Effect of Covered Metallic Stents Compared With Plastic Stents on Benign Biliary Stricture Resolution: A Randomized Clinical Trial (original) (raw)
. Author manuscript; available in PMC: 2017 Aug 5.
Published in final edited form as: JAMA. 2016 Mar 22;315(12):1250–1257. doi: 10.1001/jama.2016.2619
Abstract
IMPORTANCE
Endoscopic placement of multiple plastic stents in parallel is the first-line treatment for most benign biliary strictures; it is possible that fully covered, self-expandable metallic stents (cSEMS) may require fewer endoscopic retrograde cholangiopancreatography procedures (ERCPs) to achieve resolution.
OBJECTIVE
To assess whether use of cSEMS is noninferior to plastic stents with respect to stricture resolution.
DESIGN, SETTING, AND PARTICIPANTS
Multicenter (8 endoscopic referral centers), open-label, parallel, randomized clinical trial involving patients with treatment-naive, benign biliary strictures (N = 112) due to orthotopic liver transplant (n = 73), chronic pancreatitis (n = 35), or postoperative injury (n = 4), who were enrolled between April 2011 and September 2014 (with follow-up ending October 2015). Patients with a bile duct diameter less than 6 mm and those with an intact gallbladder in whom the cystic duct would be overlapped by a cSEMS were excluded.
INTERVENTIONS
Patients (N = 112) were randomized to receive multiple plastic stents or a single cSEMS, stratified by stricture etiology and with endoscopic reassessment for resolution every 3 months (plastic stents) or every 6 months (cSEMS). Patients were followed up for 12 months after stricture resolution to assess for recurrence.
MAIN OUTCOMES AND MEASURES
Primary outcome was stricture resolution after no more than 12 months of endoscopic therapy. The sample size was estimated based on the noninferiority of cSEMS to plastic stents, with a noninferiority margin of −15%.
RESULTS
There were 55 patients in the plastic stent group (mean [SD] age, 57 [11] years; 17 women [31%]) and 57 patients in the cSEMS group (mean [SD] age, 55 [10] years; 19 women [33%]). Compared with plastic stents (41/48, 85.4%), the cSEMS resolution rate was 50 of 54 patients (92.6%), with a rate difference of 7.2% (1-sided 95% CI, −3.0% to ∞; P < .001). Given the prespecified noninferiority margin of −15%, the null hypothesis that cSEMS is less effective than plastic stents was rejected. The mean number of ERCPs to achieve resolution was lower for cSEMS (2.14) vs plastic (3.24; mean difference, 1.10; 95% CI, 0.74 to 1.46; P < .001).
CONCLUSIONS AND RELEVANCE
Among patients with benign biliary strictures and a bile duct diameter 6 mm or more in whom the covered metallic stent would not overlap the cystic duct, cSEMS were not inferior to multiple plastic stents after 12 months in achieving stricture resolution. Metallic stents should be considered an appropriate option in patients such as these.
TRIAL REGISTRATION
clinicaltrials.gov Identifier: NCT01221311
Endoscopic retrograde cholangiopancreatography (ERCP) is the primary method for access to the pancreatobiliary system. Among the principal indications for stent placement is benign bile duct strictures, for which ERCP has become the preferred first-line treatment strategy.1 Endoscopic treatment of benign biliary strictures is significantly less morbid than surgical and percutaneous approaches and has reasonably low recurrence rates when an aggressive treatment strategy is implemented. Benign biliary strictures require intervention to treat jaundice, chronic cholestasis, and cholangitis, as well as to avoid the long-term development of secondary biliary cirrhosis.2
To maximize treatment efficacy and minimize stricture recurrence, the standard approach to endoscopic therapy is placement of multiple plastic stents in parallel after the stricture is dilated using graduated bougie-type or hydrostatic balloon catheters. Because the strictures are usually fibrotic and associated with a dilated bile duct, most benign strictures cannot be fully dilated during the initial ERCP. This obligates an average of 3 to 4 ERCPs to dilate, deploy stents, up-size, and then ultimately remove all stents once the stricture has resolved. This strategy results in very high efficacy (80%–90%) for postoperative strictures and moderately high efficacy (50%–70%) for those caused by chronic pancreatitis.3,4
Despite the high success rate of multiple plastic stent therapy, multiple treatment sessions are required. Because placement of a single, fully covered, self-expandable metallic stent (cSEMS) results in radial dilation of a stricture equivalent to that of at least 3 side-by-side plastic stents (which cannot generally be placed during the initial ERCP), preliminary studies including small clinical trials support the hypothesis that deployment of cSEMS would be beneficial in patients with benign strictures.5–9 We conducted an open-label, multicenter, randomized clinical trial to test the hypothesis that cSEMS would be noninferior to multiple plastic stents in the first-line endoscopic treatment of benign bile duct strictures. (See the trial protocol in Supplement 1 for details.)
Methods
The study was executed at 8 regional referral centers for ERCP and liver transplantation in the United States and United Kingdom, after local approval by their respective institutional review boards. The US Food and Drug Administration monitored this study under an Investigational Device Exemption (G100118) because cSEMS are not approved for use in benign bile duct strictures or for endoscopic retrieval except after immediate deployment. Written informed consent was obtained from all patients prior to the index ERCP procedure.
