p53 Functions in Endothelial Cells to Prevent Radiation-Induced Myocardial Injury in Mice (original) (raw)
. Author manuscript; available in PMC: 2013 Jan 24.
Published in final edited form as: Sci Signal. 2012 Jul 24;5(234):ra52. doi: 10.1126/scisignal.2002918
Abstract
p53 functions in the heart to promote myocardial injury after multiple types of stress. However, how p53 regulates radiation-induced myocardial injury, which develops after radiation therapy, is not well understood. Here, we utilize the Cre-loxP system to demonstrate that p53 functioned in endothelial cells to protect mice from myocardial injury after whole-heart irradiation. Mice with an endothelial cell-specific deletion of p53 succumbed to heart failure after whole-heart irradiation due to myocardial necrosis, systolic dysfunction and cardiac hypertrophy. Moreover, the onset of cardiac dysfunction was preceded by alterations in myocardial vascular permeability and density, which resulted in cardiac ischemia and myocardial hypoxia. Mechanistic studies using primary cardiac endothelial cells irradiated in vitro indicated that p53 signaling caused mitotic arrest and protected cardiac endothelial cells against radiation-induced mitotic catastrophe. Furthermore, mice lacking the cyclin-dependent kinase inhibitor p21, which is a transcriptional target of p53, were also sensitized to myocardial injury after wholeheart irradiation. Together, our results demonstrate that the p53/p21 axis functions to prevent radiation-induced myocardial injury in mice.
INTRODUCTION
The tumor suppressor protein 53 (p53) is a transcription factor that serves as a key executor of the DNA damage response to control cell survival and cell death (1, 2). In the heart, p53 functions to promote cardiac injury from pressure overload (3), ischemic injury (4), telomere attrition (5), and doxorubicin-induced oxidative stress (6–8). Therefore, blocking p53 with pharmacological inhibitors has been proposed as a promising approach to prevent cardiac injury from multiple stresses. However, the role of p53 in regulating radiation-induced myocardial injury is unknown.
Radiation-related heart disease is a well-described late effect of radiation therapy (9). In a meta-analysis from several randomized trials of women with breast cancer, mortality from heart disease was substantially increased for women who were randomized to receive adjuvant fractionated radiation therapy ranging from 35 to 65 Gy (10). Further support for the hypothesis that radiation causes heart disease in breast cancer patients comes from the observation that excess mortality from heart disease is observed in women receiving radiation therapy for left-sided breast cancer (11). A prospective study of left-sided breast cancer patients has been performed with cardiac single-photon emission computed tomography (SPECT) scans to measure blood flow to the myocardium. Patients receiving cardiac SPECT scans prior to and 6 months after radiation therapy had perfusion defects within the part of the left ventricle that received high dose irradiation (12). These perfusion defects persisted on follow-up cardiac SPECT scans 3 to 8 years after radiation therapy (13). Therefore, an important consequence of radiation therapy to the heart is decreased blood flow to the myocardium.
Damage to the microvasculature of the heart after irradiation occurs in animal models prior to pathological changes in the myocardium (14–18). For example, Fajardo and Stewart studied the pathogenesis of radiation-induced myocardial fibrosis in rabbits exposed to a single dose of 20 Gy (14, 15). In these elegant studies, focal areas of myocardial fibrosis were observed by two months after irradiation (15). From day 20 through 49 after irradiation, there was considerable damage to endothelial cells, including decreased microvessel density, within the myocardium (14). Lauk and co-workers observed similar histopathology in rats in which the heart received a single dose of 15 to 20 Gy. They found a substantial reduction in capillary density of the irradiated heart prior to any obvious histological damage to the cardiomyocytes (16). Follow-up studies comparing radiation-induced heart disease in Wistar and Sprague-Dawley rats showed that microvessel density was reduced by approximately 50% one month after a single dose of 17.5 to 20 Gy, whereas focal areas of myocardial necrosis were noted at two months (17). Seemann and colleagues reported alterations in the microvasculature in the myocardium of mice 40 weeks after a single dose of 16 Gy to the heart, with associated sudden death in one-third of the mice (18). Although it has been established that microvascular loss precedes myocardial necrosis in radiation-induced myocardial injury, the molecular mechanisms controlling the loss of the myocardial capillaries remain to be fully defined (19–21).
Radiation induces p53 in the heart in vivo (22) and in endothelial cells from various sources in vitro (23–25). In endothelial cells, whether p53 functions as a pro-survival or pro-death factor remains controversial. For example, lovastatin, a 3-hydroxy-3-methylglutaryl CoA reductase inhibitor, protects human umbilical vein endothelial cells (HUVECs) from radiation-induced cell death by apparently blocking p53 (23). This finding is further supported by the observation that the small-molecule inhibitor p53 pifithrin increases the viability of HUVECs after irradiation (23). Moreover, blocking p53 has been suggested to suppress radiation-induced damage to human dermal microvascular endothelial cells and mouse endothelial progenitor cells (24, 25). In contrast, others have reported that when p53 is inhibited or deleted in tumor-associated endothelial cells, xenograft tumors are sensitized to radiation (26), suggesting that p53 functions to protect endothelial cells against radiation. Because endothelial cell dysfunction appears to play a crucial role in initiating radiation-induced myocardial injury, clarifying how p53 regulates the radiation response of cardiac endothelial cells may reveal the molecular mechanism of radiation induced cardiac toxicity. Moreover, understanding whether p53 plays a pro-death or pro-survival role in irradiated cardiac endothelial cells will provide critical information to design clinical trials that combine radiation therapy with inhibitors of the DNA damage response pathway.
To investigate how p53 functions in cardiac endothelial cells to regulate radiation response of the heart in vivo, we deleted p53 in endothelial cells using Tie2Cre and VECadherin-Cre mice. Tie2 is a receptor tyrosine kinase present almost exclusively by endothelial cells (27–29) and VE-Cadherin is a transmembrane adhesion protein in endothelial cells (30). The promoters of both genes drive Cre expression in the endothelium of adult mice (31, 32). Deleting p53 in endothelial cells with either Tie2Cre or VE-Cadherin-Cre caused mice to be sensitized to myocardial injury after whole-heart irradiation. The onset of cardiac injury was preceded by increased endothelial cell death, increased vascular permeability and decreased microvessel density in the myocardium. In addition, cell-based studies using primary cardiac endothelial cells from these mice showed that p53-mediated cell cycle arrest protected cardiac endothelial cells from radiation-induced mitotic catastrophe. Moreover, mice lacking the cyclin-dependent kinase inhibitor p21, which is a transcriptional target of p53, also developed myocardial injury after whole-heart irradiation. Taken together, our results demonstrate that p53 functions in endothelial cells in vivo to prevent radiation-induced myocardial injury.
RESULTS
Deletion of p53 in endothelial cells by Tie2Cre sensitizes mice to radiation-induced cardiac injury
To investigate the role of p53 in endothelial cells in regulating the radiation response of the heart, we utilized Tie2Cre mice to delete p53 in endothelial cells in vivo (33). Because Tie2Cre has been reported to recombine genes in some cardiomyocytes in addition to endothelial cells (34), we examined the cell types that express Cre recombinase in the heart by crossing Tie2Cre mice to membrane-Tomato/membrane-GFP (mTmG) reporter mice (35). In Tie2Cre; mTmG/+ mice, cells expressing Cre were labeled by enhanced green fluorescent protein (EGFP), whereas cells that do not express Cre were labeled by tdTomato protein. In Tie2Cre; mTmG/+ mice, cells expressing EGFP in the myocardium are labeled by Griffonia simplicifolia isolectin B4 (GS-IB4) which marks endothelial cells (36) (Fig. S1). The pattern of Cre expression is similar in Tie2Cre mice regardless of the presence (Tie2Cre; p53 FL/+; mTmG/+) or absence (Tie2Cre; p53 FL/−; mTmG/+) of p53 in endothelial cells (Fig. S1). The results indicate that, in these Tie2Cre mice, Cre is expressed ubiquitously in endothelial cells and not in cardiomyocytes.
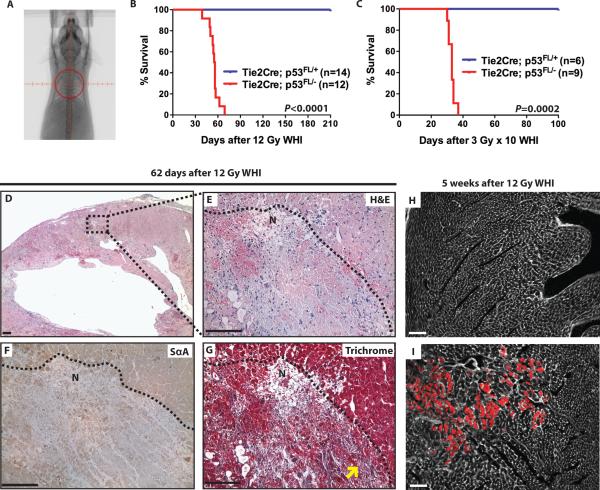
To study the radiation response of the heart, Tie2Cre; p53 FL/+ and Tie2Cre; p53 FL/− mice were treated with whole-heart irradiation using either a single dose of 12 Gy or 10 daily fractions of 3 Gy to model a dose and schedule of radiation therapy used in the clinic. Focal irradiation to the heart was performed using a micro-computed tomography (CT)/micro-irradiator so that the radiation field included the heart and a margin of normal lung tissue (Fig. 1A). Tie2Cre; p53 FL/+ mice did not show any signs of morbidity after 210 days following whole-heart irradiation, whereas all Tie2Cre; p53 FL/− mice succumbed to late effects of radiation between 39 to 69 days post-whole-heart irradiation, with the median survival of 55 days (Fig. 1B). In addition, Tie2Cre; p53 FL/− mice treated with 10 daily fractions of 3 Gy (3Gy × 10) whole-heart irradiation also succumbed to late effects of radiation, with the median survival of 33 days after the last treatment (Fig. 1C).
Fig. 1.
