Microtubule Dynamics: an interplay of biochemistry and mechanics (original) (raw)
. Author manuscript; available in PMC: 2019 Jan 1.
Published in final edited form as: Nat Rev Mol Cell Biol. 2018 Jul;19(7):451–463. doi: 10.1038/s41580-018-0009-y
Abstract
Microtubules are dynamic polymers of αβ-tubulin that are essential for intracellular organization and chromosome segregation. Microtubule growth and shrinkage occur via addition and loss of αβ-tubulin subunits — biochemical processes. Dynamic microtubules can also exert forces by pushing or pulling against a load – mechanical processes. Recent advances at the intersection of biochemistry and mechanics have revealed the existence of multiple conformations of αβ-tubulin and their central role in dictating the mechanisms of microtubule dynamics and how microtubules do work. Microtubule associated proteins selectively target specific tubulin conformations to regulate microtubule dynamics, and mechanical forces can also influence microtubule dynamics by altering the balance of tubulin conformations. Importantly, the conformational states of tubulin dimers appear to be coupled throughout the lattice, in that the conformation of one dimer affects the conformation of its nearest neighbors and beyond. This coupling provides a long-range mechanism by which MAPs and forces can modulate microtubule growth and shrinkage. These findings provide evidence that the interplay between biochemistry and mechanics is essential for the cellular functions of microtubules.
Introduction
Microtubules are long, stiff polymers of αβ-tubulin that are structurally and functionally important components of the eukaryotic cell cytoskeleton, forming the mitotic spindle, the axonemes of cilia and flagella and serving as tracks for intracellular trafficking 1. Microtubules are highly dynamic. They grow by the addition of GTP-tubulin dimers to the microtubule end, where a “stabilizing cap” is formed because GTP hydrolysis does not happen instantaneously2. This cap, which is enriched in GTP-tubulin, recruits “end-binding” (EB) proteins and a host of other microtubule-associated proteins (MAPs), including microtubule polymerases, depolymerases, and kinesins, which cooperate and compete to determine the microtubule’s fate3–5. When the stabilizing cap is lost, the microtubule shrinks rapidly in an event known as a “catastrophe”. Occasionally, a rapidly shrinking microtubule is “rescued”, switching from shrinkage back to growth. This switching behavior, known as dynamic instability 6, can be harnessed by cells to exert forces, for example during chromosome segregation in mitosis 7.
Biochemical and mechanical phenomena are intimately intertwined in microtubules: rapid turnover and dynamic instability coexist with the rigidity of a stiff polymer 8. This interplay between biochemistry and mechanics allows microtubules to be viewed from multiple perspectives. A biochemical perspective views microtubules as a collection of discrete subunits 9 that form a lattice with two main modes of interaction (Fig. 1). Recent developments in single-molecule microscopy 10 allow microtubule growth to be tracked with a resolution of ~40 nm 11; this has enabled characterization of the behaviour of the stabilizing cap immediately prior to a catastrophe 12 and of the stochastic fluctuations in length that occur during elongation 13,14. Recent measurements like these provide new insights into the biochemistry that underlies microtubule dynamics. A mechanical perspective, on the other hand, views microtubules as a continuous material 9. Indeed, seminal measurements showed that microtubule stiffness is comparable to that of plexiglass 15.
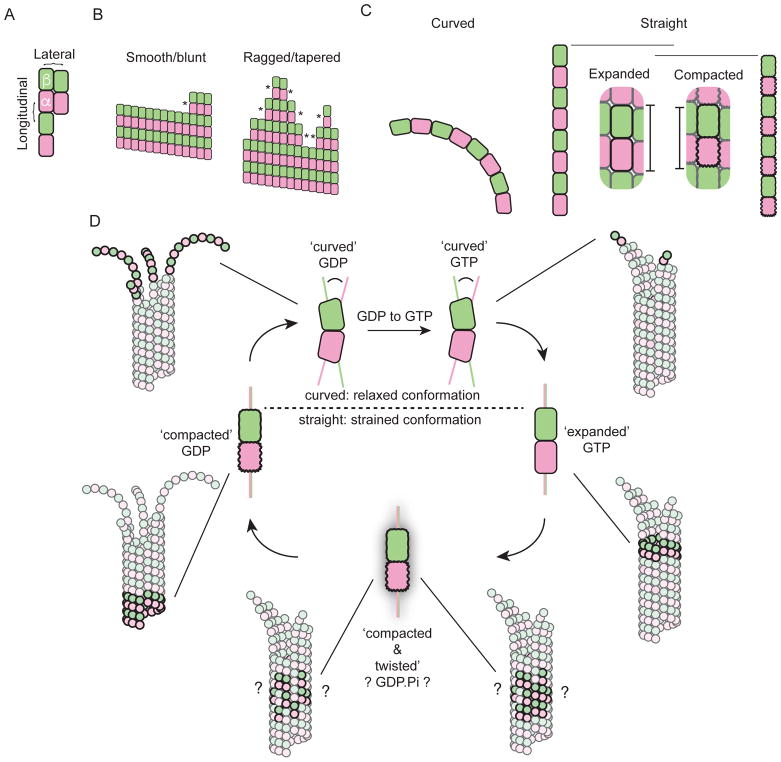
Figure 1.
Tubulin biochemistry, conformation, and mechanics. A. Cartoon illustrating the fundamental longitudinal (vertical) and lateral (horizontal) interactions between αβ-tubulins (pink and green) that make up the microtubule lattice. B. Two dimensional schematics of blunt (few ‘corner’ sites, *) and ragged/tapered (more corner sites, *) microtubule ends. Taper refers to the extension of some protofilaments beyond others; ragged describes an uneven or rough distribution of protofilament lengths. C. Cartoons of longitudinal assemblies of αβ-tubulin illustrating the three major conformations in structural detail. (left) Tubulin adopts a curved conformation in single protofilaments and when unpolymerized. (right) Tubulin adopts straight ‘expanded’ or ‘compacted’ conformations in the body of the microtubule. The horizontal bars and the inset indicate that the compacted conformation is somewhat ‘shorter’ than the expanded one. D. The tubulin conformation cycle is a mechanical cycle. Straight conformations of αβ-tubulin are strained but stabilized by interactions with the microtubule lattice (not illustrated here). Release of that strain during depolymerization can do mechanical work. The dark/bold subunits in the thumbnail cartoons of growing or shrinking microtubules indicate the region of the microtubules where the different conformations occur. The ‘glow’ indicates the compacted and twisted state, and the ?’s indicate uncertainty about whether this conformation reflects a specific nucleotide state and about the distribution of these sites in the microtubule.
