Cystic fibrosis: a mucosal immunodeficiency syndrome (original) (raw)
. Author manuscript; available in PMC: 2013 Feb 20.
Published in final edited form as: Nat Med. 2012 Apr 5;18(4):509–519. doi: 10.1038/nm.2715
Abstract
Cystic fibrosis transmembrane conductance regulator (CFTR) functions as a channel that regulates the transport of ions and the movement of water across the epithelial barrier. Mutations in CFTR, which form the basis for the clinical manifestations of cystic fibrosis, affect the epithelial innate immune function in the lung, resulting in exaggerated and ineffective airway inflammation that fails to eradicate pulmonary pathogens. Compounding the effects of excessive neutrophil recruitment, the mutant CFTR channel does not transport antioxidants to counteract neutrophil-associated oxidative stress. Whereas mutant CFTR expression in leukocytes outside of the lung does not markedly impair their function, the expected regulation of inflammation in the airways is clearly deficient in cystic fibrosis. The resulting bacterial infections, which are caused by organisms that have substantial genetic and metabolic flexibility, can resist multiple classes of antibiotics and evade phagocytic clearance. The development of animal models that approximate the human pulmonary phenotypes—airway inflammation and spontaneous infection—may provide the much-needed tools to establish how CFTR regulates mucosal immunity and to test directly the effect of pharmacologic potentiation and correction of mutant CFTR function on bacterial clearance.
Cystic fibrosis, which affects approximately 30,000 individuals in the United States, is caused by mutations in CFTR, a cAMP-regulated epithelial chloride channel (Box 1). Cystic fibrosis was originally recognized in babies with pancreatic insufficiency who failed to thrive and often succumbed to pulmonary infection in infancy or early childhood1, 2. Although there has been enormous progress made in understanding the basic biology of the CFTR chloride channel, it remains enigmatic how CFTR mutations cause enhanced susceptibility to pulmonary infection and how this susceptibility might be prevented.
BOX 1 CFTR deficiency in cystic fibrosis.
CFTR is a large glycoprotein consisting of two membrane-spanning regions and a cytoplasmic regulatory R domain178 and is expressed primarily in epithelial cells but also in many other cell types, including lymphocytes and PMNs81, 82. In addition to its role as a Cl− channel, CFTR is crucial in the regulation of ion transport, particularly Na+ and HCO3− (ref. 179). A lack of CFTR function results in sodium absorption through ENaC180, and mice with overexpression of ENaC develop lung pathology that, in some ways, mimics cystic fibrosis173, 181, although these mice do not spontaneously develop infection. There are several classes of CFTR mutations182 that correlate well with pancreatic disease, which is also a key component of cystic fibrosis, but these mutations are associated with more variable pulmonary phenotypes183. The most common CFTR mutation, ΔF508/ΔF508, results in a misfolded protein that is improperly glycosylated, is targeted for endosomal degradation and fails to reach the apical surface of the epithelium. Other CFTR mutations, such as G551D, form a partially functional channel whose activity can be potentiated165. The expression of a large number of modifier genes markedly affects the clinical manifestations of the disease184. For example, mannose-binding lectin 2 (MBL2) protein concentrations, especially in combination with high amounts of transforming growth factor β (TGF-β) production, is associated with severe pulmonary disease185. The central role of CFTR in regulating the hydration of the airways has become the focus of therapies, which seek to potentiate partially functional CFTR as well as correct the defective CFTR function attributed to specific mutations166–168.
Cystic fibrosis pulmonary disease is the most challenging problem in the management of cystic fibrosis and is the major determinant of life span and quality of life in affected individuals. Substantial clinical data have linked the recognition of bacterial infection in the lung, usually caused by Staphylococcus aureus or Pseudomonas aeruginosa, with the onset of symptomatic lung disease, which is marked by excessive airway inflammation and the eventual loss of pulmonary function. Aggressive and even prophylactic antimicrobial treatment often eradicates these infections and slows the deterioration of pulmonary function. However, the adaptive responses of the infecting organisms eventually result in isolates that are highly resistant not only to antimicrobial agents but also to the immune response that would readily eradicate these bacteria in hosts. CFTR dysfunction has long been associated with viscid mucus that causes the entrapment of bacteria in airway secretions. The prevailing hypothesis is that CFTR dysfunction, a lack of transport of chloride and accompanying water across the airway epithelium and excessive sodium reabsorption lead to dehydrated airway surface fluid, impaired mucociliary clearance, infection and inflammation3, 4. However, as we discuss below, there is accumulating evidence to suggest that CFTR dysfunction affects several components of innate immunity and that the initial predisposition to infection in infants with cystic fibrosis may represent a primary defect in local mucosal immunity.
Cystic fibrosis pulmonary disease is initiated in airways that are essentially normal at birth but that later become obstructed with mucus plugs. Early pathological findings of cystic fibrosis pulmonary disease include goblet-cell and mucus-gland hyperplasia5, 6. Even in the absence of clinically apparent infection, either viral or bacterial, there is often evidence of inflammation in cystic fibrosis airways, as evidenced by polymorphonuclear neutrophil (PMN) accumulation and excessive concentrations of interleukin-8 (IL-8) and free proteases7–11. Airway colonization and infection occur with diverse flora, which is followed by clinically apparent infection with the typical pathogens S. aureus, P. aeruginosa or both, even in infants at a very young age. Bacterial shedding of immunostimulatory pathogen–associated molecular patterns (PAMPs), such as cell-wall components, lipopolysaccharide (LPS), flagella and DNA, activate a brisk proinflammatory response. Bacterial adaptation to the airway milieu ensues, with a shift from a planktonic to a biofilm mode of growth, followed by the selection of mutants with abundant exopolysaccharide production that are resistant to phagocytosis. The intense inflammatory reaction to this airway infection consists of chemokine and cytokine expression (IL-8 and tumor necrosis factor (TNF)) and mucin secretion12, as well as PMN accumulation13 and the associated release of serine proteases14, 15, which are themselves proinflammatory stimulants16, 17. It is clear that increased airway inflammation does not result in enhanced bacterial clearance18. Airway obstruction results initially in hyperinflation, destruction of the airway walls and fibrosis, leading to decreased lung function as measured by forced expiratory volume and vital capacity.
Effects of CFTR mutation on epithelial innate immune function
The many roles of the airway epithelium in the host defense of the lung are well appreciated19. As CFTR is highly expressed in the airway epithelium, it is logical that defective CFTR function should affect the contribution of the epithelium to innate immunity. CFTR mutations have been associated with both constitutive activation of proinflammatory signaling in the absence of apparent microbial stimuli as well as exaggerated responses to bacterial products (Fig. 1). Endogenous activation of NF-κB (nuclear factor κ light-chain enhancer of activated B cells) and substantial sequestration of leukocytes has been observed in human cystic fibrosis fetal tracheal explants10 obtained before exposure to microbial flora; these observations were confirmed in pathological studies of cystic fibrosis fetuses11. Analyses of bron-choalveolar lavage fluid indicated that the induction of IL-8 and TNF in the airways of infants with cystic fibrosis is higher, often in the absence of known pathogens and elevated out of proportion to the bacterial load in airways contaminated with potential pathogens20–22 compared with normal infants. Long-term primary cultures of cystic fibrosis airway epithelial cells do not reproducibly show constitutive activation of proinflammatory signaling23, although this may be a function of clonal selection in vitro. These cultures are also influenced by epigenetic factors that are specific to the individual patients and that may influence inflammatory signaling. Whether there is, in fact, constitutive activation of proinflammatory signaling directly linked to CFTR dysfunction in humans is still unresolved and is a difficult issue to address experimentally.
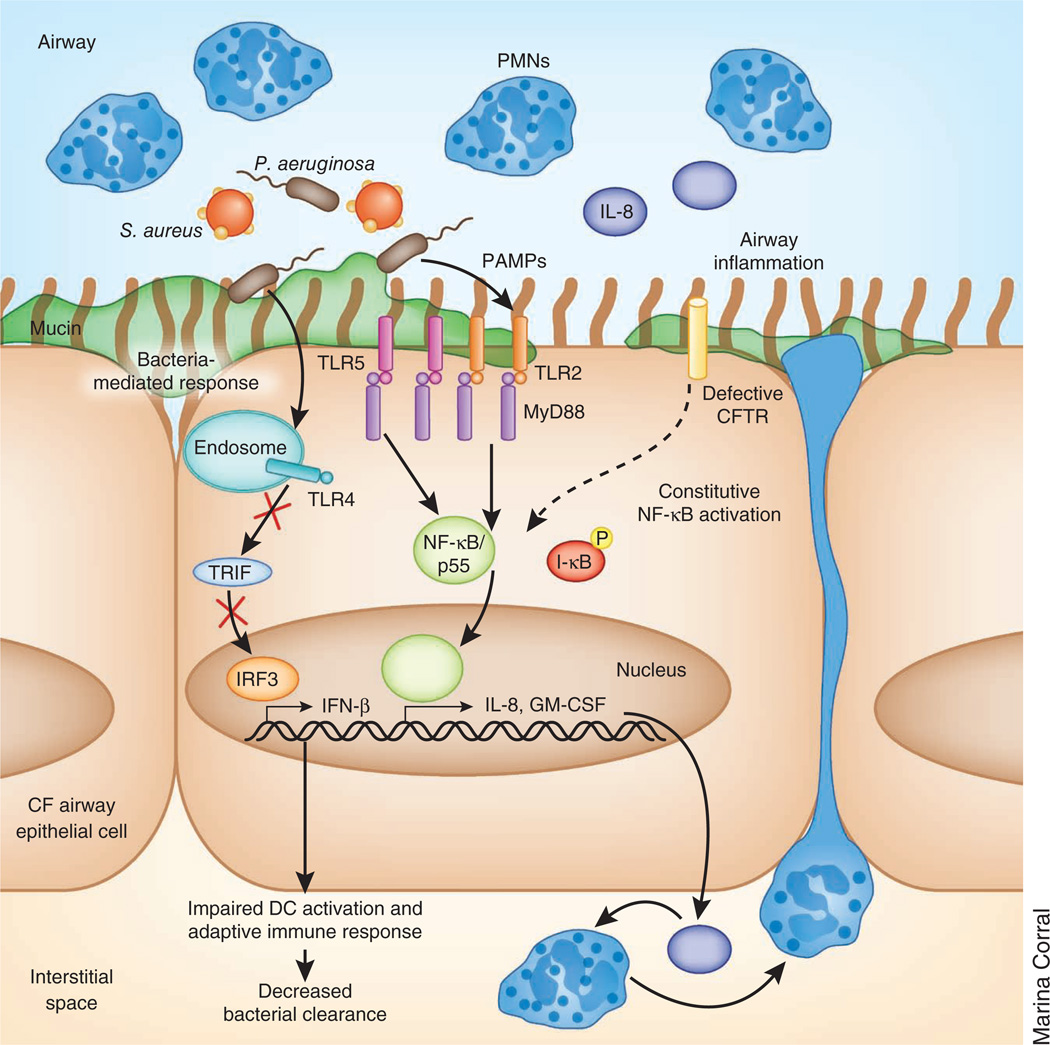
Figure 1.
Altered TLR expression and signaling in the cystic fibrosis (CF) epithelium. Expression of TLR2 and TLR5 at the apical surface is increased, whereas TLR4 expression is restricted to the endosome. NF-κB in cystic fibrosis airway epithelial cells is constitutively activated, resulting in the production of inflammatory cytokines, such as IL-8 and GM-CSF, and the recruitment of PMNs independently of TLR’s interaction with the adaptor protein MyD88. After infection, bacterial PAMPs further increase NF-κB signaling through activation of TLR-MyD88 signaling. Intracellular TLR4 activation of Trif from the endosome is impaired, preventing interferon regulatory factor 3 (IRF3) translocation to the nucleus and the activation of type I IFN gene products, which are required for the activation of dendritic cells (DCs) and the clearance of some cystic fibrosis–related pathogens.