Deletion of p53 by Tie2Cre sensitizes mice to radiation-induced myocardial injury. (A) Representative fluoroscopy image of a mouse treated with whole-heart irradiation. Whole-heart irradiation was performed with a 15 mm circular collimator (red circle) (B and C) Kaplan-Meier survival analysis of Tie2Cre; p53 FL/+ and Tie2Cre;p53 FL/– mice after a single fraction of 12 Gy or 10 daily fractions of 3 Gy whole-heart irradiation (3 Gy × 10). P value was calculated by long-rank test. (D – G) Histological analysis of the myocardium of moribund Tie2Cre; p53 FL/− mice after 12 Gy whole-heart irradiation. Representative sections of the myocardium of a Tie2Cre; p53 FL/− mouse 62 days after 12 Gy whole-heart irradiation (D) were stained with hematoxylin and eosin (H&E) (E), anti-sarcomeric α-actin IgG (SαA) (F) or Masson's trichrome (Trichrome) (G). The dashed line in (E), (F) and (G) demarcates the relatively normal appearing myocardium and the region of necrosis (labeled as N). Yellow arrows indicate necrotic cardiomyocytes replaced by blue collagen fibers. (H and I) Evans blue dye uptake (red) in Tie2Cre; p53 FL/+ (H) and Tie2Cre; p53 FL/− (I) mice 5 weeks after 12 Gy whole-heart irradiation. Cell membranes were counterstained with wheat germ agglutinin (white). Images represent four mice per group. Scale bar, 100 μm.
We performed histopathological examination of the lungs and heart in moribund Tie2Cre; p53 FL/− mice after 12 Gy or 3 Gy × 10 whole-heart irradiation. Although there was no detectable lesion in the lungs, hematoxylin and eosin-stained (H&E) sections of the heart showed multifocal areas of myocardial degeneration and necrosis throughout the myocardium (Fig. 1, D and E). Myocardial degeneration and necrosis was characterized by loss of cross striations, decreased number of cardiomyocytes, and indistinct cytoplasmic borders and nuclei. In addition, the loss of sarcomeric structure in necrotic regions was further characterized by the absence of sarcomeric α-actin filaments (Fig. 1F). Infiltration of neutrophils and macrophages as well as hemorrhage was also present within necrotic foci (Fig. 1E). As shown in Masson's trichrome-stained sections, the necrotic cardiomyocytes were replaced by small amounts of fibrous connective tissue, which indicates that the lesion was an acute process (Fig. 1G). To investigate the damage of cardiomyocytes at the cellular level, uptake of Evans blue dye of the heart was assessed in Tie2Cre; p53 FL/+ and Tie2Cre; p53 FL/− mice 5 weeks after 12 Gy whole-heart irradiation. Evans blue dye is a vital dye that penetrates into cardiomyocytes with a disrupted sarcolemma (37). Evans blue dye-positive cardiomyocytes were not present in irradiated Tie2Cre; p53 FL/+ mice; in contrast, irradiated Tie2Cre; p53 FL/− mice showed increased Evans blue dye uptake by cardiomyocytes (Fig.1, H and I). These results indicate that irradiated Tie2Cre; p53 FL/− mice showed cardiomyocyte sarcolemmal injury in the myocardium.
In addition to the pathological alterations in the myocardium, changes in the pericardium were also observed in some moribund Tie2Cre; p53 FL/− mice after 12 Gy whole-heart irradiation (Fig. S2). In these mice, certain areas of the pericardium were thickened by edema, proliferative fibroblasts, collagen fibrils and infiltration of lymphocytes. Collectively, our results suggest that deletion of p53 in endothelial cells by Tie2Cre sensitizes mice to radiation-induced cardiac injury, which is manifested as multifocal degeneration and necrosis in the myocardium as well as pericarditis.
Tie2Cre; p53 FL/− mice develop systolic dysfunction and cardiac hypertrophy following 12 Gy whole-heart irradiation
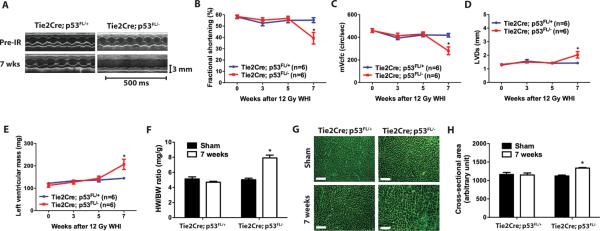
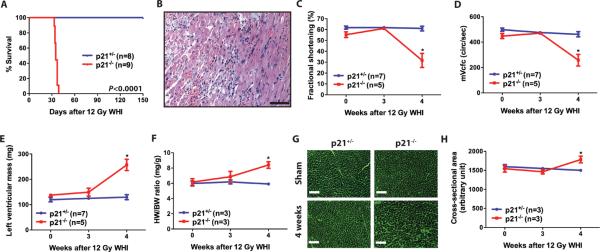
To assess the effect of 12 Gy whole-heart irradiation on cardiac function in Tie2Cre; p53 FL/+ and Tie2Cre; p53 FL/− mice, we performed serial echocardiography before and 3, 5, and 7 weeks after irradiation. Seven weeks after 12 Gy whole-heart irradiation, cardiac function of Tie2Cre; p53 FL/+ mice remained normal, whereas Tie2Cre; p53 FL/− mice developed impaired systolic function manifested by significantly decreased fractional shortening, decreased heart rate corrected mean velocity of circumferential fiber shortening, and increased left ventricular end-systolic dimension (Fig. 2, A – D and Table S1). Echocardiography also showed significantly increased mass of the left ventricle in Tie2Cre; p53 FL/− mice (Fig. 2E). Cardiac hypertrophy in Tie2Cre; p53 FL/− mice was confirmed at necropsy by a markedly increased heart weight to body weight ratio (Fig. 2F). In addition, sections of the myocardium stained with wheat germ agglutinin demonstrated that average cardiomyocyte cross-sectional area was significantly increased in Tie2Cre; p53 FL/− mice 7 weeks after 12 Gy whole-heart irradiation (Fig. 2, G and H). The results from the echocardiography and histology studies indicate that myocardial systolic dysfunction is the primary cause of morbidity in Tie2Cre; p53 FL/− mice after 12 Gy whole-heart irradiation.
Fig. 2.
Deletion of p53 by Tie2Cre sensitizes mice to systolic dysfunction and cardiac hypertrophy. (A) Representative echocardiographic recordings from Tie2Cre; p53 FL/+ and Tie2Cre; p53 FL/− mice prior to and 7 weeks after 12 Gy whole-heart irradiation. (B to E) Changes in fractional shortening (B), heart rate corrected mean velocity of circumferential fiber shortening (mVcfc) (C), left ventricular end-systolic dimension (LVDs) (D) and the left ventricular mass (E) in Tie2Cre; p53 FL/+ and Tie2Cre; p53 FL/− mice after 12 Gy whole-heart irradiation. Data are presented as mean ± SEM (n=6 mice per group). (F) Ratio of heart weight to body weight (HW/BW) of Tie2Cre; p53 FL/+ and Tie2Cre; p53 FL/− mice sham irradiated or 7 weeks after 12 Gy whole-heart irradiation. Data are presented as mean ± SEM (n=3 mice per group). (G) Representative images of wheat germ agglutinin (WGA)-stained myocardium of Tie2Cre; p53 FL/+ and Tie2Cre;p53 FL/− mice sham irradiated or 7 weeks after 12 Gy whole-heart irradiation. Scale bar, 100 μm. (H) Quantification of cardiomyocyte cross-sectional area in WGA-stained myocardium. Data are presented as mean ± SEM (n=3 mice per group). Asterisk shows individual differences (P<0.05) by two-way ANOVA with Bonferroni post-hoc test.
Radiation-induced systolic dysfunction is preceded by vascular damage and chronic ischemia in the myocardium of Tie2Cre; p53 FL/− mice
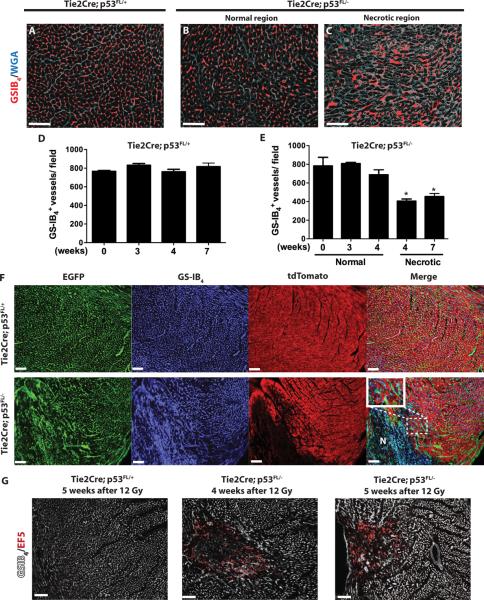
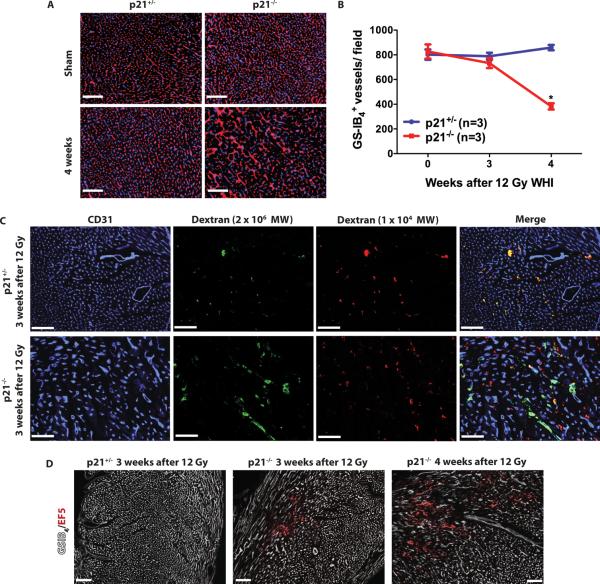
We hypothesized that cardiac dysfunction in Tie2Cre; p53 FL/− mice is preceded by the destruction of p53-deficient myocardial vessels. To test this hypothesis, we assessed the density of myocardial capillaries in Tie2Cre; p53 FL/+ and Tie2Cre; p53 FL/− mice 3, 4 and 7 weeks after 12 Gy whole-heart irradiation. Although systolic function of Tie2Cre; p53 FL/− mice remained normal 4 weeks after 12 Gy whole-heart irradiation (Fig. 2), focal areas in the myocardium of these mice showed aberrant myocardial capillary morphology characterized by dilated and disorganized vessels (Fig. 3, A – C). In addition, microvessel density was significantly decreased within the necrotic foci (Fig. 3, D and E). By 7 weeks after 12 Gy whole-heart irradiation in Tie2Cre; p53 FL/− mice, the necrotic region extended across the entire myocardium with a global decrease in microvessel density compared to unirradiated mice (Fig. 3E). Moreover, TUNEL stained myocardium showed a significant increase in dead endothelial cells in the necrotic regions of Tie2Cre; p53 FL/− mice 4 weeks after 12 Gy whole-heart irradiation (Fig. S3). To investigate functional alterations of myocardial capillaries in vivo, we assessed the permeability of the myocardial microvasculature with intravenous injection of low and high molecular weight dextrans (1 × 104 and 2 × 106, respectively) into Tie2Cre; p53 FL/+ and Tie2Cre; p53 FL/− mice 4 weeks after 12 Gy whole-heart irradiation. In Tie2Cre; p53 FL/+ mice, dextrans of both molecular weights were restricted within myocardial capillaries. In contrast, in Tie2Cre; p53 FL/− mice, focal regions of the myocardium showed increased vascular permeability characterized by leakage of the low molecular weight dextran from myocardial capillaries (Fig. S4). Collectively, our results suggest that damage to the myocardial vasculature in Tie2Cre; p53 FL/− mice after 12 Gy whole-heart irradiation precedes myocardial necrosis and systolic dysfunction.
