashimoto H, Muratani T, et al. Pituitary adenylate cyclase-activating polypeptide is required for the development of spinal sensitization and induction of neuropathic pain. J Neurosci. 2004;24:7283–7291. doi: 10.1523/JNEUROSCI.0983-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra RK, Wakade TD, Wakade AR. Vasoactive intestinal polypeptide and muscarine mobilize intracellular Ca2+ through breakdown of phosphoinositides to induce catecholamine secretion. Role of IP3 in exocytosis. J Biol Chem. 1988;263:2123–2126. [PubMed] [Google Scholar]
- Martin JL, Dietl MM, Hof PR, Palacios JM, Magistretti PJ. Autoradiographic mapping of [mono[125I]iodo-Tyr10, MetO17]vasoactive intestinal peptide binding sites in the rat brain. Neuroscience. 1987;23:539–565. doi: 10.1016/0306-4522(87)90075-3. [DOI] [PubMed] [Google Scholar]
- Martinez C, Abad C, Delgado M, Arranz A, Juarranz MG, Rodriguez-Henche N, et al. Anti-inflammatory role in septic shock of pituitary adenylate cyclase-activating polypeptide receptor. Proc Natl Acad Sci U S A. 2002;99:1053–1058. doi: 10.1073/pnas.012367999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuyama S, Matsumoto A, Hashimoto H, Shintani N, Baba A. Impaired long-term potentiation in vivo in the dentate gyrus of pituitary adenylate cyclase-activating polypeptide (PACAP) or PACAP type 1 receptor-mutant mice. Neuroreport. 2003;14:2095–2098. doi: 10.1097/00001756-200311140-00017. [DOI] [PubMed] [Google Scholar]
- Miampamba M, Germano PM, Arli S, Wong HH, Scott D, Tache Y, et al. Expression of pituitary adenylate cyclase-activating polypeptide and PACAP type 1 receptor in the rat gastric and colonic myenteric neurons. Regul Pept. 2002;105:145–154. doi: 10.1016/s0167-0115(02)00003-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata A, Arimura A, Dahl RR, Minamino N, Uehara A, Jiang L, et al. Isolation of a novel 38 residue-hypothalamic polypeptide which stimulates adenylate cyclase in pituitary cells. Biochem Biophys Res Commun. 1989;164:567–574. doi: 10.1016/0006-291x(89)91757-9. [DOI] [PubMed] [Google Scholar]
- Miyata A, Jiang L, Dahl RD, Kitada C, Kubo K, Fujino M, et al. Isolation of a neuropeptide corresponding to the N-terminal 27 residues of the pituitary adenylate cyclase activating polypeptide with 38 residues (PACAP38) Biochem Biophys Res Commun. 1990;170:643–648. doi: 10.1016/0006-291x(90)92140-u. [DOI] [PubMed] [Google Scholar]
- Modlin IM, Bloom SR, Mitchell S. VIP: the cause of the watery diarrhoea syndrome. Adv Exp Med Biol. 1978;106:195–201. doi: 10.1007/978-1-4684-7248-6_24. [DOI] [PubMed] [Google Scholar]
- Moller K, Sundler F. Expression of pituitary adenylate cyclase activating peptide (PACAP) and PACAP type I receptors in the rat adrenal medulla. Regul Pept. 1996;63:129–139. doi: 10.1016/0167-0115(96)00033-x. [DOI] [PubMed] [Google Scholar]
- Moreno D, Gourlet P, De Neef P, Cnudde J, Waelbroeck M, Robberecht P. Development of selective agonists and antagonists for the human vasoactive intestinal polypeptide VPAC2 receptor. Peptides. 2000;21:1543–1549. doi: 10.1016/s0196-9781(00)00309-0. [DOI] [PubMed] [Google Scholar]
- Moro O, Lerner EA. Maxadilan, the vasodilator from sand flies, is a specific pituitary adenylate cyclase activating peptide type I receptor agonist. J Biol Chem. 1997;272:966–970. doi: 10.1074/jbc.272.2.966. [DOI] [PubMed] [Google Scholar]