Eligible patients included those with a benign bile duct stricture located at least 2 cm below the hepatic confluence and coupled with related signs or symptoms (eg, any elevation in liver test results, jaundice, or cholangitis). A stricture was defined as any narrowing of the extrahepatic bile duct that was less than 75% of the diameter of the unaffected duct; for example, a 10-mm diameter bile duct with a 5-mm diameter stricture would be eligible. To our knowledge, no prior endoscopic studies have used a quantitative definition for benign bile duct stricture, so this was based on expert consensus of the study’s steering committee and approval from all participating investigators. The investigators chose a quantitative definition in order to define stricture resolution using the same fluoroscopic criteria: the residual diameter of the stricture must be 75% or more of the duct above and below the stricture. Patients who had undergone any treatment to the stricture within 12 months of randomization, with the exception of a single, bridging plastic stent placed within 30 days of liver transplantation or while ruling out pancreatobiliary cancers, were excluded. In addition, those having a bile duct diameter less than 6 mm above or below the stricture to avoid oversizing the bile duct (the smallest available cSEMS diameter in the United States is 8 mm), or if placement of a cSEMS would overlap the cystic duct in the setting of an intact gallbladder (due to a potential risk for stent-induced acute cholecystitis), were excluded. Other exclusion criteria are summarized in eTable 1 in Supplement 2.
Randomization
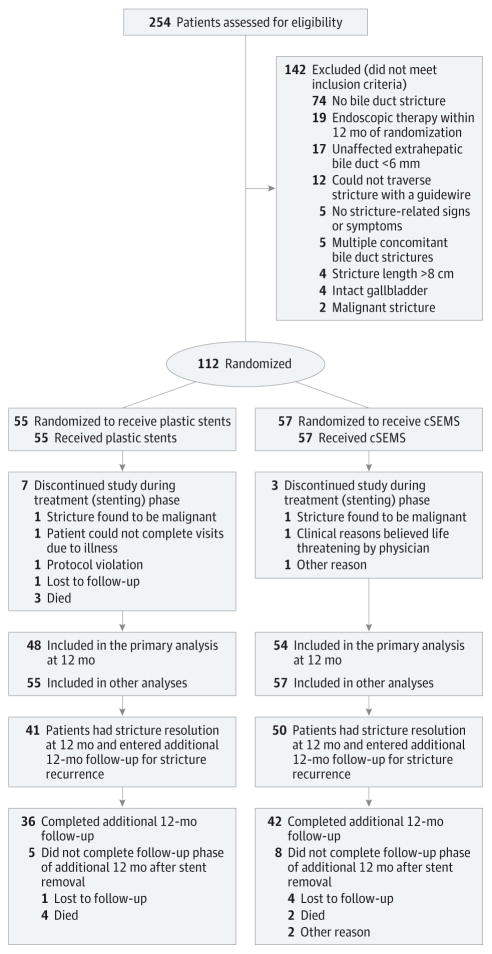
After obtaining written informed consent, the investigator determined final eligibility after performing a cholangiogram to confirm the presence of a benign bile duct stricture and to establish the absence of exclusion criteria. Because chronic pancreatitis and postoperative strictures are inherently different in their pathophysiology and response to endoscopic therapy, we stratified randomization by these etiologies and by site, in blocks of 4 to ensure balanced distribution of groups across sites. The biostatistician provided a computer-generated randomization sequence to each site, using opaque envelopes to maintain allocation concealment. Once eligibility was confirmed, the site coordinator informed the ERCP physician during the procedure of treatment allocation (Figure 1). Because follow-up was predicated on the type of stent used, patients and investigators were not blinded to group assignment.
Figure 1. Patient Flow Through the Biliary Stent Trial.
cSEMS indicates fully covered, self-expandable metallic stent.
Interventions
Patients randomized to the plastic stent group were treated using a standard algorithm (eFigure 1 in Supplement 2). Per standard of care, the stricture was dilated to the maximum safest diameter according to endoscopist judgment, and the maximum cumulative diameter of plastic stents was deployed. Repeat ERCP was performed 3 to 4 months later, when all plastic stents were removed and the stricture was assessed for resolution. If the stricture persisted, then the cumulative diameter of plastic stents was up-sized to the greatest extent feasible. Until stricture resolution, ERCPs were repeated every 3 to 4 months with plastic stent up-sizing as needed.
Among patients randomized to receive cSEMS, the endoscopist deployed a cSEMS (fully covered WallFlex, Boston Scientific) of sufficient length to traverse the stricture and the papilla (eFigure 1 in Supplement 2). The endoscopist was permitted to dilate the stricture before cSEMS deployment on an as-needed basis. To minimize cSEMS migration, an 8-mm diameter cSEMS was used for bile ducts measuring 6 to 7 mm and a 10-mm diameter cSEMS for bile ducts 8 mm or greater. Because metallic stents have superior patency to multiple plastic stents, follow-up ERCP was performed 6 months after randomization. For those with a persistent stricture at repeat ERCP, the cSEMS was replaced for another 6-month interval.
In all cases, ERCPs could be performed earlier than per protocol when there was a clinical suspicion of premature stent occlusion or migration. To ensure consistent practice across sites, whenever stricture resolution occurred per study criteria, all stents were removed and not replaced. For those with a persistent stricture, stents were replaced and up-sized.