The distinction between biochemical and mechanical perspectives is artificial9, of course, because mechanical phenomena operate at the level of discrete subunits (Fig. 1C,D). The biochemical cycle of tubulin involves GTP-tubulin binding to the microtubule end, hydrolyzing the GTP and releasing phosphate while in the lattice, and then dissociating from the microtubule as GDP-tubulin. This review will advance the argument that changes in tubulin conformation that accompany this biochemical cycle are mechanical in nature. Conformational changes associated with nucleotide hydrolysis are harnessed to do mechanical work by a wide range of oligomeric proteins (e.g. 16,17 for some recent examples). We will argue that microtubules are unique because of the structural complexity of their growing and shrinking ends. First, we discuss the dynamics of the microtubule end and describe evidence that tubulin dimers in the growing end transition through multiple conformations in response to the biochemical cycle of GTP hydrolysis. We also describe new data that emphasize the importance of the tubulin conformational cycle for understanding how microtubule dynamics generates forces and how forces can affect microtubule dynamics. We then discuss data that makes it increasingly apparent that the conformational cycle of tubulin dimers is targeted by regulatory factors that control microtubule dynamics, providing additional evidence for the intimate links between mechanical and biochemical processes in microtubule dynamics and regulation18. Finally, we propose that long-range coupling between tubulin subunits may be the key for understanding microtubule dynamics, because it provides a framework that integrates mechanical and biochemical properties in the lattice.
Tubulin cycle at the microtubule end
The transition of a GTP-tubulin dimer in solution into a GDP-tubulin dimer entombed in the microtubule lattice is a complex process that involves multiple conformational changes. Below, we describe the structure that catalyzes this transition, the microtubule end, and the mechanical phenomena that underlie this process.
Structure of the microtubule end
For growing microtubules, “the end” is often synonymous with the “stabilizing cap”, which describes the extended region near the growing end that is enriched in GTP-tubulin subunits. Although the exact distribution of nucleotide states is currently not known, the stabilizing cap contains several hundred tubulin dimers 19. In cryo-EM, growing microtubule ends have been observed in a broad range of structures, from short, blunt ends 20 to long, outwardly-curved, and flattened structures interpreted as “sheets” of tubulin 21. As a result, “the microtubule end” remains poorly defined. Our lack of certainty can be observed in the diverse schematics of microtubule ends that appear in reviews, research articles, and textbooks. These schematics range from large, elaborate sheets to simple, tapered structures that neglect the curvature of microtubule ends entirely. The most common schematics depict the growing microtubule end as a funnel. These funnels appear in undergraduate textbooks 22 and on conference posters. While scientists are not necessarily artists, and we differ in our tolerance for flourish, it’s fair to say that the field has no consensus about what a microtubule end actually looks like.
The major features of microtubule ends are nevertheless consistent across the literature: some protofilaments are longer than others, the distal protofilaments curve outward to some degree, and there is a ‘seam’ where lattice contacts are perhaps weaker because the helical symmetry is interrupted there23,24. Box 1 describes our best faith effort to illustrate average values for taper length and curvature that are consistent with findings from cryo-EM25,26 computational modelling 27,28 and fluorescence microscopy 11,13. The data indicate that the microtubule end is tapered, ragged, and variably-curved (Box 1), even if the specifics of these features remain poorly determined.
Box 1. Model for a growing microtubule end, and how it was constructed.
The structure of the microtubule end is currently elusive. Here, we show our best-faith effort to construct an atomic model of a growing microtubule end (see figure). We considered two features that distinguish growing microtubule ends from the rest of the lattice, for which atomic models exist 127. First, microtubule ends are tapered, with a “taper-length” defined as the distance between the longest protofilament and the fully-closed lattice. We chose a taper-length of 124 nm, which is consistent with the average value measured directly by cryo-EM (Fig. 3B of 25) and with estimates based on sub-pixel fitting of fluorescence images (Fig 5D of 13 and Fig. S2 of 11). Second, microtubule ends are variably curved due to the fact that GTP-tubulin adopts a curved conformation. Our model was informed by a theory wherein microtubule ends are shaped by two opposing forces: (1) the tendency to curve outward due to the intrinsic curvature of GTP-tubulin 30,33–35 and (2) the tendency to straighten due to lateral interactions between subunits that close up the lattice 125. The precise relationship between curvature of the tip and its width is not understood. We assumed: (i) that a section of the microtubule end becomes fully-straight 24 when it is at least 6 αβ-tubulins wide (Fig. 4a″ of 25); (ii) sections of the microtubule end that are 2 αβ-tubulins wide are approximately half-straightened 26; (iii) sections of the microtubule end that are 3–5 subunits wide become progressively straighter 125. Conformations with curvature in between half-straight 26 and straight 24 were approximated by interpolation (‘morphing’). Finally, we added some “raggedness” to the end, a feature that occurs frequently in computer simulations of microtubule dynamics 27,28.
Our final model used an average value for taper-length. The stochastic nature of subunit addition and loss will cause the taper length and overall curvature of growing microtubule ends to fluctuate considerably around their average values. These and other uncertainties highlight the challenge of understanding how the biochemical and mechanical properties of individual αβ-tubulin subunits dictate the structure and dynamics of the growing microtubule end.
Tubulin dimers in the cap undergo a mechano-chemical cycle
Individual dimers pass through at least three conformations during microtubule growth and shrinkage (see Figure 1C, D). These conformations are described as (1) curved (Fig. 1C), (2) expanded (Fig. 1C), and (3) compacted (Fig. 1C) based on recent cryo-EM reconstructions 24,29 and crystal structures 30. We argue that these conformations can be considered “mechanical states” because they are part of a cycle that stores and releases strain energy (Fig. 1D) 31,32. Unpolymerized GTP-tubulin dimers start out in the curved conformation 33–35 (reviewed in 18), which is characterized by a ~12° kink at the intradimer interface. After binding to the microtubule end, the dimers straighten into an expanded conformation, a process that is thought to introduce strain energy into the microtubule lattice. Following GTP hydrolysis and phosphate release, the dimers compact in the lattice, shortening in length by 3 Å through a movement of a subdomain of α-tubulin (Fig. 1C) 29. Whether microtubules from all species undergo compaction has not yet been established. Indeed, recent structures of yeast microtubules did not show compaction in the GDP lattice36,37. Regardless, after catastrophe, GDP-tubulin dimers relax back to the curved state, peeling outward into the “ram’s horns” observed by cryo-EM 20. This relaxation releases the strain energy stored in the dimer, which can be harnessed to perform work 32, and completes the mechanical cycle (Fig. 1D).