- Mounien L, Do Rego JC, Bizet P, Boutelet I, Gourcerol G, Fournier A, et al. Pituitary adenylate cyclase-activating polypeptide inhibits food intake in mice through activation of the hypothalamic melanocortin system. Neuropsychopharmacology. 2009;34:424–435. doi: 10.1038/npp.2008.73. [DOI] [PubMed] [Google Scholar]
- Neri G, Andreis PG, Prayer-Galetti T, Rossi GP, Malendowicz LK, Nussdorfer GG. Pituitary adenylate-cyclase activating peptide enhances aldosterone secretion of human adrenal gland: evidence for an indirect mechanism, probably involving the local release of catecholamines. J Clin Endocrinol Metab. 1996;81:169–173. doi: 10.1210/jcem.81.1.8550747. [DOI] [PubMed] [Google Scholar]
- Neumann JM, Couvineau A, Murail S, Lacapere JJ, Jamin N, Laburthe M. Class-B GPCR activation: is ligand helix-capping the key? Trends Biochem Sci. 2008;33:314–319. doi: 10.1016/j.tibs.2008.05.001. [DOI] [PubMed] [Google Scholar]
- Nicole P, Lins L, Rouyer-Fessard C, Drouot C, Fulcrand P, Thomas A, et al. Identification of key residues for interaction of vasoactive intestinal peptide with human VPAC1 and VPAC2 receptors and development of a highly selective VPAC1 receptor agonist. Alanine scanning and molecular modeling of the peptide. J Biol Chem. 2000;275:24003–24012. doi: 10.1074/jbc.M002325200. [DOI] [PubMed] [Google Scholar]
- Ohtaki H, Nakamachi T, Dohi K, Aizawa Y, Takaki A, Hodoyama K, et al. Pituitary adenylate cyclase-activating polypeptide (PACAP) decreases ischemic neuronal cell death in association with IL-6. Proc Natl Acad Sci U S A. 2006;103:7488–7493. doi: 10.1073/pnas.0600375103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottaway CA. Selective effects of vasoactive intestinal peptide on the mitogenic response of murine T cells. Immunology. 1987;62:291–297. [PMC free article] [PubMed] [Google Scholar]
- Otto C, Kovalchuk Y, Wolfer DP, Gass P, Martin M, Zuschratter W, et al. Impairment of mossy fiber long-term potentiation and associative learning in pituitary adenylate cyclase activating polypeptide type I receptor-deficient mice. J Neurosci. 2001a;21:5520–5527. doi: 10.1523/JNEUROSCI.21-15-05520.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto C, Martin M, Wolfer DP, Lipp HP, Maldonado R, Schutz G. Altered emotional behavior in PACAP-type-I-receptor-deficient mice. Brain Res Mol Brain Res. 2001b;92:78–84. doi: 10.1016/s0169-328x(01)00153-x. [DOI] [PubMed] [Google Scholar]
- Otto C, Hein L, Brede M, Jahns R, Engelhardt S, Grone HJ, et al. Pulmonary hypertension and right heart failure in pituitary adenylate cyclase-activating polypeptide type I receptor-deficient mice. Circulation. 2004;110:3245–3251. doi: 10.1161/01.CIR.0000147235.53360.59. [DOI] [PubMed] [Google Scholar]
- Pantaloni C, Brabet P, Bilanges B, Dumuis A, Houssami S, Spengler D, et al. Alternative splicing in the N-terminal extracellular domain of the pituitary adenylate cyclase-activating polypeptide (PACAP) receptor modulates receptor selectivity and relative potencies of PACAP-27 and PACAP-38 in phospholipase C activation. J Biol Chem. 1996;271:22146–22151. doi: 10.1074/jbc.271.36.22146. [DOI] [PubMed] [Google Scholar]
- Persson K, Ahren B. The neuropeptide PACAP contributes to the glucagon response to insulin-induced hypoglycaemia in mice. Acta Physiol Scand. 2002;175:25–28. doi: 10.1046/j.1365-201X.2002.00977.x. [DOI] [PubMed] [Google Scholar]