Follow-up and Outcomes
In all patients, treatment failure was defined as the presence of a persistent stricture after 12 months of endoscopic therapy or if there was 1 major or 2 minor stent-related adverse events that precluded study continuation (eg, stent migration that was complicated by acute cholangitis). A major or severe adverse event was defined by the need for procedural intervention or hospitalization; all other adverse events were classified as minor. A related adverse event was defined as one that was definitely or possibly related to the bile duct stents. In those who achieved stricture resolution within 12 months of randomization, follow-up continued for an additional 12 months after stent removal to assess for stricture recurrence. Follow-up included a telephone or in-person encounter to assess for signs or symptoms of a benign biliary stricture, including basic liver test results. We defined stricture recurrence using the same criteria for enrollment in the study: presence of a bile duct stricture confirmed by ERCP with associated signs or symptoms.
The primary outcome was stricture resolution rate; this was presented as an absolute rate after no more than 12 months of endoscopic therapy. Technical success was defined as successful endoscopic placement and removal of stents. Among those who achieved stricture resolution, recurrence rates were reported during the poststenting follow-up period (up to 12 months). Safety, with particular emphasis on the rates of stent migration and stent-associated strictures between groups, was also evaluated. For those who achieved stricture resolution, a process outcome was the requisite number of ERCPs to achieve resolution. A cost analysis was planned as another secondary outcome but has not yet been conducted.
Sample Size
Given the high costs and inconvenience of multiple ERCPs and infectious control concerns with duodenoscopes, and because we hypothesized that cSEMS would reduce the number of ERCPs required to achieve stricture resolution, we considered that cSEMS could replace plastic stents as the preferred first-line stent for treating benign bile duct strictures if it resulted in less than a −15% difference in treatment efficacy (non-inferiority margin). Theoretical disadvantages of metallic stents include their higher cost compared with plastic stents and difficulty with metallic stent removal during a follow-up ERCP. Assuming a −15% noninferiority margin and a resolution rate of 90% in the plastic stent group, and given no observed difference in stricture resolution rates in the cSEMS and plastic stent groups, a sample size of 112 would result in 80% power with a targeted significance level of .05. This also allowed for an attrition rate of 10% for dropouts and patients lost to follow-up. The noninferiority margin was chosen to be −15% based on the judgment of the study’s steering committee comprising 5 board-certified gastroenterologists who are experts in ERCP. The sample size was reduced to 112 from 250 after an interim analysis (77 patients randomized) requested by the data and safety monitoring board after 3 years of recruitment revealed stricture resolution rates of greater than 90% in both groups, without changing the targeted significance level (.05), statistical power (80%), or attrition rate (10%).
Statistical Analysis
The primary analysis evaluated the noninferiority of cSEMS to multiple plastic stents, which was determined based on a 2-sample binomial noninferiority test at the 5% significance level. Equivalently, the noninferiority was established if the lower bound of the 1-sided 95% confidence interval for the difference in resolution rate was −15% or greater. Patients with an incomplete treatment phase (9%) were excluded from the primary analysis because of the uncertain status of their benign biliary stricture (eg, died or lost to follow-up while stents remained in place); we chose a modified intent-to-treat approach to minimize biasing a conclusion favoring noninferiority.10,11 Sensitivity analysis was performed to examine the noninferiority of the cSEMS in the worst-case scenario, where all patients excluded from the primary analysis because of uncertain stricture status were considered to have resolution if they were in the plasticstents group and no resolution if they were in the cSEMS group.
In addition to the primary analysis of noninferiority, we performed a post hoc analysis to compare resolution rates between cSEMS and plastic stent groups using the Kaplan-Meier method for time to resolution including all randomized patients in the study. Patients who had an incomplete treatment (stenting) phase were censored at the time of study discontinuation. The median (95% CI) number of days to resolution was calculated based on the Kaplan-Meier plot. A log-rank test was performed to compare Kaplan-Meier estimates of resolution rates. Post hoc analysis included comparison of the number of days to resolution, which was performed using a Wilcoxon rank sum test. All post hoc analyses were 2-sided at the 5% significance level, and adjustment for multiple comparisons was performed to account for having 3 outcomes using the Bonferroni approach. These outcomes were also evaluated in the subgroup analyses for patients with posttransplant and chronic pancreatitis–induced strictures, where multiple comparisons were not adjusted. Safety measures were evaluated using all randomized patients. The study was not powered to detect differences in safety end points, recurrence rates, or number of ERCPs required to achieve stricture resolution.
Baseline patient characteristics were presented using mean and standard deviations for normally distributed continuous variables, median and range for nonnormally distributed continuous variables, and frequency and proportion for categorical variables. Comparisons between the cSEMS and plastic stent groups were performed using the 2-sample t test for normally distributed continuous variables and Wilcoxon rank sum test for nonnormally distributed continuous variables. For categorical variables, Pearson χ2 test was used for variables with more than 2 categories and Fisher exact test was used for binary variables. All statistical analyses were performed using SAS version 9.4 (SAS Institute).
Results
Between April 2011 and September 2014, 254 individuals were screened for eligibility and 112 were randomized to receive multiple plastic stents (n = 55; mean [SD] age, 57 (11) years; 17 women [31%]) or cSEMS (n = 57; mean [SD] age, 55 [10] years; 19 women [33%]) (Figure 1). Patient and stricture characteristics are summarized in Table 1. The etiology of bile duct stricture was balanced between treatment groups, with the majority (65%) being located at the biliary anastomosis after orthotopic liver transplantation. Per study protocol, no patients had multiple plastic stents in place at the time of enrollment while 39% had a single bridging plastic stent. During the stenting period, 7 of 57 patients (5.3%) in the plastic stent group and 3 of 57 patients (5.3%) in the cSEMS group ended the study during the treatment (stenting) phase (Figure 1). The primary outcome measure of stricture resolution for all remaining patients was available at 12 months, and these were included in the primary analysis.
