An expanded Ragulator is a GEF for the Rag GTPases that signal amino acid levels to mTORC1 (original) (raw)
. Author manuscript; available in PMC: 2013 Mar 14.
Abstract
The mTOR Complex 1 (mTORC1) pathway promotes cell growth in response to a diverse set of cues, including growth factors as well as energy and amino acid levels. Amino acids signal through the Rag GTPases to promote the translocation of mTORC1 to the lysosomal surface, its site of activation. The four mammalian Rag proteins form obligate heterodimers consisting of RagA or RagB bound to RagC or RagD. A key upstream component of the Rag GTPases is Ragulator, a trimeric complex that tethers them to the lysosome and also interacts with the v-ATPase, which is necessary for amino acid sensing by mTORC1. Amino acids stimulate the binding of GTP to RagB, a critical step in the sensing mechanism, but the factors that regulate Rag nucleotide loading are unknown. Here, we identify the proteins encoded by the HBXIP and C7orf59 genes as novel Ragulator components that are required for mTORC1 activation by amino acids. The pentameric Ragulator has nucleotide exchange activity towards RagA and RagB and interacts with the Rag heterodimers in an amino acid- and v-ATPase-dependent fashion. Thus, we provide mechanistic insight into how mTORC1 senses amino acids by revealing Ragulator to be a scaffold with guanine nucleotide exchange factor (GEF) activity for the Rag GTPases.
Introduction
Mechanistic target of rapamycin complex I (mTORC1) is a master growth regulator that couples nutrient availability to the control of cell growth and proliferation. When active, mTORC1 stimulates anabolic processes, such as translation, transcription, lipid biosynthesis, and ribosome biogenesis, and inhibits catabolic processes, such as autophagy (reviewed in (Howell and Manning, 2011; Ma and Blenis, 2009; Zoncu et al., 2011b)). Consistent with its growth-promoting function, many of the oncogenes and tumor suppressors that underlie familial tumor syndromes and sporadic cancers are upstream of mTORC1. mTORC1 responds to a variety of stimuli, including growth factors, oxygen availability, and energy levels, all of which impinge on mTORC1 through the tuberous sclerosis heterodimer (TSC1-TSC2). TSC1-TSC2 negatively regulates the mTORC1 pathway by acting as a GTPase activating protein (GAP) for Rheb1, a small GTPase that when bound to GTP is an essential activator of mTORC1 kinase activity.
One mTORC1 stimulus that does not funnel through the TSC1-TSC2-Rheb axis is amino acid sufficiency (Roccio et al., 2006; Smith et al., 2005). Recent findings indicate that amino acid signaling initiates within the lysosomal lumen (Zoncu et al., 2011a) and induces the translocation of mTORC1 to the lysosomal surface, where it comes in contact with Rheb and becomes activated. How mTORC1 moves to the lysosomal membrane is poorly understood, but another family of GTPases, known as the Rag GTPases, play an integral role (Kim et al., 2008; Sancak et al., 2008). Unique among the small GTPases, the Rags are obligate heterodimers: the highly related RagA and RagB are functionally redundant and bind to RagC or RagD, which are also very similar to each other (Hirose et al., 1998; Schurmann et al., 1995; Sekiguchi et al., 2001). The Rags localize to lysosomal membranes and bind to the raptor component of mTORC1, a process that depends on the binding of GTP to RagA or RagB. Amino acids regulate the binding of nucleotides to RagB, such that amino acid stimulation increases its GTP loading (Sancak et al., 2008). In cells expressing a RagA or RagB mutant that is constitutively bound to GTP, mTORC1 interacts with the Rags and localizes to the lysosome irrespective of amino acid levels, making the mTORC1 pathway immune to amino acid starvation (Kim et al., 2008; Sancak et al., 2008). Thus, a key event in the amino acid-dependent activation of mTORC1 is the conversion of RagA or RagB from a GDP- to GTP-bound state, yet the putative guanine nucleotide exchange factors (GEFs) that mediate this transition have yet to be identified.
Unlike the many GTPases that rely on a lipid moiety for their subcellular localization, the Rags use the recently identified Ragulator complex as their tether to the lysosomal surface. Three proteins that localize to lysosomal membranes make up Ragulator: p18, p14, and MP1, which are encoded by the LAMTOR1, LAMTOR2, and LAMTOR3 genes, respectively. In cells depleted of these proteins, the Rags and mTORC1 no longer reside at the lysosome, and, consequently, the mTORC1 pathway is inactive (Sancak et al., 2010).
The lysosomal v-ATPase is a newly characterized Ragulator-interacting complex and required for amino acid activation of mTORC1 (Zoncu et al. 2011). The mechanisms through which the v-ATPase activates the mTORC1 pathway and whether or not Ragulator has additional regulatory functions remain unknown. Here, we identify two novel components of Ragulator, the proteins encoded by the HBXIP and C7orf59 genes. These proteins interact with the Rag GTPases and together with p18, p14, and MP1 form a pentameric Ragulator complex. HBXIP and C7orf59 are necessary for both Rag and mTOR lysosomal localization and mTORC1 activation. Surprisingly, the pentameric Ragulator, but not individual subunits or the trimeric Ragulator, has GEF activity towards RagA and RagB. Furthermore, modulation of the Ragulator-Rag interaction by amino acids requires the v-ATPase, suggesting that v-ATPase activity is upstream of the GEF activity of Ragulator.
Results
HBXIP and C7orf59 encode components of an expanded Ragulator complex
We previously identified p14, MP1, and p18, collectively named Ragulator, as proteins that interact with the Rag GTPases within cells (Sancak et al., 2010). However, in cell-free assays, Rag heterodimers interact relatively weakly with purified, recombinantly-produced Ragulator (Sancak et al., 2010), suggesting that proteins responsible for stabilizing the interaction within cells are missing from our in vitro preparations. To identify such proteins, we used a purification strategy involving immunoprecipitation followed by mass spectrometry that previously led to the discovery of other mTORC1 pathway components (see Methods). Immunoprecipitates prepared from HEK-293T cells stably expressing FLAG-tagged p18, p14, or RagB, but not the Metap2 control, consistently contained HBXIP and the protein product of the C7orf59 gene, hereafter called C7orf59. Several studies implicate HBXIP in the regulation of cell cycle progression, proliferation, apoptosis, and Hepatitis B virus replication (Fujii et al., 2006; Marusawa et al., 2003; Melegari et al., 1998; Wang et al., 2007; Wen et al., 2008), while C7orf59 has no described functions.
Orthologs of HBXIP and C7orf59 exist in mammals besides humans and in Drosophila (Figure S1A). Like other Ragulator components, HBXIP and C7orf59 lack protein sequence homology with any fission or budding yeast proteins, including Ego1p and Ego3p, which tether the yeast orthologs of the Rag GTPases to the vacuole (Binda et al., 2009; Dubouloz et al., 2005; Gao and Kaiser, 2006). High-resolution crystal structures of MP1 and p14 reveal the presence of a roadblock domain in each (Kurzbauer et al., 2004; Lunin et al., 2004), and secondary structure predictions suggest that the C-terminal regions of RagB and RagC also contain this domain (Gong et al., 2011) (Figure S1B). While the function of the domain is unknown, it is interesting to note that HBXIP also contains a roadblock domain (Garcia-Saez et al., 2011), and our secondary structure analyses predict the same for C7orf59 (Figure S1B). Thus, the Rag-Ragulator complex is likely to contain six roadblock domains.
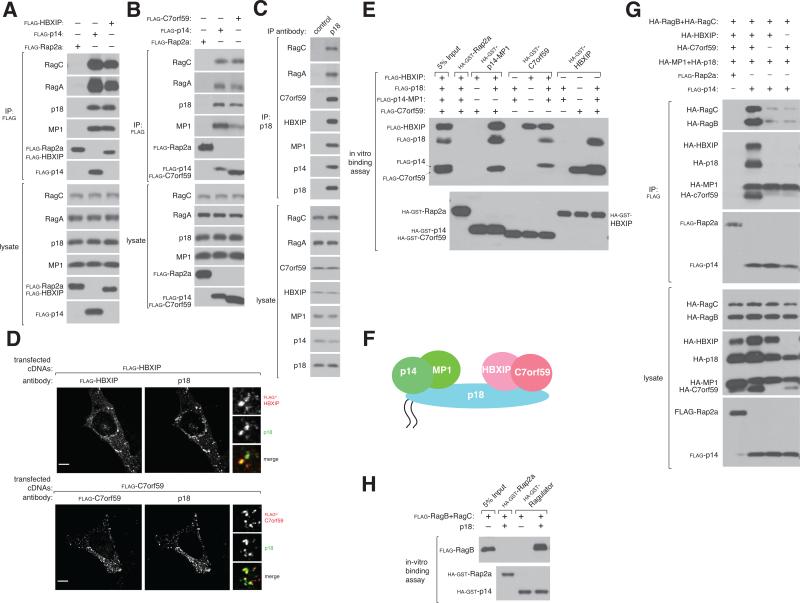
Experiments in cells and in cell-free systems indicate that that HBXIP and C7orf59 are new Ragulator components. When expressed in HEK-293T cells, FLAG-tagged HBXIP or C7orf59, but not Rap2a, co-immunoprecipitated endogenous RagA, which is highly similar but far more abundant than RagB (Figure S1D, S1E), RagC, p18, and MP1 at similar levels as FLAG-p14 (Figures 1A,B). Gratifyingly, endogenous HBXIP and C7orf59 co-immunoprecipitated with an antibody to endogenous p18, but not a control protein (Figure 1C). When co-expressed along with Ragulator proteins in HEK-293T cells, HBXIP and C7orf59 co-localized with p18 (Figure 1D), consistent with the lysosomal localization of other Ragulator components (Nada et al., 2009; Sancak et al., 2010; Wunderlich et al., 2001). In an in vitro binding assay, HBXIP bound to C7orf59 in the absence of other proteins, and the HBXIP-C7orf59 heterodimer, but neither protein alone, bound the established Ragulator components (MP1, p14 and p18) (Figure 1E). These results indicate that Ragulator is a pentameric complex in which HBXIP and C7orf59 form a heterodimer that interacts, through p18, with the MP1-p14 heterodimer (Figure 1F).
Figure 1. HBXIP and C7orf59 are components of an expanded Ragulator complex.
A) Recombinant epitope-tagged HBXIP co-immunoprecipitates endogenous MP1, p18, RagA, and RagC. Anti-FLAG immunoprecipitates were prepared from HEK-293T cells transfected with the indicated cDNAs in expression vectors. Cell lysates and immunoprecipitates were analyzed by immunoblotting for levels of indicated proteins.