- Piggins HD. Schizophrenia: zooming in on a gene. Nature. 2011;471:455–456. doi: 10.1038/471455a. [DOI] [PubMed] [Google Scholar]
- Pisegna JR, Wank SA. Molecular cloning and functional expression of the pituitary adenylate cyclase-activating polypeptide type I receptor. Proc Natl Acad Sci U S A. 1993;90:6345–6349. doi: 10.1073/pnas.90.13.6345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisegna JR, Wank SA. Cloning and characterization of the signal transduction of four splice variants of the human pituitary adenylate cyclase activating polypeptide receptor. Evidence for dual coupling to adenylate cyclase and phospholipase C. J Biol Chem. 1996;271:17267–17274. doi: 10.1074/jbc.271.29.17267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przywara DA, Guo X, Angelilli ML, Wakade TD, Wakade AR. A non-cholinergic transmitter, pituitary adenylate cyclase-activating polypeptide, utilizes a novel mechanism to evoke catecholamine secretion in rat adrenal chromaffin cells. J Biol Chem. 1996;271:10545–10550. doi: 10.1074/jbc.271.18.10545. [DOI] [PubMed] [Google Scholar]
- Rangon CM, Goursaud S, Medja F, Lelievre V, Mounien L, Husson I, et al. VPAC2 receptors mediate vasoactive intestinal peptide-induced neuroprotection against neonatal excitotoxic brain lesions in mice. J Pharmacol Exp Ther. 2005;314:745–752. doi: 10.1124/jpet.105.086405. [DOI] [PubMed] [Google Scholar]
- Raufman JP, Malhotra R, Singh L. PACAP-38, a novel peptide from ovine hypothalamus, is a potent modulator of amylase release from dispersed acini from rat pancreas. Regul Pept. 1991;36:121–129. doi: 10.1016/0167-0115(91)90200-z. [DOI] [PubMed] [Google Scholar]
- Reichlin S. Neuroendocrine significance of vasoactive intestinal polypeptide. Ann N Y Acad Sci. 1988;527:431–449. doi: 10.1111/j.1749-6632.1988.tb26998.x. [DOI] [PubMed] [Google Scholar]
- Ressler KJ, Mercer KB, Bradley B, Jovanovic T, Mahan A, Kerley K, et al. Post-traumatic stress disorder is associated with PACAP and the PAC1 receptor. Nature. 2011;470:492–497. doi: 10.1038/nature09856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reubi JC. In vitro evaluation of VIP/PACAP receptors in healthy and diseased human tissues. Clinical implications. Ann N Y Acad Sci. 2000;921:1–25. doi: 10.1111/j.1749-6632.2000.tb06946.x. [DOI] [PubMed] [Google Scholar]
- Reubi JC, Laderach U, Waser B, Gebbers JO, Robberecht P, Laissue JA. Vasoactive intestinal peptide/pituitary adenylate cyclase-activating peptide receptor subtypes in human tumors and their tissues of origin. Cancer Res. 2000;60:3105–3112. [PubMed] [Google Scholar]
- Reubi JC, Korner M, Waser B, Mazzucchelli L, Guillou L. High expression of peptide receptors as a novel target in gastrointestinal stromal tumours. Eur J Nucl Med Mol Imaging. 2004;31:803–810. doi: 10.1007/s00259-004-1476-2. [DOI] [PubMed] [Google Scholar]
- Robberecht P, Waelbroeck M, De Neef P, Tastenoy M, Gourlet P, Cogniaux J, et al. A new type of functional VIP receptor has an affinity for helodermin in human SUP-T1 lymphoblasts. FEBS Lett. 1988;228:351–355. doi: 10.1016/0014-5793(88)80030-9. [DOI] [PubMed] [Google Scholar]