Table 1.
Patient and Stricture Characteristics
| Variable | Multiple Plastic Stents (n = 55) | cSEMS (n = 57) |
|---|---|---|
| Age, mean (SD), y | 56.7 (11) | 54.5 (10.4) |
| Women, No. (%) | 17 (30.9) | 19 (33.3) |
| American Society of Anesthesiology class ≥3, No. (%)a | 42 (76.4) | 39 (68.4) |
| Karnofsky performance status, mean (SD) | 79.3 (12.7) | 78.9 (12.6) |
| Etiology of stricture, No. (%) | ||
| Postorthotopic liver transplant | 36 (65.5) | 37 (64.9) |
| Time since transplant, median (range), mo | 4 (1–96) | 3 (1–44) |
| Chronic pancreatitis | 17 (30.9) | 18 (31.6) |
| Other postoperative injury | 2 (3.6) | 2 (3.5) |
| Stricture characteristics, median (range) | ||
| Distance of top of stricture to the hepatic confluence, mm | 34 (1–85) | 36 (2.36–86) |
| Diameter of duct upstream of stricture, mm | 11 (5.7–13.0) | 10 (6–18) |
| Diameter of duct downstream of stricture, mm | 8 (5.0–8.5) | 7 (0–13.7) |
| Stricture length, mm | 5 (0.7–38) | 4 (1–32) |
| Previous cholecystectomy, No. (%) | 42 (76.4) | 43 (75.4) |
| Previous single plastic stent in place, No. (%) | 16 (29.1) | 23 (40.4) |
| Total bilirubin, median (range), mg/dL | 1.2 (0.4–20.7) | 1.4 (0.4–17.4) |
| Alkaline phosphatase, median (range), IU/L | 317 (52–1027) | 240 (14–1615) |
The majority of patients in both groups (multiple plastic stents, 90.9% and cSEMS, 93.0%; −2.1% difference; 95% CI, −12.2% to 8%) underwent a biliary sphincterotomy at the time of randomization or during an earlier ERCP (Table 2). Compared with cSEMS (14%), a significantly greater number of patients in the plastic stent group (80%, P < .001) underwent balloon dilation during their index ERCP (66.0% difference; 95% CI, 52.1% to 79.9%). Most (73%) patients in the plastic stent group received 2 or more stents at enrollment, with a median cumulative diameter of 20 F (range, 7–30 F).
Table 2.
Endoscopic Interventions During the Enrollment ERCP
| Variable | Multiple Plastic Stents (n = 55) | cSEMS (n = 57) | P Value |
|---|---|---|---|
| Biliary sphincterotomy, No. (%) | |||
| No | 5 (9.1) | 4 (7) | .49 |
| Yes | 31 (56.4) | 27 (47.4) | |
| Previous | 19 (34.5) | 26 (45.6) | |
| Passage dilation, No. (%) | 4 (7.3) | 3 (5.3) | .66 |
| Balloon dilation, No. (%) | 44 (80) | 8 (14) | <.001 |
| Device’s maximal diameter for balloon dilation, No. (%) | |||
| 4 mm | 1 (2.3) | 1 (12.5) | .15 |
| 6 mm | 15 (34.9) | 5 (62.5) | |
| 8 mm | 22 (51.2) | 2 (25) | |
| >8 mm | 5 (11.6) | 0 | |
| cSEMS diameter, No. (%) | |||
| 8 mm | 17 (29.8) | ||
| 10 mm | 40 (70.2) | ||
| Cumulative plastic stent diameter, median (range), Fa | 20 (7–30) | ||
| No. of stents, No. (%)b | |||
| 1 | 15 (27.3) | 57 (100) | |
| 2 | 35 (63.6) | 0 | |
| 3 | 5 (9.1) | 0 |
Stricture Resolution and Recurrence
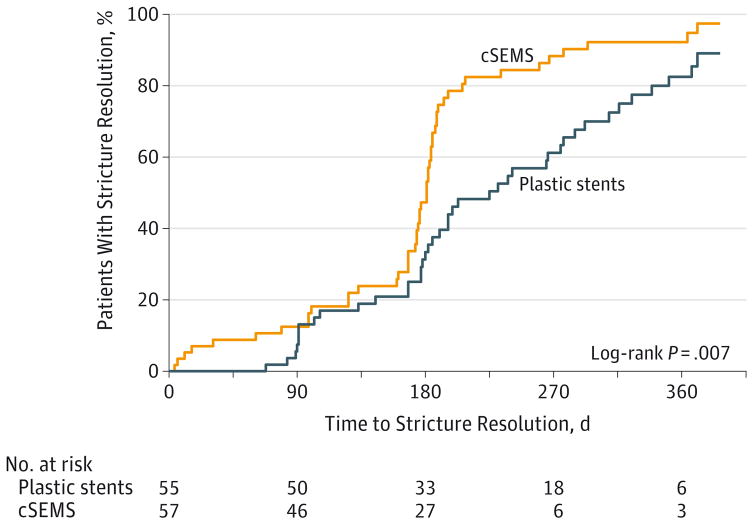
Compared with multiple plastic stents (41/48, 85.4%), the resolution rate was 50 of 54 patients (92.6%) for cSEMS with a rate difference of 7.2% (1-sided 95% CI, −3.0% to ∞; P < .001). Thus, the null hypothesis that cSEMS would be less effective than multiple plastic stents by at least −15% was rejected (because the lower bound of the 1-sided 95% CI lies above −15%). Sensitivity analysis assuming the worst-case scenario had similar findings. With a resolution rate of 87.7% (50/57) for the cSEMS group and 87.3% (48/55) for the multiple plastic stents group, the 1-sided 95% CI for the difference in resolution rates was −10% to ∞ and P = .007, again demonstrating the noninferiority of cSEMS. Despite patients in the plastic stent group having an opportunity to achieve stricture resolution earlier than patients in the cSEMS group (given their per-protocol ERCP reassessments every 3–4 months vs every 6 months for cSEMS), patients who received a cSEMS achieved stricture resolution at a significantly faster rate, as demonstrated by the Kaplan-Meier plot of time to resolution during the 12-month stenting period (Figure 2). The estimated median number of days to resolution based on the Kaplan-Meier method was 225 days (95% CI, 182 to 277 days) for the plastic stents group and 181 days (95% CI, 173 to 184 days) for the cSEMS group (log-rank P = .006). Among patients who achieved stricture resolution, the number of ERCPs required to achieve stricture resolution was significantly lower for those randomized to receive cSEMS vs multiple plastic stents (mean, 2.14 vs 3.24; mean difference, 1.10; 95% CI, 0.74 to 1.46; P < .001). These observations persisted in subgroup analyses of patients with posttransplant (n = 73) and chronic pancreatitis–induced (n = 35) strictures (eFigures 2 and 3 in Supplement 2).