B) Recombinant C7orf59 co-immunoprecipitates endogenous MP1, p18, RagA, and RagC. HEK-293T cells were transfected with the indicated cDNAs in expression vectors and analyzed as in (A).
C) Endogenous p18 co-immunoprecipitates endogenous p14, MP1, RagA, RagC, HBXIP, and C7orf59. Anti-p18 immunoprecipitates were prepared from HEK-293T cells and analyzed for the levels of the indicated proteins.
D) Images of HEK-293T cells co-immunostained for p18 (green) and FLAG-HBXIP (red) or FLAG-C7orf59 (red). Cells were co-transfected with cDNAs encoding MP1, p14, and p18 and either FLAG-HBXIP or FLAG-C7orf59 and processed for immunostaining and imaging. In all images, insets show selected fields that were magnified five times and their overlays. Scale bar represents 10 μm.
E) Ragulator is a pentameric complex. In vitro binding assay in which recombinant HA-GST-tagged-p14-MP1, -C7orf59 or -HBXIP were incubated with the indicated purified FLAG-tagged Ragulator proteins. HA-GST precipitates were analyzed for levels of the indicated proteins.
F) Schematic summarizing intra-Ragulator interactions: p18 bridges MP1-p14 with HBXIP-C7orf59.
G) The pentameric Ragulator complex co-immunoprecipitates recombinant RagB and RagC. HEK-293T cells were co-transfected with the indicated cDNAs in expression vectors and analyzed as in (A).
H) Requirement for a pentameric Ragulator complex to interact with Rags. In vitro binding assay in which recombinant HA-GST Ragulator with or without p18 was incubated with purified FLAG-RagB-RagC and analyzed as in (E). See also Figure S1.
Consistent with our initial hypothesis that the original Ragulator lacked components required to bind strongly to the Rag GTPases, in HEK-293T cells the pentameric Ragulator interacted to a much greater degree with the Rags than the trimeric one (Figure 1G, S1C). Likewise, in an in vitro binding assay, Rags interacted with an intact pentameric Ragulator, but not one lacking p18 (Figure 1H). It is likely that in previous work, the two new Ragulator components were present in binding experiments in sub-stoichiometric amounts, explaining the weaker interactions we had observed (Sancak et al., 2010). Collectively, our results show that HBXIP and C7orf59 are part of an expanded Ragulator that requires all its subunits to bind strongly to the Rag GTPases.
HBXIP and C7orf59 are necessary for TORC1 activation by amino acids in mammalian and Drosophila cells
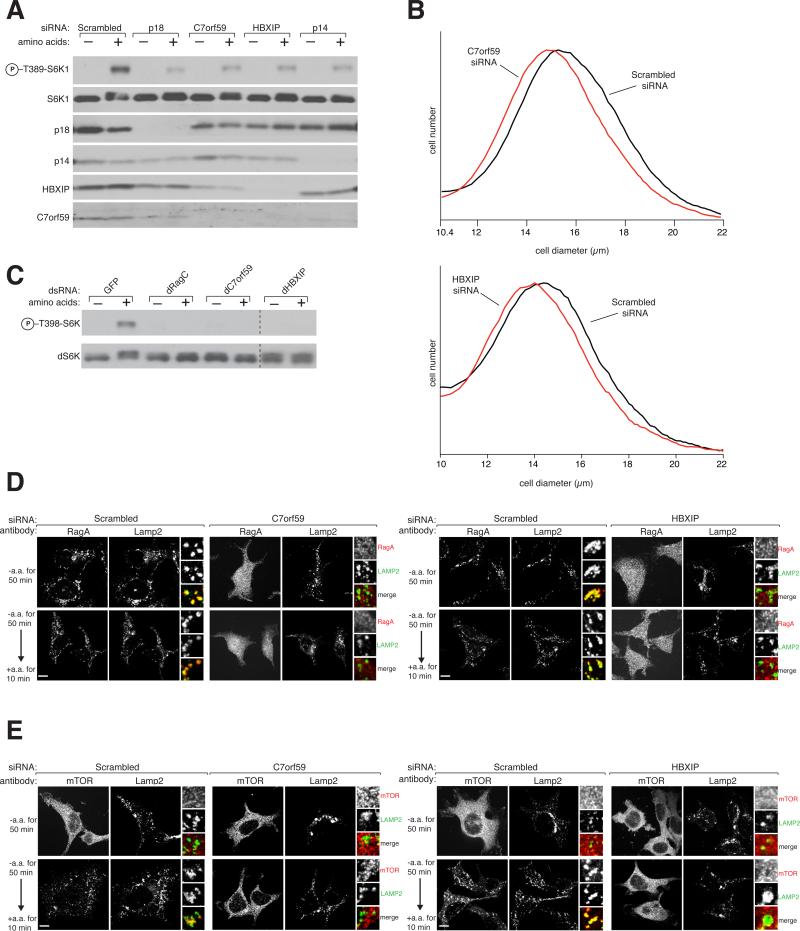
We next examined the functions of the novel Ragulator components in mTORC1 signaling. In HEK-293T cells, RNAi-mediated reductions in HBXIP or C7orf59 expression blunted mTORC1 activation by amino acids, as detected by S6K1 phosphorylation, to similar extents as knockdowns of the established Ragulator proteins p18 and p14 (Figure 2A). As expected for positive regulators of the growth-promoting mTORC1 pathway (Fingar et al., 2002; Kim et al., 2008; Sancak et al., 2010; Sancak et al., 2008; Stocker et al., 2003), reductions in HBXIP and C7orf59 levels also decreased the size of HEK-293T cells (Figure 2B). As the components of obligate heterodimers often behave (Cortez et al., 2001; Sancak et al., 2008), loss of either HBXIP or C7orf59 reduced the expression of its partner, but not of p14 (Figure 2A). Finally, consistent with the conserved functions of the Rag and Ragulator proteins in Drosophila (Kim et al., 2008; Sancak et al., 2010; Sancak et al., 2008), treatment of S2 cells with dsRNAs targeting the HBXIP (CG14812) or C7orf59 (CG14977) fly orthologs strongly inhibited dTORC1 activation by amino acids (Figure 2C). These results establish that HBXIP and C7orf59 are positive components in mammalian and Drosophila cells of the amino acid sensing branch of the TORC1 pathway.
Figure 2. HBXIP and C7orf59 are necessary for TORC1 activation by amino acids and localization of the Rag GTPases and mTOR to the lysosomal surface.
A) C7orf59 and HBXIP are necessary for the activation of the mTORC1 pathway by amino acids. HEK-293T cells, treated with siRNAs targeting the mRNAs for the indicated proteins, were starved of amino acids for 50 min, or starved and stimulated with amino acids for 10 min. Immunoblot analyses were used to measure the levels of the indicated proteins and phosphorylation states.
B) Cells depleted of C7orf59 and HBXIP are smaller than controls. Cell size distributions are shown for HEK-293T cells treated with siRNAs targeting C7orf59, HBXIP, or a scrambled non-targeting control.
C) Drosophila orthologs of HBXIP and C7orf59 are required for the activation of the TORC1 pathway. Drosophila S2 cells were transfected with a control dsRNA or dsRNAs targeting dRagC, dC7orf59, or dHBXIP, starved for amino acids for 90 min or starved and stimulated with amino acids for 30 min and analyzed as in (A).
D) Images of HEK-293T cells, treated with a non-targeting siRNA or siRNAs targeting HBXIP or C7orf59, co-immunostained for RagA (red) and LAMP2 (green). Cells were starved for amino acids or starved and stimulated for the indicated times before processing for the immunofluorescence assay and imaging.
E) Images of HEK-293T cells, treated with a non-targeting siRNA or siRNAs targeting HBXIP or C7orf59, co-immunostained for mTOR (red) and LAMP2 (green). Cells were treated and processed as in (A). In all images, insets show selected fields that were magnified five times and their overlays. Scale bars represent 10 μm. See also Figure S2.
Localization of the Rag GTPases and mTOR to the lysosomal surface requires HBXIP and C7orf59
Upon amino acid stimulation, the Rag GTPases recruit mTORC1 to the lysosomal surface (Sancak et al., 2010). In the absence of Ragulator, the Rags detach from the lysosome and cannot target mTORC1 to this organelle. The inability of amino acids to activate mTORC1 in cells depleted of HBXIP and C7orf59 suggested that HBXIP and C7orf59, like p14, MP1, and p18, might also localize the Rags, and thus mTORC1, to the lysosome. Indeed, in HEK-293T cells treated with siRNAs targeting C7orf59 and HBXIP, RagA and RagC localized in a diffuse pattern throughout the cytoplasm and did not co-localize with the lysosomal marker LAMP2 (Figure 2D, S2A). The lysosomal localization of p18 was unaffected by depletion of HBXIP or C7orf59 (Figure S2B), consistent with its function as a lysosomal tether for the Ragulator complex that does not require other Ragulator components for its own lysosomal localization (Sancak et al., 2010, Nada et al., 2009). As expected from the mis-localization of the Rags, in cells depleted of HBXIP and C7orf59, mTOR also did not co-localize with lysosomes, irrespective of whether or not they had been stimulated with amino acids (Figure 2E). These results indicate that both HBXIP and C7or59 have similar functions to p14, MP1, and p18 and confirm that the pentameric Ragulator complex acts as a scaffold for the Rag GTPases and mTORC1 at the lysosomal membrane (Sancak et al., 2010). Thus, throughout the remainder of this paper, we use the name ‘Ragulator’ to refer to the pentameric complex.