- Robberecht P, Woussen-Colle MC, De Neef P, Gourlet P, Buscail L, Vandermeers A, et al. The two forms of the pituitary adenylate cyclase activating polypeptide (PACAP (1-27) and PACAP (1-38)) interact with distinct receptors on rat pancreatic AR 4-2J cell membranes. FEBS Lett. 1991;286:133–136. doi: 10.1016/0014-5793(91)80958-6. [DOI] [PubMed] [Google Scholar]
- Robberecht P, Vertongen P, Velkeniers B, de Neef P, Vergani P, Raftopoulos C, et al. Receptors for pituitary adenylate cyclase activating peptides in human pituitary adenomas. J Clin Endocrinol Metab. 1993;77:1235–1239. doi: 10.1210/jcem.77.5.8077316. [DOI] [PubMed] [Google Scholar]
- Robberecht P, Woussen-Colle MC, Vertongen P, De Neef P, Hou X, Salmon I, et al. Expression of pituitary adenylate cyclase activating polypeptide (PACAP) receptors in human glial cell tumors. Peptides. 1994;15:661–665. doi: 10.1016/0196-9781(94)90092-2. [DOI] [PubMed] [Google Scholar]
- Said SI. Vasoactive intestinal polypeptide: biologic role in health and disease. Trends Endocrinol Metab. 1991;2:107–112. doi: 10.1016/s1043-2760(05)80006-2. [DOI] [PubMed] [Google Scholar]
- Said SI. Vasoactive intestinal peptide and nitric oxide: divergent roles in relation to tissue injury. Ann N Y Acad Sci. 1996;805:379–387. doi: 10.1111/j.1749-6632.1996.tb17498.x. discussion 387–378. [DOI] [PubMed] [Google Scholar]
- Said SI, Mutt V. Polypeptide with broad biological activity: isolation from small intestine. Science. 1970;169:1217–1218. doi: 10.1126/science.169.3951.1217. [DOI] [PubMed] [Google Scholar]
- Said SI, Mutt V. Isolation from porcine-intestinal wall of a vasoactive octacosapeptide related to secretin and to glucagon. Eur J Biochem. 1972;28:199–204. doi: 10.1111/j.1432-1033.1972.tb01903.x. [DOI] [PubMed] [Google Scholar]
- Said SI, Rattan S. The multiple mediators of neurogenic smooth muscle relaxation. Trends Endocrinol Metab. 2004;15:189–191. doi: 10.1016/j.tem.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Sharman JL, Mpamhanga CP, Spedding M, Germain P, Staels B, Dacquet C, et al. IUPHAR-DB: new receptors and tools for easy searching and visualization of pharmacological data. Nucleic Acids Res. 2011;39:D534–D538. doi: 10.1093/nar/gkq1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheward WJ, Lutz EM, Harmar AJ. The distribution of vasoactive intestinal peptide2 receptor messenger RNA in the rat brain and pituitary gland as assessed by in situ hybridization. Neuroscience. 1995;67:409–418. doi: 10.1016/0306-4522(95)00048-n. [DOI] [PubMed] [Google Scholar]
- Sheward WJ, Lutz EM, Copp AJ, Harmar AJ. Expression of PACAP, and PACAP type 1 (PAC1) receptor mRNA during development of the mouse embryo. Brain Res Dev Brain Res. 1998;109:245–253. doi: 10.1016/s0165-3806(98)00086-8. [DOI] [PubMed] [Google Scholar]
- Shioda S, Shuto Y, Somogyvari-Vigh A, Legradi G, Onda H, Coy DH, et al. Localization and gene expression of the receptor for pituitary adenylate cyclase-activating polypeptide in the rat brain. Neurosci Res. 1997;28:345–354. doi: 10.1016/s0168-0102(97)00065-5. [DOI] [PubMed] [Google Scholar]
- Shioda S, Shimoda Y, Hori T, Mizushima H, Ajiri T, Funahashi H, et al. Localization of the pituitary adenylate cyclase-activating polypeptide receptor and its mRNA in the rat adrenal medulla. Neurosci Lett. 2000;295:81–84. doi: 10.1016/s0304-3940(00)01595-0. [DOI] [PubMed] [Google Scholar]
- Shivers BD, Gorcs TJ, Gottschall PE, Arimura A. Two high affinity binding sites for pituitary adenylate cyclase-activating polypeptide have different tissue distributions. Endocrinology. 1991;128:3055–3065. doi: 10.1210/endo-128-6-3055. [DOI] [PubMed] [Google Scholar]