Figure 2. Time to Stricture Resolution.
cSEMS indicates fully covered, self-expandable metallic stent.
Of those who achieved stricture resolution, 7 of 50 patients (14%) in the cSEMS group and 2 of 41 patients (4.9%) in the plastic stent group developed a recurrent biliary stricture (P = .18); of 9 recurrences, 6 occurred in post–liver transplant cases. Among patients in the cSEMS group, 2 of 7 patients with recurrence had achieved stricture resolution after only 4 and 6 days of stent therapy, respectively. In these patients, a repeat ERCP was performed for elevated liver test results at which time the stent was removed; the bile duct stricture had resolved per study definition, so no stents were replaced per study protocol. The remaining patients with stricture recurrence in both groups had undergone no less than 3 months of stent therapy.
Adverse Events
In patients who received plastic stents and cSEMS, all were deployed successfully; there were no cases of failed cSEMS or plastic stent removal during the first follow-up ERCP (technical success 100% for both groups). Similarly, there were no cases of stent-induced strictures in either group that required treatment. The mean number of adverse events per ERCP was 0.23 for plastic stent vs 0.36 for cSEMS (−0.13 difference; 95% CI, −0.29 to 0.03; P = .31), and the number of severe adverse events per ERCP was 0.06 for plastic stents vs 0.13 for cSEMS (−0.07 difference; 95% CI, −0.17 to 0.04; P = .72). Among those randomized to receive cSEMS, there were 16 cases of stent migration in 14 patients, all of which were distal to (below) the bile duct stricture (Table 3 and eTable 2 in Supplement 2); 9 of 16 cases were observed at the time of stricture resolution, and 7 of 16 cases occurred in the setting of a persistent stricture. In 2 cases, stent migration was severe because it required an urgent repeat ERCP for overt biliary obstruction. By comparison, there were 10 cases of plastic stent migration in 9 patients; 8 of 10 cases occurred in the setting of a persistent biliary stricture, 1 of which required urgent ERCP for overt biliary obstruction. We observed low rates of post-ERCP pancreatitis (5.4%), secondary bile duct changes (6.3%, none required treatment), and postprocedure abdominal pain (15.2%) in both groups.
Table 3.
Adverse Events
| Adverse Event | Multiple Plastic Stents (n = 55) | cSEMS (n = 57) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total No. of Events | No. of Patients With Event | No. of Severe AEs,a | No. of Patients With Severe AE | No. of Related AEsb | No. of PatientsWith Related AE | Total No. of Events | No. of PatientsWith Event | No. of Severe AEs, a | No. of Patients With Severe AE | No. of Related AEsb | No. of Patients With Related AE | |
| Stent migration | 10 | 9 | 1 | 1 | 5 | 5 | 16 | 14 | 2 | 2 | 16 | 14 |
| Abdominal pain | 9 | 8 | 1 | 1 | 4 | 4 | 8 | 8 | 2 | 2 | 6 | 6 |
| Premature stent occlusion | ||||||||||||
| Cholangitis | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 2 | 2 | 2 | 2 | 2 |
| Bile duct obstruction | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 1 |
| Jaundice | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 |
| Stent-induced changes to the bile duct | 5 | 5 | 0 | 0 | 3 | 3 | 2 | 2 | 0 | 0 | 2 | 2 |
| Post-ERCP pancreatitis | 3 | 3 | 0 | 0 | 3 | 3 | 3 | 3 | 0 | 0 | 2 | 2 |
| All othersc | 7 | 7 | 5 | 5 | 0 | 0 | 9 | 6 | 5 | 4 | 1 | 1 |
| Total | 37 | 27 | 8 | 8 | 16 | 14 | 42 | 28 | 12 | 9 | 30 | 23 |
Among patients randomized to receive cSEMS (n = 57), stent migration occurred more frequently in those with posttransplant anastomotic strictures (13/14 migration cases, 93%) compared with all others (24/43 nonmigration cases, 56%; P = .02). Although the sample size was limited, strictures associated with cSEMS migration were closer to the hilum (34 mm [range, 19–86 mm] vs 40 mm [range, 2.4–86 mm]; P = .08), and the bile duct below the stricture was larger (8 mm [range, 4.8–11 mm] vs 6 mm [range, 0–13.7 mm]; P = .03). The median length of the bile duct stricture was significantly shorter in those with migration (2 mm [range, 1–10 mm] vs 9 mm [range, 1–32 mm]; P < .001).
