Interneuron Cell Types: Fit to form and formed to fit (original) (raw)
. Author manuscript; available in PMC: 2015 Mar 4.
Published in final edited form as: Nature. 2014 Jan 16;505(7483):318–326. doi: 10.1038/nature12983
Abstract
Understanding brain neural circuits begins with understanding their component parts, the cells that form them. GABAergic interneurons, although a minority of cells within the brain, are critical in the control of inhibition. While understanding their diversity has been a central goal of neurobiologists, this amazing cell type has to date defied a generalized classification system. Here we review data that supports that interneuron complexity within the telencephalon can simplified by viewing them as elaborations of a much more finite group of developmentally specified cardinal classes.
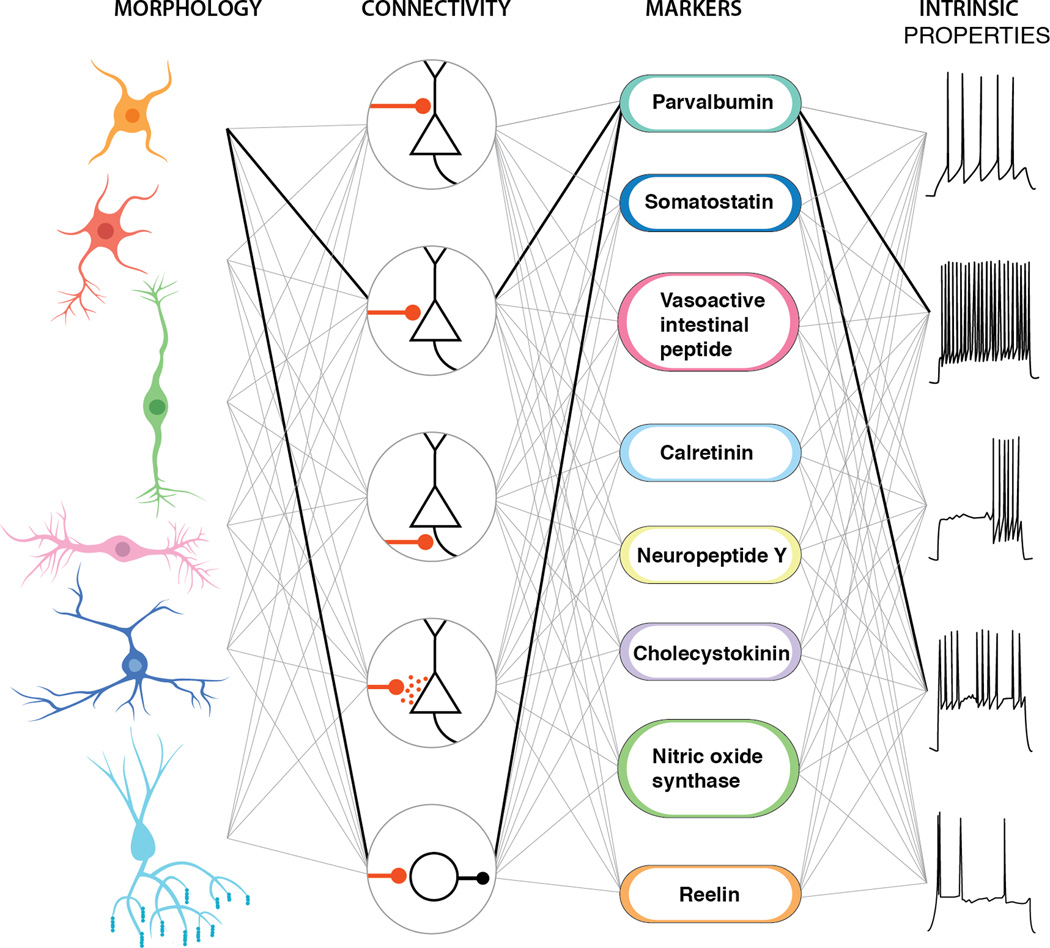
Within the forebrain, interneurons possess the largest diversity in morphology, connectivity, and physiological properties1. Up until ten years ago their classification, with a few notable exceptions2, has remained descriptive. Moreover interneuron diversity was often treated either as a quasi continuum or a diversity space with cell types numbering potentially in the hundreds3,4. The last few years of studies have coalesced into the surprising view that interneuron diversity may be fundamentally far more limited. When one considers their commonalities: at a genetic, circuit or functional level, an argument can be made for condensing large subclasses of interneurons into more finite groups. Here we suggest that based on both developmental and functional criteria interneuron diversity can be simplified and addressed experimentally. The ultimate connectivity, gene expression and physiological properties of interneurons found across the range of brain structures appear enormous (Figure 1). Nevertheless we argue this complexity arises from a small number of non-overlapping cardinal classes, which represent developmental genetic ground states that possess the ability to further specialize through their later interactions with other neurons. The ultimate goal of defining their identity through a set of computational principles remains daunting. However, with the advent of new tools that provide unprecedented specificity, coupled with the means to modulate in vivo the activity of specific targeted populations this goal is becoming attainable.
Figure 1. Schematic of interneuron diversity across the brain.
Within each of the distinct anatomical regions of the brain exist discrete interneuron populations. In some of these cases the paralog relationships between subtypes within specific regions are obvious (such as the fast-spiking basket cells), while in others such as the hippocampal CCK basket populations they appear to uniquely population specific structures.
1. Birth and specification of interneurons
How is neuronal diversity created? Developmental studies across various species14,5 and systems15,6 have suggested that cell diversity arises from specification programs established in progenitors modified to varying extents by their subsequent postmitotic interactions. The balance between these two appears to represent a compromise dictated by the organizational constraints of the particular system, Within the cortex, the pyramidal cells undergo a relatively orderly migration from the proliferative zone to the overlying cortical plate. As such cell identities are largely controlled by programs established within progenitors7. By contrast, interneuron progenitors of the telencephalon undergo incredibly complex patterns of dispersion. At the extremes, this could either be due to exquisitely precise preprograms for migration to particular structures or plasticity mechanisms that allow them to adapt to local environments.
Until the late nineties it was widely assumed that the excitatory and inhibitory neurons within the cortex shared a common lineage. The seminal breakthrough came from the realization that interneurons originated within focal subcortical proliferative zones8. This first came to light with landmark papers showing that the GABAergic populations from the ganglionic eminences migrated dorsally to populate the cortex8, as well as to all other structures within the telencephalon9,10. Following work in the spinal cord, it was conjectured that an understanding of how specific subtypes are generated would fall out of a detailed analysis of gene expression within progenitors. It was assumed that combinatorial transcriptional codes in subpallial progentiors functioned to establish distinct cortical interneuron subtypes.
The connection between developmental origins and interneuron diversity has steadily expanded over the past twenty years. Virtually all GABAergic interneurons within the telencephalon arise from one of two embryonic subcortical progenitor zones, the medial and caudal ganglionic eminences (MGE and CGE, Figure 2). Moreover, those arising from each structure represent complementary interneuron subtypes11–14. These major areas are augmented by specialized subpopulations from the lateral ganglionic eminence9 and the preoptic region15. It also became clear that there is a strong correspondence between interneuron class and the specific progenitor zones that gives rise to them. Within the cortex, the MGE gives rise to the parvalbumin (PV)-expressing fast spiking interneurons (including both basket and chandelier cells) and the somatostatin (SST)-expressing populations, of which the Martinotti cells form the largest subset11,16,17. The CGE produces the relatively rarer subtypes including the neurogliaform, bipolar and VIP-expressing multipolar interneurons12.