Fig. 3.
Deletion of p53 by Tie2Cre sensitizes mice to vascular injury and chronic ischemia in the myocardium. (A – C) Representative sections of WGA (white) and GS-IB4 (red)-stained myocardium of Tie2Cre; p53 FL/+ (A) and Tie2Cre; p53 FL/− mice (B and C) 4 weeks after 12 Gy whole-heart irradiation. (D and E) Quantification of GS-IB4 positive myocardial capillaries per high power (200×) field. For Tie2Cre; p53 FL/− mice 4 weeks after 12 Gy whole-heart irradiation, vessel density in normal and necrotic regions are scored separately. By 7 weeks, no normal appearing myocardium was present in Tie2Cre; p53 FL/− mice. Asterisk shows individual differences (P<0.05) by one-way ANOVA with Bonferroni post-hoc test compared to sham irradiated mice. Data are presented as mean ± SEM (n=3 mice per group). (F) Representative sections of the myocardium of Tie2Cre; p53 FL/+_; mTmG/+ and Tie2Cre; p53 FL/−; mTmG/_+ mice 7 weeks after 12 Gy whole-heart irradiation. Myocardial vessels were counterstained with GS-IB4. N marks an area of necrosis. High power magnification image shows disorganized vessels in the area adjacent to a necrotic region. Images represent three mice per group. (G) The hypoxia marker EF5 (red) in Tie2Cre; p53 FL/+ and Tie2Cre; p53 FL/− mice 4 and 5 weeks after 12 Gy whole-heart irradiation. EF5 positive regions correlated with loss of myocardial capillaries, shown by GS-IB4 staining (white). Images represent three mice per group. Scale bar, 100 μm.
The effect of radiation on the myocardial microvasculature was further demonstrated in Tie2Cre; p53 FL/+; mTmG/+ and Tie2Cre; p53 FL/−; mTmG/+ mice, which contain EGFP+ microvessels and tdTomato+ cardiomyocytes. Histological evaluation in Tie2Cre; p53 FL/+; mTmG/+ mice 7 weeks after 12 Gy whole-heart irradiation demonstrated tdTomato+ cardiomycytes surrounded by EGFP+ microvessels in the myocardium, which is similar to the histology of the myocardium in mice without irradiation (Fig. 3F and Fig. S1). In contrast, Tie2Cre; p53 FL/−; mTmG/+ mice had multiple regions of myocardial necrosis, which were composed of necrotic cardiomyocytes with a low tdTomato signal and disorganized EGFP+ microvessels. Adjacent to these necrotic areas, EGFP+ microvessels were dilated and disorganized although tdTomato+ cardiomyocytes remained histologically intact (Fig. 3F). These results further suggest that destruction of the microvasculature leads to myocardial injury in Tie2Cre; p53 FL/− mice after 12 Gy whole-heart irradiation.
To investigate if vascular injury in the myocardium leads to myocardial hypoxia, we used the 2-nitroimidazole agent EF5 as a surrogate to measure hypoxia (38) in the myocardium of Tie2Cre; p53 FL/+ and Tie2Cre; p53 FL/− mice 4 and 5 weeks after 12 Gy whole-heart irradiation. Although EF5 did not label the myocardium of irradiated Tie2Cre; p53 FL/+ mice, EF5 binding was observed in regions where myocardial vessels were damaged in Tie2Cre; p53 FL/− mice 4 weeks after 12 Gy whole-heart irradiation (Fig.3G). Myocardial hypoxia was also present within regions of vascular injury in Tie2Cre; p53 FL/− mice 5 weeks after 12 Gy whole-heart irradiation (Fig. 3G). Therefore, our results indicate that damage to the myocardial capillaries in Tie2Cre; p53 FL/− mice after 12 Gy whole-heart irradiation leads to cardiac ischemia and myocardial hypoxia, which precedes systolic dysfunction and heart failure.
Deletion of p53 in endothelial cells by VE-Cadherin-Cre sensitizes mice to radiation-induced damage to the myocardial vasculature
Because recombination by Tie2Cre occurs in the majority of hematopoietic cells (31, 33, 39), it is possible that deletion of p53 in hematopoietic cells in Tie2Cre mice contributes to radiation-induced myocardial injury. To investigate if the radiation-induced myocardial injury in Tie2Cre; p53 FL/− mice is specifically due to loss of p53 in endothelial cells, we utilized VE-Cadherin-Cre (VECre) mice to delete p53 in endothelial cells. Similar to reports by others (32, 40), we observed that recombination in VECre mice occurs in a small subset (10 to 15%) of hematopoietic cells (Fig. S5), which is much lower than Tie2Cre mice (approximately 84%) (39). Tissue sections from VECre; p53 FL/+; mTmG/+ and VECre; p53 FL/−; mTmG/+ mice show that Cre is expressed specifically in endothelial cells in the myocardium (Fig. S6). In contrast to Tie2Cre mice, where Cre is expressed uniformly in endothelial cells throughout the myocardium (Fig. S1), in VECre mice, Cre is predominantly expressed in endothelial cells in the epimyocardium and midmyocardium (Fig. S6).
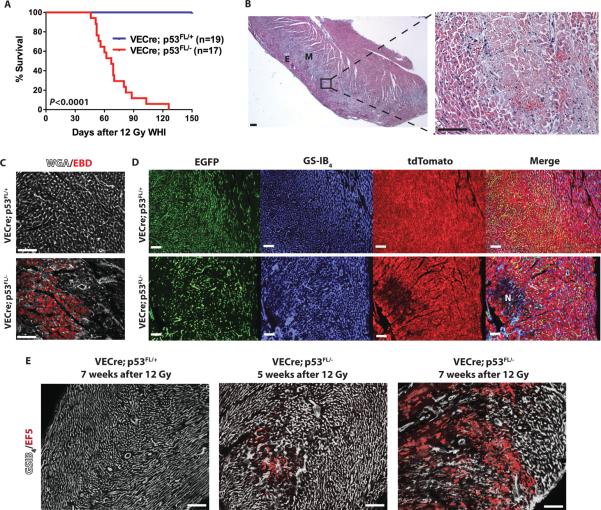
We next investigated if deletion of p53 by VECre sensitized mice to myocardial injury after exposure to a single dose of 12 Gy whole-heart irradiation. We found that VECre; p53 FL/− mice succumbed to late effects of radiation after 12 Gy whole-heart irradiation, with the median survival of 67 days (Fig. 4A). Histopathological changes in the myocardium in VECre; p53 FL/− mice were similar to the radiation-induced myocardial necrosis observed in Tie2Cre; p53 FL/− mice (Fig. 4B). 9 weeks after 12 Gy whole-heart irradiation, cardiomyocytes in irradiated VECre; p53 FL/− mice showed increased Evans blue dye uptake compared to those in irradiated VECre; p53 FL/+ mice (Fig. 4C). In addition, heart sections from VECre; p53 FL/−; mTmG/+ mice 7 weeks after 12 Gy whole-heart irradiation showed focal areas of myocardial necrosis characterized by damaged EGFP+ microvessels and loss of tdTomato+ cardiomyocytes (Fig. 4D). Furthermore, a progressive increase in myocardial hypoxia was detected in the myocardium of VECre; p53 FL/− mice 5 and 7 weeks after 12 Gy whole-heart irradiation (Fig. 4E). Collectively, the phenotypes of myocardial injury observed from VECre; p53 FL/− mice are consistent with the phenotypes of irradiated Tie2Cre; p53 FL/− mice. In contrast to the Tie2Cre; p53 FL/− mice that developed myocardial injury after 12 Gy whole-heart irradiation throughout the myocardium, most necrotic lesions in VECre; p53 FL/− mice were located in the epimyocardium and midmyocardium where Cre is expressed in cardiac endothelial cells (Fig. 4B). Therefore, these results demonstrate that specific deletion of p53 in endothelial cells in vivo sensitizes mice to radiation-induced myocardial injury.
Fig. 4.