Discussion
The invention of self-expandable metallic stents addressed an unmet need for durable biliary drainage in the setting of malignant bile duct obstruction. Given their superior patency compared with plastic stents, self-expandable metallic stents are preferred for the first-line treatment of extrahepatic malignant bile duct strictures. Now, there is burgeoning interest in using cSEMS for benign bile duct strictures because their radial force permits sustained and maximal dilation of the stricture at the time of initial ERCP as well as longer intervals between stent exchanges.
While numerous previous studies have evaluated cSEMS as salvage and first-line options, these have been limited by retrospective and nonrandomized design, small sample sizes, and inclusion of patients with partially treated strictures.8,9,12–14 In this study, cSEMS were not inferior to multiple plastic stents when used as the initial treatment strategy but achieved resolution with significantly fewer ERCPs.15 In addition, there were no failures to remove a cSEMS at the first follow-up ERCP, possibly due to the study enrollment criteria and planned 6-month indwell period. While most prior cohort studies on this topic report very high success rates with cSEMS removal, in a minority of cases endoscopic removal may be difficult and require more than 1 ERCP to remove it successfully.8,9,13,16,17 Although this study was not powered to detect differences in adverse event rates, these data support prior observations that cSEMS can be removed safely and without a higher risk of procedure-related adverse events.
Recurrent Strictures
There was no statistically significant difference in the recurrence rate among patients randomized to receive cSEMS (14% vs 5% for plastic stents; P = .15). This study was not adequately powered to detect a difference in these rates, but it is possible that the recurrence rate would be lower if cSEMS were left in place for longer than 6 months. This will require further study.
Stent Migration
Stent migration remains an important limitation of currently available cSEMS and a focus of device development.18 This occurs more frequently in postoperative strictures rather than strictures caused by chronic pancreatitis given their more focal narrowing and proximal location relative to the major papilla. There were few symptomatic migrations, but several required retreatment because the stent had migrated below the stricture, leading to recurrent obstruction. A cSEMS with antimigration properties mitigates the risk of migration but may pose a higher risk of secondary duct injury; alternatively, the ideal stent would migrate or dissolve spontaneously after adequate stricture treatment. There remains a need to develop novel, expandable stents that may be used in smaller-diameter ducts and without the need for routine follow-up ERCPs to retrieve them. Stent migration is also an issue with multiple plastic stents. When using either type of stent, a biliary sphincterotomy is almost universally performed to facilitate placement of multiple plastic stents side-by-side or a large-diameter cSEMS. There are no conclusive data that placing a cSEMS or multiple plastic stents across an intact sphincter of Oddi increases the risk of post-ERCP pancreatitis or other complications.
Limitations
This study was limited by the absence of a cost analysis comparing the 2 treatment strategies. In addition, this study was limited by its open-label design and variable period of follow-up according to treatment allocation. The steering committee determined these limitations were unavoidable; universal follow-up at 2 months would have been a suboptimal duration of cSEMS indwell whereas delayed follow-up at 6 months for patients in the plastic stent group would have delayed the often inevitable need for stent up-sizing while exposing patients to a higher risk of premature stent occlusion. To minimize outcome bias in an open-label study, a quantitative definition was used for stricture resolution, and the protocol actually favors the plastic stent strategy in terms of faster time to resolution; patients randomized to receive multiple plastic stents had an opportunity to meet the definition of stricture resolution after 3 months, whereas patients randomized to receive cSEMS generally did not return until 3 months unless there was a clinical indication to perform ERCP earlier (such as elevation in liver test results). Still, a quantitative definition of stricture resolution remains susceptible to human interpretation of the fluoroscopic images.
Because the enrollment criteria included benign biliary strictures from chronic pancreatitis and postoperative etiologies, this study was not adequately powered to conduct subgroup analyses to compare the efficacy of cSEMS vs multiple plastic stents in each entity. For those with posttransplant strictures, the observed resolution rate after 12 months of stenting was noninferior (cSEMS, 91.7% vs plastic, 93.9%), but there was a higher recurrence rate among those randomized to receive cSEMS (5/33 [15.2%] vs 1/30 [3.3%] for plastic). These observations are limited by the small number of recurrent strictures yet are in line with prior observations.19 In addition, the investigators chose a noninferiority margin of −15% after careful discussion by members of the study steering committee. A smaller noninferiority margin would have strengthened the conclusions but would have been unlikely to change them substantially because the observed margin (+7.2%) actually favored the cSEMS group.