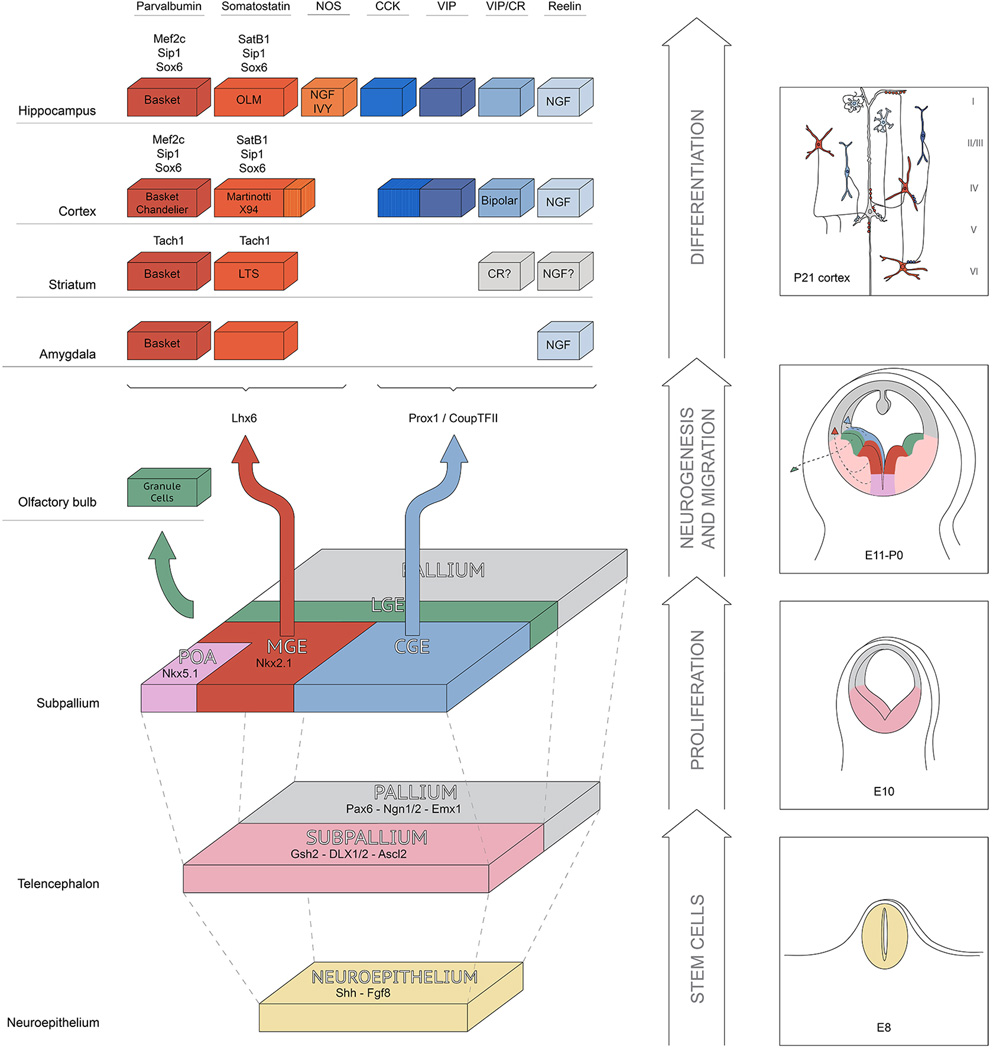
Figure 2. Interneurons subtypes are generated from discrete proliferative regions within the subpallium.
On the Left side of this figure we show the progressive development of the telencephalon from being an undifferentiated epithelium to being divided up into discrete proliferative zones that produce particular interneuron populations. On the Right side we show a more anatomically accurate cross-section of the progenitor zones and then for illustration show a schematic of interneuron diversity in the cortex in the top panel. Interestingly, while common proliferative zone produce the entire diversity of interneurons across all telencephalic structures, unique cell types and gene expression are seen in interneuron populations that reside in particular telencephalic structures.
Genetic lineage analysis within the hippocampus reinforces the idea that specific interneurons arise from specific structures but demonstrates that a simple correspondence across forebrain regions is untenable. For instance, while in the cortex neurogliaform neurons are CGE-derived, a large proportion of the corresponding population in the hippocampus arises from the MGE19. Furthermore, while some classes such as fast-spiking basket cells show marked similarities across structures, others subclasses do not yet appear to have obvious paralogs. For instance, the CCK basket cells, while a large population within the cortex and hippocampus, do not appear to be present within other brain areas18. Similarly, there appears to be at least two populations of the so called OLM cells (named in accordance with the position of their cell body and dendrites20,21) that derive from distinct sources, one which expresses the ionotropic serotonin receptor 5HT3aR and one that does not. Adding further complexity, analysis of the basal ganglia suggests that only the MGE is a major source of interneuron populations within these structures22.
These differences across areas raise two possibilities. First, there might be dedicated populations of interneuron progenitors that are committed to populating specific brain structures. Alternatively the notion of referring to an interneurons origin as deriving form a specific embryonic structure may be an imprecise proxy for gene expression. For instance, even though hippocampal neurogliaform cells arise from both the MGE and CGE, a common constellation of specification genes may be acting within both embryonic regions. Similarly, the differential expression of functional determinants such as serotonin receptors, in otherwise similar interneuron subtypes are unlikely to represent distinct cardinal classes. Rather they likely represent iterations produced by cardinal cousins or differential postmitotic interactions by members of a single cardinal class.
These details emphasize the importance of mapping interneuron diversity onto molecular mechanism. GABAergic lineages can be divided into those with long-range projections such as those in the striatum or globus pallidus and the breadth of interneuron populations that are largely locally projecting. A number of factors appear to be utilized within all GABAergic neurons (Figure 2 bottom), most notably the Dlx1/2, Ascl2 and Gsh1/2 genes that encode transcription factors that themselves form a regulatory network23–25. In the pallium (the region of the forebrain that will give rise to cortical structures), a similar cohort of transcription factors, including Emx1, Ngn1/2 and Pax6, function analogously in the specification of the excitatory populations26.
The Dlx1/2 genes in particular function at multiple stages of GABAergic maturation: in the acquisition of GABAergic identity27, the initiation and cessation of tangential migration4,28,29 and in the morphological and physiological maturation of specific subclasses29. The specific role of Dlx1/2 in these disparate developmental activities has become clearer as their transcription targets have been identified. These include Elmo1, Dlx5/6, Arx and Sip1 (Zfhx1b), each of which have been shown to be required in the control of migration and regional identity30,43,44,45 Moreover, their mutations, presumably via interneuron dysfunction, contribute to a variety of affective psychiatric disorders30.
In addition, a number of factors appear more restricted to specific subtypes. Although far from complete, a genetic hierarchy for the MGE-derived PV and SST lineages has begun to emerge. Within the MGE, the cascade begins with Nkx2.1, which acts as master regulator in promoting MGE-derived interneuron fates over CGE-derived cell types31,32. Moreover, in the cleanest example of a single gene contributing to the generation of a specific interneuron subtype, Chandelier or axo-axonic interneurons have been shown to arise relatively late in embryonic development (E15-E18 in mice) from a population of Nkx2.1 progenitors33,34. In addition, Nkx2.1 is a gene with both activator and repressor function. Its repressor function attenuates the expression of CGE-specific genes, while its activator function induces the expression of Lhx617, which is needed to promote the differentiation of both PV and SST-expressing interneurons. Lhx6 in turn drives the expression of a series of factors including Sox6 and Satb135,36 whose actions selectively affect the development of both PV- and SST-expressing interneurons. By contrast, only a few genes as yet have proven to be specific to the CGE-derived lineages. While, collectively the specific functions of these transcription factors and their targets are still a work in progress, the tools to crack this problem are at hand.
Although the emerging picture is exhilarating, rather than coalescing into an explanation for the myriad of distinct subtypes that populate all areas of the forebrain, it appears to only reveal a handful of genetic cascades. If the goal were to simply account for the neuronal markers that have been classically used to categorize interneurons then a mere six (PV, SST, VIP, NOS, REELIN, Calretinin) could collectively divide most of the range of interneurons within the forebrain. But clearly such a classification would belie the regional complexity of interneurons. Within the hippocampus alone there are easily four or five SST-expressing cell types and at least three PV-expressing populations. Similar distinct subpopulations of SST- and PV-expressing populations are being discovered in the cortex and more will likely be found. Although we believe the cardinal specification of interneurons is only the first, -- albeit critical--, step in the progressive specification of subpallial progenitors, can our cardinal identity hypothesis account for this increasing wealth of interneuron subtypes? It is certainly possible that we have grossly underestimated the cardinal subtypes. Indeed, complex maps showing intricate embryonic patterns of gene expression within the subpallium have been posited to combinatorially specify different cell types37. We however think that the cell types generated by developmental programs are unlikely to explain all the regional diversity observed. First, the loss of specific genes results in phenotypes that are invariably not restricted to specific interneuron subtypes. Second, the loss of specific genes affects the generation of interneurons across a variety of structures arguing against the existence of progenitors populations dedicated to the generation specific interneuron classes. That said, one could imagine a combinatorial gene regulation strategy in which an individual gene could be necessary for a variety of disparate differentiation programs. Hence another way to explore the question of whether regional diversity is established in progenitors is through lineal analysis.