Deletion of p53 by VE-Cadherin-Cre sensitizes mice to radiation-induced myocardial injury. (A) Kaplan-Meier survival analysis of VECre; p53 FL/+ and VECre;p53 FL/− mice after 12 Gy whole-heart irradiation. P value was calculated by long-rank test. (B) A representative hematoxylin and eosin-stained (H&E) section of the heart from VECre; p53 FL/− mice 52 days after 12 Gy whole-heart irradiation. Multifocal myocardial necrosis was mainly observed in the epicardium (E) and midmyocardium (M). (C) Evans blue dye (red) uptake in VECre; p53 FL/+ and VECre; p53 FL/− mice 9 weeks after 12 Gy whole-heart irradiation. Cell membranes were counterstained with WGA (white). Images represent three mice per group. (D) Representative sections of the myocardium of VECre; p53 FL/+_; mTmG/+ and VECre; p53 FL/−; mTmG/_+ mice 7 weeks after 12 Gy whole-heart irradiation. Myocardial vessels were counterstained with GS-IB4. An area of necrosis is marked by N. Images represent three mice per group. (E) The hypoxia marker EF5 (red) in VECre; p53 FL/+ and VECre; p53 FL/− mice 5 and 7 weeks after 12 Gy whole-heart irradiation. EF5 positive regions correlated with loss of myocardial capillaries, shown by GS-IB4 staining (white). Images represent two mice per group. Scale bar, 100 μm.
Impaired p53-mediated cell cycle arrest sensitizes cardiac endothelial cells to radiation-induced mitotic death
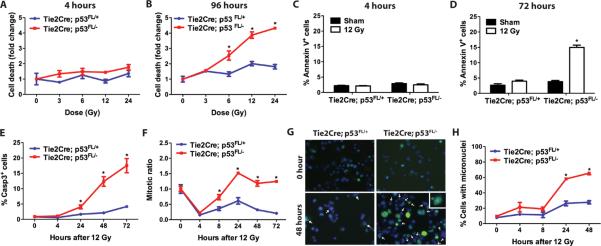
To determine if the susceptibility of radiation-induced endothelial cell damage in Tie2Cre; p53 FL/− mice is cell-autonomous, we assessed the viability of endothelial cells in vitro using primary cardiac endothelial cells isolated from the myocardium. Cardiac endothelial cells cultured in vitro displayed endothelial cell-like cobble stone morphology and surface markers of endothelial cells, CD31 and CD105, were present (Fig S7, A and B). p53 mRNA expression was lost in cardiac endothelial cells from Tie2Cre; p53 FL/− mice, whereas the abundance of p53 mRNA in cardiac endothelial cells from Tie2Cre; p53 FL/+ mice was approximately 50% compared to wild type mice (Fig. S7C). To investigate the role of p53 in mediating radiation-induced cell death, we irradiated cardiac endothelial cells from Tie2Cre; p53 FL/+ and Tie2Cre; p53 FL/− mice in vitro. Two major types of cell death have been reported in endothelial cells exposed to radiation in vitro: ceramide-mediated pre-mitotic apoptosis that occurs 4 to 6 hours post-irradiation, and post-mitotic cell death that occurs 24 to 96 hours post-irradiation (41, 42). We did not observe a significant increase in cell death 4 hours post-irradiation in cardiac endothelial cells with or without p53, but cell death was significantly increased in p53-deficient cardiac endothelial cells 96 hours post-irradiation in a dose-dependent manner (Fig. 5, A and B). In addition, a significantly higher percentage of p53-deficient cardiac endothelial cells underwent apoptosis, as shown by staining for annexin V and cleaved caspase 3, 24 to 72 hours after 12 Gy irradiation. However, no significant increase in apoptosis was detected in cardiac endothelial cells 4 hours after 12 Gy irradiation regardless of the presence or absence of p53 (Fig. 5, C – E). Collectively, these results show that p53 protects cardiac endothelial cells from post-mitotic cell death 24 to 96 hours after high dose irradiation in vitro.
Fig. 5.
p53 protects cardiac endothelial cells from radiation-induced mitotic catastrophe. Experiments were performed using cardiac endothelial cells from Tie2Cre; p53 FL/+ and Tie2Cre; p53 FL/− mice at passage 3 to 5. (A – B) Quantification of cell death 4 and 96 hours after various doses of irradiation. Cell survival was determined by propidium iodide exclusion. (C–D) Quantification of the percentage of annexin V positive cells 4 and 72 hours after 12 Gy irradiation. (E and F) Quantification of the percentage of cleaved caspase-3 (Casp3) positive (E) and mitotic ratio (F) of cardiac endothelial cells at various time points after 12 Gy irradiation. (G) Representative images of cells with micronuclei. Cells were stained with anti-γ-H2AX antibody and Hoechst 33324 to label γ-H2AX foci (green) and nuclei (blue), respectively. Arrows indicate cells with the presence of micronuclei. The majority of micronuclei contain unrepaired DNA damage indicated by positive staining for γ-H2AX foci. A representative image of a cell with micronuclei at higher magnification is shown in the inset. (H) Quantification of the percentage of cardiac endothelial cells with micronuclei at various time points after 12 Gy irradiation. Asterisk shows individual differences (P<0.05) by two-way ANOVA with Bonferroni post-hoc test. Data are presented as mean ± SEM (n=3 independent experiments).
Loss of p53-dependent cell cycle arrest in cells exposed to radiation can cause cells to proceed into mitosis with damaged DNA, resulting in mitotic catastrophe, a type of cell death following an aberrant mitosis (43, 44). Thus, we hypothesized that p53 protects cardiac endothelial cells from radiation by preventing mitotic catastrophe secondary to premature entry into mitosis. We first examined if loss of p53 alters the mitotic ratio in cardiac endothelial cells after irradiation by quantifying cells with phosphorylated histone H3. Tie2Cre; p53 FL/− cardiac endothelial cells had a significantly higher mitotic ratio 8, 24, 48 and 72 hours after 12 Gy irradiation compared to Tie2Cre; p53 FL/+ cardiac endothelial cells, indicating that p53-deficient cardiac endothelial cells have impaired cell cycle arrest after irradiation (Fig. 5F). To determine if premature entry into mitosis leads to mitotic catastrophe, we quantified the formation of micronuclei, which occurs as a result of incorrect chromosomal segregation during mitosis (43, 44). Cardiac endothelial cells from Tie2Cre; p53 FL/− mice contained a significantly higher percentage of cells with micronuclei compared to cardiac endothelial cells from Tie2Cre; p53 FL/+ mice 24 and 48 hours after 12 Gy irradiation (Fig. 5, G and H). Most cells with micronuclei also showed intense staining of γ-H2AX foci (Fig. 5G), indicating that these cells contained unrepaired DNA damage during mitosis. Taken together, these results support a model in which p53-dependent mitotic arrest regulates radiation-induced mitotic catastrophe in cardiac endothelial cells.
p53 controls the G1/S and G2/M cell cycle checkpoints at least in part by inducing the cyclin-dependent kinase inhibitor p21 (45). Thus, we hypothesized that p53 controls survival of cardiac endothelial cells through a p21-dependent mechanism. We isolated mRNA from cardiac endothelial cells from wild type, Tie2Cre; p53 FL/+, Tie2Cre; p53 FL/− and _p53_−/− mice and quantified mRNA expression of p21 as well as PUMA and Bax, which are transcriptional targets of p53 that regulates premitotic apoptosis (46, 47). Our results show that mRNA expression of p21, but not that of PUMA or Bax, was significantly induced after 12 Gy in cardiac endothelial cells containing at least one allele of p53. (Fig. S8, A – C). However, p21 mRNA was minimal at baseline in either Tie2Cre; p53 FL/− or _p53_−/− cardiac endothelial cells and was not induced by radiation (Fig. S8A). These results suggest that radiation selectively induces p53-mediated pro-survival pathways in cardiac endothelial cells in vitro. Subsequently, we examined the susceptibility of primary cardiac endothelial cells from p21+/− and _p21_−/− mice to radiation 4 and 72 hours after 12 Gy irradiation in vitro. Although cell death was not significantly induced in both types of cardiac endothelial cells 4 hours after 12 Gy irradiation, p21-deficient cardiac endothelial cells were more susceptible to radiation-induced post-mitotic cell death 72 hours after 12 Gy irradiation (Fig. S8, D and E). Collectively, these results indicate that the mechanism by which p53 protects cardiac endothelial cells against radiation is at least partially through p21.
p21-deficient mice are susceptible to radiation-induced myocardial injury
To investigate if loss of p21 potentiates radiation-induced myocardial injury in vivo, p21+/− _and p21_−/− mice were exposed to 12 Gy whole-heart irradiation and followed for the development of cardiac injury. All _p21_−/− mice were moribund 33 to 37 days after 12 Gy whole-heart irradiation (Fig. 6A). Histopathological examination indicated that _p21_−/− mice developed acute to sub-acute myocardial degeneration and necrosis (Fig. 6B), which is similar to that observed in Tie2Cre; p53 FL/− mice after 12 Gy whole-heart irradiation. Echocardiography 4 weeks after 12 Gy whole-heart irradiation revealed that _p21_−/− mice developed systolic dysfunction with significantly decreased fractional shortening and decreased mean velocity of circumferential fiber shortening (Fig. 6, C and D and Table S2). In addition, _p21_−/− mice developed cardiac hypertrophy 4 weeks after 12 Gy whole-heart irradiation indicated by an increased left ventricular mass, heart to body weight ratio, and average cardiomyocyte cross-sectional area (Fig. 6, E – H).
Fig. 6.
p21-deficient mice are susceptible to radiation-induced myocardial injury. (A) Kaplan-Meier survival analysis of p21+/− and _p21_−/− mice after 12 Gy whole-heart irradiation. (B) A representative hematoxylin and eosin-stained (H&E) section of the heart from _p21_−/− mice 34 days after 12 Gy whole-heart irradiation. Scale bar, 100 μm. (C to E) Changes in fractional shortening (C), heart rate corrected mean velocity of circumferential fiber shortening (mVcfc) (D), and left ventricular mass (E) in p21+/− and _p21_−/− mice after 12 Gy whole-heart irradiation. Data are presented as mean ± SEM. (F) Ratio of heart weight to body weight (HW/BW) of p21+/− and _p21_−/− mice 0, 3 and 4 weeks after 12 Gy whole-heart irradiation. (G) Representative images of WGA-stained myocardium in p21+/− and _p21_−/− mice sham irradiated or 7 weeks after 12 Gy whole-heart irradiation. Scale bar, 100 μm. (H) Quantification of cardiomyocyte cross-sectional area in wheat germ agglutinin-stained myocardium. Data are presented as mean ± SEM (n=3 mice per group). Asterisk shows individual differences (P<0.05) by two-way ANOVA with Bonferroni post-hoc test.