Amino acids regulate the Rag-Ragulator interaction
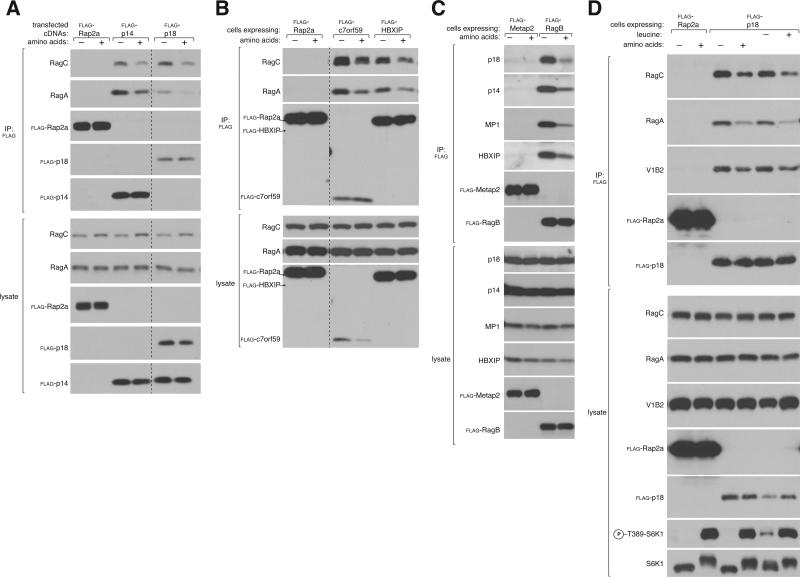
Multimeric signaling complexes often engage in regulated interactions as a mechanism to control downstream signaling events (Good et al., 2011). Because the Rag GTPases interact with mTORC1 in an amino-acid dependent manner, we wondered if the binding of Ragulator to the Rags might also be amino acid sensitive. In order to detect the endogenous Rag-Ragulator interaction using the antibodies available in the past, we had found it necessary to use cross-linked conditions that would have prevented detection of a regulated interaction (Sancak et al., 2010). Using optimized cell lysis conditions and improved antibodies, we find that amino acid starvation strengthens the interaction between endogenous Rags and the Ragulator isolated through p14, p18, HBXIP, or C7orf59 (Figures 3A, 3B, S3D, S3E). Similarly, amino acid stimulation decreased the amounts of endogenous Ragulator that co-immunoprecipitated with RagB (Figure 3C, S3F). Leucine is necessary for mTORC1 activation (Hara et al., 1998) and the Rag-Ragulator as well as the Ragulator-v-ATPase interactions, were both strengthened in cells deprived of leucine (Figure 3D), consistent with a mixture of all 20 amino acids regulating Ragulator-v-ATPase binding (Zoncu et al., 2011a). Amino acids only slightly regulated the interaction between p18 and other endogenous Ragulator proteins (Figure S3C), whereas the amount of mTORC1 that co-immunoprecipitated with Ragulator substantially increased upon amino acid stimulation (Figures S3A, S3B). Because amino acids also modulate the nucleotide loading of RagB (Sancak et al., 2008), the regulated interaction between Ragulator and the Rag heterodimers suggested that Ragulator might have additional functions towards the Rags besides simply being their lysosomal scaffold.
Figure 3. Amino acids regulate the Rag-Ragulator interaction.
A) Amino acids regulate the amounts of endogenous RagA and RagC that co-immunoprecipitate with recombinant p14 and p18. HEK-293T cells, transfected with the indicated cDNAs in expression vectors, were starved for amino acids for 2 hours or starved and stimulated with amino acids for 15 min. Anti-FLAG immunoprecipitates were prepared from cell lysates and analyzed by immunoblotting for levels of indicated proteins.
B) Amino acid starvation strengthens the interaction between endogenous Rags and recombinant C7orf59 and HBXIP. HEK-293T cells stably expressing FLAG-C7orf59 or FLAG-HBXIP were starved and re-stimulated with amino acids. Anti-FLAG immunoprecipitates were analyzed as in (A).
C) FLAG-RagB co-immunoprecipitation of endogenous Ragulator is dependent on amino acids. HEK-293T cells stably expressing FLAG-RagB were starved and re-stimulated with amino acids as in (A). Anti-FLAG immunoprecipitates were analyzed for the levels of the indicated proteins.
D) Leucine starvation strengthens the binding of Ragulator to endogenous Rags and the V1 domain of the v-ATPase. HEK-293T cells stably expressing FLAG-p18 were starved and re-stimulated with total amino acids as in (A) or starved for leucine for 2 hours or starved and stimulated with leucine for 20 min. Anti-FLAG immunoprecipitates were analyzed for the levels of the indicated proteins. See also Figure S3.
Ragulator preferentially interacts with nucleotide-free Rag GTPases
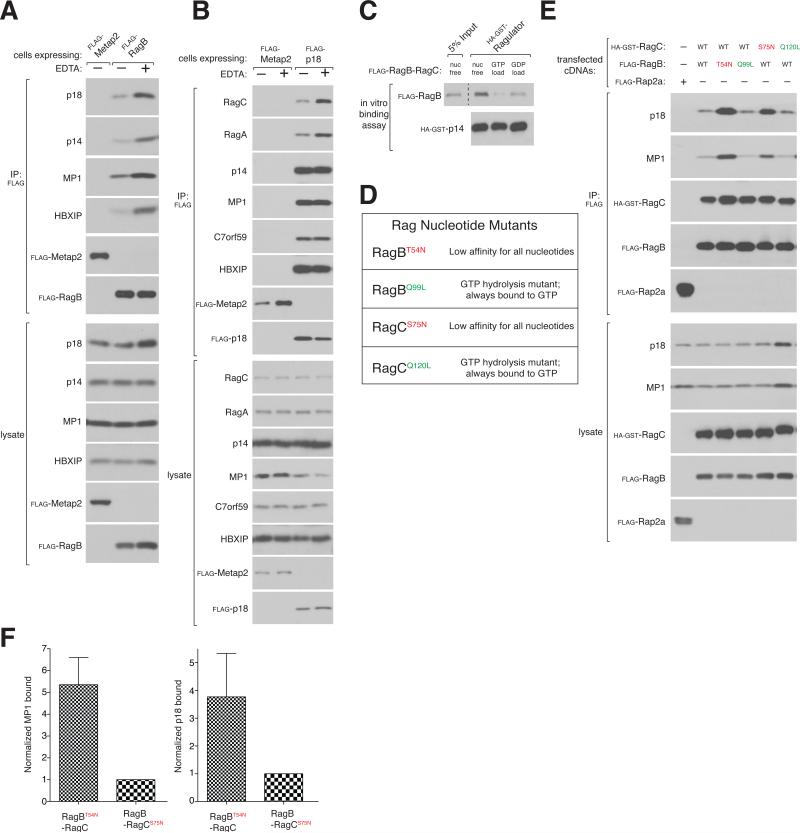
Regulation of the Rag nucleotide-binding state is not understood, but is key for amino acid signaling to mTORC1. The amino acid-sensitive interaction between Rags and Ragulator prompted us to examine whether Ragulator might also regulate nucleotide binding to the Rags. Intriguingly, many proteins that regulate nucleoside triphosphatase (NTPases) have roadblock domains (Bowman et al., 1999; Koonin and Aravind, 2000; Miertzschke et al., 2011; Wanschers et al., 2008) which four of the five Ragulator components are likely to contain. Preliminary experiments indicated that Ragulator does not have GTPase activating protein (GAP) activity towards the Rag GTPases, and so we examined whether it might have the activity of a guanine nucleotide exchange factor (GEF). A characteristic of GEFs is their strong preference for binding nucleotide-free over nucleotide-loaded GTPases (Bos et al., 2007; Feig, 1999). Incubation with buffers containing EDTA, which chelates the magnesium ion necessary for nucleotide binding, is a common way to generate largely nucleotide-free GTPases (Wang et al., 2000). Interestingly, the presence of EDTA in the cell lysis buffer significantly increased the interaction of recombinant RagB and endogenous Ragulator proteins (Figure 4A, S4A) as well as the binding of recombinant p18 to endogenous RagA and RagC (Figure 4B). In vitro binding assays proved useful in dissecting the effects of nucleotides on the Rag-Ragulator interaction. Ragulator readily bound to the Rags in vitro, likely by displacing their nucleotides (see below), but the addition of GTP significantly weakened the interaction (Figure S4B). In a complementary experiment, highly purified Ragulator had a clear preference for interacting with a recombinant Rag heterodimer stripped of its nucleotides rather than nucleotide-bound, indicating that both in cells and in vitro Ragulator prefers binding to nucleotide-free Rags (Figure 4C). It is important to note that even when nucleotide-loaded, the Rag GTPases interact to a significant extent with Ragulator, consistent with its role as a scaffold and suggesting that the Rag-Ragulator complex can exist in interaction states of differing strengths.
Figure 4. Ragulator preferentially interacts with nucleotide-free RagB.
A) EDTA increases the interaction between endogenous Ragulator and FLAG-RagB. HEK-293T cells stably expressing Flag-RagB were lysed in the absence or presence of EDTA and cell lysates and anti-FLAG immunoprecipitates analyzed by immunoblotting for the levels of the indicated proteins.
B) FLAG-p18 co-immunoprecipitates more endogenous Rags in the presence of EDTA. HEK-293T cells stably expressing FLAG-p18 were treated and analyzed as in (A).
C) Ragulator preferentially interacts with nucleotide-free Rags. In vitro binding assay in which immobilized HA-GST-Ragulator was incubated with nucleotide-free FLAG-RagB-RagC or Rag heterodimers loaded with GTP or GDP. HA-GST precipitates were analyzed for the levels of the indicated proteins.
D) Table summarizing Rag mutants used in this study.
E) The RagBT54N mutant preferentially interacts with endogenous Ragulator. Anti-FLAG immunoprecipitates were prepared from HEK-293T cells transfected with the indicated cDNAs in expression vectors and analyzed as in (A).
F) Quantification of endogenous MP1 and p18 binding to RagBT54N-RagC and RagBRagCS75N. Each value represents the normalized mean ±SD for n=3. See also Figure S4.
To study a potential regulatory function for Ragulator, it was necessary to first determine if the nucleotide binding state of RagB or RagC is the dominant determinant of the interaction between Rag heterodimers and Ragulator. To address this issue, we generated two different classes of Rag nucleotide binding mutants (Figure 4D). In the first, a critical Thr/Ser that is necessary for stabilizing magnesium was changed to Asn, resulting in mutants (RagBT54N and RagCS75N) that bind negligible amounts of nucleotides (Figure S4C). The corresponding H-Ras mutant (H-RasS17N) also binds nucleotides poorly, but, interestingly, interacts with GEFs to a greater extent than the wild type protein (Feig, 1999; Feig and Cooper, 1988; John et al., 1993). Mutants in the second class are homologous to H-RasQ61L and are constitutively bound to GTP because they lack GTPase activity (RagBQ99L and RagCQ120L) (Frech et al., 1994; Krengel et al., 1990). Within cells the heterodimer of nucleotide-free RagB and wild-type RagC (RagBT54N-RagC) interacted with Ragulator at levels 4-6 fold greater than the heterodimer of wild-type RagB and nucleotide-free RagC (RagB-RagCS75N) (Figures 4E, 4F), suggesting that the presence or absence of nucleotide on RagB largely controls the Rag-Ragulator interaction. Consistent with this interpretation, a heterodimer of GTP-bound RagB and nucleotide-free RagC (RagBQ99L-RagCS75N) interacted with Ragulator much more weakly than a heterodimer with the opposite properties (RagBT54N-RagCQ120L) (Figure S4E). Thus, the nucleotide binding state of RagB is the major determinant of the strength of the interaction between Rag heterodimers and Ragulator.