2. Lineages: families of interneurons
Are the interneuron populations that populate particular structures, such as the hippocampus or cortex, derived from dedicated progenitor pools? Two recent studies have directly explored the role of lineage in the development of cortical interneurons38,39. Both have shown that clonally related progenitors appear to be preferentially relegated to specific cortical columns or layers, supporting the idea that progenitor lineages are dedicated to producing lineages destined for particular brain structures. Given the long and convoluted paths taken by these progenitors as they transit to their mature position40,41 that clones could collectively target particular parts of cortex was stunning. Interestingly, such clones were equally likely to be comprised of mixed SST- and PV-expressing interneurons rather than one subtype. Perhaps this lack of tendency for clones to “breed true” should not come as a surprise. A wealth of lineage analysis in invertebrates42, as well as the vertebrate retina43 and spinal cord44 indicates that neuronal lineages while stereotyped do not in general produce cells of a single subtype. Moreover, it will be interesting to explore whether in addition to being clustered, lineally-related cells are also dispersed and if so to what extent45,46. If they do, it will be intriguing to assess the afferent and efferent connectivity of such clones, as this would speak to the question as to how intrinsic versus local cues impact the connectivity of interneurons.
3. How circuits nurture interneuron subtypes
The accumulated evidence supports a strong role for developmentally regulated genetic programs in the allocation of interneurons to broad “cardinal” classes. What it does not appear to explain is how interneurons from the same cardinal class are able to form connections with such a wide variety of synaptic partners. In favor of a role for local cues contributing to this process, a variety of studies have suggested that both excitatory and inhibitory signals may influence the migration and positioning of developing interneurons47,48. Recent data has shown that attenuating the activity of specific interneuron populations affects both their migration and morphological development49. Although acting in a class-specific manner, it has yet to be demonstrated that this activity is instructive rather than simply permissive. That said there appears to be growing support for the notion that local signals may direct the region specific differentiation of interneurons. In support of this, a number of genes are specific to interneurons within the cortex but not the striatum, including Zfhx1b, Dlx1, Elmo1 and Mef2c, the later three of which are activity-regulated30,49–52. While it may be that activity simply promotes the maturation of interneuron populations that are already pre-specified, it is also possible that activity directs region-specific differentiation. Extensive work has indicated that voltage-gated calcium influx may result in de novo gene expression (reviewed in53). Indeed, it is the present failure to identify genes within proliferative populations that are indicative of region specific differentiation that has led us to propose a two-phase model of interneuron specification. We envision that activity-regulated gene expression during “critical periods” may be responsible during the second phase for the allocation into cardinal classes into specific subclasses. Recent work has shown that MGE-derived cells can productively integrate into both normal and abnormal neuronal circuits54–56, This supports the idea that local cues can direct nascent interneurons to form appropriate connectivity with a variety of synaptic partners. Understanding how interneurons can form functional circuits in a variety of structures is a critical question that remains to be answered.
Until now we have taken a bottom-up developmental view, aimed at examining events by which interneuron subtypes are integrated into functional circuits. In the next section, we will take a top-down view and examine circuit specific functions of interneuron types. A prediction of our model is that the developmental genetic programs functioning in interneuron progenitors lead to the production of a relatively small number of cardinal subclasses. We believe that the much larger diversity of interneurons observed in mature brain circuits reflects later refinements imposed locally on specific subclasses. If this were true, it would predict that interneurons from the same cardinal class would within the same circuit be exposed to similar cues and hence develop similar functional properties. While the data to date is in it nascent stages, the availability of genetic driver lines57,58 to reliably target particular interneuron cohorts, has provided the means to test this hypothesis.
4. The function of cortical inhibitory interneurons
What is a meaningful measure of the function of an interneuron subtype? Because interneurons generally project locally, their firing needs to be understood in the context of the circuits they contribute to. There are two complementary ways of approaching the question of what an interneuron does (Figure 3). First, we can ask about recruitment: what drives an interneuron to fire? Under what circuit and brain state configurations or behavioral contingencies is a given neuron active? This is strongly constrained by the neuron’s afferent connectivity, which in turn is likely dictated by their developmental genetic program. However, interneurons are not hardwired. A set of afferents that drive interneurons to fire in one context may fail to do so in another. Clearly understanding their recruitment must take into account a large number of factors. We will consider two large classes of recruitment hypotheses (Figure 4). The classic idea is that interneurons serve to ‘Coordinate’ networks, such that their recruitment is best understood in reference to the local population activity. Alternatively we consider an alternative view we term the “Flow control” hypothesis, where interneurons serve to gate information flow within a given circuit and are excited at precise moments in reference to specific behavioral events. These two ideas are by no means mutually exclusive, as successful flow control must depend on the signals being suitably coordinated. Second, we can ask about circuit impact: how does the firing of an interneuron influence the activity of neurons in its local circuit? This aspect of function is strongly constrained by the efferent connectivity of a neuron, which is thought to be dependent on their developmental genetic program. In addition, the impact of an interneuron type will also greatly depend on whether their recruitment is coordinated with other neurons of the same cohort.
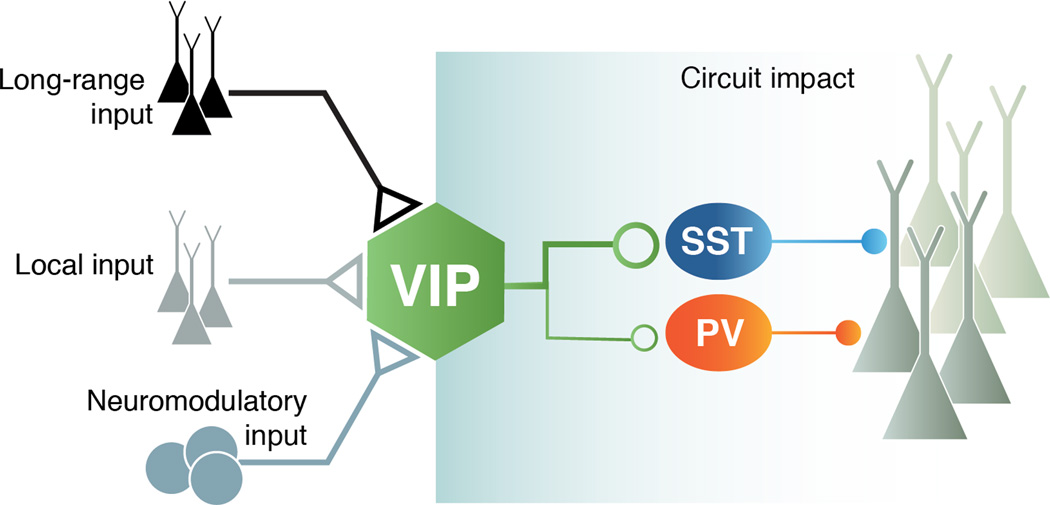
Figure 3. Two faces of interneuron function.
A cortical circuit from the perspective of a VIP interneuron. The recruitment of VIP interneurons is constrained by the inputs it receives. The afferents can be long-range from other cortical areas and also neuromodulatory from the dorsal raphe (DR) and nucleus basalis (NB) via ionotropic receptors. The circuit impact of VIP interneurons is constrained by its outputs. The efferents are mostly to SST interneurons and to a smaller degree to PV interneurons, which lead to the disinhibition of a functional subset of principal cells (black).
Figure 4. Coordination and flow control hypotheses of recruitment.
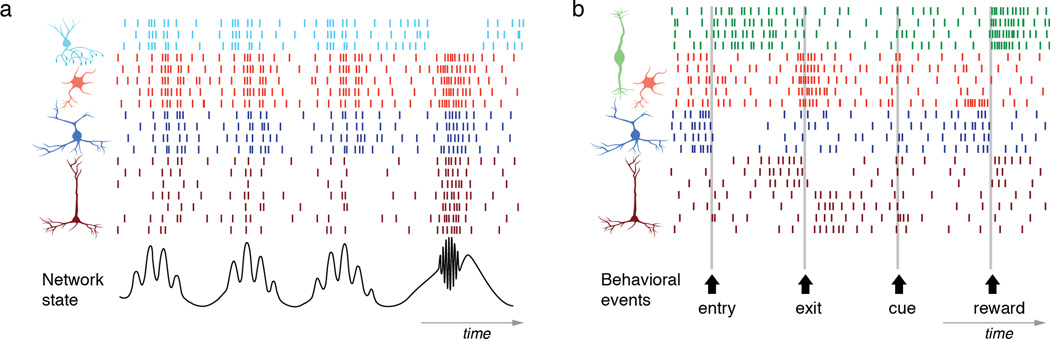
Left, Coordination hypothesis. The bottom trace shows a local field potential representing the network state in the hippocampus. The firing of different neuron types can be described in reference the LFP, both in terms of overall activity level and phase-relationship. Right, Flow control hypothesis. The bottom arrows mark the timing of three behavioral events, exit, entry and reward. The firing of different neurons can be described in reference to these events.