In vitro evidence of the effects of CFTR on NF-κB signaling was shown in Chinese hamster ovary cells expressing CFTR with a deletion of Phe508 (ΔF508 CFTR), which resulted in dose-dependent increases in NF-κB activation; this effect was not seen in cells expressing wild-type CTFR8. Numerous in vitro studies using human epithelial cystic fibrosis and control cell lines confirmed increased NF-κB signaling8, 24–26 and the involvement of several proinflammatory cascades, including activation of Ca2+-dependent signaling27, 28, increased nuclear factor of activated T cells (NFAT) transcription29 and mitogen-activated protein kinase (MAPK)-dependent activation of activator protein 1 (AP-1), in cystic fibrosis epithelia compared to control epithelia30, 31. It is crucial to recognize that the construction of wild-type and mutated cell lines with alterations in CFTR gene dosage and expression, as well as the use of CFTR inhibitors to mimic channel failure, could influence the outcome measurements32.
Additional CFTR-dependent effects on epithelia influence bacterial clearance. Glycosylation of both surface-associated and secreted epithelial glycoconjugates are affected by CFTR33, 34. Glycosylation status influences bacteria-host interactions34–36, mucus composition and rheology37–39, all properties that are key in mucociliary clearance. Despite in vitro evidence that CFTR-mediated alterations in glycosylation affect bacterial attachment, it is clear that few intact bacteria actually adhere to airway epithelial cells in vivo. Instead, organisms enmeshed in airway mucin, growing within biofilms, shed PAMPs that are recognized either by epithelial receptors on the apical surface, such as Toll-like receptors (TLR2, TLR4 and TLR5), within the cytoplasm (nucleotide-binding oligomerization domains (NODs) and retinoic acid inducible gene (RIG)) or in endosomal compartments (TLR3, TLR4 and TLR9). CFTR-associated effects on the production or distribution of these pattern-recognition receptors could be crucial in the initial pathogenesis of pulmonary infection.
Cystic fibrosis–related increases in the amount of NF-κB–dependent gene products and decreases in the amount of Trif-dependent gene products have been well documented. Although several studies suggested that cystic fibrosis and non–cystic fibrosis epithelia have similar expression of TLRs40, 41, alterations in receptor localization could result in key differences in TLR-initiated signaling between the two types of epithelia. Increased surface expression of TLR2 and TLR5 on human cystic fibrosis epithelial cells correlates with increased inflammatory responses to bacterial products40, 41. However, knockdown of myeloid differentiation primary response gene (88) (MyD88) in cystic fibrosis cells prevented bacterial-induced signaling but did not reduce baseline NF-κB signaling to the level seen in non–cystic fibrosis cells, which is consistent with some degree of constitutive activation of the NF-κB pathway in unstimulated cystic fibrosis cells. As this baseline proinflammatory status, with increased IL-8 production and neutrophil accumulation, is documented in clinical studies20, 22, 42, 43, it is unclear exactly why bacteria present in the cystic fibrosis lung evade eradication. PMNs do not phagocytose as well when bacteria are suspended in fluid as when they are associated with the cell surface44. Thus, PMNs that have accumulated in excess airway mucin may not be optimal in pathogen elimination.
Intracellular innate immune signaling is also affected by dysfunctional CFTR. Activation of toll-like receptor adaptor molecule (Trif)-dependent effectors contributes to the resolution phase of an acute inflammatory response45–47 and seems to be decreased in cystic fibrosis cells. TLR4 signals through MyD88 at the cell surface and, after internalization to the early endosome, activates Trif-dependent pathways that are linked to the type I interferons (IFNs)48. In cystic fibrosis epithelial cells, the surface expression of TLR4 is reduced49, resulting in decreased activation of both MyD88 signaling (ref. 49) and Trif signaling50. These observations were corroborated in clinical studies showing reduced expression of Trif-dependent gene products in cystic fibrosis airway fluid51–53. Limited Trif signaling would interfere with the resolution of the epithelial NF-κB–dominated inflammatory response and minimize the dendritic-cell–mediated activation of the adaptive immune system50, 54.
Epithelial cells are especially key in the activation of the type I IFN response (involving IFN-α and IFN-β), a signaling cascade that is central to the clearance of diverse airway pathogens45–47, 55, including viruses and extracellular bacteria such as Streptococcus pneumonia, P. aeruginosa and S. aureus. There are limited data detailing how CFTR deficiency affects primary viral clearance mechanisms56–58, which is in contrast to the abundant clinical data showing that viral infections exacerbate coexisting bacterial infections and cystic fibrosis lung disease59–61. Patients with cystic fibrosis seem to have no particular problems handling nonrespiratory viral infections, suggesting that the major mechanisms of viral immunity in these individuals are intact. However, cystic fibrosis epithelial cells produce reduced amounts of type I interferon in response to P. aeruginosa infection and, accordingly, are less capable of activating dendritic cell populations, which initiate the adaptive immune response50. Autocrine activation of signaling downstream of interferon production is subsequently reduced in cystic fibrosis epithelial cells in part because of increased concentrations of the protein inhibitor of activated signal transducer and activator of transcription 1 (PIAS1)52, 62, further adding to the dysregulated immune response.
CFTR dysfunction contributes to oxidative stress in the airway
Whether or not the exaggerated signals for proinflammatory signaling are caused by exogenous (microbial) stimulation, CFTR-associated alterations in signal transduction or both, the net result is an excessive accumulation of dysfunctional PMNs and their products. Constitutive activation of NF-κB signaling results in increased amounts of reactive oxygen species being generated by neutrophils accumulated in the airway. Increased amounts of reactive oxygen species (O2, H2O2 and HOCl) are associated with increased IL-8 production, increased IL-6 production in response to P. aeruginosa, defective autophagy and reduced CFTR expression63–67. Under normal conditions, CFTR contributes to the downregulation of NF-κB signaling during oxidative stress by controlling the degradation of nuclear factor of κ light polypeptide gene enhancer in B cells inhibitor, α (I-κB-α)68, a response that is lacking in the cystic fibrosis lung (Fig. 2).
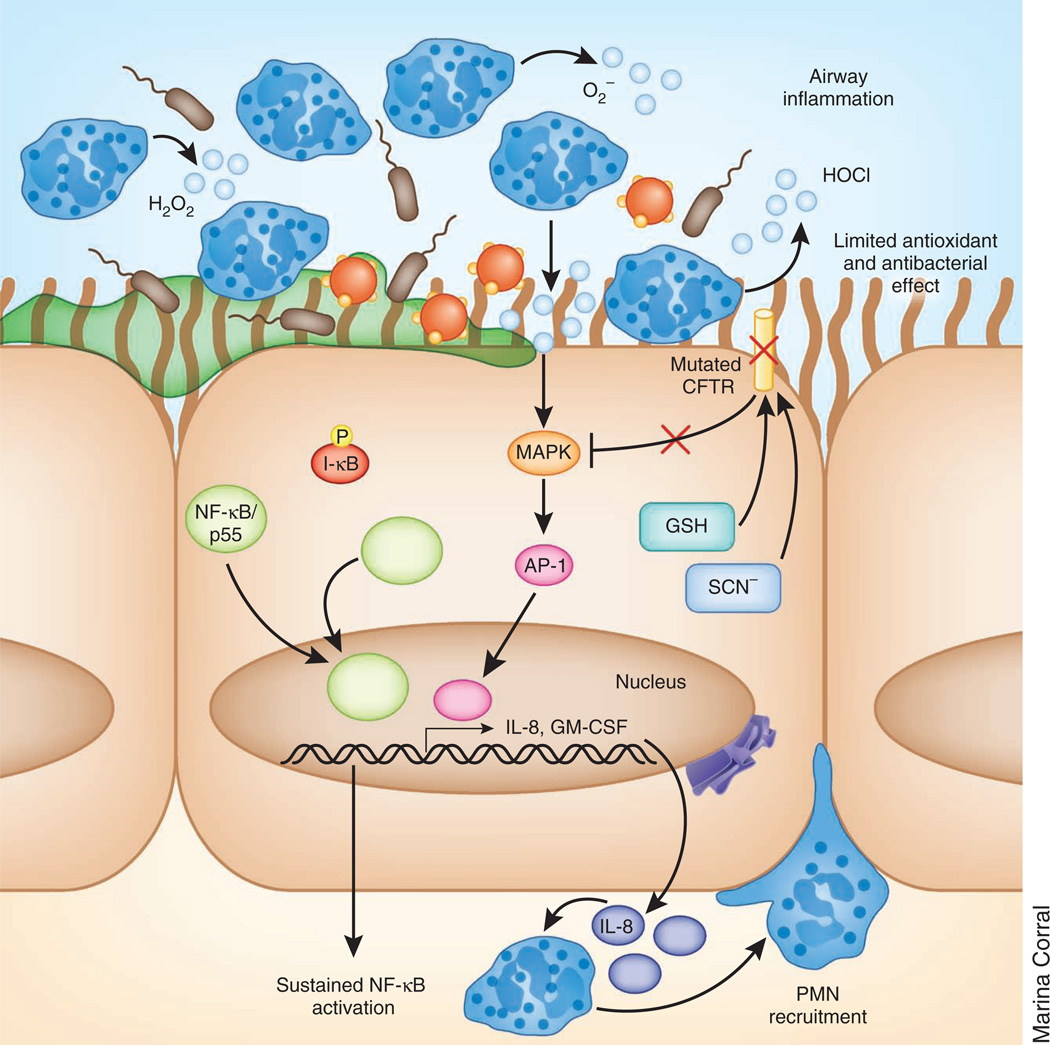
Figure 2.
Increased oxidative stress in the cystic fibrosis airway. Constitutive NF-κB–mediated production of chemokines, including IL-8, leads to PMN recruitment, which persist in the airway, increasing the oxidative burden in the lung. Oxidative stress activates MAPK signaling pathways in the cystic fibrosis epithelium, amplifying the production of IL-8 and, therefore, recruiting additional PMNs. Mutant CFTR in the epithelial cells is unable to channel the antioxidants GSH and SCN− into the airway, limiting its ability to counteract the oxidative stress. Because SCN− also has antimicrobial properties, bacterial killing in the airway is diminished as well.
Epithelial cells control oxidative damage through the production of antioxidants, such as glutathione (GSH) and thiocyanate (SCN−), that are trafficked into the epithelial-lining fluid69 and may help to modify mucus viscosity70–72. GSH secretion is markedly reduced in patients with cystic fibrosis73 (as well as in the cystic fibrosis mouse74) as a result of a protein trafficking defect in the cystic fibrosis epithelial cells75. CFTR channel dysfunction impairs the trafficking of GSH into the airway surface fluid from epithelial cells but does not impair the production of GSH76, 77. Increased oxidative stress further contributes to alterations in IL-8 gene expression64 and defects in bacterial killing. Diminished trafficking of SCN−, a potent antioxidant78, which is also associated with mutations in CFTR, similarly contributes to the diminished killing of the cystic fibrosis pathogens P. aeruginosa and S. aureus79. Thus, deficient CFTR function in the epithelium results in a diminished ability to counter the oxidative stress that is induced by both constitutive and exogenously activated inflammation.
CFTR affects immune cell function
Effective mucosal immunity is the result of the coordinated participation of epithelial cells and resident and recruited immune cells80. Particularly in the airway, in which excessive PMN accumulation interferes with pulmonary function, proinflammatory signaling is tightly regulated. The influx of immune cells, dendritic cells, macrophages and T cells is crucial in this process. Immunohistological and physiological data indicate that CFTR is both transcribed and functional in several different types of leukocytes81, 82. How CFTR dysfunction in immune cells might interfere with the regulation of innate immunity has not been well studied. Whether or not CFTR dysfunction in these cells has any biological role is unclear given the large amount of clinical data indicating that patients with cystic fibrosis have normal immune cell function at sites other than the lung. There are no clinically apparent defects in the function of cystic fibrosis macrophages or T cells, as would be suggested by the increased prevalence of infection caused by organisms usually controlled by T cells, such as Salmonella or Mycobacterium tuberculosis, in individuals with cystic fibrosis. There is also no increased incidence of abscesses or granulomatous reactions to organisms that would be suggestive of a primary defect in PMN killing in cystic fibrosis. Murine models, although lacking characteristic cystic fibrosis lung disease, nonetheless have been shown to have alterations in macrophage signaling that contribute to the exaggerated inflammatory response in the cystic fibrosis lung83. Excessive proinflammatory signaling by murine and human cystic fibrosis macrophages may caused in part by prolonged TLR4 signaling from the early endosome, resulting in the elevated activation of the NF-κB and interferon pathways84.