Ragulator is a GEF for RagA and RagB
The binding properties of Ragulator are highly consistent with it having GEF activity towards RagB. To test this possibility, it was necessary to develop a way to measure GDP dissociation from one Rag and not the other. To this end, we mutated the conserved Asp to Asn in the Rag ‘NKxD motif’ (RagBD163N and RagCD181N). This mutation changes the base specificity of a GTPase from guanine to xanthosine nucleotides (Hoffenberg et al., 1995; Schmidt et al., 1996), and we denote these mutants as RagBX or RagCX both of which bind less than 2% of the guanine nucleotides than their wild type counterparts (Figure S4D). Therefore, when we load RagBX-RagC or RagB-RagCX with GDP or GTP in vitro, we know which of the Rag GTPases in the heterodimer is bound to the guanine nucleotide.
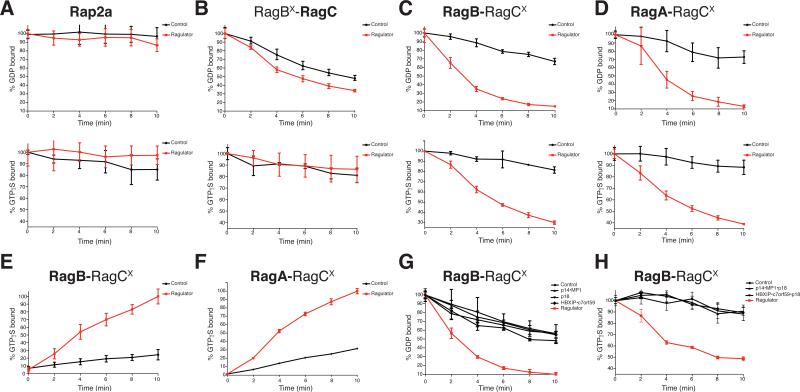
In vitro many GEFs displace GDP and GTP at similar rates from their cognate GTPases (Klebe et al., 1995; Lenzen et al., 1998; Zhang et al., 2005). Thus, we loaded RagBX-RagC or RagB-RagCX heterodimers with labeled GDP or GTP and tested the effects of Ragulator on their dissociation. Ragulator did not affect GDP or GTP dissociation from the Rap2a control GTPase (Figure 5A). When tested on RagC within the RagBX-RagC heterodimer, Ragulator modestly increased the release of GDP but not that of GTP (Figure 5B). In contrast, Ragulator greatly accelerated both GDP and GTP dissociation from RagB in the RagB-RagCX heterodimer (Figure 5C, S5A) and did so in a dose-dependent manner (Figure S5C). As expected from the very high level of homology between RagA and RagB (Figure S1D), Ragulator also greatly increased guanine nucleotide dissociation from RagA in the RagA-RagCX heterodimer (Figure 5D). Consistent with its function as a GEF, in a GTP binding assay in which we pre-bound RagB-RagCX or RagA-RagCX with unlabeled GDP and then incubated it with labeled GTP, Ragulator significantly increased GTP binding to RagB and RagA (Figure 5E, 5F, S5B).
Figure 5. Ragulator is a GEF for RagA and RagB.
A) Ragulator does not stimulate GDP or GTPγS dissociation from Rap2a. Nucleotide dissociation assay, in which Rap2a was loaded with either [3H]GDP or [35S]GTPγS, and incubated with Ragulator. Dissociation was monitored by a filter-binding assay. Each value represents the normalized mean ±SD for n=4.
B) Ragulator moderately stimulates GDP, but not GTPγS dissociation from RagC. RagBD163N-RagC was loaded, incubated with Ragulator or a control and analyzed as in (A). Each value represents the normalized mean ±SD for n=4.
C) Ragulator greatly accelerates GDP and GTPγS dissociation from RagB. RagB-RagCD181N was loaded, incubated with Ragulator or a control and analyzed as in (A). Each value represents the normalized mean ±SD for n=4.
D) Ragulator substantially increases GDP and GTPγS dissociation from RagA. RagA-RagCD181N was loaded with nucleotide, incubated with Ragulator or a control and analyzed as in (A). Each value represents the normalized mean ±SD for n=4.
E) Ragulator accelerates GTPγS binding to RagB. RagB-RagCD181N was loaded with GDP and incubated with Ragulator or a control and [35S]GTPγS. [35S]GTPγS binding was determined as in (A). Each value represents the normalized mean ±SD for n=4.
F) Ragulator potentiates GTPγS binding to RagA. RagA-RagCD181N was loaded with GDP and incubated with Ragulator or a control and [35S]GTPγS. [35S]GTPγS binding was determined as in (A). Each value represents the normalized mean ±SD for n=4.
G) Individual Ragulator subunits do not increase GDP dissociation from RagB. [3H]GDP bound RagB-RagCD181N was incubated with Ragulator, p14-MP1, HBXIP-C7orf59, or p18, and [3H]GDP dissociation monitored as in (A). Each value represents the normalized mean ±SD for n=4.
H) Trimeric Ragulator complexes do not increase GTPγS dissociation from RagB. [35S]GTPγS bound RagB-CD181N was incubated with Ragulator, p14-MP1-p18, or HBXIP-C7orf59-p18 and dissociation was monitored as in (A). Each value represents the normalized mean ±SD for n=4. See also Figure S5.
Because the Rags function as a heterodimer, we wondered whether the nucleotide binding state of RagC might alter the function of Ragulator towards RagB. When the RagB-RagCX heterodimer was co-loaded with either XDP or XTP in addition to GDP or GTP (Figure S5D, S5E), there was no difference in Ragulator-mediated GDP or GTP dissociation from RagB, suggesting that the nucleotide binding state of RagC does not alter Ragulator GEF activity towards RagB.
To determine if the exchange activity of Ragulator depends on a particular subunit, we tested p14-MP1, HBXIP-C7orf59, and p18 separately in the GDP exchange assay. Unlike the pentameric complex, none of these subassemblies increased GDP dissociation from RagB (Figure 5G). Likewise, trimeric Ragulators composed of either p14-MP1-p18 or HBXIP-C7orf59-p18 were no more effective at accelerating GTP dissociation from RagB than a control protein (Figure 5H). These results indicate that Ragulator is a GEF for RagA and RagB and that a pentameric Ragulator is required for this activity.
Recently, Vam6 was shown to act as a GEF for Gtr1p, the yeast ortholog of RagA and RagB, and to be necessary for the activation of the TORC1 pathway in yeast (Binda et al., 2009). However, we found that VPS39, the mammalian ortholog of VAM6, not only failed to interact with endogenous RagA (Figure S5F) but also did not stimulate GDP or GTP dissociation from RagB (Figure S5G). These findings suggest that VPS39 is not a GEF for RagA or RagB and that the amino acid sensing mechanisms of yeast and higher eukaryotes have diverged.
The v-ATPase controls Ragulator function in cells
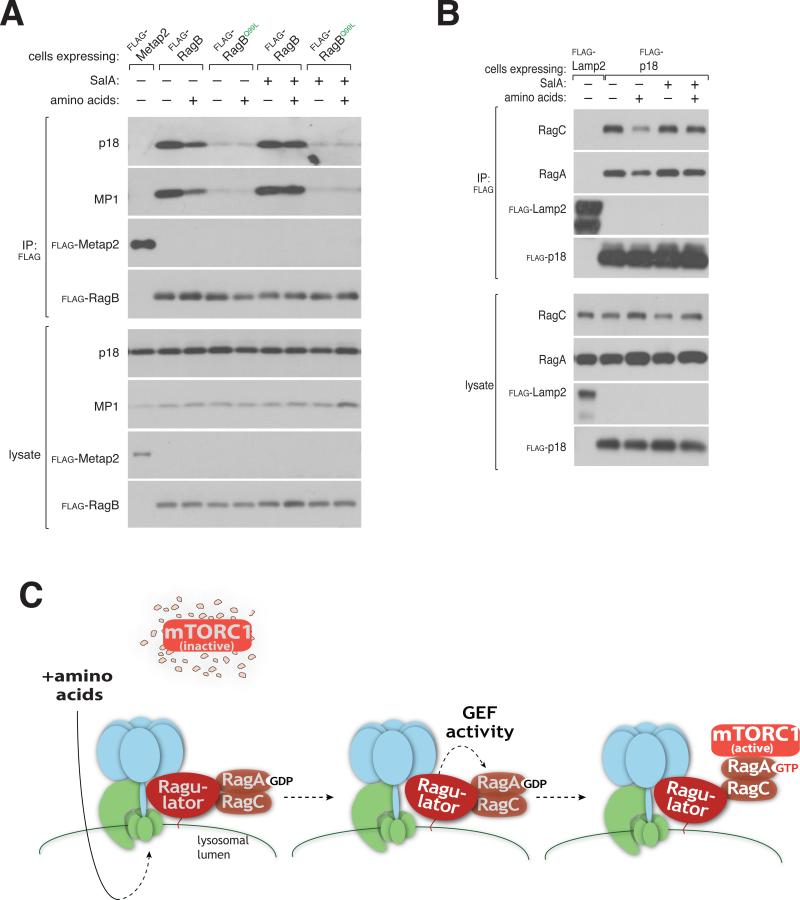
The v-ATPase is a positive regulator of the mTORC1 signaling pathway that acts downstream of amino acids and upstream of the Rags (Zoncu et al., 2011a). The v-ATPase consists of V0 and V1 domains, two multi-subunit complexes (Forgac, 2007), both of which interact with Ragulator (Zoncu et al., 2011a). Interestingly, amino acid starvation and re-stimulation strengthens and weakens the V1-Ragulator interaction, respectively, while v-ATPase inhibition prevents regulation of the interaction by locking it in the amino acid-free state. Because the v-ATPase, unlike Ragulator, is not required to maintain the Rag GTPases on the lysosomal surface (Zoncu et al., 2011a), it must have an important function distinct from the control of Rag localization. Given its regulated interaction with Ragulator, it seemed likely that the v-ATPase might regulate the GEF activity of Ragulator. To test this possibility, we used the amino acid-sensitive interaction between the Rag heterodimers and Ragulator as a marker of Ragulator GEF activity in cells. Consistent with the nucleotide loaded state of RagB determining the Rag-Ragulator interaction, in cells stably expressing the GTP-bound RagBQ99L mutant, the interaction between Ragulator and RagBQ99L was no longer regulated by amino acids and resembled the weak Rag-Ragulator interaction observed in amino acid stimulated cells (Figure 6A). Interestingly, pre-treatment of cells with the v-ATPase inhibitors Salicylihalamide A (SalA) (Xie et al., 2004) or Concanamycin A (ConA) (Bowman et al., 2004), prevented amino acid stimulation from weakening the Rag-Ragulator interaction, which remained at the strong level observed in the absence of amino acids (Figures 6A, 6B, S6A). Importantly, v-ATPase inhibition did not affect the already weak interaction between the RagBQ99L mutant and Ragulator (Figure 6A). Thus, regulation of the Rag-Ragulator interaction depends on the v-ATPase, which is necessary to transmit the amino acid signal to the GEF activity of Ragulator.