Historically, models of cortical function have focused on “circuit motifs”, repeated patterns of connectivity, to infer computational function for specific cell types59. Perhaps because inhibitory interneurons are largely local they have been generally considered to simply guard excitatory networks against runaway excitation60. In recent years our understanding of their function has become significantly more sophisticated. Among the lessons learned were that interneurons could normalize the activity of local excitatory networks as well as provide feed-forward inhibition. The later strongly influences the timing of signals and enables excitatory signals to remain subthreshold while carrying information. Of course, these are just two motifs out of a vast range of possibilities, including cross-coupling that can lead to synchronization, lateral inhibition that can segregate principal neuron populations and disinhibition generating elevated activity. Indeed, it has become clear that there are at least as many inhibitory circuit motifs as there are cell types.
Interneurons in cortical computations
How then does the diversity of interneurons contribute to neural computations? First, it’s worth noting that it would be difficult to imagine networks with only excitation. In fact from an engineering standpoint, such networks would either have to have extremely time-limited dynamics or they would become intrinsically unstable. Moreover, inhibition not only provides balance. It ensures richness in the possible dynamics within networks of principal neurons. These considerations lead to the idea that interneuron diversity allows for a vast increase in the computational power of cortical circuits61. What then are these computations? Broadly speaking, the computational functions of interneurons can be grouped into either arithmetic or timing.
A long-held idea is that different interneurons perform essentially arithmetic operations, such as subtraction or division62,63. Inhibition can provide gain control by changing the input-output relationship between the excitatory drive and the resulting firing rate in principal cells: either by decreasing the slope divisively or by a subtractive shift. In turn these elementary operations are the building blocks for cortical computations such as normalization, an operation that provides divisive gain in proportion to the summed activity in a circuit64,65. Such gain modulation might result from shunting66, synchronous67 or balanced68 inhibition. Originally proposed to explain early visual cortical responses64, it has become one of the most studied cortical computations73,74.
The advent of tools to probe interneuron function in vivo is presently allowing the contributions of different interneuron types to be explored. Several recent studies have focused on the computational functions of PV- and SST-expressing interneurons in the visual cortex. Optogenetic control of PVs can bidirectionally modulate the gain of visual responses69,70. Under some conditions optogenetic activation of PV neurons can even sharpen the tuning of cortical responses71. In contrast, SST interneurons have broader spatial tuning in visual cortex and they mediate surround suppression of visual responses72,73.
Another line of theoretical investigation has focused on the role of interneurons in controlling the timing of neural activity. More complex network functions require that neurons do not fire together. This can be achieved by dynamically balancing excitation with inhibition so that the resulting network activity becomes temporally irregular and asynchronous. These balanced networks thus provide rich dynamics and rapid responses74. Indeed, recordings from cortex often reveal finely balanced excitation and inhibition75–78, consistent with these models. When inhibition precisely tracks excitation, it can alternatively serve to increase temporal precision75,79 or decorrelate networks80.
5. Circuit impact of identified interneuron types
Understanding how computations are implemented in neural networks requires understanding how interneuron subtypes impact local networks. While traditionally studied in vitro, we will focus mostly on recent in vivo work using transgenic mice for targeting interneurons based on markers, such as parvalbumin (PV), somatostatin (SST) and vasointestinal peptide (VIP)57,58. However, these cre-driver mouse lines neither demarcate entirely homogeneous interneuron populations nor map precisely onto cardinal interneuron types. Nevertheless they provide a convenient and powerful tool for parsing interneuron heterogeneity because these three major markers target distinct non-overlapping populations and in aggregate can label ~85% of all cortical interneurons13,81,82. When combined with optogenetic modulators83,84 these have enabled researchers for the first time to test many long held theories about the roles of inhibition.
PV-expressing interneurons (either soma-targeting basket cells or chandelier cells targeting the axon initial segment) are strategically positioned to control spiking and are also strongly interconnected, which promotes their synchronous activity85–89. Recent studies showed that PVs control the timing of spikes with respect to theta oscillations in hippocampus90–92.
SST-expressing cortical Martinotti neurons are dendritic-targeting interneurons that project to layer 1 and provide inhibition to the tufts of deep layer pyramidal cells. Inhibition by Martinotti cells strongly suppresses dendritic calcium spikes and bursting93 and can mediate di-synaptic inhibition between neighboring pyramidal cells94,95. Similarly, in the hippocampus, dendritic inhibition by SST neurons controls burst firing90,91,96. However, SST-expressing neurons are anatomically diverse, with some subtypes specializing in disinhibition of local principal cells97.
VIP demarcates the third major class of interneurons, comprising ~15% of all interneurons81,82 and are mostly located in the superficial layers of cortex. VIP-expressing interneurons have been long proposed to mediate disinhibition98,99. Recent studies have shown that in four different cortical regions VIP interneurons tend to inhibit most SST and a sub-fraction of PV interneurons88,100,101 and thus disinhibits principal cells, providing a form of gain control101. These results demonstrate that VIP-expressing neurons form a disinhibitory microcircuit that is conserved across cortical regions with shared computational functions.
A final example is provided by neurons within layer 1, which are all almost all inhibitory interneurons102. Recent results found that two major interneuron types have opposing functions: neurogliaform cells inhibited layer 2/3 pyramids, while single bouquet cells inhibited other interneurons within layer 2/3, and may provide disinhibition in vivo103,104.
These studies have begun to allow for causal testing of hypotheses using optogenetics. In addition, these provide support for the hypothesis that cardinal classes of interneurons have defined circuit functions. However, these carry the caveat that optogenetically targeted neural population correspond to functional classes that “act together” that needs to be tested on a case-by-case basis by recording them during behavior.
6. Recruitment: coordination and flow control
What are the brain-state and behavior-dependent contingencies that determine when a specific interneuron type is activated? At the broadest scale, different behavioral modes are associated with large changes in global brain activity, therefore it is not surprising that different classes of inhibitory interneurons are activated in a highly state-dependent manner105–108. At a more refined scale, what is the simplest description of the conditions under which a given interneuron is activated? Is recruitment of an interneuron subtype best understood with reference to a network state or a behavioral contingency?
Network recruitment of interneurons: the coordination hypothesis
One idea is that interneurons coordinate the precise timing of principal cell activation such as the network oscillations. There is a long history of experimental and theoretical investigations proposing that the diversity of interneuronal subtypes underlies a division of labor for organizing cortical population activity at different time scales61,109–111.
The best-studied examples of the contribution of different interneurons come from the hippocampus where recordings from targeted cells have been able to correlate their firing to network oscillations106,112–115. These studies, mostly from anaesthetized animals, revealed that distinct interneuron subtypes fire during different rhythms (e.g. theta, gamma, ripple) and with distinct phase-relationships, suggesting they differentially contribute to network dynamics. These results suggest that the spike timing of different interneuron types can be referenced to specific network events61,106,116 (Figure 4, left). More generally, this work supports the “coordination hypothesis” where each interneuron subtype performs as a temporal specialist within a ‘distributed clock system’ that coordinates pyramidal cells ensembles111 (Figure 4, left).
These observations set the stage for testing the causal role of different interneuron subtypes in generating oscillations105,106. Recently two important studies probed the role of PV interneurons using either optogenetic activation117 or suppression118 and found increased and decreased gamma oscillations, respectively, suggesting that interneurons are indeed actively involved in their generation.
Behavioral correlates of interneurons: the flow control hypothesis
Alternatively, it is possible that the function of some interneuron types is better described with reference to behavioral events. Although, interneuronal identity has long been inferred in behaving animals on the basis of spike waveform and firing pattern109,119,120, it is only recently that this could be directly ascertained for a handful of genetically defined interneurons during well-controlled behaviors. Using the optogenetics-assisted identification technique in the anterior cingulate cortex (ACC) of mice performing a simple foraging task, the surprising observation was made that deep layer PV and narrow-spiking SST interneurons responded in a functionally homogeneous manner at specific-behavioral epochs87 (Figure 4, Right). Similar observations were made in rat motor cortex using juxtacellular labeling in head-fixed rats121. While pyramidal cells responded in heterogeneous ways, all PVs responded similarly at the moment of movement initiation. This shows that PV interneurons, at least in mouse frontal regions, can be thought as a functional unit. How could such functional homogeneity be achieved? One possibility is that inhibitory interneurons strongly sample local principal cell activity and their activation reflects a “summary” of local activity85. Therefore PVs might fire in a behavior and region-dependent manner, which may be the ‘leaving decision’ in ACC owing to its role in foraging122; but movement initiation in motor cortex.
Other examples of behaviorally-activated responses come from the auditory cortex, where a large fraction of interneurons in layer 1 are activated by negative reinforcers during auditory fear-conditioning104. Similarly, VIP interneurons are strongly and uniformly recruited by negative- (air puff or mild shock) or positive (water reward) reinforcement during an auditory discrimination task101 (Figure 4, Right). Although such reinforcement feedback-related signals may at first pass seem surprising in a primary sensory area, as VIP interneurons mediate disinhibition (see above) they are ideally fit to serve to gate information98 (Figure 3).