- Sorg O, Magistretti PJ. Vasoactive intestinal peptide and noradrenaline exert long-term control on glycogen levels in astrocytes: blockade by protein synthesis inhibition. J Neurosci. 1992;12:4923–4931. doi: 10.1523/JNEUROSCI.12-12-04923.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spengler D, Waeber C, Pantaloni C, Holsboer F, Bockaert J, Seeburg PH, et al. Differential signal transduction by five splice variants of the PACAP receptor. Nature. 1993;365:170–175. doi: 10.1038/365170a0. [DOI] [PubMed] [Google Scholar]
- Sreedharan SP, Huang JX, Cheung MC, Goetzl EJ. Structure, expression, and chromosomal localization of the type I human vasoactive intestinal peptide receptor gene. Proc Natl Acad Sci U S A. 1995;92:2939–2943. doi: 10.1073/pnas.92.7.2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroth N, Eiden LE. Stress hormone synthesis in mouse hypothalamus and adrenal gland triggered by restraint is dependent on pituitary adenylate cyclase-activating polypeptide signaling. Neuroscience. 2010;165:1025–1030. doi: 10.1016/j.neuroscience.2009.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C, Song D, Davis-Taber RA, Barrett LW, Scott VE, Richardson PL, et al. Solution structure and mutational analysis of pituitary adenylate cyclase-activating polypeptide binding to the extracellular domain of PAC1-RS. Proc Natl Acad Sci U S A. 2007;104:7875–7880. doi: 10.1073/pnas.0611397104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabadfi K, Atlasz T, Kiss P, Danyadi B, Tamas A, Helyes Z, et al. Mice deficient in pituitary adenylate cyclase activating polypeptide (PACAP) are more susceptible to retinal ischemic injury in vivo. Neurotox Res. 2012;21:41–48. doi: 10.1007/s12640-011-9254-y. [DOI] [PubMed] [Google Scholar]
- Tan YV, Couvineau A, Murail S, Ceraudo E, Neumann JM, Lacapere JJ, et al. Peptide agonist docking in the N-terminal ectodomain of a class II G protein-coupled receptor, the VPAC1 receptor. Photoaffinity, NMR, and molecular modeling. J Biol Chem. 2006;281:12792–12798. doi: 10.1074/jbc.M513305200. [DOI] [PubMed] [Google Scholar]
- Tan YV, Abad C, Lopez R, Dong H, Liu S, Lee A, et al. Pituitary adenylyl cyclase-activating polypeptide is an intrinsic regulator of Treg abundance and protects against experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A. 2009;106:2012–2017. doi: 10.1073/pnas.0812257106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatemoto K, Mutt V. Isolation and characterization of the intestinal peptide porcine PHI (PHI-27), a new member of the glucagon – secretin family. Proc Natl Acad Sci U S A. 1981;78:6603–6607. doi: 10.1073/pnas.78.11.6603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatsuno I, Uchida D, Tanaka T, Saeki N, Hirai A, Saito Y, et al. Maxadilan specifically interacts with PAC1 receptor, which is a dominant form of PACAP/VIP family receptors in cultured rat cortical neurons. Brain Res. 2001;889:138–148. doi: 10.1016/s0006-8993(00)03126-7. [DOI] [PubMed] [Google Scholar]
- Tomimoto S, Hashimoto H, Shintani N, Yamamoto K, Kawabata Y, Hamagami K, et al. Overexpression of pituitary adenylate cyclase-activating polypeptide in islets inhibits hyperinsulinemia and islet hyperplasia in agouti yellow mice. J Pharmacol Exp Ther. 2004;309:796–803. doi: 10.1124/jpet.103.062919. [DOI] [PubMed] [Google Scholar]