Assessment of microvessel density in the myocardium 4 weeks after 12 Gy whole-heart irradiation showed decreased density of myocardial capillaries in _p21_−/− mice (Fig. 7, A and B). This global decrease in microvessel density in the myocardium was preceded by focal increased vascular permeability (Fig. 7C). Furthermore, _p21_−/− mice showed focal myocardial hypoxia 3 weeks after 12 Gy whole-heart irradiation, and the hypoxic areas further increased in size and number at 4 weeks (Fig. 7D). Collectively, our results indicate that _p21_−/− mice phenocopy the sensitivity of Tie2Cre; p53 FL/− mice to radiation-induced myocardial injury because after 12 Gy whole-heart irradiation, _p21_−/− mice developed (i) myocardial degeneration and necrosis, (ii) systolic dysfunction, (iii) a reduction in microvessel density, and (iv) increased vascular permeability and myocardial hypoxia prior to the onset of cardiac dysfunction. Taken together, our results demonstrate that p53 functions in endothelial cells to prevent radiation-induced myocardial injury through a mechanism at least partially regulated by p21.
Fig. 7.
Loss of p21 sensitizes mice to microvascular injury and chronic ischemia in the myocardium. (A) Representative sections of the myocardium stained with Hoechst 33324 (blue) and GS-IB4 (red). Scale bar, 100 μm. (B) Quantification of GS-IB4 positive capillaries per high power (200×) field in the myocardium of p21+/− and _p21_−/− mice. Asterisk shows individual differences (P<0.05) by two-way ANOVA with Bonferroni post-hoc test. Data are presented as mean ± SEM (n=3 mice for each group). (C) Measurement of vascular permeability in the myocardium of p21+/− and _p21_−/− mice 3 weeks after 12 Gy whole-heart irradiation. Mice were injected with a mixture of high (2 × 106) and low (1 × 104) molecular weight (molecular weight) dextrans. Blood vessels were stained by anti-CD31 IgG. Images represent three mice per genotype. These results show that dextrans of both low (red) and high (green) MWs are restricted in blood vessels in irradiated p21+/− mice. In contrast, in irradiated _p21_−/− mice, the high MW dextran is retained in blood vessels, whereas the low MW dextran (red) leaks out of blood vessels in the damaged regions. Images represent three mice per group. (D) The hypoxia marker EF5 (red) in p21+/− and _p21_−/− mice 3 and 4 weeks after 12 Gy whole-heart irradiation. EF5 positive regions correlated with loss of myocardial capillaries, shown by GS-IB4 staining (white). Images represent two mice per group. Scale bar, 100 μm.
DISCUSSION
In the heart, p53 promotes cardiac injury from pressure overload (3), ischemic injury (4), telomere attrition (5), and doxorubicin treatment (6–8). In contrast, in the present study, we demonstrate that p53 functions to protect mice from myocardial injury caused by radiation. Because radiation-related heart disease is a major cause of treatment-associated mortality for cancer patients after thoracic radiotherapy (9), our results suggest that combining radiation therapy with inhibitors of p53 (48) or other components of the DNA damage response that regulate mitotic arrest (49) may increase the risk of cardiac injury if the heart is in the field of radiation. However, it is important to note that complete deletion of p53 in cardiac endothelial cells is necessary to sensitize mice to radiation-induced myocardial injury. Therefore, the deleterious effects of reversibly blocking p53 by chemical inhibitors in combination with radiation therapy may be less than we have observed with complete deletion of p53.
Previous histological and ultrastructural studies of radiation-induced myocardial injury in the rabbit and rat suggest that loss of cardiac endothelial cells preceded damage to cardiomyocytes (14, 16). Despite these elegant experiments, the role of the vasculature in mediating late effects from radiation has been controversial (50) because it has been difficult to separate injury to the vasculature from injury to the parenchymal cells of an organ. By demonstrating that Tie2Cre mice or VECre mice with an endothelial cell-specific deletion of p53 are sensitized to radiation-induced cardiac injury in vivo (Fig. 1 and 4), we provide compelling genetic evidence that endothelial cells play a critical role in regulating radiation-induced injury to the heart. In addition, we demonstrate that damage in the myocardial vasculature results in cardiac ischemia and myocardial hypoxia (Fig. 3G and 4E), which leads to systolic dysfunction and heart failure. This system may also be useful for investigating the role of the vasculature in mediating other late effects of radiation.
Our experiments also clarify how p53 regulates the response of endothelial cells in the heart to radiation. Although HUVECs appear to undergo radiation-induced apoptosis that can be blocked by a p53 inhibitor (23), we find that cardiac endothelial cells do not undergo p53-dependent apoptosis 4 hours after radiation up to doses of 24 Gy. In contrast, cardiac endothelial cells undergo a delayed mitotic death following irradiation, which is potentiated by deletion of p53 (Fig. 5). This conclusion is consistent with a report that blocking p53 in tumor-associated endothelial cells can sensitize xenograft tumors to radiation (26). As additional evidence to support a model in which p53 functions in cardiac endothelial cells after irradiation to activate cell cycle checkpoints to prevent mitotic death and subsequent myocardial injury, we observed that mice lacking the p53 target gene p21 are also sensitized to radiation-induced cardiac injury (Fig. 6). Because p53 mediates increased abundance of the cyclin dependent kinase inhibitor p21 in cardiac endothelial cells (Fig. S8), our results indicate that the p53–p21 cell cycle arrest pathway is a mechanism by which cardiac endothelial cells survive radiation.
Our results do not exclude that cell types other than cardiac endothelial cells play a role in radiation-induced cardiac injury. After 12 Gy whole-heart irradiation, both Tie2Cre; p53 FL/− and _p21_−/− mice showed destruction of the capillary network throughout the myocardium. Thus, loss of p53-dependent signaling may not only promote radiation-induced mitotic death in endothelial cells, but may also impair vessel regeneration in the myocardium. p21 plays an important role in regulating the turnover of mature endothelial cells and endothelial progenitor cells: _p21_−/− mice show impaired neovascularization despite increased proliferation of mature endothelial cells and endothelial progenitor cells (51). In addition, p53 and p21 inhibit stromal-derived factor-1 (SDF-1) signaling through chemokine receptor 4 (CXCR4), which promotes inflammatory responses (52–54). After vascular wire injury, _p21_−/− mice show impaired would healing and increased migration and trafficking of inflammatory cells into areas of vascular damage as a consequence of increased abundance of vascular SDF-1 proteins (54). These results suggest that the p53–p21 axis may also regulate vessel regeneration after radiation. Indeed, the more rapid onset of myocardial necrosis in mice lacking p21 throughout the entire animal compared to mice lacking p53 specifically in endothelial cells (compare Fig. 1B and Fig. S6B with Fig. 6A) suggests that p21 may also function in cells other than endothelial cells to prevent radiation-induced myocardial injury. Therefore, how p53-dependent signaling functions in other stromal cells or in the cardiomyocytes to control radiation-induced heart disease warrants further investigation.
This study has several strengths. First, whole heart irradiation (whole-heart irradiation) was utilized with a micro-CT/micro-irradiator to deliver focal irradiation to the heart (Fig. 1A). Therefore, the potential contribution of radiation-induced damage from other tissues, such as the gastrointestinal tract or bone marrow, to the observed myocardial injury is limited. Second, we performed serial echocardiography on mice before and after whole-heart irradiation and documented systolic dysfunction and cardiac hypertrophy in mice lacking p53 in the endothelial cells or in _p21_−/− mice (Fig. 2 and 6). These physiological measurements documented cardiac dysfunction independently of the histological endpoints thereby demonstrating that mice in this study succumbed to late effects of cardiac injury. Finally, we also studied the effect of deleting p53 in endothelial cells on radiation-induced cardiac injury in two independent Cre lines. By deleting p53 in endothelial cells with either Tie2Cre (Fig. 1) or VECre (Fig. 4), mice were sensitized to radiation-induced myocardial injury from whole-heart irradiation. Although multifocal myocardial necrosis was present throughout the myocardium of Tie2Cre; p53 FL/− mice (Fig. 1D), myocardial necrosis in VECre; p53 FL/− mice was prominent in the epimyocardium and midmyocardium (Fig. 4). Because the VECre mice did not have substantial necrosis in the endomyocardium after whole-heart irradiation in a region where the endothelial cells show poor expression of Cre recombinase (Fig. S6), the endomyocardium serves as an internal control to demonstrate that loss of p53 in endothelial cells, rather than other cell types, sensitizes mice to myocardial injury after whole-heart irradiation.
In addition to causing decreased blood supply to the myocardium, radiation to the heart also causes pericardial disease in humans and animal models (18, 55). In the pericardium of some mice lacking p53 in endothelial cells, we also observed increased edema, proliferative fibroblasts, collagen fibrils and infiltration of lymphocytes (Fig. S2). These findings suggest that, in addition to myocardial injury, damage to endothelial cells may also precede the pathological alterations in the pericardium. In the future, we plan to conduct studies to irradiate part of the pericardium of Tie2Cre; p53 FL/− or VE-Cre; p53 FL/− mice with tangent radiation fields, which may provide further insight into how damage to endothelial cells may regulate the progression of radiation-induced pericardial disease.