Figure 6. The v-ATPase controls the function of Ragulator.
A) The v-ATPase functions upstream of the regulated binding between Rags and Ragulator. HEK-293T cells stably expressing FLAG-RagB or FLAG-RagBQ99L were starved for 2 hours or starved and stimulated with amino acids for 15 min in the absence or presence of the v-ATPase inhibitor SalA. Cell lysates and anti-FLAG immunoprecipitates were analyzed by immunoblotting for the levels of the indicated proteins.
B) Inactivation of the v-ATPase blocks the amino acid dependent Rag-Ragulator interaction. HEK-293T cells stably expressing FLAG-p18 were treated as in (B). Anti-FLAG immunoprecipitates were analyzed for the levels of the indicated proteins.
C) Model for amino-acid induced mTORC1 activation. In the absence of amino acids the v-ATPase, Ragulator, and Rags exist in a tightly bound super complex and mTORC1 cannot associate with the lysosomal surface and remains inactive. Amino acid accumulation in the lysosomal lumen generates an activating signal that is transmitted in a v-ATPase-dependent fashion to activate the GEF activity of Ragulator towards RagA. Upon RagA-GTP loading, the Rag-Ragulator interaction weakens and mTORC1 is recruited to the lysosomal surface where it interacts with Rheb and becomes activated. Rheb is not shown.
Discussion
In this study we identify HBXIP and C7orf59 as two novel components of the mTORC1 pathway. In association with known Ragulator proteins (p18, p14 and MP1), HBXIP and C7orf59 form a pentameric complex that is essential for localizing the Rag GTPases to the lysosomal surface and activating mTORC1 in response to amino acids. In addition to being a scaffold, Ragulator promotes nucleotide exchange of RagB and of the highly related RagA. Thus, we identify a key link in the signaling cascade that converts a signal emanating from amino acids into the nucleotide loading of the Rags and ultimately the recruitment of mTORC1 to the lysosomal surface. We suggest that C7orf59 and HBXIP be renamed LAMTOR4 and LAMTOR5, respectively, to reflect their critical roles in regulating the mTORC1 pathway and to be consistent with the naming convention of other Ragulator components.
Our in vitro binding results and secondary structure predictions, combined with available structural data, support the following molecular architecture for pentameric Ragulator: p18 is a lysosome-associated scaffold protein that binds two roadblock-containing heterodimers, p14-MP1 and HBXIP-C7orf59, and thereby tethers them to the lysosome. In vitro and in vivo data suggest that all five members of Ragulator must be present to efficiently interact with the Rag heterodimers, although the stoichiometry between the two complexes is unknown. The recently reported crystal structure of a Gtr1p-Gtr2p heterodimer, the yeast orthologs of mammalian Rags, reveals the presence of roadblock domains in the C-terminal portion of both GTPases, a structural feature that the C-terminal domains of the Rags are also predicted to have (Gong et al., 2011). Thus, the roadblock domain may represent the basic architectural element of the Ragulator-Rag complex.
Several proteins that interact with NTPases have a roadblock domain, suggesting that it may have regulatory functions as well as structural ones (Koonin and Aravind, 2000). The recently solved crystal structure of the bacterial GAP, MglB, shows that it contains a roadblock domain and may promote GTP hydrolysis through stabilization of the catalytic machinery of its cognate GTPase (Miertzschke et al., 2011). Consistent with a possible regulatory role for proteins with this domain, Ragulator prefers to bind to nucleotide-free rather than nucleotide-bound RagB. These binding properties are characteristic of other GEF-GTPase interactions and suggested that Ragulator might be a GEF for RagA and RagB. To test this hypothesis, it was necessary to develop a system for monitoring the nucleotide bound to an individual Rag in a heterodimer containing two of them. To this end, we made use of Rag complexes containing a wild type Rag that binds guanine nucleotides and a RagX mutant that cannot. We suggest that the RagX mutants may be useful reagents for the identification of other factors that control the nucleotide-loading state of the Rags. Ragulator greatly increases both GDP and GTP dissociation from RagB and RagA but not RagC. The preferential GEF activity of Ragulator for RagB and RagA likely stems from differences between the RagA/B and the RagC switch I/II regions, which are known to serve as a critical recognition motif on a GTPase for its cognate GEF (Fiegen et al., 2006; Jonathan, 1998). In addition, the intrinsic rapid GDP dissociation capacity of RagC suggests that a GEF might not be necessary for it. Rather, other regulators, namely guanine dissociation inhibitors, which block GDP dissociation (Garcia-Mata et al., 2011; Jennings and Pavitt, 2010), might have a more prominent role in the regulation of GTP binding by RagC.
Protein scaffolds encompass one of the most diverse sets of signaling molecules in cells. Recent studies have suggested that in addition to bringing multiple proteins together, some scaffolds also have catalytic functions. E. coli uses the catalytic scaffold EspG to inhibit host intracellular trafficking by bringing together the Arf1 GTPase and Pak2 kinase as well as blocking Arf1-GAP assisted-GTP hydrolysis and activating Pak2 kinase activity (Selyunin et al., 2011). Similarly, by binding to both Rags and the v-ATPase, Ragulator not only physically connects two major regulators of mTORC1 but also transmits the amino acid signal from the v-ATPase to the Rags through its GEF activity.
Our inability to detect GEF activity in partial assemblies of Ragulator implies that multiple surfaces, which exist only on the pentameric Ragulator are required to endow it with exchange activity. Recently, TRAPPI, a multi-protein tethering complex was identified as a GEF for YPT1 (Jones et al., 2000; Wang et al., 2000). Like Ragulator, the GEF activity of the TRAPPI complex is not contained in one subunit, but requires the presence of multiple components (Cai et al., 2008).
The likely presence of roadblock domains in the C-terminal regions of all four Rags raises the tantalizing possibility that one Rag may directly regulate the nucleotide cycle of the other. The solution of a Rag-Ragulator structure will greatly enhance our understanding of the function of the roadblock domain in this system and the precise mechanism by which Ragulator activates RagA and RagB. While Ragulator is the first factor identified that directly regulates Rag nucleotide binding, we anticipate the identification of other Rag regulatory proteins such as GAPs that will help explain how amino acid starvation inactivates the Rags and by extension the mTORC1 pathway.
The regulated interaction between Rags and Ragulator depends on amino acids and the nucleotide binding state of RagA and RagB and provides an in-cell output for the activity of Ragulator and the pathway downstream of it. Using this assay, we find that inhibition of the v-ATPase inactivates Ragulator. The fact that the v-ATPase is required for mTORC1 activation, functions downstream of amino acids but upstream of RagA/B nucleotide loading, and interacts with Ragulator, suggests a model in which the v-ATPase links an amino acid-generated signal to the activation of the Ragulator GEF activity (Figure 6C).
There are many possible functions for the regulated interaction between the Rags and Ragulator. In one model, the Rag-Ragulator complex exists in two conformations that are determined by amino acid availability: a tightly bound state, which cannot interact with mTORC1, and an open one that favors mTORC1 recruitment to the lysosomal surface. Upon amino acid stimulation, Ragulator promotes GTP loading of RagA/B, leading to a weakening of Ragulator-Rag binding and a conformation that may expose an mTORC1-binding surface on the Rag GTPases. A precedent for such a nutrient-dependent conformational change exists within mTORC1. Conditions that inhibit the mTORC1 pathway result in a stronger association between mTOR and raptor with a concomitant decrease in in vitro kinase activity, and conditions that activate result in a weaker interaction and greater in vitro activity (Kim et al., 2002).
Alternatively, or in addition to the first model, the regulated interaction might be necessary for the Rags to reversibly leave the lysosomal surface. During starvation conditions, Ragulator would hold Rags at the lysosome. Upon amino acid stimulation, Rags may dissociate from Ragulator when RagA/B binds GTP, capture mTORC1 in the cytoplasm, and then shuttle it back to the lysosome by re-associating with Ragulator. Many GTPases are known to cycle on and off membranes in a nucleotide dependent manner (Hutagalung and Novick, 2011), and Rag cycling may provide a physical means to ferry mTORC1 to the lysosome. Future work combining structural studies and dynamic live cell imaging will clarify the mechanistic aspects of the regulation of Rag-mTORC1 binding, and how mTORC1 is ferried to the lysosome.
Materials and Methods
Materials
Reagents were obtained from the following sources: antibodies to ATP6V1B2 and LAMP2 from Abcam; antibodies to phospho-T389 S6K1, S6K1, RagA, RagC, p14, p18, MP1, C7orf59, HBXIP mTOR, phospho-T398 dS6K, and the FLAG epitope from Cell Signaling Technology; HRP-labeled anti-mouse, and anti-rabbit secondary antibodies from Santa Cruz Biotechnology; antibody to the HA tag from Bethyl laboratories; RPMI, FLAG M2 affinity gel, GTPγS, GDP, Chaps, Triton, and amino acids from Sigma Aldrich; [3H]GDP and [35S]GTPγS from Perkin Elmer; protein G-sepharose and immobilized glutathione beads from Pierce; FuGENE 6 and Complete Protease Cocktail from Roche; Alexa 488 and 568-conjugated secondary antibodies, Schneider's media, Express Five Drosophila-SFM, and Inactivated Fetal Calf Serum (IFS) from Invitrogen; amino acid-free RPMI, and amino acid free Schneider's media from US Biological; siRNAs targeting indicated genes and siRNA transfection reagent from Dharmacon; human cDNA encoding HBXIP from Open Biosystems; Concanamycin A from A. G. Scientific; nitrocellulose membrane filters from Advantec; calf-alkaline phosphatase from NEB. The dS6K antibody was a generous gift from Mary Stewart (North Dakota State University). Salicylihalamide A (SalA) was a generous gift from Jeff DeBrabander (UT Southwestern).