Mechanics in the tubulin cycle
Direct evidence that the conformational cycle of tubulin is mechanical can be found in studies of how microtubules generate and respond to force. A growing microtubule generates a pushing force when it encounters a barrier 38; this pushing force can center microtubule asters 39, mitotic spindles 40, and nuclei 41. Conversely, a shrinking microtubule generates a pulling force on objects that are “coupled” to the microtubule end. The signature “coupler” is the kinetochore 32, a large molecular machine that attaches chromosomes to the growing and shrinking ends of a microtubule. Pulling forces arise from the outward peeling of protofilaments, which either push directly on the coupler 42 or bias its diffusion 43 on the microtubule lattice. Importantly, compressive stress and tensile stress generated by these forces can affect the dynamic instability of microtubules themselves.
The response to tensile stresses generated by pulling forces provides a clear example of forces altering the mechanical state of tubulin. Tensile stresses occur when a shrinking microtubule pulls on a “coupler” such as a kinetochore or, conversely, when the coupler pulls on a growing microtubule end. Both cases of tensile stress can be reconstituted in vitro and studied using optical tweezers. The couplers in these in vitro assays have included direct linkages 42, purified kinetochore proteins 43–46, as well as intact kinetochore particles from budding yeast 47. Remarkably, applying tension to a growing microtubule via an intact kinetochore particle causes the microtubule to grow faster 47. Similarly, applying tension via a microtubule polymerase in the XMAP215 family also caused faster growth48 although other coupling proteins did not45. Perhaps tension increases the enzymatic cycle of XMAP215 family polymerases? Kinetochores contain a subunit with microtubule polymerase activity (e.g., in budding yeast the polymerase is Stu2 49, an XMAP215 homolog), but, surprisingly, kinetochore particles completely depleted of Stu2 retain the ability to make microtubules grow faster 50. So why does tension applied through intact kinetochore particles lead to faster microtubule growth? The explanation may be that tension applied to the microtubule end may alter the distribution of tubulin conformations at the microtubule end and/or its taper and curvature. According to this view, pulling on a microtubule might promote the straightening of curved dimers at the end or antagonize the compaction of expanded dimers in the stabilizing cap. It remains unclear how such changes would lead to faster growth, and why intact kinetochores particles and XMAP215 do this while other couplers do not.
The key to understanding the relationship between microtubule dynamics and forces will be continued measurements of the relationship between the two. As with the experiments above, optical trapping currently provides the best way to do this. In a recent study, the amount of work performed by a shrinking microtubule was measured using optical tweezers 51, building upon a related assay pioneered by others 42. In this assay, the depolymerizing microtubules “push” on a bead, and the magnitude of the bead deflection reflects the shape of the curling protofilaments. Interestingly, increasing tension on the bead led to reduced deflections, indicating that tension was reducing the curvature of the outwardly peeling protofilaments. The work output per tubulin dimer was estimated at ~4.7 _k_BT, which is approximately 25% of the free energy available from GTP hydrolysis. This efficiency of this conversion of free energy to work is similar to that demonstrated for molecular motors like kinesin and dynein 52,53. It is commonly assumed that the strain energy released as work is what makes microtubules shrink rapidly after a catastrophe. However, microtubules harbouring a slow-shrinking β-tubulin mutant (T238V, see Box 2) were shown to perform the same work as wild-type 51. This counter-intuitive result could be explained by a physical model in which the T238V mutant makes stronger lateral bonds and the shrinking rate is determined by the breaking of lateral bonds rather than by the dissociation of dimers 51. But the T238V mutation is buried and far from tubulin:tubulin interfaces, so its stronger lateral bonds cannot be explained by direct perturbation of that interface. Instead, the stronger lateral bonds must arise from an allosteric effect. Indeed, mutations at T238 alter the conformational cycle of αβ-tubulin dimers in the lattice 51,54. Therefore, one hypothesis is that T238 mutants adopt a more GTP-like conformation in the GDP lattice, and this shift in conformational preference gives rise to stronger lateral bonds. Testing hypotheses like these will require structures of mutant tubulins. Whatever the precise structural mechanism, these optical trapping studies demonstrate that the balance of αβ-tubulin conformations in the lattice influences microtubule dynamics by tuning the strength of lateral bonds.
Box 2. Recombinant αβ-tubulin: a new tool to study microtubule dynamics.
The current understanding of microtubule biochemistry has relied almost exclusively on αβ-tubulin derived from mammalian (mostly bovine) brain preparations. While ‘brain preps’ have large yields, the resulting αβ-tubulin is a mixture of multiple α- and β-tubulin isotypes and varies in its degree of post-translational modifications128. The path to recombinant αβ-tubulin began with its purification from genetically tractable model organisms129,130. Subsequent work showed that fusing an affinity tag to β-tubulin could improve purification without compromising function131, showed isotype-specific polymerization dynamics132, and probed the binding sites for taxol133 and kinesin134,135. The potential value of recombinant tubulin was clear.
These early methods had poor yields and were restricted to viable mutants. Inducible overexpression in yeast136 improved yields and expanded the scope of purifiable mutants to include non-viable mutants. Methods for purifying recombinant human αβ-tubulin from baculovirus-infected insect cells soon followed137–139.
Illuminating mechanisms of MAPs and motors
A polymerization-blocked mutant of yeast tubulin136 led to structures of complexes between αβ-tubulin and the TOG1 or TOG2 domains from Stu2 (see main text)81,82. Recombinant point mutants of tubulin created microtubules that separated the motility and depolymerase activities of the kinesin-8 family Kip373. Motor proteins generally interact with the unstructured C-termini of αβ-tubulin, which previously could only be removed by proteolytic cleavage. Recombinant, chimeric microtubules in which human C-terminal tails were fused to a yeast tubulin core, demonstrated that specific tubulin tails or post-translational modifications affected different motor proteins in distinct ways140.
Revealing properties of individual tubulin isotypes
Humans have 7 α-tubulin genes and 8 β-tubulin genes, some only expressed in specific cell types. Studies with recombinant, single-isotype mammalian αβ-tubulin discovered different polymerization dynamics for the isotypes relative to bovine brain tubulin138,139,141, echoing observations for different yeast isotypes132. Although αβ-tubulin isotypes differ mainly in the sequence of their C-terminal tails, in at least one case the differences in dynamics could be attributed to sequence variation in the more conserved structured core of the protein141. Thus, all positions, not just those in the tails, can contribute to the functional divergence of tubulin genes.
Insights into allosteric properties of the lattice
Insight into the connection between the αβ-tubulin conformational cycle and polymerization dynamics came from studies of the buried T238A mutation in the yeast β-tubulin54. β:T238 mutations suppress catastrophe, slow microtubule shrinking, and increase spontaneous nucleation, with little effect on microtubule growth rates54. β:T238 αβ-tubulin retains a GTP-like conformation in the GDP lattice51,54, providing a structural correlate to the observed changes in dynamics. Point mutations in β-tubulin associated with a human genetic disorder altered microtubule growth rates and/or catastrophe frequencies, even though the mutations are not found at polymerization interfaces138, but the underlying mechanisms remain to be clarified.