Accumulating data from mouse models reveal that activation of p53 by radiation in vivo can be either pro-survival or pro-death depending on the cell-type (1, 2). The diverse cell-type specific outcome of p53 activation after irradiation may be influenced by the ability of p53 to selectively activate its transcriptional targets through differential DNA binding cooperativity in a cell-type specific manner. Selectivity of p53 binding can be regulated by co-factors or by distinct post-translational modifications of p53 (56). For example, co-factors of p53 such as Hzf and Miz1 interact with the DNA binding domain of p53 to promote the expression of p21, but prevent the induction of the pro-apoptotic targets such as PUMA and Bax (57–59). In addition, whether p53 activation can induce premitotic apoptosis may depend on whether radiation causes p53 to tip the balance between pro-apoptotic and anti-apoptotic proteins. For example, in the hematopoietic system, radiation induces pro-apoptotic genes such as PUMA and Bax in both granulocyte and macrophage progenitors (GMPs) and hematopoietic stem cells (HSCs) (60). However, premitotic apoptosis is preferentially activated by radiation in GMPs compared to HSCs. This phenomenon can be at least partially explained because the expression of anti-apoptotic genes such as Bcl-xl, Mcl-1 and A1 (60) is higher in HSCs, which may inhibit the pro-apoptotic function of p53 target genes, such as PUMA and Bax or p53 itself acting at the mitochondria (61). In the present study, our results show that radiation does not induce premitotic apoptosis in cardiac endothelial cells in vitro. Consistent with this finding, mRNA expression analyses reveal that radiation selectively induces p21 rather than pro-apoptotic genes PUMA and Bax (Fig. S8). Therefore, further studies are warranted to investigate whether cardiac endothelial cells harbor distinct co-factors or modifiers of p53 that regulate cell fate after activation in response to radiation.
In summary, we have demonstrated that p53 functions in endothelial cells to protect mice from radiation-induced myocardial injury. This finding may have important implications for cancer therapy. Several pharmacological inhibitors of proteins that regulate cell cycle checkpoints are now in clinical development including radiosensitizers, such as checkpoint kinases inhibitors (49), and radioprotectors, such as p53 inhibitors (48). Our findings raise the possibility that when these inhibitors are combined with radiation therapy, patients may experience increased late effects, such as radiation-related heart disease.
MATERIALS AND METHODS
Mouse strains
All animal procedures for this study were approved by the Institutional Animal Care and Use Committee (IACUC) at Duke University. All of the mouse strains used in this study have been described previously including _p21_−/−, _p53_−/−, p53FL/FL, Tie2Cre, VECre, mTmG and LSL-YFP mice (32, 33, 35, 62). The p53FL/FL mice were kindly provided by Anton Berns and the _p21_−/− and _p53_−/− mice were kindly provided by Tyler Jacks. Tie2Cre, VECre, mTmG and LSL-YFP mice were obtained from Jackson laboratories. Experiments were carried out with mice that were between 8 to 10 weeks old and were on mixed genetic backgrounds. For every experiment, age-matched, littermate controls were utilized to minimize the effect of genetic background. Therefore, potential genetic modifiers of the response to radiation would be randomly distributed among the experimental and control groups. Because Cre expression can occur in the germ line of female Tie2Cre mice (63), Tie2Cre; p53+/− males were crossed to p53FL/FL females to avoid non-specific deletion of floxed alleles in the germ line.
Radiation treatment
Whole-heart irradiation was performed using a small-field biological irradiator, the X-RAD 225Cx (Precision X-Ray, Inc). The system was commissioned as previously described (64). The heart was localized to isocenter (source to subject distance is approximately 30.76 cm) with fluoroscopy at 40 kVp, 2.5 mA with a 2 mm Al filter. Mice were irradiated with parallel-opposed anterior and posterior fields. Irradiation was performed with a collimating cone to produce a circular radiation field of 15 mm at treatment isocenter with an average dose rate of 300 cGy/min at target depth with a 225 kVp, 13 mA beam and a 0.3 mm Cu filter. Endothelial cells were irradiated with ionizing radiation in vitro using an X-RAD 320 Biological Irradiator (Precision X-ray, Inc). Irradiation was performed 50 cm from the radiation source with a dose rate of 200 cGy/min with 320 kVp X-rays, using 12.5 mA and a filter consisting of 2.5 mm Al and 0.1 mm Cu. The dose rate was measured with an ion chamber by members of the Radiation Safety Division at Duke University.
Echocardiography
Serial transthoracic echocardiography was performed on conscious mice for all groups with a Vevo 2100 high-resolution image system (VisualSonics) as previously described (65)
Histological analyses
To prepare paraffin-embedded tissues, tissues specimens were fixed in 10% neutralized formalin overnight, preserved in 70% ethanol and then embedded in paraffin. To prepare frozen tissues, tissue specimens were fixed in 4% paraformaldehyde in PBS for 2 hours at 4°C and transferred to 30% sucrose in PBS overnight at 4°C. Tissues were immersed in OCT compound (Sakura Finetek), snap frozen in dry ice/isopentane slurry, and stored at -80°C. H&E and Masson's trichrome-stained sections were analyzed by a veterinary pathologist (YK) blinded to genotype and treatment.
Evans blue dye (10 mg/ml in PBS; Sigma Aldrich) was administered to mice through intraperitoneal injection at the volume of 10 μl/ g body weight and the hearts were collected 24 hours after injection. Frozen sections were prepared for histological analyses.
The 2-nitroimidazole agent EF5 was kindly provided by Cameron Koch. EF5 (10 mM in PBS) was administered to mice through intraperitoneal injection at the volume of 26.5 μl/ g body weight and the hearts were collected 3 hours after injection. Frozen sections were prepared for histological analyses. EF5 was detected by staining with Cy3-conjugated anti-EF5 IgG (provided by Cameron Koch).
For quantification of the averaged cardiomyocyte cross-sectional area and microvessel density, sections cut from paraffin embedded tissues were deparaffinized with xylene and rehydrated with a graded series of ethanol and water washes. Antigens were retrieved by Antigen Unmasking Solution (Vector Laboratories) according to the manufacturer's instructions. Sections were stained with Alexa Fluor 488-conjugated WGA (5 μg/mL) and Alexa Fluor 647-conjugated GS-IB4 (10 μg/mL) (Invitrogen) 2 hours at room temperature.
For TUNEL staining, sections from the same tissues were used. Antigens were retrieved by proteinase K (0.8 U/mL, Sigma-Aldrich) for 15 min at room temperature. TUNEL staining was performed using In Situ Cell Death Detection Kit, TMR red (Roche) according to the manufacturer's instructions. Sections were counterstained with Alexa Fluor 488-conjugated WGA (5 μg/mL) and Alexa Fluor 647-conjugated GS-IB4 (10 μg/mL) (Invitrogen) overnight at 4°C. Six images (200×) per slide were randomly taken from the myocardium with similar cross sections in all mice except Tie2Cre; p53 FL/− mice 4 weeks after 12 Gy. In these mice, 6 fields (200×) were chosen from representative non-necrotic regions, and 3 to 6 fields (200×) were chosen from representative necrotic regions. Necrotic regions were identified by abnormal morphology of cardiomyocytes and microvessels. The microvessel density, the averaged cardiomyocyte cross-sectional area and the percentage of microvessels containing TUNEL positive cells were quantified by MetaMorph (Molecular Devices). Data of all images from the same mouse were averaged.
Vascular permeability assay
Mice were injected intravenously with a mixture of TMR-conjugated high molecular weight (2 × 106 molecular weight, 1 mg/ml) and Alexa Fluor 647-conjugated low molecular weight (1 × 104 molecular weight, 0.4 mg/ml) dextrans (Invitrogen). Mice were euthanized 1.5 hours later. Ten μm sections were cut from frozen tissues and stained with rat anti-mouse CD31 IgG (BD Pharmigen) diluted 1:50 in 5% rabbit serum overnight at 4°C. After washing with PBS, sections were incubated with Alexa Fluor 488-conjugated donkey anti-rat (Invitrogen) diluted 1:400 in 2% mouse serum for 30 min at room temperature.
Primary culture of cardiac endothelial cells
Isolation of cardiac endothelial cells was performed as described previously (66) with slight modifications. Primary cardiac endothelial cells were isolated from ventricles of 4 to 6-week-old mice. Ventricles were digested using Hank's Balanced Salt Solution (Invitrogen) with 200 U/ml of collagenase I (Worthington Biochemical Corporation) for 45 min at 37°C. Cardiac endothelial cells were captured using rat IgG magnetic beads (Invitrogen) coated with rat anti-mouse CD31 IgG (BD Pharmigen). After the initial purification, cardiac endothelial cells were cultured in DMEM supplemented with 25 mM HEPES, 20% FBS, penicillin/ streptomycin, 2 mM L-glutamine, 100 μg/ml heparin, nonessential amino acid (1×), sodium pyruvate (1×) and 100 μg/ml endothelial cell growth stimulant (Biomedical Technologies) on cell culture dishes coated with 0.1% of gelatin (Sigma). cardiac endothelial cells were grown to 70% to 80% confluency followed by a second isolation using magnetic beads coated with rat anti-mouse CD102 IgG (BD Pharmigen) to further improve the purity. cardiac endothelial cells were used for experiments from passage 1 to 4 after the secondary purification (total passage 3 to 6). The purity of cardiac endothelial cells was assessed by flow cytometry using FITC-conjugated anti-mouse CD31 and PE-conjugated anti-mouse CD105 antibodies (eBioscience).
Quantification of cell death by flow cytometry
Cardiac endothelial cells were irradiated at approximately 60% confluency and harvested at time points post-irradiation as indicated. Cells were washed with PBS and stained with either propidum iodide (5 μg/ml, Sigma-Aldrich) alone or with FITC annexin V (BD Pharmigen) and propidium iodide according to the manufacturers' instructions. Data were collected by FACScan (BD Pharmingen) and analyzed by Flowjo (Tree Star, Inc).
Quantification of cleaved caspase-3 and pHH3 positive cells by flow cytometry
Cardiac endothelial cells were irradiated at approximately 60% confluency and harvested at time points post-irradiation as indicated. Cells were fixed with cold 70% ethanol and stored at −20°C. To quantify the percentage of cells positive for phosphorylated histone H3 (pHH3), cells were stained with rabbit anti-mouse pHH3 IgG (Abcam) diluted 1:500 in PBS/0.25% Triton-X 100/3% BSA for 1 hour at room temperature followed by staining with Alexa Fluor 488-conjugated anti-rabbit IgG (1:1000) (Invitrogen) at room temperature for 1 hour. Cleaved caspase-3 staining was performed using PE Active Caspase-3 Apoptosis Kit (BD Pharmingen) according to the manufacturer's instructions. Data were collected by FACScan (BD Pharmingen) and analyzed by Flowjo (Tree Star, Inc).