As we stated above, because interneurons are embedded in a highly interconnected network, their functions need to be understood in the context of local networks123. In this light, the observations that some interneuron types are recruited at specific behavioral events may seem puzzling. Indeed, consistent with the “coordination” hypothesis111,116, one would expect that the majority of the responses would be constrained by the state of the local network, on a time scale of milliseconds, and not by behavioral contingencies. Indeed mechanistically, the observed behaviorally specific activation may reflect local network activity, which itself is tied to specific behavioral contingencies. We suspect that this explains the homogeneous activation of deep layer PV neurons. Nevertheless their function is most parsimoniously described by temporal reference to specific behavioral events. Alternatively, specific classes of interneurons may be activated by strong long-range inputs. For instance, neuromodulatory systems can provide behavior-dependent inputs to specific interneuron classes. Interestingly, VIP neurons have ionotropic receptors for the neuromodulators acetylcholine and serotonin, which likely drive reinforcement signals in these neurons98,124.
At present, the behavioral recruitment of many interneuron types remains unexplored and different mechanisms may apply to each type. In contrast to the coordination hypothesis, these early results support the “flow control” hypothesis (Figure 4) proposing that distinct interneuron types specialize in controlling information flow in and out of a local circuits by suppressing or boosting principal cell pathways and modulating response gain during specific behavioral contingencies and thus acting much like controllers to a state-machine. This suggests that the recruitment of an interneuron type is linked with behavioral scale requirements of local circuits. Moreover, the observations that genetically-targeted interneuron classes show similar recruitment suggests they do indeed act as functional units, supporting the existence of a small number of cardinal interneuron types.
7. Conclusions and Outlook
We are in the midst of an exciting era where new data on the development and function of interneurons are being weekly brought to light. In our perspective we suggest that the large diversity in interneuron classes may originate from a handful of cardinal cell types. Such an assertion could be misinterpreted as a statement claiming that interneuron classes are in fact not diverse or that divisions into further subtypes is not warranted. The incredible work in areas such as the hippocampus show us that this is patently incorrect. What we are trying to provide is a framework that will help direct future studies by consolidating interneuron diversity into cardinal classes with specific ground states. Therefore if one wishes to explore questions such as the intrinsic physiological properties, axonal targeting or general target selection then understanding the ground state established in progenitors is a good place to start. However, if one wishes to explore the circuit properties, connectivity or computational contributions of a subclass of interneurons, one needs to consider the interplay between cardinal cells and the local cues received from the circuits they contribute to. From a functional point of view, if we confine ourselves to specific circuits, such as VIP interneurons, a cardinal class will share important aspects of their function. Hence the tools to genetically target cardinal classes will prove invaluable for parsing the function of the different and quite exotic interneuron subtypes they ultimately give rise to.
It is intriguing to contemplate why such a mechanism is used to create cellular diversity. It may be that the strategy used by natural selection favors simple programs to provide stability, and combinatorial assembly to provide complexity. Although they are ultimately incorporated in a wide breadth of circuits, cardinal interneuron classes share critical combinations of features that enable their function. Genetic program direct the receptors they express, the cell types and subcellular compartment they innervate, as well as their firing properties. These features in turn strongly constrain their recruitment and circuit impact. In short, we may ultimately find interneurons exist as cardinal classes because nature has conspired to bestow on them generalized computational function that necessitated the presence of common biophysical and hodological properties. Despite these commonalities, it is self-evident that neural circuits allow for a remarkable array of behavioral outcomes. Harnessing biology’s ability to use limited set of building blocks, to create enormous diversity circuits holds the real promise that we may soon begin understand the means by which brain circuits self-assemble and initiate function.
Acknowledgements
Work in the authors’ laboratories is supported by grants from the National Institute of Health (R01NS075531 to A.K. and MH071679, MH095147, NS074972, NS081297 to G.F.) and generous support from the Simons Foundation (G.F.). We are grateful to Gyorgy Buzsaki, Chris McBain, Bernardo Rudy, Michael Long, Richard Tsien and members of our labs for discussions and comments. We thank Jordane Demidschstein for creating figure 2.
References
- 1.Group PIN, et al. Petilla terminology: nomenclature of features of GABAergic interneurons of the cerebral cortex. Nat. Rev. Neurosci. 2008;9:557–68. doi: 10.1038/nrn2402. (Comment: the best effort to date by physiologists, anatomists and developmental neurobiologists to come to a common nomenclature for GABAergic interneurons).
- 2.Freund TF, Buzsáki G. Interneurons of the hippocampus. Hippocampus. 1996;6:347–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 3.Markram H, et al. Interneurons of the neocortical inhibitory system. Nat. Rev. Neurosci. 2004;5:793–807. doi: 10.1038/nrn1519. [DOI] [PubMed] [Google Scholar]
- 4.Parra P, Gulyás AI, Miles R. How Many Subtypes of Inhibitory Cells in the Hippocampus? Neuron. 1998;20:983–993. doi: 10.1016/s0896-6273(00)80479-1. [DOI] [PubMed] [Google Scholar]
- 5.Brody T, Odenwald WF. Regulation of temporal identities during Drosophila neuroblast lineage development. Current Opinion in Cell Biology. 2005;17:672–675. doi: 10.1016/j.ceb.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 6.Leone DP, Srinivasan K, Chen B, Alcamo E, McConnell SK. The determination of projection neuron identity in the developing cerebral cortex. Current Opinion in Neurobiology. 2008;18:28–35. doi: 10.1016/j.conb.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O'Leary DDM, Borngasser D. Cortical Ventricular Zone Progenitors and Their Progeny Maintain Spatial Relationships and Radial Patterning during Preplate Development Indicating an Early Protomap. Cereb. Cortex. 2006;16:i46–i56. doi: 10.1093/cercor/bhk019. [DOI] [PubMed] [Google Scholar]
- 8.Anderson SA, Eisenstat DD, Shi L, Rubenstein JL. Interneuron migration from basal forebrain to neocortex: dependence on Dlx genes. Science. 1997;278:474–6. doi: 10.1126/science.278.5337.474. (Comment: the paper to demonstrate that cortical interneurons are derived subpallially)
- 9.Wichterle H, Turnbull DH, Nery S, Fishell G, Alvarez-Buylla A. In utero fate mapping reveals distinct migratory pathways and fates of neurons born in the mammalian basal forebrain. Development. 2001;128:3759–3771. doi: 10.1242/dev.128.19.3759. (Comment: this paper provided in vivo evidence that specific interneurons are derived from specific embryonic progenitor zones).
- 10.Nery S, Fishell G, Corbin JG. The caudal ganglionic eminence is a source of distinct cortical and subcortical cell populations. Nat. Neurosci. 2002;5:1279–1287. doi: 10.1038/nn971. [DOI] [PubMed] [Google Scholar]
- 11.Butt SJ, et al. The temporal and spatial origins of cortical interneurons predict their physiological subtype. Neuron. 2005;48:591–604. doi: 10.1016/j.neuron.2005.09.034. [DOI] [PubMed] [Google Scholar]
- 12.Lee S, Hjerling-Leffler J, Zagha E, Fishell G, Rudy B. The largest group of superficial neocortical GABAergic interneurons expresses ionotropic serotonin receptors. J. Neurosci. 2010;30:16796–16808. doi: 10.1523/JNEUROSCI.1869-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miyoshi G, et al. Genetic fate mapping reveals that the caudal ganglionic eminence produces a large and diverse population of superficial cortical interneurons. J. Neurosci. 2010;30:1582–1594. doi: 10.1523/JNEUROSCI.4515-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu Q, Cobos I, La Cruz De E, Rubenstein JL, Anderson SA. Origins of cortical interneuron subtypes. J. Neurosci. 24:2612–2622. doi: 10.1523/JNEUROSCI.5667-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gelman D, Griveau A, Dehorter N. A wide diversity of cortical GABAergic interneurons derives from the embryonic preoptic area. The Journal of …. 2011 doi: 10.1523/JNEUROSCI.4068-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fogarty M, et al. Spatial genetic patterning of the embryonic neuroepithelium generates GABAergic interneuron diversity in the adult cortex. J. Neurosci. 2007;27:10935–10946. doi: 10.1523/JNEUROSCI.1629-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Du T, Xu Q, Ocbina PJ, Anderson SA. NKX2.1 specifies cortical interneuron fate by activating Lhx6. Development. 2008;135:1559–1567. doi: 10.1242/dev.015123. [DOI] [PubMed] [Google Scholar]
- 18.Armstrong C, Soltesz I. Basket cell dichotomy in microcircuit function. The Journal of Physiology. 2012;590:683–694. doi: 10.1113/jphysiol.2011.223669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tricoire L, et al. Common origins of hippocampal Ivy and nitric oxide synthase expressing neurogliaform cells. J. Neurosci. 2010;30:2165–76. doi: 10.1523/JNEUROSCI.5123-09.2010. (Comment: Comparison between this paper to references 11–15 demonstrated that similar interneuron subtypes in cortex versus hippocampus could be derived from distinct progenitor zones)
- 20.McBain CJ, Fisahn AE. Interneurons unbound. Nat. Rev. Neurosci. 2001;2:11–23. doi: 10.1038/35049047. [DOI] [PubMed] [Google Scholar]
- 21.Chittajallu R, et al. Dual origins of functionally distinct O-LM interneurons revealed by differential 5-HT3AR expression. Nat. Neurosci. 2013;16:1598–1607. doi: 10.1038/nn.3538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marín O, Anderson SA, Rubenstein JLR. Origin and molecular specification of striatal interneurons. J. Neurosci. 2000;20:6063–6076. doi: 10.1523/JNEUROSCI.20-16-06063.2000. (Comment: This paper provides to date our best understanding of the origins of striatal interneurons).