A phagocytic defect associated with CFTR mutations that would explain the failure to clear inhaled bacteria has long been sought85–87. Unfortunately, analyses of PMN function in cells harvested from the peripheral blood of patients with cystic fibrosis are complicated by the exposure of these patients to circulating concentrations of LPS and to activated lymphocytes and their products. PMNs85, 86 and macrophages87 isolated from patients with cystic fibrosis have a modest reduction in the ability to kill phagocytosed bacteria in vitro, but such data have not been uniformly reproduced88. In contrast, a recent study of PMNs harvested from newborns with cystic fibrosis identified by neonatal screening studies did not find appreciable differences in PMN phagocytic function between these newborns and normal controls89. Less controversial are studies showing that PMNs isolated from patients with cystic fibrosis have a slower rate of apoptosis than PMNs from control individuals without cystic fibrosis90–92 as a result of a resistance to the proapoptotic cytokine TNF-α and the effects of anti-apoptotic granulocyte-macrophage colony-stimulating factor (GM-CSF), an NF-κB–dependent cytokine that is produced in greater quantity by cystic fibrosis epithelial cells than in non–cystic fibrosis cells93. As a result, cystic fibrosis PMNs persist in the airway, further contributing to airway inflammation. The abundance of PMN-derived proteases also thwarts efficient phagocytosis94, 95, resulting in the cleavage of macrophage receptors and causing inefficient opsonization and impaired bacterial killing96. Thus, even if CFTR does not directly contribute to phagocytic function, it may influence the kinetics of PMN degradation, protease and DNA release to further exacerbate the hyperinflammatory state in the cystic fibrosis airway.
T cell function is also affected by defects in CFTR, although the specific mechanisms involved in this effect have not been defined. Diminished recruitment of T cells in response to S. aureus9 by cystic fibrosis epithelial cells, as well as altered T cell responses to Aspergillus fumigatus in both human97 and murine98 infections, have been documented. Several studies have suggested that CFTR dysfunction results in a T helper type 2 (TH2) cell bias in response to respiratory pathogens99, 100, although more recent data reflect the key role of TH17 signaling in the host response to extracellular pathogens in the lung, including P. aeruginosa101. IL-17 participates in the regulation of neutrophil recruitment by several mechanisms, including inducing the expression of IL-8, and is necessary for an effective host response to P. aeruginosa102–107. Increased TH17 signaling contributes to cystic fibrosis lung pathology, and cystic fibrosis sputa have elevated amounts of IL-17, further enhancing neutrophil recruitment in the cystic fibrosis lung108, 109. Both T cell and neutrophil production of IL-17 are increased in cystic fibrosis compared to control lung specimens110, 111, but there is no evidence that increased production of IL-17 is a direct consequence of CFTR dysfunction.
Effective clearance of inhaled bacteria from the respiratory tract involves the integrated activities of both the epithelium and the immune cells. Clinical observations suggest that induction of the PMN-dominated inflammatory responses in the cystic fibrosis airway is not normally regulated; there is a relative lack of IL-10 production112, diminished lipoxin production113–115 and a failure of the normal progression from PMN predominance to monocytes with the expected resolution phase of acute inflammation in the cystic fibrosis airway95, 116. Thus, even if the primary CFTR-dependent alteration in innate immunity is primarily an epithelial defect, there is a failure of the recruited and resident leukocytes to adequately control the hyperinflammatory state in the airway, and there is ample documentation that the presence of activated PMNs in the airway does not correlate with bacterial eradication.
Bacterial adaptation to the host
Having blamed the failure to eradicate inhaled bacteria on a presumed CFTR-associated defect in mucosal immunity, it is also crucial to recognize the prodigious capabilities of typical cystic fibrosis pathogens to adapt to and flourish within the cystic fibrosis lung. The vast majority of patients with cystic fibrosis eventually become infected with opportunistic pathogens, often P. aeruginosa and S. aureus, in addition to a growing list of both cultivatable and non-cultivatable bacterial species, including anaerobes117, 118. Infection is caused not only by host defects in innate immunity but also by the selection of bacteria that are able to evade immune clearance. The successful cystic fibrosis pathogens share a genetic flexibility and an ability to adapt to the pressures imposed by mucosal immunity (Fig. 3). Even within individual clones from one patient, there is tremendous heterogeneity, such that numerous different gene expression profiles are expressed by the bacteria colonizing the lung, allowing for the selection of optimally fit clones119, 120. Planktonic organisms aspirated from the environment upregulate gene expression for motility, proteolytic activity and carbon utilization, whereas the pathways involved in immune evasion and iron scavenging are increasingly expressed during infection in vivo. Sequential P. aeruginosa strains isolated from chronically infected patients with cystic fibrosis have alterations in the expression of numerous virulence factors (O-antigen biosynthesis, type III secretion and mobility) and of multidrug-resistance genes121, as well as of genes required for survival within the nitrogen-rich, nutrient-deficient cystic fibrosis lung122–124.
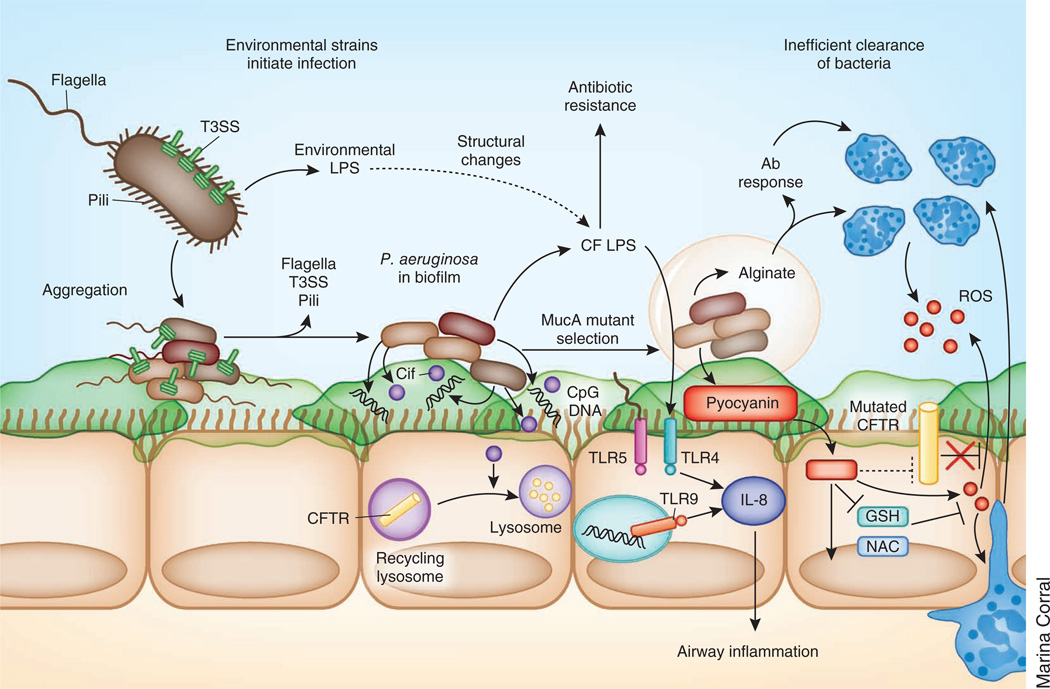
Figure 3.
Adaptation of inhaled bacteria to the cystic fibrosis airway. Inhaled bacteria expressing flagella, pili and a type 3 secretion system (T3SS) aggregate within the cystic fibrosis lung, resulting in the formation of biofilm. Within the biofilm, bacteria lose flagella, pili and the T3SS, increase alginate production, release CpG DNA and express a diverse range of virulence factors promoting evasion of the host immune system. P. aeruginosa also releases outer membrane vesicles containing Cif, a protein that inhibits the recycling of CFTR in the host. Furthermore, the lipid A structure of the LPS is altered through the addition of palmitate and aminoarabinose, resulting in increased antibiotic (Ab) resistance and increased induction of IL-8 production by host cells.
Changes in LPS structure contribute to the proinflammatory milieu in the cystic fibrosis lung. LPS from P. aeruginosa isolated from chronically infected patients with cystic fibrosis (cystic fibrosis LPS) is structurally distinct from the environmental strains that initiate infection. The lipid A portion of cystic fibrosis LPS is typically penta-acylated, as opposed to the more common hexa-acylated structure, and contains substantially more palmitate and aminoarabinose than non–cystic fibrosis LPS125, 126. These alterations correlate with increased induction of IL-8 from human endothelial cells. Worth noting is that human TLR4 responds to these changes in LPS structure, whereas murine TLR4 does not127, a factor that may be relevant to the failure of the murine model of cystic fibrosis to reflect human pulmonary pathology.
Once a critical mass of bacteria is present in the airways, in vivo data indicate that they form biofilms128, 129. This then facilitates the coordinate expression of numerous genes throughout the microbial population through secretion of highly soluble quorum sensors, such as the Pseudomonas homoserine lactones and the quinolones, which act in concert with specific transcriptional activators130. The switch to the biofilm phenotype does not necessarily indicate a reduction of proinflammatory stimulation. P. aeruginosa in biofilms is associated with the expression of more inflammatory LPS131, as mentioned above, and the enrichment of extracellular DNA132, both of which contribute to the activation of TLR-mediated proinflammatory signaling in the airways. When serially isolated cystic fibrosis strains of P. aeruginosa were instilled into mice, isolates from late-stage infection did not cause lethal infection, as the early isolates did, but were equally capable of inducing excessive lung inflammation and establishing infection133. Thus, the adaptation to the host does not necessarily imply a failure to activate local mucosal signaling but does suggest a combined failure of first the innate, and then the adaptive, immune response to clear infection.
From within this large bacterial population there is also the spontaneous selection of MucA mutants that overexpress the exopolysaccharide alginate134, 135. These mucoid organisms elude phagocytosis and have been virtually pathognomonic for cystic fibrosis136. Although alginate is immunogenic and elicits antibody production, this amplification of the inflammatory immune response further contributes to oxidative stress without resulting in the clearance of the organisms137, 138. An indication of well established P. aeruginosa infection, the predominance of alginate-producing organisms represents the end result of failed innate immunity and an effective bacterial adaptation to the host.
Bacterial products further contribute to the adaptation of organisms to the milieu within the cystic fibrosis airways. Pyocyanin, a phenazine pigment produced by all P. aeruginosa, particularly those growing in biofilms139, activates proinflammatory signaling140 and inhibits the activity of the antioxidants GSH and _N_-acetylcysteinie by blocking the dual-oxidase–based antimicrobial system141. Pyocyanin may also affect CFTR function by blocking Cl− transport in human bronchial cells142. The P. aeruginosa toxin CFTR inhibitory factor (Cif) redirects CFTR from recycling endosomes to the lysosome, where it is degraded143, compounding the deficiency associated with the major CFTR mutations; however, the in vivo role of this activity in cells that already have mutant CFTR targeted for degradation is not clear. These direct modifications of CFTR function in the host further potentiate the local immune defects associated with cystic fibrosis.
S. aureus has long been recognized as a major cystic fibrosis pathogen, and infection with the epidemic community-acquired methicillin-resistant S. aureus strains has been associated with decreased survival in individuals with cystic fibrosis144. Staphylococci adapt to the cystic fibrosis lung, proliferate as a biofilm and modulate proinflammatory activity, as monitored by cytokine and MAPK activation145. Similar to P. aeruginosa, S. aureus adapt their gene expression during colonization to enhance their fitness in the cystic fibrosis lung146. Within the S. aureus biofilm, the slower growing small-colony variants are more persistent or become resistant to antibiotics and evade host recognition by downregulating numerous virulence factors147–149. Subpopulations of the biomass revert to planktonic growth, further stimulating inflammation in the airways. Culture-independent as well as traditional methodologies have identified numerous other cystic fibrosis pathogens, especially streptococci and anaerobes that have successfully adapted to the cystic fibrosis lung and whose presence has been correlated with clinical symptomatology150. Thus, the major cystic fibrosis pathogens share key characteristics, one of which is the genetic flexibility to adapt to the milieu within the human airway, including resistance to antibiotic pressure, metabolic versatility and the ability to evade innate immune clearance mechanisms. These properties help to explain why the cystic fibrosis lung is infected by a relatively discrete group of organisms.