It is not clear how long to keep stents in place. Longer indwell periods for cSEMS may increase the risk for secondary bile duct injuries, migration, and stent removal, while prolonged indwell periods for plastic stents (after stricture resolution) increase the risk of premature stent occlusion and excess utilization of ERCPs. The optimal indwell period likely depends on the etiology of biliary stricture. Because the objective was to study first-line treatments for bile duct stricture and avoided refractory or recurrent strictures that often obligate more aggressive and longer periods of stenting, the study protocol reflects standard practice.1 In addition, the observed resolution and recurrence rates were comparable with those in previous studies.3,20
The generalizability of these findings was compromised by the study’s strict enrollment criteria, particularly avoiding patients with small (<6 mm) extrahepatic bile ducts and those in whom the cystic duct might have been occluded by an overlapping cSEMS. While the risk of SEMS-induced acute cholecystitis is controversial in studies evaluating SEMS for malignant biliary strictures, the authors determined that a more cautious approach in this patient population would be prudent. The safety of cSEMS in these 2 settings will require further study.
The poststenting follow-up period of 12 months may be inadequate for determining overall stricture recurrence rates. We chose a 12-month follow-up period because of assumptions that delayed stricture recurrence rates would be less likely to differ across treatment strategies. This will require further study.
Conclusions
Among patients with benign biliary strictures of 6 mm or larger in whom the covered metallic stent would not overlap the cystic duct, cSEMS were not inferior to multiple plastic stents in achieving stricture resolution after no more than 12 months of endoscopic therapy. Metallic stents should be considered an appropriate option in patients such as these.
Supplementary Material
Supplemental materials
Acknowledgments
Funding/Support: Research reported in this publication was supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health (R21DK090708). This research was also supported by an American Society for Gastrointestinal Endoscopy Career Development Award (Dr Coté).
Footnotes
Role of the Funder/Sponsor: Neither sponsor had a role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Additional Contributions: We thank the members of the data and safety monitoring board, none of whom were compensated for their contributions: Robert H. Hawes, MD, Florida Hospital, Orlando; Lois G. Bucksot, BS, Indiana University Health, Indianapolis; and Debra J. Helper, MD (chairperson), Indiana University School of Medicine, Indianapolis. Also, we are grateful to the research coordinators at each of the participating centers for their diligent efforts. No one received compensation for their contributions: Kathleen Bauer, BS, University of Pittsburgh Medical School, Pittsburgh, Pennsylvania; Nancy L. Rosales, AS, Dallas Methodist Medical Center, Dallas, Texas; Sheryl J. Korsnes, MA, University of Michigan, Ann Arbor; Marie Green, BS, Royal Wolverhampton Trust, Wolverhampton, United Kingdom; April Wood, BS, Medical University of South Carolina, Charleston; Teresa L. Radake, BS, Washington University in St Louis, St Louis, Missouri; Ann Koons, BS, University of Chicago, Chicago, Illinois. In addition, we are grateful to the patients who participated in this study.
Author Contributions: Drs Coté and Xu had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Coté, Fogel, McHenry, Watkins, Xu, Sherman.
Acquisition, analysis, or interpretation of data: Coté, Slivka, Tarnasky, Mullady, Elmunzer, Elta, Fogel, Lehman, McHenry, Romagnuolo, Menon, Siddiqui, Lynch, Denski, Xu, Sherman.
Drafting of the manuscript: Coté, Xu.
Critical revision of the manuscript for important intellectual content: Coté, Slivka, Tarnasky, Mullady, Elmunzer, Elta, Fogel, Lehman, McHenry, Romagnuolo, Menon, Siddiqui, Watkins, Xu, Sherman.
Statistical analysis: Xu.
Obtained funding: Coté, Sherman.
Administrative, technical, or material support: Coté, Elmunzer, McHenry, Watkins, Denski.
Study supervision: Coté, Denski, Xu, Sherman.
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr Coté reported having been a consultant for Boston Scientific, Cook Medical, and Olympus America. Dr Slivka reported having been a consultant for and having received grants from Boston Scientific and having received other support from Mauna Kea Technology and Pinnacle Biologics. Dr Tarnasky reported having been a speaker and consultant for Boston Scientific. Dr Elta reported having been a consultant for Olympus Medical. Dr Lehman reported having received grants or other support from Cook Endoscopy, Olympus, Boston Scientific, and Medigus. Dr McHenry reported having received grants or other support from Cook and Conmed. Dr Romagnuolo reported having received a grant for his institution from the American Society for Gastrointestinal Endoscopy. Dr Sherman reported having received support from Boston Scientific and Olympus. No other disclosures were reported.