Cell lysis and immunoprecipitation
Cells were rinsed once with ice-cold PBS and lysed with Chaps lysis buffer (0.3% Chaps, 10 mM β-glycerol phosphate, 10 mM pyrophosphate, 40 mM Hepes pH 7.4, 2.5 mM MgCl2 and 1 tablet of EDTA-free protease inhibitor [Roche] per 25 ml). Where specified in the figures, Chaps lysis buffer was supplemented with 12.5 mM EDTA. When only cell lysates were required (i.e., no immunoprecipitation was to be performed), 1% Triton X-100 was substituted for Chaps. When the interaction between Ragulator and mTORC1 was interrogated, in cell cross-linking with DSP was preformed as described in (Sancak et al., 2008) prior to cell lysis. The soluble fractions of cell lysates were isolated by centrifugation at 13,000 rpm in a microcentrifuge for 10 minutes. For immunoprecipitations, primary antibodies were added to the cleared lysates and incubated with rotation for 1.5 hours at 4°C. 60 μl of a 50% slurry of protein G-sepharose was then added and the incubation continued for an additional 1 hour. Immunoprecipitates were washed three times with lysis buffer containing 150 mM NaCl. Immunoprecipitated proteins were denatured by the addition of 20 μl of sample buffer and boiling for 5 minutes, resolved by 8%–16% SDS-PAGE, and analyzed by immunoblotting as described (Kim et al., 2002). For anti-FLAG-immunoprecipitations, the FLAG-M2 affinity gel was washed with lysis buffer 3 times. 20 μl of a 50% slurry of the affinity gel was then added to cleared cell lysates and incubated with rotation for 2 hours at 4°C. The beads were washed 3 times with lysis buffer containing 150 mM NaCl. Immunoprecipitated proteins were denatured by the addition of 50 μl of sample buffer and boiling for 5 minutes.
For co-transfection experiments, 2,000,000 HEK293T cells were plated in 10 cm culture dishes. Twenty-four hours later, cells were transfected with the pRK5-based cDNA expression plasmids indicated in the figures in the following amounts: 100 ng or 1000 ng FLAG- or HA-HBXIP; 100 ng or 1000 ng FLAG- or HA-HBXIP; 100 ng or 1000 ng FLAG-p14; 100 ng HA-MP1; 100 ng or 1000 ng FLAG- or HA-p18; 100 ng or 1000 ng FLAG-Rap2a; 300 ng Flag-Metap2; 300 ng Flag-VPS39; 100 ng Flag- or HA-RagB and 100 ng HA- or HA-GST-RagC. The total amount of plasmid DNA in each transfection was normalized to 2 μg with empty pRK5. Thirty-six hours after transfection, cells were lysed as described above.
Identification of HBXIP and C7orf59
Immunoprecipitates from HEK-293T cells stably expressing FLAG-p18, FLAG-p14, FLAG-RagB or FLAG-Metap2 were prepared using Chaps lysis buffer as described above. Proteins were eluted with the FLAG peptide (sequence DYKDDDDK) from the FLAG-M2 affinity gel, resolved on 4-12% NuPage gels (Invitrogen), and stained with simply blue stain (Invitrogen). Each gel lane was sliced into 10-12 pieces and the proteins in each gel slice digested overnight with trypsin. The resulting digests were analyzed by mass spectrometry as described (Sancak et al., 2008). Peptides corresponding to HBXIP and C7orf59 were identified in the FLAG-p14, FLAG-p18 and FLAG-RagB immunoprecipitates, while no peptides were detected in negative control immunoprecipitates of FLAG-Metap2.
Total amino acid starvation and stimulation, leucine starvation and stimulation, Salicylihalamide A and Concanamycin A treatment
HEK-293T cells in culture dishes or coated glass cover slips were rinsed with and incubated in amino acid-free RPMI or leucine-free RPMI for either 50 minutes or 2 hours, and stimulated with a 10X mixture of total amino acids or 10X leucine for 10-20 minutes, respectively. After stimulation, the final concentration of amino acids in the media was the same as in RPMI. The 10X mixture of total amino acids was prepared from individual powders of amino acids. Where drug treatment was performed, cells were incubated with 2.5 μm of Salicylihalamide A or 2.5 μm of Concanamycin A during the 2 hour starvation period and the 15 min stimulation period.
RNAi in mammalian cells
On day one, 200,000 HEK-293T cells were plated in a 6 well plate. Twenty-fours hours later, the cells were transfected with 250 nM of a pool of siRNAs [Dharmacon] targeting HBXIP or C7orf59, a non-targeting pool, or 125 nM of siRNAs targeting p14 or p18. On day four, the cells were transfected again but this time with double the amount of siRNAs. On day five, the cells were either split onto coated glass cover slips or rinsed with ice-cold PBS, lysed and subjected to immunobloting as described above.
RNAi in Drosophila S2 cells
dsRNAs against Drosophila HBXIP and C7orf59 genes were designed as described in (Sancak et al., 2008). Primer sequences used to amplify DNA templates for dsRNA synthesis for dHBXIP and, dC7orf59 including underlined 5’ and 3’ T7 promoter sequences, are as follows:
dHBXIP (CG14812)
Forward primer: GAATTAATACGACTCACTATAGGGAGAGGAGAAAGTCCTAGCGGAAATC
Reverse primer: GAATTAATACGACTCACTATAGGGAGAGCTTGAAGATAACGCCTGTGAT
dC7orf59 (CG14977)
Forward primer: GAATTAATACGACTCACTATAGGGAGACTGATACTAAAGGAAGATGGAGCAG
Reverse primer: GAATTAATACGACTCACTATAGGGAGAGTATATTCTACGGTTGGACATGCAG
dsRNAs targeting GFP and dRagC were used as positive and negative controls, respectively. On day one, 4,000,000 S2 cells were plated in 6-cm culture dishes in 5 ml of Express Five SFM media. Cells were transfected with 1 μg of dsRNA per million cells using Fugene (Roche). Two days later, a second round of dsRNA transfection was performed. On day five, cells were rinsed once with amino acid-free Schneider's medium, and starved for amino acids by replacing the media with amino acid-free Schneider's medium for 1.5 hours. To stimulate with amino acids, the amino acid-free medium was replaced with complete Schneider's medium for 30 minutes. Cells were then washed with ice cold PBS, lysed, and subjected to immunoblotting for phospho-T398 dS6K and total dS6K.
Immunofluorescence assays
Immunofluorescence assays were performed as described in (Sancak et al., 2010). Briefly, 200,000 HEK-293T cells were plated on fibronectin-coated glass coverslips in 12-well tissue culture plates. Twenty-four hours later, the slides were rinsed with PBS once and fixed for 15 min with 4% paraformaldehyde in PBS at room temperature. The slides were rinsed twice with PBS and cells were permeabilized with 0.05% Triton X-100 in PBS for 5 min. After rinsing twice with PBS, the slides were incubated with primary antibody in 5% normal donkey serum for 1 hr at room temperature, rinsed four times with PBS, and incubated with secondary antibodies produced in donkey (diluted 1:1000 in 5% normal donkey serum) for 45 min at room temperature in the dark and washed four times with PBS. Slides were mounted on glass coverslips using Vectashield (Vector Laboratories) and imaged on a spinning disk confocal system (Perkin Elmer) or a Zeiss Laser Scanning Microscope (LSM) 710.
In immunofluorescence assays where HBXIP or C7orf59 were co-localized with p18, HEK-293T cells were seeded and processed as described above with the following exceptions. Immediately after seeding, cells were transfected with the following constructs (all cDNAs were expressed from pRK5 expression plasmid): 50 ng Flag-HBXIP, 50 ng HA-p14, 50 ng HA-MP1, 50 ng HA-C7orf59 and 50 ng HA-p18; or 50 ng Flag-C7orf59, 50 ng HA-p14, 50 ng HA-MP1, 50 ng HA-HBXIP and 50 ng HA-p18. The cells were processed the following day.
Cell size determinations
For measurements of cell size, HEK-293T cells treated with siRNAs as described above were harvested by trypsinization in a 4 ml volume and diluted 1:20 with counting solution (Isoton II Diluent, Beckman Coulter). Cell diameters were determined with a particle size counter (Coulter Z2, Beckman Coulter) running Coulter Z2 AccuComp software.
Protein purification of recombinant Rag heterodimers and Ragulator
To produce protein complexes used for GEF or in vitro binding assays, 4,000,000 HEK-293T cells were plated in 15 cm culture dishes. Forty-eight hours later, cells were transfected separately with the following constructs (all cDNAs were expressed from pRK5 expression plasmid). For pentameric Ragulator: 4 μg Flag-p14, 8 μg HA-MP1, 8 μg HA-p18G2A (a lipidation defective mutant), 8 μg HA-HBXIP, and 8 μg HA-C7orf59. For trimeric Ragulator complexes: 8 μg FLAG-p14, 16 μg HA-MP1 and 16 μg HA-p18G2A; or 8 μg FLAG-HBXIP, 16 μg HA-C7orf59 and 16 μg HA-p18G2A. For dimeric complexes: 8 μg FLAG-p14 and 16 μg HA-MP1; 8 μg FLAG-HBXIP and 16 μg HA-C7orf59; 8 μg of HA-GST-p14 and 16 μg of MP1; 8 μg FLAG-RagBD163N and 16 μg HA-RagC; 8 μg FLAG-RagCD181N and 16 μg HA-RagB; or 8 μg FLAG-RagB and 16 μg HA-RagC. For individual proteins: 10 μg Flag-p18G2A; 10 μg Flag-Metap2; 15 μg Flag-VPS39; 10 μg HA-GST-HBXIP, 10 μg HA-GST-C7orf59; or 10 μg HAGST-Rap2a
Thirty-six hours post transfection cell lysates were prepared as described above and either 200 μl of a 50% slurry of glutathione affinity beads or 200 μl of a 50% slurry of FLAG-M2 affinity gel were added to lysates from cells expressing HA-GST-tagged or FLAG-tagged proteins, respectively. Recombinant proteins were immunoprecipitated for 3 hours at 4°C. Each sample was washed once with Triton lysis buffer, followed by 3 washes with Triton lysis buffer supplemented with 500 mM NaCl. Samples containing FLAG-tagged proteins were eluted from the FLAG-M2 affinity gel with a competing FLAG peptide as described above.