Implications for therapy
Whether cystic fibrosis is an innate or an acquired immune deficiency, the clinical benefits of targeting both the pathological immune response as well as the infecting organisms in this disease have been well documented in controlled clinical trials151, 152. Longitudinal studies of infants with cystic fibrosis in the first 2 years of life have documented loss of lung function, even in the absence of P. aeruginosa or S. aureus infection, both of which accelerate such a decline153. Antimicrobial therapy, in addition to the mechanical loosening of secretions and improved nutrition, have long been the mainstays of cystic fibrosis therapy, and longevity has paralleled the development of highly active antibiotics to P. aeruginosa (http://www.cff.org/). Recognition that young infants have airway inflammation that is out of proportion to the recovery of bacterial pathogens13, 22 led to clinical trials of anti-inflammatory agents such as systemic and inhaled steroids154, 155 and ibuprofen156, but each trial was limited by substantial toxicities. Azithromycin, a macrolide antibiotic that has a major anti-inflammatory activity, is effective in preventing loss of lung function in patients with cystic fibrosis157, even in those individuals who are not infected with susceptible bacteria, and is now a part of routine cystic fibrosis care at most centers. However, the accumulation of azithromycin within macrophages may also contribute to an increasing susceptibility to atypical mycobacterial infection in patients with cystic fibrosis who are treated with this drug158.
An alternative approach to cystic fibrosis therapy is based on targeting the dehydrated airway surface fluid present in individuals with the disease. Such strategies to counteract the physiological consequences of defective CFTR seem physiologically valid but have not been optimal thus far in controlled clinical trials. One approach to correct the defect in Cl− and water transport across the airway epithelium using a purinergic receptor P2Y purinoceptor 2 (P2Y2) receptor agonist to activate alternative Cl− channels was expected to result in hydrated airway surface fluid and improved mucociliary clearance. Despite promising initial results159, a large clinical trial using this drug failed to ameliorate lung disease in patients with a mild cystic fibrosis160. Whether these results were caused by a failure to target the innate immune defect, which is presumably CFTR dependent, is not clear. Similarly, the use of osmotic agents such as hypertonic saline or mannitol (Bronchitol) have had mixed results in clinical trials161–164.
The pharmacological potentiation of mutant CFTR function or the correction of mutant CFTR trafficking is a more desirable and realistic goal that, if achieved early enough in childhood, might prevent chronic infection and inflammation and irreversible airway damage. In vitro characterization of such compounds is currently in progress165. As clinical trials in human neonates are fraught with ethical and technical hazards, the newly developed _CFTR_−/− pig and _CFTR_−/− ferret models may be especially crucial in establishing whether pharmacologic therapy early in life can prevent cystic fibrosis lung disease (Box 2). Sequential bronchial biopsies could be done to ascertain whether endogenous upregulation of NF-κB activity is normalized, the amount of IL-8 and TNF in the airway is corrected and the numbers of recruited immune cells in the bronchoalveolar lavage fluid are normalized. Drugs to correct specific defects in CFTR biology, either by increasing the trafficking of mutant CFTR to the apical surface of the cell or by increasing Cl− transport through the mutant channel or both (by the use of two drugs in combination) are currently in clinical trials in patients with cystic fibrosis with specific genotypes166–168. However, there have not been any data published thus far that show that pharmacologic correction of channel function in vivo corrects abnormalities in innate immunity or prevents infection. This outcome should be a reasonable expectation, as in vitro correction of mutant CFTR decreases proinflammatory signaling in numerous model systems169, 170. Ideally, treatment of infants with cystic fibrosis with drugs that target specific CFTR genotypes would not only correct the defect in electrolyte transport in these infants but would also restore normal epithelial innate immune function, diminish endogenous proinflammatory signaling inflammation and prevent infection by correcting the many abnormalities detailed above. Such therapy has recently become available with the licensing of Kalydeco (ivacaftor) in January 2012, a CFTR potentiator that targets the CFTR G551D mutant, which has been found in approximately 1,200 people in the United States. Phase 3 clinical trials showed that the patients with cystic fibrosis carrying the G551D mutation (>6 years of age) who received this drug had improved pulmonary function. In the presence of established infection, as is the case in many patients with cystic fibrosis who are old enough to participate in clinical trials, a realistic goal of CFTR correctors and potentiators would be to stabilize lung function and decrease proinflammatory signaling. The availability of the porcine and, perhaps, the ferret models of cystic fibrosis should greatly expedite the evaluation of these new CFTR pharmacotherapeutics and facilitate the identification of optimal drug combinations, doses and methods of delivery.
BOX 2 Animal models of cystic fibrosis.
A tractable animal model of cystic fibrosis would be a tremendous tool in understanding how CFTR is linked to infection and for assessing the efficacy of therapy to correct CFTR dysfunction, particularly in young infants. The ideal animal model would accurately reflect the salient characteristics of human lung disease, namely excessive airway inflammation, spontaneous development of bacterial infection and progression to chronic infection with characteristic biofilm formation (Table 1). Although CFTR correction is typically monitored by the restoration of a cAMP-mediated Cl− current186, an animal model could be used to establish the clinically relevant outcome measurement, namely, the amount of CFTR correction that is necessary to prevent inflammation and infection. The much heralded _Cftr_-deficient mouse that was developed in 1992 (refs. 174, 187) unfortunately did not fulfill these goals; cystic fibrosis mice did not develop spontaneous lung disease, and an accurate reflection of cystic fibrosis pancreatic disease was dependent on the strain of mouse used175. Although these mice and, later, gut-corrected (_Cftrtm1Unc_-TgN(FABPCFTR) mice did show excessive proinflammatory signaling with increased chemokine (C-X-C motif) ligand 1 (CXCL1 or KC), TNF and PMN recruitment into the airways and increased weight loss during the course of infection, they spontaneously cleared even large inocula of typical cystic fibrosis pathogens. Thus, these mice have not been useful for testing the efficacy of antimicrobial regimens or of anti-inflammatory therapy or for monitoring effects of pharmacological correction of CFTR channel activity on inflammation or infection.
Recently developed _CFTR_-deficient171 and ΔF508/ΔF508 pigs188 spontaneously develop lung disease that is characterized by inflammation, mucus overproduction, airway obstruction and infection189. These features, which are typical of human disease, develop very early in life in these pigs. The _CFTR_-deficient pig fails to clear staphylococcal infection, a characteristic that may help to clarify the roles of CFTR expression in innate immunity. Physiological studies have suggested that the biology of porcine and human airway surface fluids are similar176. Notably, the antimicrobial activity of the airway surface fluid from cystic fibrosis pigs is not impaired172. _CFTR_-deficient pigs manifest the predicted defect in chloride and bicarbonate transport that is typical of human CFTR mutations, but they do not hyperabsorb sodium nor do they show diminished amounts of airway surface fluid. These results suggest that decreased hydration of airway surface fluid may not be central to the development of infection and inflammation190. Similarly, the cystic fibrosis ferret177 also develops lung infection very early in life, which is severe enough to require antibiotic treatment191. These models support the hypothesis that there is a direct role of CFTR in mucosal immunity beyond its contribution to the hydration of the airway surface fluids. These animal models may provide a useful model system to test the pharmacologic agents under development to correct specific CFTR mutations.
Concluding remarks
A tremendous amount of progress has been made in understanding the protean manifestations of cystic fibrosis: the genetic basis for cystic fibrosis and the influence of modifier genes in the disease has been established, the structure of the CFTR Cl− channel has been defined, and much of the complex physiology of CFTR and its central role in epithelial electrolyte transport has been explained. Cystic fibrosis pigs and ferrets are being developed that seem to reproduce human disease, providing model systems to explore the pathogenesis of cystic fibrosis and potential therapeutics, even in neonates. Clinical trials are ongoing to develop potent therapies based on this information to correct the multiple CFTR-associated abnormalities in epithelial function that together result in such a major defect in mucosal immune function. The involvement of CFTR in so many epithelial functions that are relevant to the host-pathogen interaction (constitutive activation of NF-κB, dysregulated TLR4 trafficking and signaling, associated defects in type I IFN regulation, impaired epithelial antioxidant activity and the failure of appropriate immune regulation) indicate that there is not a single ‘cause’ of the defective mucosal immunity associated with cystic fibrosis.
However, major questions remain unanswered. (i) Exactly how does CFTR participate in innate immune signaling? Is CFTR function involved in pathogen recognition, PAMP trafficking or the afferent limbs of signal transduction? (ii) Why do activated neutrophils in the cystic fibrosis airway fail to clear the initial P. aeruginosa infection? (iii) Does CFTR dysfunction affect dendritic cells, T cells or the development of adaptive immunity in the lung? (iv) What amount of CFTR ‘correction’ or ‘potentiation’ is necessary to restore normal innate immune function? (v) How early must therapy to correct CFTR function commence to prevent infection?
We anticipate that pharmacological correctors and potentiators of CFTR function, currently defined by restoration of chloride channel activity, will also ameliorate defects in mucosal immunity and diminish proinflamatory signaling and infection and the subsequent pulmonary pathology. Nonetheless, there are many unresolved issues that merit intensive investigation.
Table 1.
Characteristics of cystic fibrosis in human disease and animal models
| Mouse | ||||||
|---|---|---|---|---|---|---|
| Human | CFTR−/− andCFTRΔF508/ΔF505 | ENaC overexpression | Ferret | Pig | References | |
| Spontaneousinfection | P. aeruginosa; _S. aureus;_streptococci; anaerobes | – | – | Streptococci;staphylococcienterococci | Streptococci;staphylococci | 116, 117 171, 172 |
| Airwayinflammation | PMNs; NF-κBactivation; IL-8;TNF-α | Macrophage recruitment(strain dependent) | PMNs; NF-κBactivation | Uncharacterized | PMNs | 7–12, 173–176 |
| Mucusaccumulationin airway | Submucosal glandsecretion;mucus dehydration | – | Mucus plugs | Uncharacterized lesionsin newborn lungs | Mucus (hydrated);expanded submucosalglands | 5, 6, 173, 172, 176 177 |
ACKNOWLEDGMENTS
Work in the laboratory is supported by US National Institutes of Health grants 5R21AI083491, 2R01HL079395 and 5R01HL073989 (A.P.) and a Parker B. Francis Fellowship (T.S.C.).