Together, these studies emphasize the mechanical nature of the αβ-tubulin conformational cycle. Microtubules push and pull, and the energetics of individual dimers are altered by the resulting compressive and tensile stresses.
Conformational cycle and the role of MAPs
The conformational cycle of tubulin establishes the size and composition of the microtubule’s stabilizing cap, loss of which is the cause of dynamic instability 6. In recent years, enabled by improved fluorescence microscopy assays 10, studies of the EB proteins 4,11,12,19,55–60 have provided a wealth of new insights into the size, composition, and dynamics of the stabilizing cap. Studies of EBs and other MAPs have revealed that MAPs take advantage of the tubulin conformational cycle in order to regulate microtubule dynamics. Yet knowledge about the distributions of tubulin conformations and nucleotide states in the stabilizing cap, and how they relate to each other, remains an important gap in understanding. In this section we will discuss how MAPs interact with the mechanical cycle of tubulin, providing evidence that biochemical regulation of microtubule dynamics through MAPs is strongly tied to the conformational changes of tubulin dimers.
Interplay between EB proteins and tubulin conformations
Autonomous plus-end tracking by an EB protein was first reconstituted in vitro using Mal3, the S. pombe EB ortholog 19. Mal3 marked a “comet” region hundreds of nm in length (Fig. 2A) and also increased the frequency of microtubule catastrophe 19. Similar observations followed for vertebrate EBs 57. Single-molecule and kinetic analyses revealed the high-affinity EB-binding site on the microtubule to be transient, with a lifetime of about 10s 19. Simultaneous imaging of the comet and the microtubule demonstrated that the comet region does not extend all the way to the very end of the growing microtubule 11. Instead, there is an “EB-free” zone at the very end (Fig. 2A) 11 that might overlap with the partially curved, tapered region 25. The presence of this zone indicates that the high-affinity EB binding site takes time to develop.
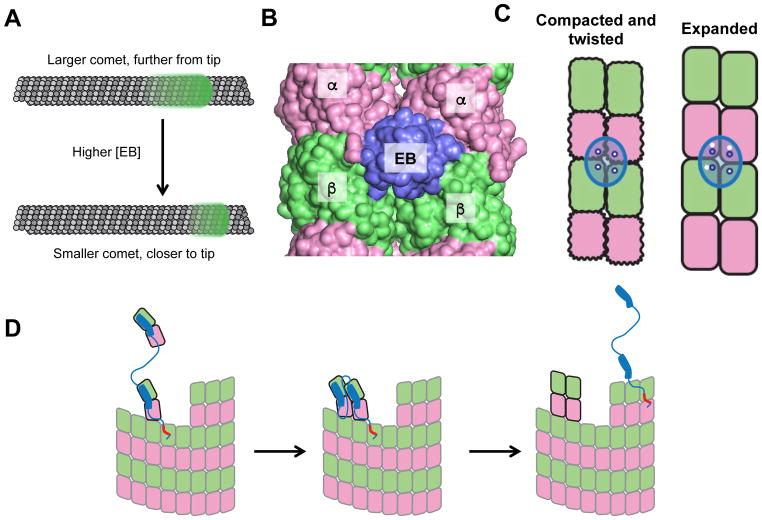
Figure 2.
Regulatory proteins and the tubulin conformation cycle. A. EBs form a ‘comet’ (green glow) near but somewhat behind the growing microtubule end (top). Increasing the concentration of EB (arrow) reduces the size of the comet and moves it closer to the growing end (bottom). B. EB (purple) binds at a vertex of 4 αβ-tubulin dimers (α: pink; β: green) in the lattice. C. EB (purple oval; the 4 empty blue circles illustrate the arrangement of EB’s tubulin-binding epitopes) binds most tightly to a ‘compacted and twisted’ conformation of αβ-tubulin. Red circles indicate the EB-contacting surfaces on the lattice (white circles). EB binds poorly to the expanded lattice because the EB-contacting epitopes are improperly spaced (mismatch between white and blue circles). D. Conformation-based mechanism of a microtubule polymerase. Linked TOG domains like those in Stu2 bind selectively to curved tubulin and increase the rate of tubulin:microtubule associations by concentrating unpolymerized tubulin near the microtubule end via a tethering mechanism (left). The tethered TOG-bound tubulin associates faster with the microtubule end, and lateral interactions between TOG-bound tubulins on the microtubule end (middle) leads to straightening, which releases the TOGs (right) for another round of incorporation. The red segment indicates a basic region that mediates lattice binding.
Interestingly, increasing the concentration of EB decreases the size of the EB comet and causes it to shift closer to the microtubule end (Fig. 2A) 11, thereby reducing the size of the “EB-free” zone. Thus, it seems clear that EBs both recognize particular sites on the lattice and influence the creation and destruction of these sites. These EB concentration-dependent effects have led to the hypothesis that EBs are “maturation factors” that increase the rate of GTP hydrolysis in the lattice 11,24. This hypothesis explains how increasing the concentration of EB can increase the catastrophe frequency, because faster GTP hydrolysis should lead to smaller stabilizing caps. By revealing and quantifying a transient and distinctive region at the growing end of MTs that could be marked and altered by EBs, these studies provided provocative new insight into the molecular events occurring near the growing microtubule end.
Although the dynamics of EB comets have been measured with increasing accuracy and precision 11, the nature of the high-affinity EB site remains poorly defined. Important clues have come from experiments using GTP analogs. EBs bind with comparable affinity to comets and to static microtubules containing GTPγS, a hydrolysis-resistant GTP analog, so GTPγS microtubules are thought to mimic the comet region of growing microtubules 58. In contrast, EBs bind less tightly to microtubules containing GMPCPP, a different hydrolysis-resistant GTP analog that yields an ‘expanded’ conformation of tubulin in the lattice 29. GMPCPP is thought to provide the best model for the GTP state of the lattice because it supports microtubule polymerization better than GTPγS or other analogs 61. These experiments have been taken to suggest that EB proteins bind preferentially to the GDP-Pi state.