- 23.Wang B, Waclaw RR, Allen ZJ, Guillemot F, Campbell K. Ascl1 is a required downstream effector of Gsx gene function in the embryonic mouse telencephalon. Neural Dev. 2009;4:5. doi: 10.1186/1749-8104-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang B, et al. Loss of Gsx1 and Gsx2 function rescues distinct phenotypes in Dlx1/2 mutants. J. Comp. Neurol. 2013;521:1561–1584. doi: 10.1002/cne.23242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Long JE, Cobos I, Potter GB, Rubenstein JL. Dlx1&2 and Mash1 transcription factors control MGE and CGE patterning and differentiation through parallel and overlapping pathways. Cereb. Cortex. 2009;19(Suppl 1):i96–i106. doi: 10.1093/cercor/bhp045. IS - PB - CY - T2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schuurmans C, Guillemot F. Molecular mechanisms underlying cell fate specification in the developing telencephalon. Current Opinion in Neurobiology. 2002;12:26–34. doi: 10.1016/s0959-4388(02)00286-6. [DOI] [PubMed] [Google Scholar]
- 27.Stühmer T, Anderson SA, Ekker M, Rubenstein JLR. Ectopic expression of the Dlx genes induces glutamic acid decarboxylase and Dlx expression. Development. 2002;129:245–252. doi: 10.1242/dev.129.1.245. [DOI] [PubMed] [Google Scholar]
- 28.Cobos I, Borello U, Rubenstein JL. Dlx transcription factors promote migration through repression of axon and dendrite growth. Neuron. 2007;54:873–888. doi: 10.1016/j.neuron.2007.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cobos I, et al. Mice lacking Dlx1 show subtype-specific loss of interneurons, reduced inhibition and epilepsy. Nat. Neurosci. 2005;8:1059–1068. doi: 10.1038/nn1499. [DOI] [PubMed] [Google Scholar]
- 30.Colombo E, et al. Inactivation of Arx, the murine ortholog of the X-linked lissencephaly with ambiguous genitalia gene, leads to severe disorganization of the ventral telencephalon with impaired neuronal migration and differentiation. J. Neurosci. 2007;27:4786–4798. doi: 10.1523/JNEUROSCI.0417-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sussel L, Marín O, Kimura S, Rubenstein JL. Loss of Nkx2.1 homeobox gene function results in a ventral to dorsal molecular respecification within the basal telencephalon: evidence for a transformation of the pallidum into the striatum. Development. 1999;126:3359–3370. doi: 10.1242/dev.126.15.3359. [DOI] [PubMed] [Google Scholar]
- 32.Butt SJ, et al. The requirement of Nkx2-1 in the temporal specification of cortical interneuron subtypes. Neuron. 2008;59:722–732. doi: 10.1016/j.neuron.2008.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taniguchi H, Lu J, Huang ZJ. The spatial and temporal origin of chandelier cells in mouse neocortex. Science. 2013 doi: 10.1126/science.1227622. (Comment: the previous two papers provide support for Nkx2.1 functioning as a master regulatory of the fate of specific cortical interneuron identities).
- 34.Inan M, Welagen J, Anderson SA. Spatial and Temporal Bias in the Mitotic Origins of Somatostatin- and Parvalbumin-Expressing Interneuron Subgroups and the Chandelier Subtype in the Medial Ganglionic Eminence. Cereb. Cortex. 2012;22:820–827. doi: 10.1093/cercor/bhr148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Denaxa M, et al. Maturation-promoting activity of SATB1 in MGE-derived cortical interneurons. Cell Rep. 2012;2:1351–1362. doi: 10.1016/j.celrep.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Close J, et al. Satb1 Is an Activity-Modulated Transcription Factor Required for the Terminal Differentiation and Connectivity of Medial Ganglionic Eminence-Derived Cortical Interneurons. Journal of Neuroscience. 2012;32:17690–17705. doi: 10.1523/JNEUROSCI.3583-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Flames N, et al. Delineation of multiple subpallial progenitor domains by the combinatorial expression of transcriptional codes. Journal of Neuroscience. 2007;27:9682–9695. doi: 10.1523/JNEUROSCI.2750-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ciceri G, et al. Lineage-specific laminar organization of cortical GABAergic interneurons. Nat. Neurosci. 2013;16:1199–1210. doi: 10.1038/nn.3485. [DOI] [PubMed] [Google Scholar]
- 39.Brown KN, et al. Clonal production and organization of inhibitory interneurons in the neocortex. Science. 2011;334:480–486. doi: 10.1126/science.1208884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Corbin JG, Nery S, Fishell G. Telencephalic cells take a tangent: non-radial migration in the mammalian forebrain. Nat. Neurosci. 2001;4:1177–1182. doi: 10.1038/nn749. [DOI] [PubMed] [Google Scholar]
- 41.Marín O, Rubenstein JLR. A long, remarkable journey: Tangential migration in the telencephalon. Nat. Rev. Neurosci. 2001;2:780–790. doi: 10.1038/35097509. [DOI] [PubMed] [Google Scholar]
- 42.Hobert O. Specification of the nervous system. 2005 doi: 10.1895/wormbook.1.12.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bassett EA, Wallace VA. Cell fate determination in the vertebrate retina. Trends in Neurosciences. 2012;35:565–573. doi: 10.1016/j.tins.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 44.Leber SM, Breedlove SM, Sanes JR. Lineage, arrangement, and death of clonally related motoneurons in chick spinal cord. Journal of Neuroscience. 1990;10:2451–2462. doi: 10.1523/JNEUROSCI.10-07-02451.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Walsh C, Cepko CL. Clonally related cortical cells show several migration patterns. Science. 1988;241:1342–1345. doi: 10.1126/science.3137660. [DOI] [PubMed] [Google Scholar]
- 46.Beier KT, Samson ME, Matsuda T, Cepko CL. Conditional expression of the TVA receptor allows clonal analysis of descendents from Cre-expressing progenitor cells. Dev. Biol. 2011;353:309–320. doi: 10.1016/j.ydbio.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cancedda L, Fiumelli H, Chen K, Poo MM. Excitatory GABA action is essential for morphological maturation of cortical neurons in vivo. J. Neurosci. 2007;27:5224–5235. doi: 10.1523/JNEUROSCI.5169-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bortone D, Polleux F. KCC2 expression promotes the termination of cortical interneuron migration in a voltage-sensitive calcium-dependent manner. Neuron. 2009;62:53–71. doi: 10.1016/j.neuron.2009.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.De Marco García NV, Karayannis T, Fishell G. Neuronal activity is required for the development of specific cortical interneuron subtypes. Nature. 2011;472:351–355. doi: 10.1038/nature09865. (Comment: the previous two papers provide the best evidence to date for a role for activity in the positioning and maturation of cortical interneurons).