- Tse DL, Pang RT, Wong AO, Chan SM, Vaudry H, Chow BK. Identification of a potential receptor for both peptide histidine isoleucine and peptide histidine valine. Endocrinology. 2002;143:1327–1336. doi: 10.1210/endo.143.4.8714. [DOI] [PubMed] [Google Scholar]
- Tsukiyama N, Saida Y, Kakuda M, Shintani N, Hayata A, Morita Y, et al. PACAP centrally mediates emotional stress-induced corticosterone responses in mice. Stress. 2011;14:368–375. doi: 10.3109/10253890.2010.544345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsumi M, Claus TH, Liang Y, Li Y, Yang L, Zhu J, et al. A potent and highly selective VPAC2 agonist enhances glucose-induced insulin release and glucose disposal: a potential therapy for type 2 diabetes. Diabetes. 2002;51:1453–1460. doi: 10.2337/diabetes.51.5.1453. [DOI] [PubMed] [Google Scholar]
- Uchida D, Tatsuno I, Tanaka T, Hirai A, Saito Y, Moro O, et al. Maxadilan is a specific agonist and its deleted peptide (M65) is a specific antagonist for PACAP type 1 receptor. Ann N Y Acad Sci. 1998;865:253–258. doi: 10.1111/j.1749-6632.1998.tb11185.x. [DOI] [PubMed] [Google Scholar]
- Usdin TB, Bonner TI, Mezey E. Two receptors for vasoactive intestinal polypeptide with similar specificity and complementary distributions. Endocrinology. 1994;135:2662–2680. doi: 10.1210/endo.135.6.7988457. [DOI] [PubMed] [Google Scholar]
- Vacic V, McCarthy S, Malhotra D, Murray F, Chou HH, Peoples A, et al. Duplications of the neuropeptide receptor gene VIPR2 confer significant risk for schizophrenia. Nature. 2011;471:499–503. doi: 10.1038/nature09884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Rampelbergh J, Gourlet P, De Neef P, Robberecht P, Waelbroeck M. Properties of the pituitary adenylate cyclase-activating polypeptide I and II receptors, vasoactive intestinal peptide1, and chimeric amino-terminal pituitary adenylate cyclase-activating polypeptide/vasoactive intestinal peptide1 receptors: evidence for multiple receptor states. Mol Pharmacol. 1996;50:1596–1604. [PubMed] [Google Scholar]
- Vaudry D, Gonzalez BJ, Basille M, Anouar Y, Fournier A, Vaudry H. Pituitary adenylate cyclase-activating polypeptide stimulates both c-fos gene expression and cell survival in rat cerebellar granule neurons through activation of the protein kinase A pathway. Neuroscience. 1998;84:801–812. doi: 10.1016/s0306-4522(97)00545-9. [DOI] [PubMed] [Google Scholar]
- Vaudry D, Gonzalez BJ, Basille M, Fournier A, Vaudry H. Neurotrophic activity of pituitary adenylate cyclase-activating polypeptide on rat cerebellar cortex during development. Proc Natl Acad Sci U S A. 1999;96:9415–9420. doi: 10.1073/pnas.96.16.9415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaudry D, Gonzalez BJ, Basille M, Pamantung TF, Fontaine M, Fournier A, et al. The neuroprotective effect of pituitary adenylate cyclase-activating polypeptide on cerebellar granule cells is mediated through inhibition of the CED3-related cysteine protease caspase-3/CPP32. Proc Natl Acad Sci U S A. 2000;97:13390–13395. doi: 10.1073/pnas.97.24.13390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaudry D, Pamantung TF, Basille M, Rousselle C, Fournier A, Vaudry H, et al. PACAP protects cerebellar granule neurons against oxidative stress-induced apoptosis. Eur J Neurosci. 2002a;15:1451–1460. doi: 10.1046/j.1460-9568.2002.01981.x. [DOI] [PubMed] [Google Scholar]