The first cryo-EM reconstruction of EB-bound GTPγS microtubules showed that EBs bind at the vertex of four αβ-tubulin dimers (Fig. 2B) 59. At higher resolution, αβ-tubulin in the EB-bound GTPγS lattice adopted a “twisted and compacted” conformation that differs from the compacted, GDP-bound conformation with respect to the relative rotation between αβ-tubulins along a protofilament (Fig. 2C) 24. Consistent with this structural difference, EBs bind to the GDP lattice about 10-fold less tightly than they bind to the comet region or GTPγS microtubules 58. EB binding can also influence the conformation of tubulin in the lattice 36. The EB-GTPγS structure may indicate that EBs bind preferentially to a “post-GTPase” nucleotide state of the lattice such as GDP-Pi 24. It is not yet clear whether EBs from all species induce/prefer a “twisted and compacted” state; the structure of Mal3 bounds to S. pombe microtubules was distinct37. Another caveat is that EB proteins have some affinity for all lattices58,60, a fact which may explain why EBs have been observed by cryo-EM within the “EB-free zone25.” Finally, whether GTPγS faithfully mimics a GDP. Pi state is not known. Nevertheless, preferential binding to a post-GTPase state of the lattice would also explain the “EB-free zone” at the very end of a microtubule, because in this zone tubulin would be in its expanded conformation.
This “post-GTPase” hypothesis is at odds with the observation that increasing the concentration of EB decreases the size of the EB comet 11; this effect on comet size has been proposed to occur because EB binding increases the rate of GTP hydrolysis in the microtubule 11,24. However, to increase the rate of GTP hydrolysis, EBs should bind to the lattice before hydrolysis (pre-GTPase), not after. Thus, there is an apparent conflict between EB’s preference for a “twisted and compacted” GTPγS lattice (supposedly a post-GTPase phenomenon) and the concentration dependence of EB comet size (rationalized as a pre-GTPase phenomenon). One way to resolve this conflict is to move away from the viewpoint that the conformation of tubulin is only determined by its nucleotide state. As will be discussed in the final section of the article, we argue that long-range mechanical coupling between subunits in the lattice could explain how EBs can stimulate GTP hydrolysis while binding preferentially to what appears to be a post-GTPase state of the lattice.
The conformation cycle underlies regulation by microtubule polymerases and depolymerases
EB proteins currently provide one of the best-understood regulatory mechanisms for microtubule dynamics, but recent studies of other MAPs indicate that, like for EBs, they bind selectively to specific conformations of tubulin and thereby modulate the tubulin conformation cycle.
Whereas EB proteins modulate the transitions between expanded and compacted conformations within the microtubule lattice, a growing number of proteins regulate transitions between straight and curved conformations of αβ-tubulin at the growing microtubule end. The first protein shown to bind preferentially to the curved conformation of αβ-tubulin was Op18/stathmin 62,63, which uses this activity to sequester unpolymerized αβ-tubulin. It is now clear that microtubule depolymerases of the kinesin-13 family also bind preferentially to, and can induce, curved αβ-tubulin 64 on the microtubule end 65,66. Kinesin-13’s use this activity to trigger catastrophe 67–69. Binding preferentially to curved tubulin may be a universal feature of microtubule depolymerases. Indeed, a recent study of the cooperative microtubule depolymerase Kip3 70–72 from the kinesin-8 family indicates that it also interacts preferentially with curved αβ-tubulin (see also Box 2) 73, and that this property underlies its depolymerase activity 73. Despite sharing the same overall kinesin fold, Kip3 and MCAK evolved distinct ways to bind preferentially to curved αβ-tubulin 64,73–75. These studies demonstrate that microtubule dynamics depends on the balance between straight and curved conformations on the microtubule end and that depolymerases act by favouring curved conformations.
Surprisingly, preferential binding to curved αβ-tubulin can also be used to promote microtubule growth. Indeed, microtubule polymerases in the Stu2/XMAP215 family make microtubules grow faster 76–80 using TOG domains that bind preferentially to curved αβ-tubulin 81,82. Recent advances in the mechanistic understanding of these proteins further illuminate the central role of mechanical coupling and conformational transitions in microtubule dynamics and regulation.
In the absence of unpolymerized αβ-tubulin, isolated polymerase TOG domains will depolymerize stabilized microtubules, presumably by stabilizing a curved, more dissociation-prone conformation of αβ-tubulin on the microtubule end 54. Yet when linked in the intact protein, in the presence of unpolymerized αβ-tubulin multiple TOGs catalyze polymerization. How can this be? While unpolymerized αβ-tubulin is curved and binds tightly to TOGs, lattice interactions induce straighter conformations that bind weakly to TOGs. As long as the straightening-inducing interactions with the lattice are stronger than the TOG:αβ-tubulin interactions they compete with, the TOG domains will ‘hand-off’ their bound tubulins to the lattice, allowing the polymerase to promote growth by accelerating the delivery of new subunits.
These examples drew mainly on studies of how selected MAPs control microtubule dynamics by influencing and being influenced by the conformation of tubulin subunits. It will be fascinating to discover if related logic underlies the activity of less understood regulatory proteins like Kinesin-family polymerases 83–86, TOG-based rescue factors (e.g. CLASPs 87,88), anti-catastrophe factors like TPX2 (see below), and others 89,90. Combining these MAPs together with αβ-tubulin mutants that have altered conformational cycles (54, see also Box 2) should yield more direct insight into the mechanical underpinnings of microtubule regulation.
Implications for nucleation and rescue
In the previous sections, we focused on how the conformational cycle of tubulin dimers is linked to microtubule growth and catastrophe. Notably, similar processes also operate during microtubule nucleation 91,92 and rescue 93.
Microtubules can nucleate spontaneously (form ‘de novo’) when unpolymerized αβ-tubulin oligomerizes in solution (Fig. 3A). Oligomers below a certain size are unstable but growth becomes favourable after a “critical nucleus” is formed 94,95. Because tubulin oligomers grow from soluble GTP-tubulin, which is curved, these small oligomers must also have curvature and may resemble growing microtubule ends. The kinetics of microtubule nucleation show a high degree of cooperativity 96,97, suggesting that the critical nucleus requires multiple, kinetically unfavourable steps to form.
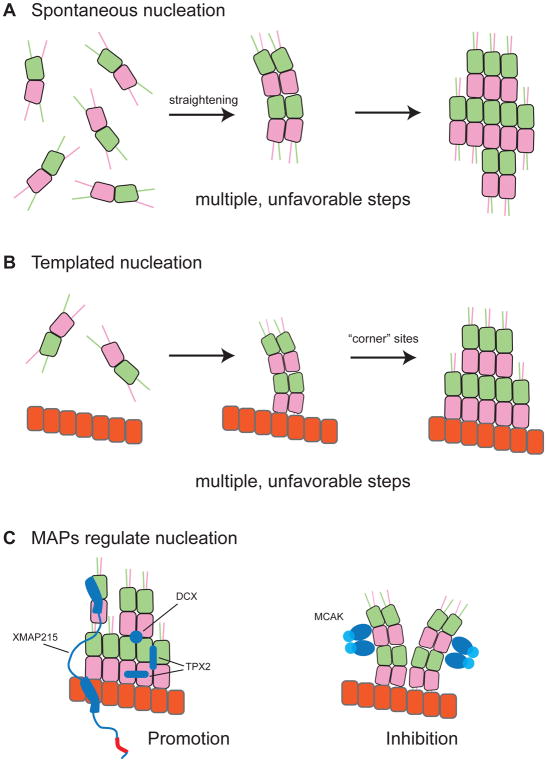
Figure 3.