In vitro binding assays
For the binding reactions, 20 μl of a 50% slurry containing immobilized HA-GST-tagged proteins were incubated in binding buffer (1% Triton X-100, 2.5mM MgCl2, 40 mM Hepes pH 7.4, 2 mM DTT and 1mg/ml BSA) with 2 μg of FLAG-tagged proteins in a total volume of 50 μl for 1 hour and 30 minutes at 4°C. In binding assays where HA-GST-Ragulator was used, HA-GST-p14-MP1 was pre-bound to FLAG-HBXIP-HA-C7orf59 and FLAG-p18 for 5 minutes at 4°C prior to the addition of other FLAG-tagged proteins. In experiments where the Flag-RagB-HA-RagC heterodimer was loaded with nucleotides, 2 μg of FLAG-RagB-HA-RagC was incubated at 25°C for 10 minutes in Rag loading buffer (0.3% Chaps, 40 mM Hepes pH 7.4, 5 mM EDTA, 2 mM DTT and 1 mg/ml BSA) supplemented with either 1 mM GTPγS or 1 mM GDP in a total volume of 10 μl. The Rag-nucleotide complex was stabilized by the addition of 20 mM MgCl2 and incubated for an additional 5 minutes at 25°C. In assays with nucleotide free Rags, 2 μg of FLAG-RagB-HA-RagC was added to the binding assay with 3 μl of Calf-alkaline phosphatase (NEB). Binding assays in which Ragulator was incubated with nucleotide-loaded or -free Rags were conducted at 4°C for 45 minutes. For the nucleotide competition assay, 2 μg FLAG-RagB-HA-RagC was pre-bound to Ragulator proteins for 30 minutes followed by the addition of 1 mM GTPγS and further incubated for 1 hour and 30 minutes at 4°C. To terminate all binding assays, samples were washed 3 times with 1 ml of ice-cold binding buffer supplemented with 150 mM NaCl followed by the addition of 50 μl of sample buffer.
Nucleotide exchange (GEF) assays
40 pmols of FLAG-RagBD163N-HA-RagC, FLAG-RagCD181N-HA-RagB or FLAG-Rap2a were loaded with either 2 μM of [3H]GDP (25-50 Ci/mmol), 10 μCi of [35S]GTPγS (1250 Ci/mmol), 2 mM GDP (for GTP binding assays), or co-loaded with guanine nucleotides and either 50 nM of XTPγS or 50 nM XDP (Ragulator GEF activity was maintained between a range of 5-500 nM xanthine nucleotide) in a total volume of 100 μl of Rag loading buffer as described above. The GTPase-[3H]GDP-XDP/ XTPγS or GTPase-[35S]GTPγS-XDP/ XTPγS and GTPase-GDP complexes were stabilized by addition of 20 mM MgCl2 followed by a further incubation at 4°C for 12 hours or 25°C for 5 minutes, respectively. To initiate the GEF assay, 40 pmols of pentameric Ragulator, the indicated Ragulator subcomplexes or a control (FLAG-Metap2, FLAG-VPS39, or FLAG-HBXIP-HA-C7orf59) were added along with 200 μM GTPγS or 5 μCi of [35S]GTPγS (for GTP binding assays) and incubated at 25°C. Samples were taken every 2 minutes and spotted on nitrocellulose filters, which were washed with 2 ml of wash buffer (40 mM Hepes pH 7.4, 150 mM NaCl and 5 mM MgCl2). Filter-associated radioactivity was measured using a TriCarb scintillation counter (Perkin Elmer).
Monomeric Rag GDP loading
40 pmols of FLAG-RagB, FLAG-RagBS54N, FLAG-RagBD163N, FLAG-RagC, FLAG-RagCT75N or FLAG-RagCD181N were loaded with 2 μM of [3H]GDP as described for Rag heterodimers, but MgCl2 stabilization lasted for 5 min at 25°C. The amount of [3H]GDP bound to monomeric Rags was determined with a filter binding assay and was normalized to [3H]GDP binding by wild type RagB or RagC.
Supplementary Material
Supplementary Data
Acknowledgements
We thank all members of the Sabatini Lab for helpful suggestions and Eric Spooner for mass spectrometric analyses of samples. This work was supported by grants from the NIH (CA103866 and AI47389) and Department of Defense (W81XWH-07-0448) to D.M.S., awards from the LAM Foundation to D.M.S, and fellowship support from the LAM Foundation and from the Jane Coffin Childs Memorial Fund for Medical Research to R.Z. D.M.S. is an investigator of the Howard Hughes Medical Institute.
References
- Binda M, PÈli-Gulli M-P, Bonfils G, Panchaud N, Urban J, Sturgill TW, Loewith R, De Virgilio C. The Vam6 GEF Controls TORC1 by Activating the EGO Complex. Mol Cell. 2009;35:563–573. doi: 10.1016/j.molcel.2009.06.033. [DOI] [PubMed] [Google Scholar]
- Bos JL, Rehmann H, Wittinghofer A. GEFs and GAPs: critical elements in the control of small G proteins. Cell. 2007;129:865–877. doi: 10.1016/j.cell.2007.05.018. [DOI] [PubMed] [Google Scholar]
- Bowman AB, Patel-King RS, Benashski SE, McCaffery JM, Goldstein LS, King SM. Drosophila roadblock and Chlamydomonas LC7: a conserved family of dynein-associated proteins involved in axonal transport, flagellar motility, and mitosis. J Cell Biol. 1999;146:165–180. [PMC free article] [PubMed] [Google Scholar]
- Bowman EJ, Graham LA, Stevens TH, Bowman BJ. The bafilomycin/concanamycin binding site in subunit c of the V-ATPases from Neurospora crassa and Saccharomyces cerevisiae. J Biol Chem. 2004;279:33131–33138. doi: 10.1074/jbc.M404638200. [DOI] [PubMed] [Google Scholar]
- Cai Y, Chin HF, Lazarova D, Menon S, Fu C, Cai H, Sclafani A, Rodgers DW, De La Cruz EM, Ferro-Novick S, et al. The Structural Basis for Activation of the Rab Ypt1p by the TRAPP Membrane-Tethering Complexes. Cell. 2008;133:1202–1213. doi: 10.1016/j.cell.2008.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortez D, Guntuku S, Qin J, Elledge SJ. ATR and ATRIP: Partners in Checkpoint Signaling. Science. 2001;294:1713–1716. doi: 10.1126/science.1065521. [DOI] [PubMed] [Google Scholar]
- Dubouloz F, Deloche O, Wanke V, Cameroni E, De Virgilio C. The TOR and EGO protein complexes orchestrate microautophagy in yeast. Mol Cell. 2005;19:15–26. doi: 10.1016/j.molcel.2005.05.020. [DOI] [PubMed] [Google Scholar]
- Feig LA. Tools of the trade: use of dominant-inhibitory mutants of Ras-family GTPases. Nat Cell Biol. 1999;1:E25–27. doi: 10.1038/10018. [DOI] [PubMed] [Google Scholar]
- Feig LA, Cooper GM. Inhibition of NIH 3T3 cell proliferation by a mutant ras protein with preferential affinity for GDP. Mol Cell Biol. 1988;8:3235–3243. doi: 10.1128/mcb.8.8.3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiegen D, Dvorsky R, Ahmadian MR. Structural Principles of Ras Interaction with Regulators and Effectors. 2006.
- Der C, editor. RAS Family GTPases. Springer; Netherlands: pp. 45–66. [Google Scholar]
- Fingar DC, Salama S, Tsou C, Harlow E, Blenis J. Mammalian cell size is controlled by mTOR and its downstream targets S6K1 and 4EBP1/eIF4E. Genes Dev. 2002;16:1472–1487. doi: 10.1101/gad.995802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forgac M. Vacuolar ATPases: rotary proton pumps in physiology and pathophysiology. Nat Rev Mol Cell Biol. 2007;8:917–929. doi: 10.1038/nrm2272. [DOI] [PubMed] [Google Scholar]
- Frech M, Darden TA, Pedersen LG, Foley CK, Charifson PS, Anderson MW, Wittinghofer A. Role of glutamine-61 in the hydrolysis of GTP by p21H-ras: an experimental and theoretical study. Biochemistry. 1994;33:3237–3244. doi: 10.1021/bi00177a014. [DOI] [PubMed] [Google Scholar]
- Fujii R, Zhu C, Wen Y, Marusawa H, Bailly-Maitre B, Matsuzawa S, Zhang H, Kim Y, Bennett CF, Jiang W, et al. HBXIP, cellular target of hepatitis B virus oncoprotein, is a regulator of centrosome dynamics and cytokinesis. Cancer Res. 2006;66:9099–9107. doi: 10.1158/0008-5472.CAN-06-1886. [DOI] [PubMed] [Google Scholar]
- Gao M, Kaiser CA. A conserved GTPase-containing complex is required for intracellular sorting of the general amino-acid permease in yeast. Nat Cell Biol. 2006;8:657–667. doi: 10.1038/ncb1419. [DOI] [PubMed] [Google Scholar]
- Garcia-Mata R, Boulter E, Burridge K. The ‘invisible hand’: regulation of RHO GTPases by RHOGDIs. Nat Rev Mol Cell Biol. 2011;12:493–504. doi: 10.1038/nrm3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Saez I, Lacroix FB, Blot D, Gabel F, Skoufias DA. Structural characterization of HBXIP: the protein that interacts with the anti-apoptotic protein survivin and the oncogenic viral protein HBx. J Mol Biol. 2011;405:331–340. doi: 10.1016/j.jmb.2010.10.046. [DOI] [PubMed] [Google Scholar]
- Gong R, Li L, Liu Y, Wang P, Yang H, Wang L, Cheng J, Guan KL, Xu Y. Crystal structure of the Gtr1p-Gtr2p complex reveals new insights into the amino acid-induced TORC1 activation. Genes Dev. 2011;25:1668–1673. doi: 10.1101/gad.16968011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good MC, Zalatan JG, Lim WA. Scaffold proteins: hubs for controlling the flow of cellular information. Science. 2011;332:680–686. doi: 10.1126/science.1198701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara K, Yonezawa K, Weng QP, Kozlowski MT, Belham C, Avruch J. Amino acid sufficiency and mTOR regulate p70 S6 kinase and eIF-4E BP1 through a common effector mechanism. J Biol Chem. 1998;273:14484–14494. doi: 10.1074/jbc.273.23.14484. [DOI] [PubMed] [Google Scholar]
- Hirose E, Nakashima N, Sekiguchi T, Nishimoto T. RagA is a functional homologue of S. cerevisiae Gtr1p involved in the Ran/Gsp1-GTPase pathway. J Cell Sci. 1998;111(Pt 1):11–21. doi: 10.1242/jcs.111.1.11. [DOI] [PubMed] [Google Scholar]
- Hoffenberg S, Nikolova L, Pan JY, Daniel DS, Wessling-Resnick M, Knoll BJ, Dickey BF. Functional and structural interactions of the Rab5 D136N mutant with xanthine nucleotides. Biochem Biophys Res Commun. 1995;215:241–249. doi: 10.1006/bbrc.1995.2459. [DOI] [PubMed] [Google Scholar]