- Vaudry D, Rousselle C, Basille M, Falluel-Morel A, Pamantung TF, Fontaine M, et al. Pituitary adenylate cyclase-activating polypeptide protects rat cerebellar granule neurons against ethanol-induced apoptotic cell death. Proc Natl Acad Sci U S A. 2002b;99:6398–6403. doi: 10.1073/pnas.082112699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaudry D, Falluel-Morel A, Basille M, Pamantung TF, Fontaine M, Fournier A, et al. Pituitary adenylate cyclase-activating polypeptide prevents C2-ceramide-induced apoptosis of cerebellar granule cells. J Neurosci Res. 2003;72:303–316. doi: 10.1002/jnr.10530. [DOI] [PubMed] [Google Scholar]
- Vaudry D, Falluel-Morel A, Bourgault S, Basille M, Burel D, Wurtz O, et al. Pituitary adenylate cyclase-activating polypeptide and its receptors: 20 years after the discovery. Pharmacol Rev. 2009;61:283–357. doi: 10.1124/pr.109.001370. [DOI] [PubMed] [Google Scholar]
- Vertongen P, Devalck C, Sariban E, De Laet MH, Martelli H, Paraf F, et al. Pituitary adenylate cyclase activating peptide and its receptors are expressed in human neuroblastomas. J Cell Physiol. 1996;167:36–46. doi: 10.1002/(SICI)1097-4652(199604)167:1<36::AID-JCP4>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Vertongen P, Schiffmann SN, Gourlet P, Robberecht P. Autoradiographic visualization of the receptor subclasses for vasoactive intestinal polypeptide (VIP) in rat brain. Peptides. 1997;18:1547–1554. doi: 10.1016/s0196-9781(97)00229-5. [DOI] [PubMed] [Google Scholar]
- Villalba M, Bockaert J, Journot L. Pituitary adenylate cyclase-activating polypeptide (PACAP-38) protects cerebellar granule neurons from apoptosis by activating the mitogen-activated protein kinase (MAP kinase) pathway. J Neurosci. 1997;17:83–90. doi: 10.1523/JNEUROSCI.17-01-00083.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voice JK, Dorsam G, Lee H, Kong Y, Goetzl EJ. Allergic diathesis in transgenic mice with constitutive T cell expression of inducible vasoactive intestinal peptide receptor. FASEB J. 2001;15:2489–2496. doi: 10.1096/fj.01-0671com. [DOI] [PubMed] [Google Scholar]
- Voice JK, Dorsam G, Chan RC, Grinninger C, Kong Y, Goetzl EJ. Immunoeffector and immunoregulatory activities of vasoactive intestinal peptide. Regul Pept. 2002;109:199–208. doi: 10.1016/s0167-0115(02)00182-9. [DOI] [PubMed] [Google Scholar]
- Voice JK, Grinninger C, Kong Y, Bangale Y, Paul S, Goetzl EJ. Roles of vasoactive intestinal peptide (VIP) in the expression of different immune phenotypes by wild-type mice and T cell-targeted type II VIP receptor transgenic mice. J Immunol. 2003;170:308–314. doi: 10.4049/jimmunol.170.1.308. [DOI] [PubMed] [Google Scholar]
- Waschek JA, Casillas RA, Nguyen TB, DiCicco-Bloom EM, Carpenter EM, Rodriguez WI. Neural tube expression of pituitary adenylate cyclase-activating peptide (PACAP) and receptor: potential role in patterning and neurogenesis. Proc Natl Acad Sci U S A. 1998;95:9602–9607. doi: 10.1073/pnas.95.16.9602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T, Masuo Y, Matsumoto H, Suzuki N, Ohtaki T, Masuda Y, et al. Pituitary adenylate cyclase activating polypeptide provokes cultured rat chromaffin cells to secrete adrenaline. Biochem Biophys Res Commun. 1992;182:403–411. doi: 10.1016/s0006-291x(05)80159-7. [DOI] [PubMed] [Google Scholar]