Barriers to spontaneous and templated nucleation. A. Spontaneous nucleation involves multiple, unfavorable steps. Unpolymerized tubulin is curved (right) and must straighten to form small oligomers (middle). The growth of these oligomers (left) requires increased straightening for newly added tubulins and of those already in the oligomer. B. Templated nucleation also involves multiple, unfavourable steps. A blunt template (right) presents fewer high-affinity “corner” sites than a tapered growing end; curved tubulin binding to the blunt template may also have to straighten more than at the partially curved microtubule end (middle). The transition of the blunt template into a growing microtubule end requires many tubulins to straighten (left). C. MAPs regulate microtubule nucleation by altering the conformation of tubulin. Nucleation-promoting MAPs like XMAP215, TPX2, and DCX help form a nascent plus end (left). Nucleation-inhibiting MAPs like MCAK prevent this formation (right).
Cells are thought to bypass these unfavourable steps by using templates like the γ-tubulin ring complex (γ-TuRC) 98,99 or newly severed microtubule ends (e.g., 100) to nucleate microtubules. In vitro, templates like the γ-TuRC or stabilized microtubule ‘seeds’ do accelerate microtubule formation and reduce the tubulin concentration required to observe elongation, but this ‘templated nucleation’ still shows a time lag 101. The presence of this lag, which is unexpected 102, indicates that templates imperfectly mimic the microtubule end (Fig. 3B).
Why might blunt templates be imperfect substrates for elongation compared to a growing microtubule end? One source of imperfection may be that templates like the γ-TuRCs are blunt rings rather than the tapered, variably-curved arrangements of αβ-tubulin normally found at growing microtubule ends (Fig. 3B; also see Box 1). αβ-tubulin may associate onto a blunt template with lower affinity because blunt templates require more straightening of the incoming subunits, and/or because blunt templates lack the ‘raggedness’ that generates favourable binding sites. The maturation of a blunt template into a tapered, variably-curved microtubule end can be accelerated in vitro by MAPs that interact preferentially with partially curved tubulin conformations, that stabilize αβ-tubulin:αβ-tubulin interfaces, or that accelerate the association of αβ-tubulin 101.
Several MAPs have been shown to regulate nucleation (e.g. 103–105, and new data shows they use conformation-based mechanisms to do so. XMAP215 family proteins provide an example of how accelerating the binding of αβ-tubulin to microtubule ends can promote nucleation 101,105,106, which depends on XMAP215’s conformation-specific interactions with tubulin dimers (Fig. 3C, left). TPX2 (targeting factor for Xklp2) is a spindle assembly factor in the chromatin-mediated microtubule nucleation pathway 107–109. TPX2 suppresses catastrophe and slows post-catastrophe shrinking in vitro 101,106,110. TPX2 also reduces the lag for templated nucleation 101, which may be critical to its function given that TPX2 also interacts directly with γ-TuRCs during branching nucleation 111,112. TPX2 can also stabilize “stubs” (small oligomers) of tubulin that act as nuclei for microtubule formation in vitro 106. Suppressing catastrophe and stabilizing small oligomers of αβ-tubulin indicates that TPX2 stabilizes an αβ-tubulin:αβ-tubulin interface, and a recent cryo-EM structure showed TPX2 binding across both longitudinal and lateral interfaces 113. Interestingly, TPX2 also binds preferentially to partially curved tubulin structures at microtubule ends 106. The unrelated protein doublecortin (DCX), which is expressed in developing neurons, provides another example of a MAP that regulates nucleation in vitro 114. DCX also binds to partially curved tubulin structures 115 by binding at the vertex of 4 αβ-tubulins 116. DCX nucleates microtubules spontaneously with extraordinary efficacy 117,118,119. Thus, two MAPs that stabilize αβ-tubulin:αβ-tubulin interfaces in partially curved tubulin assemblies at microtubule ends (Fig. 3C, right) show strong nucleation activity, indicating that microtubule ends and nucleation intermediates are structurally similar. Interacting with conformations found in these intermediates may be the key to regulating nucleation.
Depending on the conformation being stabilized, MAPs can also inhibit nucleation (Fig. 3C, right). For example, EB1 and MCAK each reduce the rate of templated nucleation and increase the concentration of tubulin required to observe elongation 101. As discussed above, EB1 may accomplish this by promoting a twisted and compacted state of αβ-tubulin 24 in the GTP lattice that may have higher GTPase activity and/or be a less favourable substrate for elongation. MCAK antagonizes nucleation because it prefers fully curved conformations of tubulin that are not compatible with the variably-curved structures present at the growing microtubule end and in nucleation intermediates (Fig. 3C, right).
The lag associated with nucleation on a blunt template thus reflects an energetic barrier to develop a properly structured microtubule end. By modulating what must be a delicate balance between different conformations of tubulin in the lattice, MAPs can either promote or inhibit this development.
The transition from a blunt template to a “true”, persistently-growing microtubule end is hard. It must be even harder to rescue a shrinking microtubule by converting the outwardly peeling protofilaments back into a growing microtubule end. This difficulty may explain why rescues are rarely observed in vitro. In contrast, rescues are readily observed in tissue culture cells likely owing to the presence of “rescue factors”. Perhaps the best-characterized rescue factor is Cls1, the CLASP family protein in S. pombe 88. CLASP-family proteins 87 contain TOG domains, but some of these TOGs appear to prefer a different conformation of tubulin 120 compared to the TOGs from microtubule polymerases. Thus, CLASPs may facilitate rescue by interacting with a specific conformation of tubulin that occurs during protofilament peeling. However, little is currently known about how the CLASP TOGs actually contribute to rescue.
An unexpected connection between rescue and microtubule mechanics comes from recent work examining how microtubules respond to bending stress 121, which exemplifies how mechanical forces can alter the biochemical properties of tubulin in the lattice. In this work, microtubules were bent repeatedly by controlled fluid flow in a microfluidics chamber; the microtubule became increasingly flexible with each bend. Normal stiffness could be restored over time in the presence of soluble tubulin, suggesting that the mechanical stress from repeated bending damaged the lattice, and that fresh tubulin could enter and repair these damaged sites. Sites of photodamage can also be repaired by fresh tubulin 121. Interestingly, microtubule rescues cluster at sites of repair in vitro 122. In cells, sites of damage also appear to undergo repair and facilitate rescue 122. Indeed, cells may protect their microtubules from damage via acetylation 123,124, but whether and how acetylation is linked to mechanics remains unclear.. Although we do not understand what “repair” entails, rescues may occur near repaired sites because the fresh tubulin stabilizes the region and reduces or eliminates protofilament peeling. Under normal circumstances, regardless of nucleotide state, tubulin below the cap and in the body of the lattice is thought to be so tightly incorporated that it does not dissociate appreciably. By demonstrating that the mechanical deformations accompanying bending can significantly alter how strongly tubulin is bound in the lattice, this line of work provides clear new evidence for the intimate connections between material properties of microtubules and the biochemical properties of the individual subunits.