Footnotes
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
References
- 1.Wilmers MJ, Mackay HM, Anderson IM. Five cases of cystic fibrosis of the pancreas. Proc. R. Soc. Med. 1950;43:829–832. [PMC free article] [PubMed] [Google Scholar]
- 2.Gugler E, Pallavicini JC, Swedlow H, Zipkin I, Agnese PA. Immunological studies of submaxillary saliva from patients with cystic fibrosis and from normal children. J. Pediatr. 1968;73:548–559. doi: 10.1016/s0022-3476(68)80270-7. [DOI] [PubMed] [Google Scholar]
- 3.Knowles MR, et al. Ion composition of airway surface liquid of patients with cystic fibrosis as compared with normal and disease-control subjects. J. Clin. Invest. 1997;100:2588–2595. doi: 10.1172/JCI119802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matsui H, et al. Evidence for periciliary liquid layer depletion, not abnormal ion composition, in the pathogenesis of cystic fibrosis airways disease. Cell. 1998;95:1005–1015. doi: 10.1016/s0092-8674(00)81724-9. [DOI] [PubMed] [Google Scholar]
- 5.Martens CJ, et al. Mucous solids and liquid secretion by airways: studies with normal pig, cystic fibrosis human, and non-cystic fibrosis human bronchi. Am. J. Physiol. Lung Cell. Mol. Physiol. 2011;301:L236–L246. doi: 10.1152/ajplung.00388.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burgel PR, Montani D, Danel C, Dusser DJ, Nadel JA. A morphometric study of mucins and small airway plugging in cystic fibrosis. Thorax. 2007;62:153–161. doi: 10.1136/thx.2006.062190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Heeckeren AM, Schluchter MD, Drumm ML, Davis PB. Role of Cftr genotype in the response to chronic Pseudomonas aeruginosa lung infection in mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004;287:L944–L952. doi: 10.1152/ajplung.00387.2003. [DOI] [PubMed] [Google Scholar]
- 8.Weber AJ, Soong G, Bryan R, Saba S, Prince A. Activation of NF-κB in airway epithelial cells is dependent on CFTR trafficking and Cl− channel function. Am. J. Physiol. Lung Cell. Mol. Physiol. 2001;281:L71–L78. doi: 10.1152/ajplung.2001.281.1.L71. [DOI] [PubMed] [Google Scholar]
- 9.Al Alam D, et al. Impaired interleukin-8 chemokine secretion by staphylococcus aureus -activated epithelium and T-cell chemotaxis in cystic fibrosis. Am. J. Respir. Cell Mol. Biol. 2010;42:644–650. doi: 10.1165/rcmb.2008-0021OC. [DOI] [PubMed] [Google Scholar]
- 10.Tirouvanziam R, et al. Inflammation and infection in naive human cystic fibrosis airway grafts. Am. J. Respir. Cell Mol. Biol. 2000;23:121–127. doi: 10.1165/ajrcmb.23.2.4214. [DOI] [PubMed] [Google Scholar]
- 11.Verhaeghe C, Delbecque K, de Leval L, Oury C, Bours V. Early inflammation in the airways of a cystic fibrosis foetus. J. Cyst. Fibros. 2007;6:304–308. doi: 10.1016/j.jcf.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 12.Li JD, et al. Transcriptional activation of mucin by Pseudomonas aeruginosa lipopolysaccharide in the pathogenesis of cystic fibrosis lung disease. Proc. Natl. Acad. Sci. USA. 1997;94:967–972. doi: 10.1073/pnas.94.3.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sagel SD, et al. Induced sputum inflammatory measures correlate with lung function in children with cystic fibrosis. J. Pediatr. 2002;141:811–817. doi: 10.1067/mpd.2002.129847. [DOI] [PubMed] [Google Scholar]
- 14.Weldon S, et al. Decreased levels of secretory leucoprotease inhibitor in the Pseudomonas -infected cystic fibrosis lung are due to neutrophil elastase degradation. J. Immunol. 2009;183:8148–8156. doi: 10.4049/jimmunol.0901716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quinn DJ, Weldon S, Taggart CC. Antiproteases as therapeutics to target inflammation in cystic fibrosis. Open Respir. Med. J. 2010;4:20–31. doi: 10.2174/1874306401004010020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Birrer P, et al. Protease-antiprotease imbalance in the lungs of children with cystic fibrosis. Am. J. Respir. Crit. Care Med. 1994;150:207–213. doi: 10.1164/ajrccm.150.1.7912987. [DOI] [PubMed] [Google Scholar]
- 17.Cosgrove S, Chotirmall SH, Greene CM, McElvaney NG. Pulmonary proteases in the cystic fibrosis lung induce interleukin 8 expression from bronchial epithelial cells via a heme/meprin/epidermal growth factor receptor/Toll-like receptor pathway. J. Biol. Chem. 2011;286:7692–7704. doi: 10.1074/jbc.M110.183863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mizgerd JP, et al. Nuclear factor-κB p50 limits inflammation and prevents lung injury during Escherichia coli pneumonia. Am. J. Respir. Crit. Care Med. 2003;168:810–817. doi: 10.1164/rccm.200303-412OC. [DOI] [PubMed] [Google Scholar]
- 19.Parker D, Prince A. Innate immunity in the respiratory epithelium. Am. J. Respir. Cell Mol. Biol. 2011;45:189–201. doi: 10.1165/rcmb.2011-0011RT. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khan TZ, et al. Early pulmonary inflammation in infants with cystic fibrosis. Am. J. Respir. Crit. Care Med. 1995;151:1075–1082. doi: 10.1164/ajrccm/151.4.1075. [DOI] [PubMed] [Google Scholar]
- 21.Rosenfeld M, et al. Early pulmonary infection, inflammation, and clinical outcomes in infants with cystic fibrosis. Pediatr. Pulmonol. 2001;32:356–366. doi: 10.1002/ppul.1144. [DOI] [PubMed] [Google Scholar]
- 22.Muhlebach MS, Noah TL. Endotoxin activity and inflammatory markers in the airways of young patients with cystic fibrosis. Am. J. Respir. Crit. Care Med. 2002;165:911–915. doi: 10.1164/ajrccm.165.7.2107114. [DOI] [PubMed] [Google Scholar]
- 23.Fulcher ML, et al. Novel human bronchial epithelial cell lines for cystic fibrosis research. Am. J. Physiol. Lung Cell. Mol. Physiol. 2009;296:L82–L91. doi: 10.1152/ajplung.90314.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vij N, Mazur S, Zeitlin PL. CFTR is a negative regulator of NFκB mediated innate immune response. PLoS ONE. 2009;4:e4664. doi: 10.1371/journal.pone.0004664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Venkatakrishnan A, et al. Exaggerated activation of nuclear factor-κB and altered IκB-β processing in cystic fibrosis bronchial epithelial cells. Am. J. Respir. Cell Mol. Biol. 2000;23:396–403. doi: 10.1165/ajrcmb.23.3.3949. [DOI] [PubMed] [Google Scholar]
- 26.Perez A, et al. CFTR inhibition mimics the cystic fibrosis inflammatory profile. Am. J. Physiol. Lung Cell. Mol. Physiol. 2007;292:L383–L395. doi: 10.1152/ajplung.00403.2005. [DOI] [PubMed] [Google Scholar]
- 27.Ribeiro CM, Paradiso AM, Carew MA, Shears SB, Boucher RC. Cystic fibrosis airway epithelial Ca2+ i signaling: the mechanism for the larger agonist-mediated Ca2+ i signals in human cystic fibrosis airway epithelia. J. Biol. Chem. 2005;280:10202–10209. doi: 10.1074/jbc.M410617200. [DOI] [PubMed] [Google Scholar]
- 28.Ribeiro CM, et al. Chronic airway infection/inflammation induces a Ca2+ i-dependent hyperinflammatory response in human cystic fibrosis airway epithelia. J. Biol. Chem. 2005;280:17798–17806. doi: 10.1074/jbc.M410618200. [DOI] [PubMed] [Google Scholar]
- 29.Waters V, et al. The effect of cyclosporin A on airway cell proinflammatory signaling and pneumonia. Am. J. Respir. Cell Mol. Biol. 2005;33:138–144. doi: 10.1165/rcmb.2005-0005OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Verhaeghe C, et al. Role of IKK and ERK pathways in intrinsic inflammation of cystic fibrosis airways. Biochem. Pharmacol. 2007;73:1982–1994. doi: 10.1016/j.bcp.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 31.Bhattacharyya S, et al. MAPK signaling pathways regulate IL-8 mRNA stability and IL-8 protein expression in cystic fibrosis lung epithelial cell lines. Am. J. Physiol. Lung Cell. Mol. Physiol. 2011;300:L81–L87. doi: 10.1152/ajplung.00051.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Machen TE. Innate immune response in CF airway epithelia: hyperinflammatory? Am. J. Physiol. Cell Physiol. 2006;291:C218–C230. doi: 10.1152/ajpcell.00605.2005. [DOI] [PubMed] [Google Scholar]
- 33.Barasch J, et al. Defective acidification of intracellular organelles in cystic fibrosis. Nature. 1991;352:70–73. doi: 10.1038/352070a0. [DOI] [PubMed] [Google Scholar]
- 34.Saiman L, Prince A. Pseudomonas aeruginosa pili bind to asialoGM1 which is increased on the surface of cystic fibrosis epithelial cells. J. Clin. Invest. 1993;92:1875–1880. doi: 10.1172/JCI116779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Davies JC, et al. CFTR gene transfer reduces the binding of Pseudomonas aeruginosa to cystic fibrosis respiratory epithelium. Am. J. Respir. Cell Mol. Biol. 1997;16:657–663. doi: 10.1165/ajrcmb.16.6.9191467. [DOI] [PubMed] [Google Scholar]
- 36.Pier GB, Grout M, Zaidi TS. Cystic fibrosis transmembrane conductance regulator is an epithelial cell receptor for clearance of Pseudomonas aeruginosa from the lung. Proc. Natl. Acad. Sci. USA. 1997;94:12088–12093. doi: 10.1073/pnas.94.22.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jayaraman S, Joo NS, Reitz B, Wine JJ, Verkman AS. Submucosal gland secretions in airways from cystic fibrosis patients have normal [Na+] and pH but elevated viscosity. Proc. Natl. Acad. Sci. USA. 2001;98:8119–8123. doi: 10.1073/pnas.131087598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Puchelle E, Jacquot J, Beck G, Zahm JM, Galabert C. Rheological and transport properties of airway secretions in cystic fibrosis—relationships with the degree of infection and severity of the disease. Eur. J. Clin. Invest. 1985;15:389–394. doi: 10.1111/j.1365-2362.1985.tb00290.x. [DOI] [PubMed] [Google Scholar]
- 39.Lethem MI, James SL, Marriott C. The role of mucous glycoproteins in the rheologic properties of cystic fibrosis sputum. Am. Rev. Respir. Dis. 1990;142:1053–1058. doi: 10.1164/ajrccm/142.5.1053. [DOI] [PubMed] [Google Scholar]
- 40.Muir A, et al. Toll-like receptors in normal and cystic fibrosis airway epithelial cells. Am. J. Respir. Cell Mol. Biol. 2004;30:777–783. doi: 10.1165/rcmb.2003-0329OC. [DOI] [PubMed] [Google Scholar]
- 41.Greene CM, et al. TLR-induced inflammation in cystic fibrosis and non-cystic fibrosis airway epithelial cells. J. Immunol. 2005;174:1638–1646. doi: 10.4049/jimmunol.174.3.1638. [DOI] [PubMed] [Google Scholar]
- 42.Noah TL, Black HR, Cheng PW, Wood RE, Leigh MW. Nasal and bronchoalveolar lavage fluid cytokines in early cystic fibrosis. J. Infect. Dis. 1997;175:638–647. doi: 10.1093/infdis/175.3.638. [DOI] [PubMed] [Google Scholar]
- 43.Gangell C, et al. Inflammatory responses to individual microorganisms in the lungs of children With cystic fibrosis. Clin. Infect. Dis. 2011;53:425–432. doi: 10.1093/cid/cir399. [DOI] [PubMed] [Google Scholar]
- 44.Colucci-Guyon E, Tinevez JY, Renshaw SA, Herbomel P. Strategies of professional phagocytes in vivo : unlike macrophages, neutrophils engulf only surface-associated microbes. J. Cell Sci. 2011;124:3053–3059. doi: 10.1242/jcs.082792. [DOI] [PubMed] [Google Scholar]
- 45.Carrigan SO, et al. IFN regulatory factor 3 contributes to the host response during Pseudomonas aeruginosa lung infection in mice. J. Immunol. 2010;185:3602–3609. doi: 10.4049/jimmunol.0903429. [DOI] [PubMed] [Google Scholar]
- 46.Martin FJ, et al. Staphylococcus aureus activates type I IFN signaling in mice and humans through the Xr repeated sequences of protein A. J. Clin. Invest. 2009;119:1931–1939. doi: 10.1172/JCI35879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Power MR, Li B, Yamamoto M, Akira S, Lin TJ. A role of Toll-IL-1 receptor domain-containing adaptor-inducing IFN-β in the host response to Pseudomonas aeruginosa lung infection in mice. J. Immunol. 2007;178:3170–3176. doi: 10.4049/jimmunol.178.5.3170. [DOI] [PubMed] [Google Scholar]