Quantification of micronuclei
Cardiac endothelial cells were irradiated at approximately 60% confluency and harvested at time points post-irradiation as indicated. Cells were fixed with cold 70% ethanol and stored at −20°C. Approximately 40,000 fixed cells were cytospun on poly-L-lysine coated slides (Azer scientific) at 500 rpm for 5 min. Slides were dried overnight at room temperature prior to staining. For γH2AX staining, cells were permeabilized with 0.2% Triton ×-100 in PBS, blocked with 3% goat serum in PBS and stained with anti-γH2AX (Ser 139) antibody (Millipore) (1:500 dilution) for 1 hour at 37°C. Slides were then washed with PBS and stained with Alexa Fluor 488-conjugated goat anti-mouse IgG1 (Invitrogen) for 1 hour at 37°C. Hoechst 33342 (Sigma) was used for nuclear counterstaining. Cells with micronuclei were counted by examining the morphology of nuclei counterstained with Hoechst 33342. A hundred cells were counted per slide by a single observer (EJM).
Quantitative RT-PCR
Total RNA was extracted from the cardiac endothelial cells using the TRI reagent (Ambion) and RNeasy Mini kit (QIAGEN) following the manufacturer's protocol. RNA yield and quantity were determined by measuring absorbencies at 260 nm and 280 nm using NanoDrop (Thermo Scientific). Six hundred ng of total RNA were used for in vitro reverse transcription using iScript cDNA synthesis Kit (Bio-Rad). The expression of p53, p21, PUMA, Bax and β2-microglobulin (B2m) mRNA was quantified by qRT-PCR with Taqman probes (Applied Biosystems, Mm01731290_g1 for p53, Mm00432448_m1 for p21, Mm00519268_m1 for PUMA, Mm00432050_m1 for Bax and Mm00437762_m1 for B2m). B2m was used as an internal control to correct for the concentration of cDNA in different samples.
Statistics
Results are presented as mean ± SEM. Student's _t_-test (two-tailed) was performed to compare the means of two groups, and one-way ANOVA was performed to compare the means of three or more groups. Two-way ANOVA followed by Bonferroni post-hoc test was performed to examine the interaction between genotypes and radiation treatment. If the normality test failed, data were log-transformed prior to applying statistical tests. For survival studies, Kaplan-Meier analysis was performed followed by the log-rank test. Significance was assumed at P<0.05. GraphPad Prism 5 (GraphPad Software, Inc) was used for the calculation of statistics.
Microscopy
Pictures were taken using a Leica DFC 340FX fluorescent microscope (Leica Microsystems). Images were captured by Leica suite software (Leica Microsystems).
Supplementary Material
Supplementary Data
Acknowledgements
We thank Anton Berns for providing the p53 FL/FL mice and Tyler Jacks for providing the _p21_−/− and _p53_−/− mice. We thank Cameron Koch for providing the EF5 and anti-EF5 antibody. We thank Jeff Mito for assistance with intravenous injections and Barbara Williams for assistance with analyzing the echocardiography results.
Funding: These studies were supported by NIAID grant R01 AI080488 (D.G.K) and NHLBI grant R01 HL56687 (H.A.R). I.W. is a recipient of the HATs Summer Minority Fellowship Award from ASCO. C-.L.L. is a recipient of the Hung Taiwan-Duke University Fellowship.
Footnotes
Author contributions: C-.L.L and D.G.K. designed the study, analyzed the data and wrote the paper. C-.L.L., E.J.M., K.C.C., Y.L., J.M.S., L.M., and I.W. performed the experiments. L.B.J. and R.C.R. assisted with animal studies. Y.M. processed histological specimens. Y.K. performed pathohistological diagnosis. Y.L. and S.D. commissioned the micro-irradiatror. C.D.K. designed the study. L.M. and H.A.R. analyzed the results of echocardiography.
Data and materials availability: A materials transfer agreement is required by HHMI for p21−/− and _p53_−/− mice. A materials transfer agreement is required by NKI for p53FL/FL mice.
REFERENCES AND NOTES
- 1.Gudkov AV, Komarova EA. The role of p53 in determining sensitivity to radiotherapy. Nat Rev Cancer. 2003;3:117. doi: 10.1038/nrc992. [DOI] [PubMed] [Google Scholar]
- 2.Gudkov AV, Komarova EA. Pathologies associated with the p53 response. Cold Spring Harb Perspect Biol. 2010;2:a001180. doi: 10.1101/cshperspect.a001180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sano M, et al. p53-induced inhibition of Hif-1 causes cardiac dysfunction during pressure overload. Nature. 2007;446:444. doi: 10.1038/nature05602. [DOI] [PubMed] [Google Scholar]
- 4.Matsusaka H, et al. Targeted deletion of p53 prevents cardiac rupture after myocardial infarction in mice. Cardiovas Res. 2006;70:457. doi: 10.1016/j.cardiores.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 5.Leri A, et al. Ablation of telomerase and telomere loss leads to cardiac dilatation and heart failure associated with p53 upregulation. The EMBO journal. 2003;22:131. doi: 10.1093/emboj/cdg013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shizukuda Y, Matoba S, Mian OY, Nguyen T, Hwang PM. Targeted disruption of p53 attenuates doxorubicin-induced cardiac toxicity in mice. Molecular and Cellular Biochemistry. 2005;273:25. doi: 10.1007/s11010-005-5905-8. [DOI] [PubMed] [Google Scholar]
- 7.Liu X, et al. Pifithrin-alpha protects against doxorubicin-induced apoptosis and acute cardiotoxicity in mice. American journal of physiology. Heart and circulatory physiology. 2004;286:H933. doi: 10.1152/ajpheart.00759.2003. [DOI] [PubMed] [Google Scholar]
- 8.Zhu W, et al. Acute doxorubicin cardiotoxicity is associated with p53-induced inhibition of the mammalian target of rapamycin pathway. Circulation. 2009;119:99. doi: 10.1161/CIRCULATIONAHA.108.799700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Darby SC, et al. Radiation-related heart disease: current knowledge and future prospects. Int J Radiat Oncol Biol Phys. 2010;76:656. doi: 10.1016/j.ijrobp.2009.09.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.EBCTCG Favourable and unfavourable effects on long-term survival of radiotherapy for early breast cancer: an overview of the randomised trials. Early Breast Cancer Trialists' Collaborative Group. Lancet. 2000;355:1757. [PubMed] [Google Scholar]
- 11.Darby SC, McGale P, Taylor CW, Peto R. Long-term mortality from heart disease and lung cancer after radiotherapy for early breast cancer: prospective cohort study of about 300,000 women in US SEER cancer registries. Lancet Oncol. 2005;6:557. doi: 10.1016/S1470-2045(05)70251-5. [DOI] [PubMed] [Google Scholar]
- 12.Marks LB, et al. The incidence and functional consequences of RT-associated cardiac perfusion defects. Int J Radiat Oncol Biol Phys. 2005;63:214. doi: 10.1016/j.ijrobp.2005.01.029. [DOI] [PubMed] [Google Scholar]
- 13.Prosnitz RG, et al. Prospective assessment of radiotherapy-associated cardiac toxicity in breast cancer patients: analysis of data 3 to 6 years after treatment. Cancer. 2007;110:1840. doi: 10.1002/cncr.22965. [DOI] [PubMed] [Google Scholar]
- 14.Fajardo LF, Stewart JR. Pathogenesis of radiation-induced myocardial fibrosis. Lab Invest. 1973;29:244. [PubMed] [Google Scholar]
- 15.Fajardo LF, Stewart JR. Experimental radiation-induced heart disease. I. Light microscopic studies. Am J Pathol. 1970;59:299. [PMC free article] [PubMed] [Google Scholar]
- 16.Lauk S, Kiszel Z, Buschmann J, Trott KR. Radiation-induced heart disease in rats. Int J Radiat Oncol Biol Phys. 1985;11:801. doi: 10.1016/0360-3016(85)90314-1. [DOI] [PubMed] [Google Scholar]
- 17.Yeung TK, Lauk S, Simmonds RH, Hopewell JW, Trott KR. Morphological and functional changes in the rat heart after X irradiation: strain differences. Radiat Res. 1989;119:489. [PubMed] [Google Scholar]
- 18.Seemann I, et al. Irradiation induced modest changes in murine cardiac function despite progressive structural damage to the myocardium and microvasculature. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 2012;103:143. doi: 10.1016/j.radonc.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 19.Stewart FA, Hoving S, Russell NS. Vascular damage as an underlying mechanism of cardiac and cerebral toxicity in irradiated cancer patients. Radiat Res. 2010;174:865. doi: 10.1667/RR1862.1. [DOI] [PubMed] [Google Scholar]
- 20.Boerma M, Hauer-Jensen M. Preclinical research into basic mechanisms of radiation-induced heart disease. Cardiol Res Pract. 2010;2011 doi: 10.4061/2011/858262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schultz-Hector S, Trott KR. Radiation-induced cardiovascular diseases: is the epidemiologic evidence compatible with the radiobiologic data? Int J Radiat Oncol Biol Phys. 2007;67:10. doi: 10.1016/j.ijrobp.2006.08.071. [DOI] [PubMed] [Google Scholar]
- 22.Midgley CA, et al. Coupling between gamma irradiation, p53 induction and the apoptotic response depends upon cell type in vivo. Journal of Cell Science. 1995;108(Pt 5)):1843. doi: 10.1242/jcs.108.5.1843. [DOI] [PubMed] [Google Scholar]
- 23.Nubel T, Damrot J, Roos WP, Kaina B, Fritz G. Lovastatin protects human endothelial cells from killing by ionizing radiation without impairing induction and repair of DNA double-strand breaks. Clin Cancer Res. 2006;12:933. doi: 10.1158/1078-0432.CCR-05-1903. [DOI] [PubMed] [Google Scholar]
- 24.Kumar P, et al. Bcl-2 protects endothelial cells against gamma-radiation via a Raf-MEK-ERK-survivin signaling pathway that is independent of cytochrome c release. Cancer Res. 2007;67:1193. doi: 10.1158/0008-5472.CAN-06-2265. [DOI] [PubMed] [Google Scholar]