Long range mechanical coupling in the lattice
The complex structure of the microtubule end and the presence of expanded, compacted, and variably-curved conformations of tubulin therein that differently interact with each other and that are modulated by MAPs raise a number of questions about how the mechanical states of tubulin affect the biochemistry of tubulin–tubulin interfaces and vice versa. For example, what drives the straightening of curved GTP-tubulin dimers when they assemble into the lattice at the growing end or in nucleation intermediates? It has been proposed that straightening would be driven by the alignment of lateral interfaces between neighboring dimers 34. The presence of variably-curved conformations at the end implies that the straightening of one dimer both requires and facilitates the straightening of its neighbors 125 (Fig. 4B). These interactions describe a kind of allostery, wherein the effect of one tubulin binding to another is transmitted to other interfaces via changes in curvature.
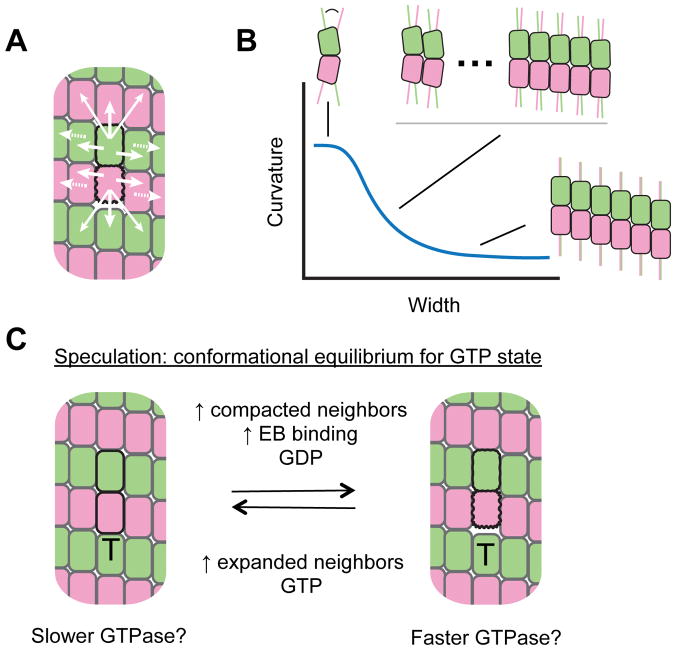
Figure 4.
Mechanical coupling in the microtubule lattice. A. Cartoon illustrating a rationale for mechanical coupling in the lattice. A compacted tubulin (dark outline) is shown in a majority expanded lattice (light outlines). The mismatch between expanded and compacted conformations is likely resolved through conformational changes near the mismatch (white arrows). The periodic nature of the lattice means that such accommodation could propagate beyond nearest-neighbors (dashed arrows). B. At the microtubule end or in nucleation intermediates, tubulin curvature is thought to vary with width of the taper, affecting the strength of tubulin:tubulin interactions. Colored lines on the αβ-tubulin cartoons provide a visual reference for the amount of curvature. This provides another example of long-range mechanical coupling. C. Model for conformational control of GTPase that was inspired by observations about EB proteins. The model speculates that expanded tubulins may have slower GTPase than compacted tubulins. ‘T’ on the cartoons indicates GTP nucleotide state. Combined with long-range coupling, such a model is expected to display threshold-type behaviours and could give rise to cooperative, positive-feedback enhancement of GTPase activity in the stabilizing cap.
Likewise, how does the compaction of a single tubulin dimer affect its neighbors? The expanded GTP-tubulin lattice and compacted GDP-tubulin lattice are incommensurate 29. The mismatch between these conformations must introduce structural conflicts near the microtubule end where the lattice contains a mixture of nucleotide states. Resolution of these conflicts, wherein tubulin dimers change conformation to accommodate their mismatched neighbors, may give rise to long-range coupling in the lattice: the effects of a conformational transition in one tubulin may propagate beyond its immediate neighbors to alter the conformation or biochemistry of more distant subunits in the lattice. For example, when one αβ-tubulin compacts in an otherwise expanded GTP-lattice, the resulting structural mismatch likely creates a kind of stress that makes neighboring tubulins more likely to compact, which would in turn make their neighbours more likely to compact, and so on (Fig. 4A). This conformational coupling could in principle over-ride or modulate the effect of nucleotide state on tubulin conformation. Because of this mechanical coupling in the lattice, the conformation of an individual αβ-tubulin might not be uniquely determined by its nucleotide state but rather also by the conformation of its neighbors. Such long-range mechanical coupling would make the microtubule end a highly-cooperative structure capable of abrupt transitions.
Long-range coupling in the lattice provides a new way to think about how regulatory proteins like EBs could interact with microtubules and influence tubulin conformation in the lattice. Recall the pre-GTPase vs. post-GTPase paradox: EBs appear to accelerate the rate of GTP hydrolysis while binding preferentially to what has been thought to be a post-GTPase conformation in the lattice. These behaviours are hard to reconcile with a view in which an individual αβ-tubulin’s conformation is uniquely determined by its nucleotide state. Long-range coupling in the lattice may resolve this paradox (Fig. 4C). An interesting recent study of XMAP215 and EB1 revealed synergistic action that may result from long-range coupling in the lattice 126. Indeed, when EBs bind to and stabilize a “twisted and compacted” conformation at one site, long-range coupling means the EB-induced change in conformation could propagate some distance through the lattice. If the corresponding changes in conformation also elevate the rate of GTP hydrolysis at remote sites in the stabilizing cap, then EB’s preference for a “twisted and compacted” conformation at one site has the potential to accelerate GTP hydrolysis elsewhere. This hypothesis, which is speculative, should ultimately be testable using αβ-tubulin mutants with altered conformational cycles. The idea that long-range mechanical coupling might influence GTP hydrolysis throughout the stabilizing cap also has implications for the mechanism of microtubule catastrophe: mechanical coupling creates the possibility for positive feedback and threshold effects, wherein increasing compaction within the stabilizing cap drives GTP hydrolysis and cap loss. We speculate that whether acting through GTPase activity or not, lattice-propagated modulation of αβ-tubulin conformation and biochemistry, and the threshold-dependent effects that may accompany this long-range coupling, will be important for understanding fundamental mechanisms of microtubule dynamics in general.