References
- 1.Dumonceau JM, Tringali A, Blero D, et al. European Society of Gastrointestinal Endoscopy. Biliary stenting: indications, choice of stents and results: European Society of Gastrointestinal Endoscopy (ESGE) clinical guideline. Endoscopy. 2012;44(3):277–298. doi: 10.1055/s-0031-1291633. [DOI] [PubMed] [Google Scholar]
- 2.Warshaw AL, Schapiro RH, Ferrucci JT, Jr, Galdabini JJ. Persistent obstructive jaundice, cholangitis, and biliary cirrhosis due to common bile duct stenosis in chronic pancreatitis. Gastroenterology. 1976;70(4):562–567. [PubMed] [Google Scholar]
- 3.Costamagna G, Pandolfi M, Mutignani M, Spada C, Perri V. Long-term results of endoscopic management of postoperative bile duct strictures with increasing numbers of stents. Gastrointest Endosc. 2001;54(2):162–168. doi: 10.1067/mge.2001.116876. [DOI] [PubMed] [Google Scholar]
- 4.Draganov P, Hoffman B, Marsh W, Cotton P, Cunningham J. Long-term outcome in patients with benign biliary strictures treated endoscopically with multiple stents. Gastrointest Endosc. 2002;55(6):680–686. doi: 10.1067/mge.2002.122955. [DOI] [PubMed] [Google Scholar]
- 5.Rossi P, Bezzi M, Salvatori FM, Maccioni F, Porcaro ML. Recurrent benign biliary strictures: management with self-expanding metallic stents. Radiology. 1990;175(3):661–665. doi: 10.1148/radiology.175.3.2343110. [DOI] [PubMed] [Google Scholar]
- 6.Deviere J, Cremer M, Baize M, Love J, Sugai B, Vandermeeren A. Management of common bile duct stricture caused by chronic pancreatitis with metal mesh self expandable stents. Gut. 1994;35(1):122–126. doi: 10.1136/gut.35.1.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Berkel AM, Cahen DL, van Westerloo DJ, Rauws EA, Huibregtse K, Bruno MJ. Self-expanding metal stents in benign biliary strictures due to chronic pancreatitis. Endoscopy. 2004;36(5):381–384. doi: 10.1055/s-2004-814319. [DOI] [PubMed] [Google Scholar]
- 8.Haapamäki C, Kylänpää L, Udd M, et al. Randomized multicenter study of multiple plastic stents vs covered self-expandable metallic stent in the treatment of biliary stricture in chronic pancreatitis. Endoscopy. 2015;47(7):605–610. doi: 10.1055/s-0034-1391331. [DOI] [PubMed] [Google Scholar]
- 9.Devière J, Nageshwar Reddy D, Püspök A, et al. Benign Biliary Stenoses Working Group. Successful management of benign biliary strictures with fully covered self-expanding metal stents. Gastroenterology. 2014;147(2):385–395. doi: 10.1053/j.gastro.2014.04.043. [DOI] [PubMed] [Google Scholar]
- 10.Piaggio G, Elbourne DR, Pocock SJ, Evans SJ, Altman DG CONSORT Group. Reporting of noninferiority and equivalence randomized trials: extension of the CONSORT 2010 statement. JAMA. 2012;308(24):2594–2604. doi: 10.1001/jama.2012.87802. [DOI] [PubMed] [Google Scholar]
- 11.Abraha I, Montedori A. Modified intention to treat reporting in randomised controlled trials: systematic review. BMJ. 2010;340:c2697. doi: 10.1136/bmj.c2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saxena P, Diehl DL, Kumbhari V, et al. A US multicenter study of safety and efficacy of fully covered self-expandable metallic stents in benign extrahepatic biliary strictures. Dig Dis Sci. 2015;60(11):3442–3448. doi: 10.1007/s10620-015-3653-5. [DOI] [PubMed] [Google Scholar]
- 13.Siiki A, Helminen M, Sand J, Laukkarinen J. Covered self-expanding metal stents may be preferable to plastic stents in the treatment of chronic pancreatitis-related biliary strictures: a systematic review comparing 2 methods of stent therapy in benign biliary strictures. J Clin Gastroenterol. 2014;48(7):635–643. doi: 10.1097/MCG.0000000000000020. [DOI] [PubMed] [Google Scholar]
- 14.Kao D, Zepeda-Gomez S, Tandon P, Bain VG. Managing the post-liver transplantation anastomotic biliary stricture: multiple plastic versus metal stents: a systematic review. Gastrointest Endosc. 2013;77(5):679–691. doi: 10.1016/j.gie.2013.01.015. [DOI] [PubMed] [Google Scholar]
- 15.Kaffes A, Griffin S, Vaughan R, et al. A randomized trial of a fully covered self-expandable metallic stent versus plastic stents in anastomotic biliary strictures after liver transplantation. Therap Adv Gastroenterol. 2014;7(2):64–71. doi: 10.1177/1756283X13503614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kahaleh M, Brijbassie A, Sethi A, et al. Multicenter trial evaluating the use of covered self-expanding metal stents in benign biliary strictures: time to revisit our therapeutic options? J Clin Gastroenterol. 2013;47(8):695–699. doi: 10.1097/MCG.0b013e31827fd311. [DOI] [PubMed] [Google Scholar]
- 17.Traina M, Tarantino I, Barresi L, et al. Efficacy and safety of fully covered self-expandable metallic stents in biliary complications after liver transplantation: a preliminary study. Liver Transpl. 2009;15(11):1493–1498. doi: 10.1002/lt.21886. [DOI] [PubMed] [Google Scholar]
- 18.Walter D, Laleman W, Jansen JM, et al. A fully covered self-expandable metal stent with antimigration features for benign biliary strictures: a prospective, multicenter cohort study. Gastrointest Endosc. 2015;81(5):1197–1203. doi: 10.1016/j.gie.2014.10.026. [DOI] [PubMed] [Google Scholar]
- 19.Alazmi WM, Fogel EL, Watkins JL, et al. Recurrence rate of anastomotic biliary strictures in patients who have had previous successful endoscopic therapy for anastomotic narrowing after orthotopic liver transplantation. Endoscopy. 2006;38(6):571–574. doi: 10.1055/s-2006-925027. [DOI] [PubMed] [Google Scholar]
- 20.Tabibian JH, Asham EH, Han S, et al. Endoscopic treatment of postorthotopic liver transplantation anastomotic biliary strictures with maximal stent therapy (with video) Gastrointest Endosc. 2010;71(3):505–512. doi: 10.1016/j.gie.2009.10.023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental materials