- 25.Lee MO, et al. Effect of Ionizing Radiation Induced Damage of Endothelial Progenitor Cells in Vascular Regeneration. Arteriosclerosis, thrombosis, and vascular biology. 2011;32:343. doi: 10.1161/ATVBAHA.111.237651. [DOI] [PubMed] [Google Scholar]
- 26.Burdelya LG, et al. Inhibition of p53 response in tumor stroma improves efficacy of anticancer treatment by increasing antiangiogenic effects of chemotherapy and radiotherapy in mice. Cancer Res. 2006;66:9356. doi: 10.1158/0008-5472.CAN-06-1223. [DOI] [PubMed] [Google Scholar]
- 27.Davis S, et al. Isolation of angiopoietin-1, a ligand for the TIE2 receptor, by secretion-trap expression cloning. Cell. 1996;87:1161. doi: 10.1016/s0092-8674(00)81812-7. [DOI] [PubMed] [Google Scholar]
- 28.Suri C, et al. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell. 1996;87:1171. doi: 10.1016/s0092-8674(00)81813-9. [DOI] [PubMed] [Google Scholar]
- 29.Vikkula M, et al. Vascular dysmorphogenesis caused by an activating mutation in the receptor tyrosine kinase TIE2. Cell. 1996;87:1181. doi: 10.1016/s0092-8674(00)81814-0. [DOI] [PubMed] [Google Scholar]
- 30.Lampugnani MG, et al. The molecular organization of endothelial cell to cell junctions: differential association of plakoglobin, beta-catenin, and alpha-catenin with vascular endothelial cadherin (VE-cadherin) The Journal of cell biology. 1995;129:203. doi: 10.1083/jcb.129.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koni PA, et al. Conditional vascular cell adhesion molecule 1 deletion in mice: impaired lymphocyte migration to bone marrow. J Exp Med. 2001;193:741. doi: 10.1084/jem.193.6.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alva JA, et al. VE-Cadherin-Cre-recombinase transgenic mouse: a tool for lineage analysis and gene deletion in endothelial cells. Dev Dyn. 2006;235:759. doi: 10.1002/dvdy.20643. [DOI] [PubMed] [Google Scholar]
- 33.Kirsch DG, et al. p53 controls radiation-induced gastrointestinal syndrome in mice independent of apoptosis. Science. 2010;327:593. doi: 10.1126/science.1166202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kisanuki YY, et al. Low blood pressure in endothelial cell-specific endothelin 1 knockout mice. Hypertension. 2010;56:121. doi: 10.1161/HYPERTENSIONAHA.109.138701. [DOI] [PubMed] [Google Scholar]
- 35.Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L. A global double-fluorescent Cre reporter mouse. Genesis. 2007;45:593. doi: 10.1002/dvg.20335. [DOI] [PubMed] [Google Scholar]
- 36.Laitinen L. Griffonia simplicifolia lectins bind specifically to endothelial cells and some epithelial cells in mouse tissues. The Histochemical journal. 1987;19:225. doi: 10.1007/BF01680633. [DOI] [PubMed] [Google Scholar]
- 37.Danialou G, et al. Dystrophin-deficient cardiomyocytes are abnormally vulnerable to mechanical stress-induced contractile failure and injury. FASEB Journal. 2001;15:1655. doi: 10.1096/fj.01-0030fje. [DOI] [PubMed] [Google Scholar]
- 38.Evans SM, et al. Patterns and levels of hypoxia in head and neck squamous cell carcinomas and their relationship to patient outcome. Int J Radiat Oncol Biol Phys. 2007;69:1024. doi: 10.1016/j.ijrobp.2007.04.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tang Y, Harrington A, Yang X, Friesel RE, Liaw L. The contribution of the Tie2+ lineage to primitive and definitive hematopoietic cells. Genesis. 2010;48:563. doi: 10.1002/dvg.20654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zovein AC, et al. Fate tracing reveals the endothelial origin of hematopoietic stem cells. Cell Stem Cell. 2008;3:625. doi: 10.1016/j.stem.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bonnaud S, et al. Sphingosine-1-phosphate protects proliferating endothelial cells from ceramide-induced apoptosis but not from DNA damage-induced mitotic death. Cancer Res. 2007;67:1803. doi: 10.1158/0008-5472.CAN-06-2802. [DOI] [PubMed] [Google Scholar]
- 42.Yazlovitskaya EM, Linkous AG, Thotala DK, Cuneo KC, Hallahan DE. Cytosolic phospholipase A2 regulates viability of irradiated vascular endothelium. Cell Death Differ. 2008;15:1641. doi: 10.1038/cdd.2008.93. [DOI] [PubMed] [Google Scholar]
- 43.Castedo M, et al. Cell death by mitotic catastrophe: a molecular definition. Oncogene. 2004;23:2825. doi: 10.1038/sj.onc.1207528. [DOI] [PubMed] [Google Scholar]
- 44.Vakifahmetoglu H, Olsson M, Zhivotovsky B. Death through a tragedy: mitotic catastrophe. Cell Death Differ. 2008;15:1153. doi: 10.1038/cdd.2008.47. [DOI] [PubMed] [Google Scholar]
- 45.Bunz F, et al. Requirement for p53 and p21 to sustain G2 arrest after DNA damage. Science. 1998;282:1497. doi: 10.1126/science.282.5393.1497. [DOI] [PubMed] [Google Scholar]
- 46.Nakano K, Vousden KH. PUMA, a novel proapoptotic gene, is induced by p53. Molecular Cell. 2001;7:683. doi: 10.1016/s1097-2765(01)00214-3. [DOI] [PubMed] [Google Scholar]
- 47.Miyashita T, Reed JC. Tumor suppressor p53 is a direct transcriptional activator of the human bax gene. Cell. 1995;80:293. doi: 10.1016/0092-8674(95)90412-3. [DOI] [PubMed] [Google Scholar]
- 48.Gudkov AV, Komarova EA. Prospective therapeutic applications of p53 inhibitors. Biochem Biophys Res Commun. 2005;331:726. doi: 10.1016/j.bbrc.2005.03.153. [DOI] [PubMed] [Google Scholar]
- 49.Begg AC, Stewart FA, Vens C. Strategies to improve radiotherapy with targeted drugs. Nat Rev Cancer. 2011;11:239. doi: 10.1038/nrc3007. [DOI] [PubMed] [Google Scholar]
- 50.Hopewell J, Withers HR. Proposition: long-term changes in irradiated tissues are due principally to vascular damage in the tissues. Medical Physics. 1998;25:2265. doi: 10.1118/1.598455. [DOI] [PubMed] [Google Scholar]
- 51.Bruhl T, et al. p21Cip1 levels differentially regulate turnover of mature endothelial cells, endothelial progenitor cells, and in vivo neovascularization. Circ Res. 2004;94:686. doi: 10.1161/01.RES.0000119922.71855.56. [DOI] [PubMed] [Google Scholar]
- 52.Moskovits N, Kalinkovich A, Bar J, Lapidot T, Oren M. p53 Attenuates cancer cell migration and invasion through repression of SDF-1/CXCL12 expression in stromal fibroblasts. Cancer Res. 2006;66:10671. doi: 10.1158/0008-5472.CAN-06-2323. [DOI] [PubMed] [Google Scholar]
- 53.Mehta SA, et al. Negative regulation of chemokine receptor CXCR4 by tumor suppressor p53 in breast cancer cells: implications of p53 mutation or isoform expression on breast cancer cell invasion. Oncogene. 2007;26:3329. doi: 10.1038/sj.onc.1210120. [DOI] [PubMed] [Google Scholar]
- 54.Olive M, et al. p21Cip1 modulates arterial wound repair through the stromal cell-derived factor-1/CXCR4 axis in mice. J Clin Invest. 2008;118:2050. doi: 10.1172/JCI31244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Adams MJ, et al. Radiation-associated cardiovascular disease: manifestations and management. Seminars in radiation oncology. 2003;13:346. doi: 10.1016/S1053-4296(03)00026-2. [DOI] [PubMed] [Google Scholar]
- 56.Schlereth K, Charles JP, Bretz AC, Stiewe T. Life or death: p53-induced apoptosis requires DNA binding cooperativity. Cell cycle. 2010;9:4068. doi: 10.4161/cc.9.20.13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Das S, et al. Hzf Determines cell survival upon genotoxic stress by modulating p53 transactivation. Cell. 2007;130:624. doi: 10.1016/j.cell.2007.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Herold S, et al. Negative regulation of the mammalian UV response by Myc through association with Miz-1. Molecular Cell. 2002;10:509. doi: 10.1016/s1097-2765(02)00633-0. [DOI] [PubMed] [Google Scholar]
- 59.Miao L, et al. ARF antagonizes the ability of Miz-1 to inhibit p53-mediated transactivation. Oncogene. 2010;29:711. doi: 10.1038/onc.2009.372. [DOI] [PubMed] [Google Scholar]
- 60.Mohrin M, et al. Hematopoietic stem cell quiescence promotes error-prone DNA repair and mutagenesis. Cell Stem Cell. 2010;7:174. doi: 10.1016/j.stem.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mihara M, et al. p53 has a direct apoptogenic role at the mitochondria. Molecular Cell. 2003;11:577. doi: 10.1016/s1097-2765(03)00050-9. [DOI] [PubMed] [Google Scholar]
- 62.Srinivas S, et al. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC developmental biology. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.de Lange WJ, Halabi CM, Beyer AM, Sigmund CD. Germ line activation of the Tie2 and SMMHC promoters causes noncell-specific deletion of floxed alleles. Physiological genomics. 2008;35:1. doi: 10.1152/physiolgenomics.90284.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Newton J, et al. Commissioning a small-field biological irradiator using point, 2D, and 3D dosimetry techniques. Medical Physics. 2011;38:6754. doi: 10.1118/1.3663675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Noma T, et al. Beta-arrestin-mediated beta1-adrenergic receptor transactivation of the EGFR confers cardioprotection. J Clin Invest. 2007;117:2445. doi: 10.1172/JCI31901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lucinskas Y.-C. L. a. F. W. Isolation and Culture of Murine Heart and Lung Endothelial Cells for In Vitro Model Systems. Methods Mol Biol. 2006;341:141. doi: 10.1385/1-59745-113-4:141. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Data