- 50.McKinsey GL, Lindtner S, Trzcinski B, Visel A. Dlx1&2-Dependent Expression of Zfhx1b (Sip1, Zeb2) Regulates the Fate Switch between Cortical and Striatal Interneurons. Neuron. 2011 doi: 10.1016/j.neuron.2012.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van den Berghe V, et al. Directed Migration of Cortical Interneurons Depends on the Cell-Autonomous Action of Sip1. Neuron. 2013;77:70–82. doi: 10.1016/j.neuron.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 52.Lyons MR, Schwarz CM, West AE. Members of the myocyte enhancer factor 2 transcription factor family differentially regulate Bdnf transcription in response to neuronal depolarization. J. Neurosci. 2012;32:12780–12785. doi: 10.1523/JNEUROSCI.0534-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.West AE, Greenberg ME. Neuronal activity-regulated gene transcription in synapse development and cognitive function. Cold Spring Harb Perspect Biol. 2011;3 doi: 10.1101/cshperspect.a005744. undefined. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Southwell DG, Froemke RC, Alvarez-Buylla A, Stryker MP, Gandhi SP. Cortical plasticity induced by inhibitory neuron transplantation. Science. 2010;327:1145–1148. doi: 10.1126/science.1183962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bráz JM, et al. Forebrain GABAergic neuron precursors integrate into adult spinal cord and reduce injury-induced neuropathic pain. Neuron. 2012;74:663–675. doi: 10.1016/j.neuron.2012.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Martínez-Cerdeño V, et al. Embryonic MGE precursor cells grafted into adult rat striatum integrate and ameliorate motor symptoms in 6-OHDA-lesioned rats. Cell Stem Cell. 2010;6:238–250. doi: 10.1016/j.stem.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Taniguchi H, et al. A Resource of Cre Driver Lines for Genetic Targeting of GABAergic Neurons in Cerebral Cortex. Neuron. 2011;71:995–1013. doi: 10.1016/j.neuron.2011.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hippenmeyer S, et al. A Developmental Switch in the Response of DRG Neurons to ETS Transcription Factor Signaling. PLoS Biol. 2005;3:e159. doi: 10.1371/journal.pbio.0030159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Douglas RJ, Martin KA. A functional microcircuit for cat visual cortex. The Journal of Physiology. 1991 doi: 10.1113/jphysiol.1991.sp018733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Douglas RJ, Koch C, Mahowald M, Martin KA. Recurrent excitation in neocortical circuits. Science. 1995 doi: 10.1126/science.7638624. [DOI] [PubMed] [Google Scholar]
- 61.Buzsáki G, Draguhn A. Neuronal oscillations in cortical networks. Science. 2004 doi: 10.1126/science.1099745. [DOI] [PubMed] [Google Scholar]
- 62.Silver RA. Neuronal arithmetic. Nat. Rev. Neurosci. 2010;11:474–489. doi: 10.1038/nrn2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Holt GR, Koch C. Shunting Inhibition Does Not Have a Divisive Effect on Firing Rates. doi: 10.1162/neco.1997.9.5.1001. [DOI] [PubMed] [Google Scholar]
- 64.Carandini M, Heeger DJ. Normalization as a canonical neural computation. Nat. Rev. Neurosci. 2011 doi: 10.1038/nrn3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schwartz O, Simoncelli EP. Natural signal statistics and sensory gain control. Nat. Neurosci. 2001 doi: 10.1038/90526. [DOI] [PubMed] [Google Scholar]
- 66.Mitchell SJ, Silver RA. Shunting inhibition modulates neuronal gain during synaptic excitation. Neuron. 2003 doi: 10.1016/s0896-6273(03)00200-9. [DOI] [PubMed] [Google Scholar]
- 67.Tiesinga P, Sejnowski TJ. Rapid temporal modulation of synchrony by competition in cortical interneuron networks. Neural computation. 2004 doi: 10.1162/089976604322742029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chance FS, Abbott LF, Reyes AD. Gain modulation from background synaptic input. Neuron. 2002 doi: 10.1016/s0896-6273(02)00820-6. [DOI] [PubMed] [Google Scholar]
- 69.Wilson NR, Runyan CA, Wang FL, Sur M. Division and subtraction by distinct cortical inhibitory networks in vivo. Nature. 2012;488:343–348. doi: 10.1038/nature11347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Atallah BV, Bruns W, Carandini M, Scanziani M. Parvalbumin-Expressing Interneurons Linearly Transform Cortical Responses to Visual Stimuli. Neuron. 2012;73:159–170. doi: 10.1016/j.neuron.2011.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lee S-H, et al. Activation of specific interneurons improves V1 feature selectivity and visual perception. Nature. 2012;488:379–383. doi: 10.1038/nature11312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Adesnik H, Scanziani M. Lateral competition for cortical space by layer-specific horizontal circuits. Nature. 2010;464:1155–1160. doi: 10.1038/nature08935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Taniguchi H, Huang ZJ, Callaway EM. Contrast Dependence and Differential Contributions from Somatostatin-and Parvalbumin-Expressing Neurons to Spatial Integration in Mouse V1. The Journal of …. 2013 doi: 10.1523/JNEUROSCI.5320-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.van Vreeswijk C, Sompolinsky H. Chaos in neuronal networks with balanced excitatory and inhibitory activity. Science. 1996 doi: 10.1126/science.274.5293.1724. [DOI] [PubMed] [Google Scholar]
- 75.Wehr M, Zador AM. Balanced inhibition underlies tuning and sharpens spike timing in auditory cortex. Nature. 2003 doi: 10.1038/nature02116. [DOI] [PubMed] [Google Scholar]
- 76.Haider B, Duque A, Hasenstaub AR. Neocortical network activity in vivo is generated through a dynamic balance of excitation and inhibition. The Journal of …. 2006 doi: 10.1523/JNEUROSCI.5297-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Okun M, Lampl I. Instantaneous correlation of excitation and inhibition during ongoing and sensory-evoked activities. Nat. Neurosci. 2008 doi: 10.1038/nn.2105. [DOI] [PubMed] [Google Scholar]
- 78.Haider B, Häusser M, Carandini M. Inhibition dominates sensory responses in the awake cortex. Nature. 2012;493:97–100. doi: 10.1038/nature11665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pouille F, poui & Scanziani M. Enforcement of temporal fidelity in pyramidal cells by somatic feed-forward inhibition. Science. 2001;293:1159–1163. doi: 10.1126/science.1060342. [DOI] [PubMed] [Google Scholar]
- 80.Renart A, la Rocha de J, Bartho P, Hollender L. The asynchronous state in cortical circuits. Science. 2010 doi: 10.1126/science.1179850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rudy B, Fishell G, Lee S, Hjerling-Leffler J. Three groups of interneurons account for nearly 100% of neocortical GABAergic neurons. Devel Neurobio. 2010;71:45–61. doi: 10.1002/dneu.20853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Xu X, Roby KD, Callaway EM. Immunochemical characterization of inhibitory mouse cortical neurons: three chemically distinct classes of inhibitory cells. Journal of Comparative …. 2010 doi: 10.1002/cne.22229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat. Neurosci. 2005;8:1263–1268. doi: 10.1038/nn1525. [DOI] [PubMed] [Google Scholar]
- 84.Zhang F, Aravanis AM, Adamantidis A, de Lecea L, Deisseroth K. Circuit-breakers: optical technologies for probing neural signals and systems. Nat. Rev. Neurosci. 2007;8:577–581. doi: 10.1038/nrn2192. [DOI] [PubMed] [Google Scholar]
- 85.Somogyi P, Tamas G, Lujan R, Buhl EH. Salient features of synaptic organisation in the cerebral cortex. Brain Research Reviews. 1998 doi: 10.1016/s0165-0173(97)00061-1. [DOI] [PubMed] [Google Scholar]
- 86.Hestrin S, Galarreta M. A network of fast-spiking cells in the neocortex connected by electrical synapses : Abstract : Nature. Nature. 1999;402:72–75. doi: 10.1038/47029. [DOI] [PubMed] [Google Scholar]
- 87.Kvitsiani D, et al. Distinct behavioural and network correlates of two interneuron types in prefrontal cortex. Nature. 2013;498:363–366. doi: 10.1038/nature12176. (Comment: provides evidence that genetically-identified interneuron classes are recruited at specific behavioral epochs)
- 88.Pfeffer CK, Xue M, He M, Huang ZJ, Scanziani M. Inhibition of inhibition in visual cortex: the logic of connections between molecularly distinct interneurons. Nat. Neurosci. 2013 doi: 10.1038/nn.3446. (Comment: Defines the rules of connectivity for marker-defined interneuron classes.)
- 89.Szabadics J, Lorincz A, Tamás G. Beta and gamma frequency synchronization by dendritic gabaergic synapses and gap junctions in a network of cortical interneurons. Journal of Neuroscience. 2001;21:5824–5831. doi: 10.1523/JNEUROSCI.21-15-05824.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Royer S, et al. Control of timing, rate and bursts of hippocampal place cells by dendritic and somatic inhibition. Nat. Neurosci. 2012;15:769–775. doi: 10.1038/nn.3077. (Comment: First direct demonstration of the distinct roles of PV and SST interneurons in awake hippocampus.)