- 48.Kagan JC, et al. TRAM couples endocytosis of Toll-like receptor 4 to the induction of interferon-β. Nat. Immunol. 2008;9:361–368. doi: 10.1038/ni1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.John G, Yildirim AO, Rubin BK, Gruenert DC, Henke MO. TLR-4- mediated innate immunity is reduced in cystic fibrosis airway cells. Am. J. Respir. Cell Mol. Biol. 2010;42:424–431. doi: 10.1165/rcmb.2008-0408OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Parker D, et al. Induction of type I interferon signaling by Pseudomonas aeruginosa is diminished in cystic fibrosis epithelial cells. Am. J. Respir. Cell. Mol. Biol. 2011;46:6–13. doi: 10.1165/rcmb.2011-0080OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schwiebert LM, Estell K, Propst SM. Chemokine expression in CF epithelia: implications for the role of CFTR in RANTES expression. Am. J. Physiol. 1999;276:C700–C710. doi: 10.1152/ajpcell.1999.276.3.C700. [DOI] [PubMed] [Google Scholar]
- 52.Zheng S, et al. Impaired nitric oxide synthase-2 signaling pathway in cystic fibrosis airway epithelium. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004;287:L374–L381. doi: 10.1152/ajplung.00039.2004. [DOI] [PubMed] [Google Scholar]
- 53.Estell K, et al. Plasma membrane CFTR regulates RANTES expression via its C-terminal PDZ-interacting motif. Mol. Cell. Biol. 2003;23:594–606. doi: 10.1128/MCB.23.2.594-606.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zheng S, et al. Impaired innate host defense causes susceptibility to respiratory virus infections in cystic fibrosis. Immunity. 2003;18:619–630. doi: 10.1016/s1074-7613(03)00114-6. [DOI] [PubMed] [Google Scholar]
- 55.Parker D, et al. Streptococcus pneumoniae DNA initiates type I interferon signaling in the respiratory tract. MBio. 2011;2:e00016–e00011. doi: 10.1128/mBio.00016-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sutanto EN, et al. Innate inflammatory responses of pediatric cystic fibrosis airway epithelial cells: effects of nonviral and viral stimulation. Am. J. Respir. Cell Mol. Biol. 2011;44:761–767. doi: 10.1165/rcmb.2010-0368OC. [DOI] [PubMed] [Google Scholar]
- 57.Johnson JS, et al. AAV exploits subcellular stress associated with inflammation, endoplasmic reticulum expansion, and misfolded proteins in models of cystic fibrosis. PLoS Pathog. 2011;7:e1002053. doi: 10.1371/journal.ppat.1002053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kieninger E, et al. Lack of an exaggerated inflammatory response upon virus infection in cystic fibrosis. Eur. Respir. J. 2011;39:297–304. doi: 10.1183/09031936.00054511. [DOI] [PubMed] [Google Scholar]
- 59.de Vrankrijker AM, et al. Respiratory syncytial virus infection facilitates acute colonization of Pseudomonas aeruginosa in mice. J. Med. Virol. 2009;81:2096–2103. doi: 10.1002/jmv.21623. [DOI] [PubMed] [Google Scholar]
- 60.Wang EE, Prober CG, Manson B, Corey M, Levison H. Association of respiratory viral infections with pulmonary deterioration in patients with cystic fibrosis. N. Engl. J. Med. 1984;311:1653–1658. doi: 10.1056/NEJM198412273112602. [DOI] [PubMed] [Google Scholar]
- 61.van Ewijk BE, et al. Prevalence and impact of respiratory viral infections in young children with cystic fibrosis: prospective cohort study. Pediatrics. 2008;122:1171–1176. doi: 10.1542/peds.2007-3139. [DOI] [PubMed] [Google Scholar]
- 62.Kelley TJ, Elmer HL. In vivo alterations of IFN regulatory factor-1 and PIAS1 protein levels in cystic fibrosis epithelium. J. Clin. Invest. 2000;106:403–410. doi: 10.1172/JCI9560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cantin AM, Bilodeau G, Ouellet C, Liao J, Hanrahan JW. Oxidant stress suppresses CFTR expression. Am. J. Physiol. Cell Physiol. 2006;290:C262–C270. doi: 10.1152/ajpcell.00070.2005. [DOI] [PubMed] [Google Scholar]
- 64.Bartling TR, Drumm ML. Oxidative stress causes IL8 promoter hyperacetylation in cystic fibrosis airway cell models. Am. J. Respir. Cell Mol. Biol. 2009;40:58–65. doi: 10.1165/rcmb.2007-0464OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bérubé J, Roussel L, Nattagh L, Rousseau S. Loss of cystic fibrosis transmembrane conductance regulator function enhances activation of p38 and ERK MAPKs, increasing interleukin-6 synthesis in airway epithelial cells exposed to Pseudomonas aeruginosa. J. Biol. Chem. 2010;285:22299–22307. doi: 10.1074/jbc.M109.098566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Luciani A, et al. Defective CFTR induces aggresome formation and lung inflammation in cystic fibrosis through ROS-mediated autophagy inhibition. Nat. Cell Biol. 2010;12:863–875. doi: 10.1038/ncb2090. [DOI] [PubMed] [Google Scholar]
- 67.Blackwell TS, Blackwell TR, Holden EP, Christman BW, Christman JW. In vivo antioxidant treatment suppresses nuclear factor-κB activation and neutrophilic lung inflammation. J. Immunol. 1996;157:1630–1637. [PubMed] [Google Scholar]
- 68.Boncoeur E, et al. Cystic fibrosis transmembrane conductance regulator controls lung proteasomal degradation and nuclear factor-κB activity in conditions of oxidative stress. Am. J. Pathol. 2008;172:1184–1194. doi: 10.2353/ajpath.2008.070310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cantin AM, North SL, Hubbard RC, Crystal RG. Normal alveolar epithelial lining fluid contains high levels of glutathione. J. Appl. Physiol. 1987;63:152–157. doi: 10.1152/jappl.1987.63.1.152. [DOI] [PubMed] [Google Scholar]
- 70.Kelly FJ. Gluthathione: in defense of the lung. Food Chem. Toxicol. 1999;37:963–966. doi: 10.1016/s0278-6915(99)00087-3. [DOI] [PubMed] [Google Scholar]
- 71.Hudson VM. Rethinking cystic fibrosis pathology: the critical role of abnormal reduced glutathione (GSH) transport caused by CFTR mutation. Free Radic. Biol. Med. 2001;30:1440–1461. doi: 10.1016/s0891-5849(01)00530-5. [DOI] [PubMed] [Google Scholar]
- 72.Rahman I, MacNee W. Oxidative stress and regulation of glutathione in lung inflammation. Eur. Respir. J. 2000;16:534–554. doi: 10.1034/j.1399-3003.2000.016003534.x. [DOI] [PubMed] [Google Scholar]
- 73.Roum JH, Buhl R, McElvaney NG, Borok Z, Crystal RG. Systemic deficiency of glutathione in cystic fibrosis. J. Appl. Physiol. 1993;75:2419–2424. doi: 10.1152/jappl.1993.75.6.2419. [DOI] [PubMed] [Google Scholar]
- 74.Velsor LW, van Heeckeren A, Day BJ. Antioxidant imbalance in the lungs of cystic fibrosis transmembrane conductance regulator protein mutant mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 2001;281:L31–L38. doi: 10.1152/ajplung.2001.281.1.L31. [DOI] [PubMed] [Google Scholar]
- 75.Ringe D, Petsko GA. Cystic fibrosis. A transport problem? Nature. 1990;346:312–313. doi: 10.1038/346312a0. [DOI] [PubMed] [Google Scholar]
- 76.Gao L, Kim KJ, Yankaskas JR, Forman HJ. Abnormal glutathione transport in cystic fibrosis airway epithelia. Am. J. Physiol. 1999;277:L113–L118. doi: 10.1152/ajplung.1999.277.1.L113. [DOI] [PubMed] [Google Scholar]
- 77.Kogan I, et al. CFTR directly mediates nucleotide-regulated glutathione flux. EMBO J. 2003;22:1981–1989. doi: 10.1093/emboj/cdg194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xu Y, Szep S, Lu Z. The antioxidant role of thiocyanate in the pathogenesis of cystic fibrosis and other inflammation-related diseases. Proc. Natl. Acad. Sci. USA. 2009;106:20515–20519. doi: 10.1073/pnas.0911412106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Moskwa P, et al. A novel host defense system of airways is defective in cystic fibrosis. Am. J. Respir. Crit. Care Med. 2007;175:174–183. doi: 10.1164/rccm.200607-1029OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Andonegui G, et al. Endothelium-derived Toll-like receptor-4 is the key molecule in LPS-induced neutrophil sequestration into lungs. J. Clin. Invest. 2003;111:1011–1020. doi: 10.1172/JCI16510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.McDonald TV, Nghiem PT, Gardner P, Martens CL. Human lymphocytes transcribe the cystic fibrosis transmembrane conductance regulator gene and exhibit CF-defective cAMP-regulated chloride current. J. Biol. Chem. 1992;267:3242–3248. [PubMed] [Google Scholar]
- 82.Yoshimura K, et al. Expression of the cystic fibrosis transmembrane conductance regulator gene in cells of non-epithelial origin. Nucleic Acids Res. 1991;19:5417–5423. doi: 10.1093/nar/19.19.5417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bruscia EM, et al. Macrophages directly contribute to the exaggerated inflammatory response in cystic fibrosis transmembrane conductance regulator−/− mice. Am. J. Respir. Cell Mol. Biol. 2009;40:295–304. doi: 10.1165/rcmb.2008-0170OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bruscia EM, et al. Abnormal trafficking and degradation of TLR4 underlie the elevated inflammatory response in cystic fibrosis. J. Immunol. 2011;186:6990–6998. doi: 10.4049/jimmunol.1100396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Painter RG, et al. The role of chloride anion and CFTR in killing of Pseudomonas aeruginosa by normal and CF neutrophils. J. Leukoc. Biol. 2008;83:1345–1353. doi: 10.1189/jlb.0907658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hartl D, et al. Cleavage of CXCR1 on neutrophils disables bacterial killing in cystic fibrosis lung disease. Nat. Med. 2007;13:1423–1430. doi: 10.1038/nm1690. [DOI] [PubMed] [Google Scholar]
- 87.Di A, et al. CFTR regulates phagosome acidification in macrophages and alters bactericidal activity. Nat. Cell Biol. 2006;8:933–944. doi: 10.1038/ncb1456. [DOI] [PubMed] [Google Scholar]
- 88.Haggie PM, Verkman AS. Cystic fibrosis transmembrane conductance regulator-independent phagosomal acidification in macrophages. J. Biol. Chem. 2007;282:31422–31428. doi: 10.1074/jbc.M705296200. [DOI] [PubMed] [Google Scholar]
- 89.Kingma P. CFTR and Neutrophil function: our children may have the answers. Pediatr. Pulmonol. 2010;45(suppl. 33):1–76. [Google Scholar]
- 90.Moriceau S, Lenoir G, Witko-Sarsat V. In cystic fibrosis homozygotes and heterozygotes, neutrophil apoptosis is delayed and modulated by diamide or roscovitine: evidence for an innate neutrophil disturbance. J. Innate Immun. 2010;2:260–266. doi: 10.1159/000295791. [DOI] [PubMed] [Google Scholar]
- 91.Moriceau S, et al. Coronin-1 is associated with neutrophil survival and is cleaved during apoptosis: potential implication in neutrophils from cystic fibrosis patients. J. Immunol. 2009;182:7254–7263. doi: 10.4049/jimmunol.0803312. [DOI] [PubMed] [Google Scholar]
- 92.McKeon DJ, et al. Prolonged survival of neutrophils from patients with F508 CFTR mutations. Thorax. 2008;63:660–661. doi: 10.1136/thx.2008.096834. [DOI] [PubMed] [Google Scholar]
- 93.Saba S, Soong G, Greenberg S, Prince A. Bacterial stimulation of epithelial G-CSF and GM-CSF expression promotes PMN survival in CF airways. Am. J. Respir. Cell Mol. Biol. 2002;27:561–567. doi: 10.1165/rcmb.2002-0019OC. [DOI] [PubMed] [Google Scholar]
- 94.Sedor J, et al. Cathepsin-G interferes with clearance of Pseudomonas aeruginosa from mouse lungs. Pediatr. Res. 2007;61:26–31. doi: 10.1203/01.pdr.0000250043.90468.c2. [DOI] [PubMed] [Google Scholar]