- Wei Y, Mojsov S. Tissue specific expression of different human receptor types for pituitary adenylate cyclase activating polypeptide and vasoactive intestinal polypeptide: implications for their role in human physiology. J Neuroendocrinol. 1996;8:811–817. doi: 10.1046/j.1365-2826.1996.05191.x. [DOI] [PubMed] [Google Scholar]
- Xia M, Sreedharan SP, Bolin DR, Gaufo GO, Goetzl EJ. Novel cyclic peptide agonist of high potency and selectivity for the type II vasoactive intestinal peptide receptor. J Pharmacol Exp Ther. 1997;281:629–633. [PubMed] [Google Scholar]
- Yada T, Sakurada M, Ihida K, Nakata M, Murata F, Arimura A, et al. Pituitary adenylate cyclase activating polypeptide is an extraordinarily potent intra-pancreatic regulator of insulin secretion from islet beta-cells. J Biol Chem. 1994;269:1290–1293. [PubMed] [Google Scholar]
- Yamamoto K, Hashimoto H, Tomimoto S, Shintani N, Miyazaki J, Tashiro F, et al. Overexpression of PACAP in transgenic mouse pancreatic β-cells enhances insulin secretion and ameliorates streptozotocin-induced diabetes. Diabetes. 2003;52:1155–1162. doi: 10.2337/diabetes.52.5.1155. [DOI] [PubMed] [Google Scholar]
- Yiangou Y, Di Marzo V, Spokes RA, Panico M, Morris HR, Bloom SR. Isolation, characterization, and pharmacological actions of peptide histidine valine 42, a novel prepro-vasoactive intestinal peptide-derived peptide. J Biol Chem. 1987;262:14010–14013. [PubMed] [Google Scholar]
- Yon L, Breault L, Contesse V, Bellancourt G, Delarue C, Fournier A, et al. Localization, characterization, and second messenger coupling of pituitary adenylate cyclase-activating polypeptide receptors in the fetal human adrenal gland during the second trimester of gestation. J Clin Endocrinol Metab. 1998;83:1299–1305. doi: 10.1210/jcem.83.4.4690. [DOI] [PubMed] [Google Scholar]
- Zaben M, Sheward WJ, Shtaya A, Abbosh C, Harmar AJ, Pringle AK, et al. The neurotransmitter VIP expands the pool of symmetrically dividing postnatal dentate gyrus precursors via VPAC2 receptors or directs them toward a neuronal fate via VPAC1 receptors. Stem Cells. 2009;27:2539–2551. doi: 10.1002/stem.184. [DOI] [PubMed] [Google Scholar]
- Zeng N, Athmann C, Kang T, Lyu RM, Walsh JH, Ohning GV, et al. PACAP type I receptor activation regulates ECL cells and gastric acid secretion. J Clin Invest. 1999;104:1383–1391. doi: 10.1172/JCI7537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou CJ, Shioda S, Shibanuma M, Nakajo S, Funahashi H, Nakai Y, et al. Pituitary adenylate cyclase-activating polypeptide receptors during development: expression in the rat embryo at primitive streak stage. Neuroscience. 1999;93:375–391. doi: 10.1016/s0306-4522(99)00108-6. [DOI] [PubMed] [Google Scholar]
- Zupan V, Hill JM, Brenneman DE, Gozes I, Fridkin M, Robberecht P, et al. Involvement of pituitary adenylate cyclase-activating polypeptide II vasoactive intestinal peptide 2 receptor in mouse neocortical astrocytogenesis. J Neurochem. 1998;70:2165–2173. doi: 10.1046/j.1471-4159.1998.70052165.x. [DOI] [PubMed] [Google Scholar]
- Zusev M, Gozes I. Differential regulation of activity-dependent neuroprotective protein in rat astrocytes by VIP and PACAP. Regul Pept. 2004;123:33–41. doi: 10.1016/j.regpep.2004.05.021. [DOI] [PubMed] [Google Scholar]