- 91.Lovett-Barron M, et al. Regulation of neuronal input transformations by tunable dendritic inhibition. Nat. Neurosci. 2012;15:423–430. doi: 10.1038/nn.3024. [DOI] [PubMed] [Google Scholar]
- 92.Losonczy A, Zemelman BV, Vaziri A, Magee JC. Network mechanisms of theta related neuronal activity in hippocampal CA1 pyramidal neurons. Nat. Neurosci. 2010 doi: 10.1038/nn.2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Murayama M, Pérez-Garci E, Nevian T, Bock T, Senn W. Dendritic encoding of sensory stimuli controlled by deep cortical interneurons. Nature. 2009 doi: 10.1038/nature07663. (Comment: Reveals how a specific interneuron type gates bursting in layer V pyramidal cells.)
- 94.Berger TK, Perin R, Silberberg G, Markram H. Frequency-dependent disynaptic inhibition in the pyramidal network: a ubiquitous pathway in the developing rat neocortex. The Journal of Physiology. 2009;587:5411–5425. doi: 10.1113/jphysiol.2009.176552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kapfer C, Glickfeld LL, Atallah BV, Scanziani M. Supralinear increase of recurrent inhibition during sparse activity in the somatosensory cortex. Nat. Neurosci. 2007 doi: 10.1038/nn1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Miles R, Tóth K, Gulyas AI, Hájos N, Freund TF. Differences between somatic and dendritic inhibition in the hippocampus. Neuron. 1996 doi: 10.1016/s0896-6273(00)80101-4. [DOI] [PubMed] [Google Scholar]
- 97.Xu H, Jeong HY, Tremblay R, Rudy B. Neocortical somatostatin-expressing GABAergic interneurons disinhibit the thalamorecipient layer 4. Neuron. 2013 doi: 10.1016/j.neuron.2012.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hájos N, Acsády L, Freund TF. Target Selectivity and Neurochemical Characteristics of VIP-immunoreactive Interneurons in the Rat Dentate Gyrus. Eur J Neurosci. 1996;8:1415–1431. doi: 10.1111/j.1460-9568.1996.tb01604.x. [DOI] [PubMed] [Google Scholar]
- 99.Acsady L, Görcs TJ, Freund TF. Different populations of vasoactive intestinal polypeptide-immunoreactive interneurons are specialized to control pyramidal cells or interneurons in the hippocampus. Neuroscience. 1996;73:317–334. doi: 10.1016/0306-4522(95)00609-5. [DOI] [PubMed] [Google Scholar]
- 100.Lee S, Kruglikov I, Huang ZJ, Fishell G, Rudy B. A disinhibitory circuit mediates motor integration in the somatosensory cortex. Nat. Neurosci. 2013;16:1662–1670. doi: 10.1038/nn.3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pi H-J, et al. Cortical interneurons that specialize in disinhibitory control. Nature. 2013 doi: 10.1038/nature12676. (Comment: Direct demonstration that VIP-expressing interneurons are disinhibitory and are recruited by behavioral reinforcers. Together with ref. 88, 100 reveals that this function is supported by a microciruit conserved across regions)
- 102.Hestrin S, Armstrong WE. Morphology and physiology of cortical neurons in layer I. J. Neurosci. 1996 doi: 10.1523/JNEUROSCI.16-17-05290.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jiang X, Wang G, Lee AJ, Stornetta RL, Zhu JJ. The organization of two new cortical interneuronal circuits. Nat. Neurosci. 2013 doi: 10.1038/nn.3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Letzkus JJ, et al. A disinhibitory microcircuit for associative fear learning in the auditory cortex. Nature. 2011;480:331–335. doi: 10.1038/nature10674. (Comment: Demonstrates a functionally-relevant disinhibitory circuit in auditory cortex.)
- 105.Lapray D, et al. Behavior-dependent specialization of identified hippocampal interneurons. Nat. Neurosci. 2012;15:1265–1271. doi: 10.1038/nn.3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Varga C, Golshani P, Soltesz I. Frequency-invariant temporal ordering of interneuronal discharges during hippocampal oscillations in awake mice. Proceedings of the National Academy of Sciences. 2012;109:E2726–E2734. doi: 10.1073/pnas.1210929109. (Comment: Demonstrates the hippocampal recruitment of distinct interneuron types in awake mice.)
- 107.Gentet LJ, et al. Unique functional properties of somatostatin-expressing GABAergic neurons in mouse barrel cortex. Nat. Neurosci. 2012;15:607–612. doi: 10.1038/nn.3051. [DOI] [PubMed] [Google Scholar]
- 108.Gentet LJ, Avermann M, Matyas F, Staiger JF, Petersen CCH. Membrane Potential Dynamics of GABAergic Neurons in the Barrel Cortex of Behaving Mice. Neuron. 2010;65:422–435. doi: 10.1016/j.neuron.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 109.Csicsvari J, Hirase H, Czurko A, Buzsáki G. Reliability and state dependence of pyramidal cell-interneuron synapses in the hippocampus: an ensemble approach in the behaving rat. Neuron. 1998;21:179–189. doi: 10.1016/s0896-6273(00)80525-5. [DOI] [PubMed] [Google Scholar]
- 110.Whittington MA, Traub RD. Interneuron Diversity series: Inhibitory interneurons and network oscillations in vitro. Trends in Neurosciences. 2003;26:676–682. doi: 10.1016/j.tins.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 111.Buzsáki G, Chrobak JJ. Temporal structure in spatially organized neuronal ensembles: a role for interneuronal networks. Current Opinion in Neurobiology. 1995;5:504–510. doi: 10.1016/0959-4388(95)80012-3. [DOI] [PubMed] [Google Scholar]
- 112.Klausberger T, Magill PJ, Márton LF, Roberts J. Brain-state-and cell-type-specific firing of hippocampal interneurons in vivo. Nature. 2003 doi: 10.1038/nature01374. (Comment: First demonstration how different interneuron types specialize in specific network oscillations.)
- 113.Klausberger T, Márton LF, Baude A, Roberts J. Spike timing of dendrite-targeting bistratified cells during hippocampal network oscillations in vivo. Nature. 2003 doi: 10.1038/nn1159. [DOI] [PubMed] [Google Scholar]
- 114.Klausberger T, Márton LF, O'Neill J. Complementary roles of cholecystokinin-and parvalbumin-expressing GABAergic neurons in hippocampal network oscillations. The Journal of …. 2005 doi: 10.1523/JNEUROSCI.3269-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hartwich K, Somogyi P, Klausberger T. Cell type-specific tuning of hippocampal interneuron firing during gamma oscillations in vivo. The Journal of …. 2007 doi: 10.1523/JNEUROSCI.1685-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Klausberger T, Somogyi P. Neuronal diversity and temporal dynamics: the unity of hippocampal circuit operations. Science. 2008 doi: 10.1126/science.1149381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cardin JA, Carlen M, Meletis K, Knoblich U, Zhang F. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature. 2009 doi: 10.1038/nature08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sohal VS, Zhang F, Yizhar O, Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature. 2009 doi: 10.1038/nature07991. (Comment: These last two references provided the first causal evidence for the role of PV interneurons in gamma oscillations.)
- 119.Mountcastle VB, Talbot WH, Sakata H, Hyvarinen J. Cortical neuronal mechanisms in flutter-vibration studied in unanesthetized monkeys: Neuronal periodicity and frequency discrimination. Journal of Neurophysiology. 1969 doi: 10.1152/jn.1969.32.3.452. [DOI] [PubMed] [Google Scholar]
- 120.Mitchell JF, Sundberg KA, Reynolds JH. Differential Attention-Dependent Response Modulation across Cell Classes in Macaque Visual Area V4. Neuron. 2007;55:131–141. doi: 10.1016/j.neuron.2007.06.018. [DOI] [PubMed] [Google Scholar]
- 121.Isomura Y, Harukuni R, Takekawa T, Aizawa H, Fukai T. Microcircuitry coordination of cortical motor information in self-initiation of voluntary movements. Nat. Neurosci. 2009;12:1586–1593. doi: 10.1038/nn.2431. [DOI] [PubMed] [Google Scholar]
- 122.Hayden BY, Pearson JM, Platt ML. Neuronal basis of sequential foraging decisions in a patchy environment. Nat. Neurosci. 2011 doi: 10.1038/nn.2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Carandini M. From circuits to behavior: a bridge too far? Nat. Neurosci. 2012 doi: 10.1038/nn.3043. [DOI] [PubMed] [Google Scholar]
- 124.Alitto HJ, Dan Y. Cell-type-specific modulation of neocortical activity by basal forebrain input. Front. Syst. Neurosci. 2013;6 doi: 10.3389/fnsys.2012.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]