- 95.Vandivier RW, et al. Elastase-mediated phosphatidylserine receptor cleavage impairs apoptotic cell clearance in cystic fibrosis and bronchiectasis. J. Clin. Invest. 2002;109:661–670. doi: 10.1172/JCI13572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Berger M, et al. Tissue-specific Fcγ and complement receptor expression by alveolar macrophages determines relative importance of IgG and complement in promoting phagocytosis of Pseudomonas aeruginosa . Pediatr. Res. 1994;35:68–77. doi: 10.1203/00006450-199401000-00015. [DOI] [PubMed] [Google Scholar]
- 97.Kreindler JL, et al. Vitamin D3 attenuates Th2 responses to Aspergillus fumigatus mounted by CD4+ T cells from cystic fibrosis patients with allergic bronchopulmonary aspergillosis. J. Clin. Invest. 2010;120:3242–3254. doi: 10.1172/JCI42388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mueller C, et al. Lack of cystic fibrosis transmembrane conductance regulator in CD3+ lymphocytes leads to aberrant cytokine secretion and hyperinflammatory adaptive immune responses. Am. J. Respir. Cell Mol. Biol. 2011;44:922–929. doi: 10.1165/rcmb.2010-0224OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hartl D, et al. Pulmonary T(H)2 response in Pseudomonas aeruginosa -infected patients with cystic fibrosis. J. Allergy Clin. Immunol. 2006;117:204–211. doi: 10.1016/j.jaci.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 100.Xu Y, et al. Transcriptional adaptation to cystic fibrosis transmembrane conductance regulator deficiency. J. Biol. Chem. 2003;278:7674–7682. doi: 10.1074/jbc.M210277200. [DOI] [PubMed] [Google Scholar]
- 101.McAleer JP, Kolls JK. Mechanisms controlling Th17 cytokine expression and host defense. J. Leukoc. Biol. 2011;90:263–270. doi: 10.1189/jlb.0211099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Liu Y, et al. IL-17A and TNF-α exert synergistic effects on expression of CXCL5 by alveolar type II cells in vivo and in vitro . J. Immunol. 2011;186:3197–3205. doi: 10.4049/jimmunol.1002016. [DOI] [PubMed] [Google Scholar]
- 103.Laan M, et al. Neutrophil recruitment by human IL-17 via C-X-C chemokine release in the airways. J. Immunol. 1999;162:2347–2352. [PubMed] [Google Scholar]
- 104.Kolls JK, Linden A. Interleukin-17 family members and inflammation. Immunity. 2004;21:467–476. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 105.Jones CE, Chan K. Interleukin-17 stimulates the expression of interleukin-8, growth-related oncogene-α, and granulocyte-colony-stimulating factor by human airway epithelial cells. Am. J. Respir. Cell Mol. Biol. 2002;26:748–753. doi: 10.1165/ajrcmb.26.6.4757. [DOI] [PubMed] [Google Scholar]
- 106.Dubin PJ, Kolls JK. IL-23 mediates inflammatory responses to mucoid Pseudomonas aeruginosa lung infection in mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 2007;292:L519–L528. doi: 10.1152/ajplung.00312.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Liu J, et al. Early production of IL-17 protects against acute pulmonary Pseudomonas aeruginosa infection in mice. FEMS Immunol. Med. Microbiol. 2011;61:179–188. doi: 10.1111/j.1574-695X.2010.00764.x. [DOI] [PubMed] [Google Scholar]
- 108.McAllister F, et al. Role of IL-17A, IL-17F, and the IL-17 receptor in regulating growth-related oncogene-α and granulocyte colony-stimulating factor in bronchial epithelium: implications for airway inflammation in cystic fibrosis. J. Immunol. 2005;175:404–412. doi: 10.4049/jimmunol.175.1.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Decraene A, et al. Elevated expression of both mRNA and protein levels of IL-17A in sputum of stable cystic fibrosis patients. Respir. Res. 2010;11:177. doi: 10.1186/1465-9921-11-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dubin PJ, Kolls JK. IL-17 in cystic fibrosis: more than just Th17 cells. Am. J. Respir. Crit. Care Med. 2011;184:155–157. doi: 10.1164/rccm.201104-0617ED. [DOI] [PubMed] [Google Scholar]
- 111.Tan HL, et al. The th17 pathway in cystic fibrosis lung disease. Am. J. Respir. Crit. Care Med. 2011;184:252–258. doi: 10.1164/rccm.201102-0236OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bonfield TL, et al. Normal bronchial epithelial cells constitutively produce the anti-inflammatory cytokine interleukin-10, which is downregulated in cystic fibrosis. Am. J. Respir. Cell Mol. Biol. 1995;13:257–261. doi: 10.1165/ajrcmb.13.3.7544594. [DOI] [PubMed] [Google Scholar]
- 113.Starosta V, Ratjen F, Rietschel E, Paul K, Griese M. Anti-inflammatory cytokines in cystic fibrosis lung disease. Eur. Respir. J. 2006;28:581–587. doi: 10.1183/09031936.06.00071405. [DOI] [PubMed] [Google Scholar]
- 114.Karp CL, et al. Defective lipoxin-mediated anti-inflammatory activity in the cystic fibrosis airway. Nat. Immunol. 2004;5:388–392. doi: 10.1038/ni1056. [DOI] [PubMed] [Google Scholar]
- 115.Karp CL, Flick LM, Yang R, Uddin J, Petasis NA. Cystic fibrosis and lipoxins. Prostaglandins Leukot. Essent. Fatty Acids. 2005;73:263–270. doi: 10.1016/j.plefa.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 116.Liu G, et al. High mobility group protein-1 inhibits phagocytosis of apoptotic neutrophils through binding to phosphatidylserine. J. Immunol. 2008;181:4240–4246. doi: 10.4049/jimmunol.181.6.4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lipuma JJ. The changing microbial epidemiology in cystic fibrosis. Clin. Microbiol. Rev. 2010;23:299–323. doi: 10.1128/CMR.00068-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rogers GB, et al. Bacterial diversity in cases of lung infection in cystic fibrosis patients: 16S ribosomal DNA (rDNA) length heterogeneity PCR and 16S rDNA terminal restriction fragment length polymorphism profiling. J. Clin. Microbiol. 2003;41:3548–3558. doi: 10.1128/JCM.41.8.3548-3558.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mowat E, et al. Pseudomonas aeruginosa population diversity and turnover in cystic fibrosis chronic infections. Am. J. Respir. Crit. Care Med. 2011;183:1674–1679. doi: 10.1164/rccm.201009-1430OC. [DOI] [PubMed] [Google Scholar]
- 120.Jelsbak L, et al. Molecular epidemiology and dynamics of Pseudomonas aeruginosa populations in lungs of cystic fibrosis patients. Infect. Immun. 2007;75:2214–2224. doi: 10.1128/IAI.01282-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Smith EE, et al. Genetic adaptation by Pseudomonas aeruginosa to the airways of cystic fibrosis patients. Proc. Natl. Acad. Sci. USA. 2006;103:8487–8492. doi: 10.1073/pnas.0602138103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hoboth C, et al. Dynamics of adaptive microevolution of hypermutable Pseudomonas aeruginosa during chronic pulmonary infection in patients with cystic fibrosis. J. Infect. Dis. 2009;200:118–130. doi: 10.1086/599360. [DOI] [PubMed] [Google Scholar]
- 123.Lee KM, Yoon MY, Park Y, Lee JH, Yoon SS. Anaerobiosis-induced loss of cytotoxicity is due to inactivation of quorum sensing in Pseudomonas aeruginosa . Infect. Immun. 2011;79:2792–2800. doi: 10.1128/IAI.01361-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Huse HK, et al. Parallel evolution in Pseudomonas aeruginosa over 39,000 generations in vivo . MBio. 2010;1:e00199-10. doi: 10.1128/mBio.00199-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ernst RK, et al. Unique lipid a modifications in Pseudomonas aeruginosa isolated from the airways of patients with cystic fibrosis. J. Infect. Dis. 2007;196:1088–1092. doi: 10.1086/521367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ernst RK, et al. Specific lipopolysaccharide found in cystic fibrosis airway Pseudomonas aeruginosa . Science. 1999;286:1561–1565. doi: 10.1126/science.286.5444.1561. [DOI] [PubMed] [Google Scholar]
- 127.Hajjar AM, Ernst RK, Tsai JH, Wilson CB, Miller SI. Human Toll-like receptor 4 recognizes host-specific LPS modifications. Nat. Immunol. 2002;3:354–359. doi: 10.1038/ni777. [DOI] [PubMed] [Google Scholar]
- 128.Davies DG, et al. The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science. 1998;280:295–298. doi: 10.1126/science.280.5361.295. [DOI] [PubMed] [Google Scholar]
- 129.Singh PK, et al. Quorum-sensing signals indicate that cystic fibrosis lungs are infected with bacterial biofilms. Nature. 2000;407:762–764. doi: 10.1038/35037627. [DOI] [PubMed] [Google Scholar]
- 130.Wade DS, et al. Regulation of Pseudomonas quinolone signal synthesis in Pseudomonas aeruginosa . J. Bacteriol. 2005;187:4372–4380. doi: 10.1128/JB.187.13.4372-4380.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ciornei CD, et al. Biofilm-forming Pseudomonas aeruginosa bacteria undergo lipopolysaccharide structural modifications and induce enhanced inflammatory cytokine response in human monocytes. Innate Immun. 2010;16:288–301. doi: 10.1177/1753425909341807. [DOI] [PubMed] [Google Scholar]
- 132.Fuxman Bass JI, et al. Extracellular DNA: a major proinflammatory component of Pseudomonas aeruginosa biofilms. J. Immunol. 2010;184:6386–6395. doi: 10.4049/jimmunol.0901640. [DOI] [PubMed] [Google Scholar]
- 133.Bragonzi A, et al. Pseudomonas aeruginosa microevolution during cystic fibrosis lung infection establishes clones with adapted virulence. Am. J. Respir. Crit. Care Med. 2009;180:138–145. doi: 10.1164/rccm.200812-1943OC. [DOI] [PubMed] [Google Scholar]
- 134.Silo-Suh L, Suh SJ, Sokol PA, Ohman DE. A simple alfalfa seedling infection model for Pseudomonas aeruginosa strains associated with cystic fibrosis shows AlgT (σ-22) and RhlR contribute to pathogenesis. Proc. Natl. Acad. Sci. USA. 2002;99:15699–15704. doi: 10.1073/pnas.242343999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Martin DW, et al. Mechanism of conversion to mucoidy in Pseudomonas aeruginosa infecting cystic fibrosis patients. Proc. Natl. Acad. Sci. USA. 1993;90:8377–8381. doi: 10.1073/pnas.90.18.8377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bjarnsholt T, et al. Pseudomonas aeruginosa biofilms in the respiratory tract of cystic fibrosis patients. Pediatr. Pulmonol. 2009;44:547–558. doi: 10.1002/ppul.21011. [DOI] [PubMed] [Google Scholar]
- 137.Pedersen SS. Lung infection with alginate-producing, mucoid Pseudomonas aeruginosa in cystic fibrosis. APMIS Suppl. 1992;28:1–79. [PubMed] [Google Scholar]
- 138.Schiøtz PO, Nielsen H, Hoiby N, Glikmann G, Svehag SE. Immune complexes in the sputum of patients with cystic fibrosis suffering from chronic Pseudomonas aeruginosa lung infection. Acta Pathol. Microbiol. Scand. [C] 1978;86:37–40. doi: 10.1111/j.1699-0463.1978.tb02555.x. [DOI] [PubMed] [Google Scholar]
- 139.Schaber JA, et al. Analysis of quorum sensing-deficient clinical isolates of Pseudomonas aeruginosa . J. Med. Microbiol. 2004;53:841–853. doi: 10.1099/jmm.0.45617-0. [DOI] [PubMed] [Google Scholar]
- 140.Look DC, et al. Pyocyanin and its precursor phenazine-1-carboxylic acid increase IL-8 and intercellular adhesion molecule-1 expression in human airway epithelial cells by oxidant-dependent mechanisms. J. Immunol. 2005;175:4017–4023. doi: 10.4049/jimmunol.175.6.4017. [DOI] [PubMed] [Google Scholar]
- 141.Rada B, Lekstrom K, Damian S, Dupuy C, Leto TL. The Pseudomonas toxin pyocyanin inhibits the dual oxidase-based antimicrobial system as it imposes oxidative stress on airway epithelial cells. J. Immunol. 2008;181:4883–4893. doi: 10.4049/jimmunol.181.7.4883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Schwarzer C, et al. Oxidative stress caused by pyocyanin impairs CFTR Cl− transport in human bronchial epithelial cells. Free Radic. Biol. Med. 2008;45:1653–1662. doi: 10.1016/j.freeradbiomed.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Bomberger JM, et al. A Pseudomonas aeruginosa toxin that hijacks the host ubiquitin proteolytic system. PLoS Pathog. 2011;7:e1001325. doi: 10.1371/journal.ppat.1001325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Dasenbrook EC, et al. Association between respiratory tract methicillin-resistant Staphylococcus aureus and survival in cystic fibrosis. J. Am. Med. Assoc. 2010;303:2386–2392. doi: 10.1001/jama.2010.791. [DOI] [PubMed] [Google Scholar]