- Howell JJ, Manning BD. mTOR couples cellular nutrient sensing to organismal metabolic homeostasis. Trends Endocrinol Metab. 2011;22:94–102. doi: 10.1016/j.tem.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutagalung AH, Novick PJ. Role of Rab GTPases in membrane traffic and cell physiology. Physiol Rev. 2011;91:119–149. doi: 10.1152/physrev.00059.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings MD, Pavitt GD. eIF5 has GDI activity necessary for translational control by eIF2 phosphorylation. Nature. 2010;465:378–381. doi: 10.1038/nature09003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John J, Rensland H, Schlichting I, Vetter I, Borasio GD, Goody RS, Wittinghofer A. Kinetic and structural analysis of the Mg(2+)-binding site of the guanine nucleotide-binding protein p21H-ras. J Biol Chem. 1993;268:923–929. [PubMed] [Google Scholar]
- Jonathan G. Structural Basis for Activation of ARF GTPase: Mechanisms of Guanine Nucleotide Exchange and GTP,ÄìMyristoyl Switching. Cell. 1998;95:237–248. doi: 10.1016/s0092-8674(00)81754-7. [DOI] [PubMed] [Google Scholar]
- Jones S, Newman C, Liu F, Segev N. The TRAPP complex is a nucleotide exchanger for Ypt1 and Ypt31/32. Mol Biol Cell. 2000;11:4403–4411. doi: 10.1091/mbc.11.12.4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D-H, Sarbassov DD, Ali SM, King JE, Latek RR, Erdjument-Bromage H, Tempst P, Sabatini DM. mTOR Interacts with Raptor to Form a Nutrient-Sensitive Complex that Signals to the Cell Growth Machinery. Cell. 2002;110:163–175. doi: 10.1016/s0092-8674(02)00808-5. [DOI] [PubMed] [Google Scholar]
- Kim E, Goraksha-Hicks P, Li L, Neufeld TP, Guan KL. Regulation of TORC1 by Rag GTPases in nutrient response. Nat Cell Biol. 2008;10:935–945. doi: 10.1038/ncb1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebe C, Prinz H, Wittinghofer A, Goody RS. The kinetic mechanism of Ran--nucleotide exchange catalyzed by RCC1. Biochemistry. 1995;34:12543–12552. doi: 10.1021/bi00039a008. [DOI] [PubMed] [Google Scholar]
- Koonin EV, Aravind L. Dynein light chains of the Roadblock/LC7 group belong to an ancient protein superfamily implicated in NTPase regulation. Curr Biol. 2000;10:R774–776. doi: 10.1016/s0960-9822(00)00774-0. [DOI] [PubMed] [Google Scholar]
- Krengel U, Schlichting I, Scherer A, Schumann R, Frech M, John J, Kabsch W, Pai EF, Wittinghofer A. Three-dimensional structures of H-ras p21 mutants: molecular basis for their inability to function as signal switch molecules. Cell. 1990;62:539–548. doi: 10.1016/0092-8674(90)90018-a. [DOI] [PubMed] [Google Scholar]
- Kurzbauer R, Teis D, de Araujo ME, Maurer-Stroh S, Eisenhaber F, Bourenkov GP, Bartunik HD, Hekman M, Rapp UR, Huber LA, et al. Crystal structure of the p14/MP1 scaffolding complex: how a twin couple attaches mitogen-activated protein kinase signaling to late endosomes. Proc Natl Acad Sci U S A. 2004;101:10984–10989. doi: 10.1073/pnas.0403435101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenzen C, Cool RH, Prinz H, Kuhlmann J, Wittinghofer A. Kinetic analysis by fluorescence of the interaction between Ras and the catalytic domain of the guanine nucleotide exchange factor Cdc25Mm. Biochemistry. 1998;37:7420–7430. doi: 10.1021/bi972621j. [DOI] [PubMed] [Google Scholar]
- Lunin VV, Munger C, Wagner J, Ye Z, Cygler M, Sacher M. The structure of the MAPK scaffold, MP1, bound to its partner, p14. A complex with a critical role in endosomal map kinase signaling. J Biol Chem. 2004;279:23422–23430. doi: 10.1074/jbc.M401648200. [DOI] [PubMed] [Google Scholar]
- Ma XM, Blenis J. Molecular mechanisms of mTOR-mediated translational control. Nat Rev Mol Cell Biol. 2009;10:307–318. doi: 10.1038/nrm2672. [DOI] [PubMed] [Google Scholar]
- Marusawa H, Matsuzawa S, Welsh K, Zou H, Armstrong R, Tamm I, Reed JC. HBXIP functions as a cofactor of survivin in apoptosis suppression. Embo J. 2003;22:2729–2740. doi: 10.1093/emboj/cdg263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melegari M, Scaglioni PP, Wands JR. Cloning and characterization of a novel hepatitis B virus x binding protein that inhibits viral replication. J Virol. 1998;72:1737–1743. doi: 10.1128/jvi.72.3.1737-1743.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miertzschke M, Koerner C, Vetter IR, Keilberg D, Hot E, Leonardy S, Sogaard-Andersen L, Wittinghofer A. Structural analysis of the Ras-like G protein MglA and its cognate GAP MglB and implications for bacterial polarity. Embo J. 2011;30:4185–4197. doi: 10.1038/emboj.2011.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nada S, Hondo A, Kasai A, Koike M, Saito K, Uchiyama Y, Okada M. The novel lipid raft adaptor p18 controls endosome dynamics by anchoring the MEK-ERK pathway to late endosomes. Embo J. 2009;28:477–489. doi: 10.1038/emboj.2008.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roccio M, Bos JL, Zwartkruis FJ. Regulation of the small GTPase Rheb by amino acids. Oncogene. 2006;25:657–664. doi: 10.1038/sj.onc.1209106. [DOI] [PubMed] [Google Scholar]
- Sancak Y, Bar-Peled L, Zoncu R, Markhard AL, Nada S, Sabatini DM. Ragulator-Rag complex targets mTORC1 to the lysosomal surface and is necessary for its activation by amino acids. Cell. 2010;141:290–303. doi: 10.1016/j.cell.2010.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancak Y, Peterson TR, Shaul YD, Lindquist RA, Thoreen CC, Bar-Peled L, Sabatini DM. The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science. 2008;320:1496–1501. doi: 10.1126/science.1157535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt G, Lenzen C, Simon I, Deuter R, Cool RH, Goody RS, Wittinghofer A. Biochemical and biological consequences of changing the specificity of p21ras from guanosine to xanthosine nucleotides. Oncogene. 1996;12:87–96. [PubMed] [Google Scholar]
- Schurmann A, Brauers A, Massmann S, Becker W, Joost HG. Cloning of a novel family of mammalian GTP-binding proteins (RagA, RagBs, RagB1) with remote similarity to the Ras-related GTPases. J Biol Chem. 1995;270:28982–28988. doi: 10.1074/jbc.270.48.28982. [DOI] [PubMed] [Google Scholar]
- Sekiguchi T, Hirose E, Nakashima N, Ii M, Nishimoto T. Novel G proteins, Rag C and Rag D, interact with GTP-binding proteins, Rag A and Rag B. J Biol Chem. 2001;276:7246–7257. doi: 10.1074/jbc.M004389200. [DOI] [PubMed] [Google Scholar]
- Selyunin AS, Sutton SE, Weigele BA, Reddick LE, Orchard RC, Bresson SM, Tomchick DR, Alto NM. The assembly of a GTPase-kinase signalling complex by a bacterial catalytic scaffold. Nature. 2011;469:107–111. doi: 10.1038/nature09593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EM, Finn SG, Tee AR, Browne GJ, Proud CG. The tuberous sclerosis protein TSC2 is not required for the regulation of the mammalian target of rapamycin by amino acids and certain cellular stresses. J Biol Chem. 2005;280:18717–18727. doi: 10.1074/jbc.M414499200. [DOI] [PubMed] [Google Scholar]
- Stocker H, Radimerski T, Schindelholz B, Wittwer F, Belawat P, Daram P, Breuer S, Thomas G, Hafen E. Rheb is an essential regulator of S6K in controlling cell growth in Drosophila. Nat Cell Biol. 2003;5:559–565. doi: 10.1038/ncb995. [DOI] [PubMed] [Google Scholar]
- Wang FZ, Sha L, Zhang WY, Wu LY, Qiao L, Li N, Zhang XD, Ye LH. Involvement of hepatitis B X-interacting protein (HBXIP) in proliferation regulation of cells. Acta Pharmacol Sin. 2007;28:431–438. doi: 10.1111/j.1745-7254.2007.00531.x. [DOI] [PubMed] [Google Scholar]
- Wang W, Sacher M, Ferro-Novick S. TRAPP stimulates guanine nucleotide exchange on Ypt1p. J Cell Biol. 2000;151:289–296. doi: 10.1083/jcb.151.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanschers B, van de Vorstenbosch R, Wijers M, Wieringa B, King SM, Fransen J. Rab6 family proteins interact with the dynein light chain protein DYNLRB1. Cell Motil Cytoskeleton. 2008;65:183–196. doi: 10.1002/cm.20254. [DOI] [PubMed] [Google Scholar]
- Wen Y, Golubkov VS, Strongin AY, Jiang W, Reed JC. Interaction of hepatitis B viral oncoprotein with cellular target HBXIP dysregulates centrosome dynamics and mitotic spindle formation. J Biol Chem. 2008;283:2793–2803. doi: 10.1074/jbc.M708419200. [DOI] [PubMed] [Google Scholar]
- Wunderlich W, Fialka I, Teis D, Alpi A, Pfeifer A, Parton RG, Lottspeich F, Huber LA. A novel 14-kilodalton protein interacts with the mitogen-activated protein kinase scaffold mp1 on a late endosomal/lysosomal compartment. J Cell Biol. 2001;152:765–776. doi: 10.1083/jcb.152.4.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie XS, Padron D, Liao X, Wang J, Roth MG, De Brabander JK. Salicylihalamide A inhibits the V0 sector of the V-ATPase through a mechanism distinct from bafilomycin A1. J Biol Chem. 2004;279:19755–19763. doi: 10.1074/jbc.M313796200. [DOI] [PubMed] [Google Scholar]
- Zhang B, Zhang Y, Shacter E, Zheng Y. Mechanism of the guanine nucleotide exchange reaction of Ras GTPase--evidence for a GTP/GDP displacement model. Biochemistry. 2005;44:2566–2576. doi: 10.1021/bi048755w. [DOI] [PubMed] [Google Scholar]
- Zoncu R, Bar-Peled L, Efeyan A, Wang S, Sancak Y, Sabatini DM. mTORC1 senses lysosomal amino acids through an inside-out mechanism that requires the vacuolar H(+)-ATPase. Science. 2011a;334:678–683. doi: 10.1126/science.1207056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011b;12:21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Data