- 145.Secor PR, et al. Staphylococcus aureus biofilm and planktonic cultures differentially impact gene expression, MAPK phosphorylation, and cytokine production in human keratinocytes. BMC Microbiol. 2011;11:143. doi: 10.1186/1471-2180-11-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Goerke C, et al. Increased frequency of genomic alterations in Staphylococcus aureus during chronic infection is in part due to phage mobilization. J. Infect. Dis. 2004;189:724–734. doi: 10.1086/381502. [DOI] [PubMed] [Google Scholar]
- 147.Kahl BC, et al. Thymidine-dependent Staphylococcus aureus small-colony variants are associated with extensive alterations in regulator and virulence gene expression profiles. Infect. Immun. 2005;73:4119–4126. doi: 10.1128/IAI.73.7.4119-4126.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Besier S, et al. Prevalence and clinical significance of Staphylococcus aureus small-colony variants in cystic fibrosis lung disease. J. Clin. Microbiol. 2007;45:168–172. doi: 10.1128/JCM.01510-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Mitchell G, Grondin G, Bilodeau G, Cantin AM, Malouin F. Infection of polarized airway epithelial cells by normal and small-colony variant strains of Staphylococcus aureus is increased in cells with abnormal CFTR function and is influenced by NF-κB. Infect. Immun. 2011;79:3541–3551. doi: 10.1128/IAI.00078-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sibley CD, et al. The Streptococcus milleri population of a cystic fibrosis clinic reveals patient specificity and intraspecies diversity. J. Clin. Microbiol. 2010;48:2592–2594. doi: 10.1128/JCM.00414-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Saiman L, et al. Effect of azithromycin on pulmonary function in patients with cystic fibrosis uninfected with Pseudomonas aeruginosa : a randomized controlled trial. J. Am. Med. Assoc. 2010;303:1707–1715. doi: 10.1001/jama.2010.563. [DOI] [PubMed] [Google Scholar]
- 152.Pilcer G, et al. Pharmacoscintigraphic and pharmacokinetic evaluation of tobramycin DPI formulations in cystic fibrosis patients. Eur. J. Pharm. Biopharm. 2008;68:413–421. doi: 10.1016/j.ejpb.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 153.Pillarisetti N, et al. Infection, inflammation and lung function decline in infants with cystic fibrosis. Am. J. Respir. Crit. Care. Med. 2011;184:75–81. doi: 10.1164/rccm.201011-1892OC. [DOI] [PubMed] [Google Scholar]
- 154.Auerbach HS, Williams M, Kirkpatrick JA, Colten HR. Alternate-day prednisone reduces morbidity and improves pulmonary function in cystic fibrosis. Lancet. 1985;2:686–688. doi: 10.1016/s0140-6736(85)92929-0. [DOI] [PubMed] [Google Scholar]
- 155.Ren CL, et al. Relationship between inhaled corticosteroid therapy and rate of lung function decline in children with cystic fibrosis. J. Pediatr. 2008;153:746–751. doi: 10.1016/j.jpeds.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 156.Konstan MW, Byard PJ, Hoppel CL, Davis PB. Effect of high-dose ibuprofen in patients with cystic fibrosis. N. Engl. J. Med. 1995;332:848–854. doi: 10.1056/NEJM199503303321303. [DOI] [PubMed] [Google Scholar]
- 157.Saiman L, et al. Azithromycin in patients with cystic fibrosis chronically infected with Pseudomonas aeruginosa : a randomized controlled trial. J. Am. Med. Assoc. 2003;290:1749–1756. doi: 10.1001/jama.290.13.1749. [DOI] [PubMed] [Google Scholar]
- 158.Renna M, et al. Azithromycin blocks autophagy and may predispose cystic fibrosis patients to mycobacterial infection. J. Clin. Invest. 2011;121:3554–3563. doi: 10.1172/JCI46095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Deterding RR, et al. Phase 2 randomized safety and efficacy trial of nebulized denufosol tetrasodium in cystic fibrosis. Am. J. Respir. Crit. Care Med. 2007;176:362–369. doi: 10.1164/rccm.200608-1238OC. [DOI] [PubMed] [Google Scholar]
- 160.Accurso FJ, et al. Denufosol tetrasodium in patients with cystic fibrosis and normal to mildly impaired lung function. Am. J. Respir. Crit. Care Med. 2011;183:627–634. doi: 10.1164/rccm.201008-1267OC. [DOI] [PubMed] [Google Scholar]
- 161.Teper A, Jaques A, Charlton B. Inhaled mannitol in patients with cystic fibrosis: a randomised open-label dose response trial. J. Cyst. Fibros. 2011;10:1–8. doi: 10.1016/j.jcf.2010.08.020. [DOI] [PubMed] [Google Scholar]
- 162.Minasian C, Wallis C, Metcalfe C, Bush A. Bronchial provocation testing with dry powder mannitol in children with cystic fibrosis. Pediatr. Pulmonol. 2008;43:1078–1084. doi: 10.1002/ppul.20903. [DOI] [PubMed] [Google Scholar]
- 163.Laube BL, Sharpless G, Carson KA, Kelly A, Mogayzel PJ. Jr. Acute inhalation of hypertonic saline does not improve mucociliary clearance in all children with cystic fibrosis. BMC Pulm. Med. 2011;11:45. doi: 10.1186/1471-2466-11-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Reeves EP, Williamson M, O’Neill SJ, Greally P, McElvaney NG. Nebulized hypertonic saline decreases IL-8 in sputum of patients with cystic fibrosis. Am. J. Respir. Crit. Care Med. 2011;183:1517–1523. doi: 10.1164/rccm.201101-0072OC. [DOI] [PubMed] [Google Scholar]
- 165.Van Goor F, et al. Rescue of CF airway epithelial cell function in vitro by a CFTR potentiator, VX-770. Proc. Natl. Acad. Sci. USA. 2009;106:18825–18830. doi: 10.1073/pnas.0904709106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Clancy JP, et al. Results of a phase IIa study of VX-809, an investigational CFTR corrector compound, in subjects with cystic fibrosis homozygous for the F508del- CFTR mutation. Thorax. 2011;67:12–18. doi: 10.1136/thoraxjnl-2011-200393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Accurso FJ, et al. Effect of VX-770 in persons with cystic fibrosis and the G551D-CFTR mutation. N. Engl. J. Med. 2010;363:1991–2003. doi: 10.1056/NEJMoa0909825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Ramsey BW, et al. A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N. Engl. J. Med. 2011;365:1663–1672. doi: 10.1056/NEJMoa1105185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Dechecchi MC, et al. MPB-07 reduces the inflammatory response to Pseudomonas aeruginosa in cystic fibrosis bronchial cells. Am. J. Respir. Cell Mol. Biol. 2007;36:615–624. doi: 10.1165/rcmb.2006-0200OC. [DOI] [PubMed] [Google Scholar]
- 170.Dechecchi MC, et al. Anti-inflammatory effect of miglustat in bronchial epithelial cells. J. Cyst. Fibros. 2008;7:555–565. doi: 10.1016/j.jcf.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 171.Rogers CS, et al. Production of CFTR-null and CFTR-F508 heterozygous pigs by adeno-associated virus-mediated gene targeting and somatic cell nuclear transfer. J. Clin. Invest. 2008;118:1571–1577. doi: 10.1172/JCI34773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Pezzulo A, et al. Production of antimicrobial peptides is preserved in the airways of pigs with cystic fibrosis. Pediatr. Pulmonol. 2011;46(supp. 34):269. [Google Scholar]
- 173.Mall M, Grubb BR, Harkema JR, O’Neal WK, Boucher RC. Increased airway epithelial Na+ absorption produces cystic fibrosis-like lung disease in mice. Nat. Med. 2004;10:487–493. doi: 10.1038/nm1028. [DOI] [PubMed] [Google Scholar]
- 174.Clarke LL, et al. Defective epithelial chloride transport in a gene-targeted mouse model of cystic fibrosis. Science. 1992;257:1125–1128. doi: 10.1126/science.257.5073.1125. [DOI] [PubMed] [Google Scholar]
- 175.Rozmahel R, et al. Modulation of disease severity in cystic fibrosis transmembrane conductance regulator deficient mice by a secondary genetic factor. Nat. Genet. 1996;12:280–287. doi: 10.1038/ng0396-280. [DOI] [PubMed] [Google Scholar]
- 176.Song Y, et al. Airway surface liquid depth measured in ex vivo fragments of pig and human trachea: dependence on Na+ and Cl− channel function. Am. J. Physiol. Lung Cell. Mol. Physiol. 2009;297:L1131–L1140. doi: 10.1152/ajplung.00085.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Sun X, et al. Disease phenotype of a ferret CFTR-knockout model of cystic fibrosis. J. Clin. Invest. 2010;120:3149–3160. doi: 10.1172/JCI43052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Riordan JR, et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989;245:1066–1073. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- 179.Coakley RD, et al. Abnormal surface liquid pH regulation by cultured cystic fibrosis bronchial epithelium. Proc. Natl. Acad. Sci. USA. 2003;100:16083–16088. doi: 10.1073/pnas.2634339100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Stutts MJ, Rossier BC, Boucher RC. Cystic fibrosis transmembrane conductance regulator inverts protein kinase A-mediated regulation of epithelial sodium channel single channel kinetics. J. Biol. Chem. 1997;272:14037–14040. doi: 10.1074/jbc.272.22.14037. [DOI] [PubMed] [Google Scholar]
- 181.Mall MA, et al. Airway surface liquid volume regulation determines different airway phenotypes in liddle compared with βENaC-overexpressing mice. J. Biol. Chem. 2010;285:26945–26955. doi: 10.1074/jbc.M110.151803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.McKone EF, Emerson SS, Edwards KL, Aitken ML. Effect of genotype on phenotype and mortality in cystic fibrosis: a retrospective cohort study. Lancet. 2003;361:1671–1676. doi: 10.1016/S0140-6736(03)13368-5. [DOI] [PubMed] [Google Scholar]
- 183.de Gracia J, et al. Genotype-phenotype correlation for pulmonary function in cystic fibrosis. Thorax. 2005;60:558–563. doi: 10.1136/thx.2004.031153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Drumm ML, et al. Genetic modifiers of lung disease in cystic fibrosis. N. Engl. J. Med. 2005;353:1443–1453. doi: 10.1056/NEJMoa051469. [DOI] [PubMed] [Google Scholar]
- 185.Dorfman R, et al. Complex two-gene modulation of lung disease severity in children with cystic fibrosis. J. Clin. Invest. 2008;118:1040–1049. doi: 10.1172/JCI33754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Rich DP, et al. Effect of deleting the R domain on CFTR-generated chloride channels. Science. 1991;253:205–207. doi: 10.1126/science.1712985. [DOI] [PubMed] [Google Scholar]
- 187.Snouwaert JN, et al. An animal model for cystic fibrosis made by gene targeting. Science. 1992;257:1083–1088. doi: 10.1126/science.257.5073.1083. [DOI] [PubMed] [Google Scholar]
- 188.Ostedgaard LS, et al. The F508 mutation causes CFTR misprocessing and cystic fibrosis-like disease in pigs. Sci. Transl. Med. 2011;3:74ra24. doi: 10.1126/scitranslmed.3001868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Stoltz DA, et al. Cystic fibrosis pigs develop lung disease and exhibit defective bacterial eradication at birth. Sci. Transl. Med. 2010;2:29ra31. doi: 10.1126/scitranslmed.3000928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Chen JH, et al. Loss of anion transport without increased sodium absorption characterizes newborn porcine cystic fibrosis airway epithelia. Cell. 2010;143:911–923. doi: 10.1016/j.cell.2010.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Keiser NW, Engelhardt JF. New animal models of cystic fibrosis: what are they teaching us? Curr. Opin. Pulm. Med. 2011;17:478–483. doi: 10.1097/MCP.0b013e32834b14c9. [DOI] [PMC free article] [PubMed] [Google Scholar]