Conclusion and perspective
The field has made remarkable progress advancing the biochemical and mechanical understanding of microtubule polymerization dynamics. New structural states of αβ-tubulin have been defined in atomic detail 24,29. We are beginning to appreciate how the transitions between these states create the phenomenon of dynamic instability 54, and how MAPs can manipulate these transitions to modulate microtubule dynamics 73,74,81,82,90,113. Our understanding of material properties of microtubules – how they generate and respond to force, and transmit information over nearest-neighbour and longer distances – has increased apace 42,51,126. However, we still lack an understanding of how biochemistry determines mechanics and of how mechanics modulates biochemistry. Integrating these two perspectives remains a major challenge for the future.
An integrated understanding of microtubule dynamics and regulation that unifies mechanical and biochemical perspectives will require deeper insight into the conformations of αβ-tubulin and their relative energetics, new knowledge about how regulatory factor binding can modulate αβ-tubulin conformation and energetics, and the ability to detect and quantify the contribution of mechanical coupling effects. Although formidable challenges remain, the remarkable recent leaps forward leave us optimistic about the prospects for substantial progress in the near future.
Acknowledgments
We thank S. Chaaban for his masterful work rendering the microtubule end in Box 1. We thank members of the Brouhard and Rice labs for helpful discussions and feedback on the manuscript. Work GJB’s lab is supported by the Canadian Institutes of Health Research (PJT-148702 & MOP-137055), the Natural Sciences and Engineering Research Council of Canada (RGPIN-2014-03791), the Fonds de recherche du Québec – Nature et technologies (FRQ-NT 191128), the Canadian Foundation for Innovation, and McGill University. LMR is the Thomas O. Hicks Scholar in Medical Research; work in his lab is supported by the NIH (R01-GM098543), the NSF (MCB-1615938), and the Robert A. Welch Foundation (I-1908).
Glossary
Microtubule plus end
The end of the microtubule that is crowned by β-tubulin subunits. This more dynamic end of the polymer typically grows from the middle of the cell toward the periphery.
Microtubule minus end
The end of the microtubule that is crowned by α-tubulin subunits. This less dynamic end of the polymer is typically capped or anchored in a microtubule organizing structure like the centrosome.
Catastrophe
The switch from microtubule growing to microtubule shrinking.
Rescue
The switch from microtubule shrinking to microtubule growing.
Nucleation
The process of forming a microtubule de novo (from unpolymerized αβ-tubulin subunits) or on a template such as the γ-tubulin ring complex or a severed microtubule end.
γ-Tubulin ring complex (γ-TuRC)
A conical oligomer of γ-tubulins and gamma-ring complex proteins (GCPs) that creates a template for microtubule nucleation.
TPX2
A spindle-assembly factor that functions in the Ran-GTP pathway. TPX2 is released from importins in the vicinity of chromosomes, where it stimulates microtubule nucleation and interacts with motor proteins.
Compaction
A conformational change posited to occur upon GTP hydrolysis in the microtubule lattice, based on distinct conformations of αβ-tubulin observed in structures of mammalian microtubules.
End-binding (EB) protein
a family of evolutionarily conserved proteins that autonomously track the growing microtubule end. EB proteins regulate microtubule dynamics and are also responsible for recruiting the majority of other plus-end tracking proteins to the growing microtubule end.
Microtubule polymerase
Regulatory factor that promotes microtubule elongation, typically by making microtubules grow faster.
XMAP215/Stu2
The best-studied family of microtubule polymerases. Polymerases in this family are the major factors that promote fast microtubule growth in cells, and their activity is important for proper formation of the mitotic spindle.
Microtubule depolymerase
Regulatory factor that promotes microtubule depolymerisation. Well-studied microtubule depolymerases include the MCAK/kinesin-13 and the Kip3/kinesin-8 families.
MCAK/kinesin-13
Kinesin-family proteins that act as microtubule depolymerases. Kinesin-13’s use ATP hydrolysis to induce outward curvature in protofilaments, triggering catastrophes.
Kip3/kinesin-8
Microtubule depolymerases that combine the motility of conventional kinesins with depolymerase activity. Because of this unique combination, some kinesin-8’s have been shown to depolymerize microtubules in a length-dependent manner.
Rescue factor
Regulatory factor that increases the likelihood of microtubule rescue. The best-studied rescue factors belong to the CLASP family.
Free energy
a thermodynamic quantity that describes the amount of energy that can do work in a given system. The change in free energy associated with a chemical reaction provides the amount of work that is released by that reaction, or that must be input to drive that reaction.
Conformational strain
Describes internal energy stored by a molecule when that molecule is held in a conformation that differs from the strain-free conformation.
Optical tweezers
Device in which radiation pressure from a laser beam focused by a microscope objective is used to trap a small bead or other object. Optical tweezers provide a sensitive way to measure both the position of the trapped object and the forces experienced by the trapped object.
‘Morphing’ (interpolation)
A computational procedure that creates a series of structural intermediates between two known conformations of a molecule. Intermediate conformations generated by morphing provide a useful way to visualize steps along a conformational change, but do not always capture the actual pathway for the conformational change.
References
- 1.Desai A, Mitchison TJ. Annu Rev Cell Dev Biol. 1997;13:83–117. doi: 10.1146/annurev.cellbio.13.1.83. [DOI] [PubMed] [Google Scholar]
- 2.Carlier MF, Pantaloni D. Biochemistry. 1981;20:1918–1924. doi: 10.1021/bi00510a030. [DOI] [PubMed] [Google Scholar]
- 3.Akhmanova A, Steinmetz MO. Tracking the ends: a dynamic protein network controls the fate of microtubule tips. Nature reviews. Molecular cell biology. 2008;9:309–322. doi: 10.1038/nrm2369. [DOI] [PubMed] [Google Scholar]
- 4.Akhmanova A, Steinmetz MO. Control of microtubule organization and dynamics: two ends in the limelight. Nature reviews. Molecular cell biology. 2015;16:711–726. doi: 10.1038/nrm4084. [DOI] [PubMed] [Google Scholar]
- 5.Howard J, Hyman AA. Microtubule polymerases and depolymerases. Current opinion in cell biology. 2007;19:31–35. doi: 10.1016/j.ceb.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 6.Mitchison T, Kirschner M. Dynamic instability of microtubule growth. Nature. 1984;312:237–242. doi: 10.1038/312237a0. [DOI] [PubMed] [Google Scholar]
- 7.Inoue S, Salmon ED. Force generation by microtubule assembly/disassembly in mitosis and related movements. Molecular biology of the cell. 1995;6:1619–1640. doi: 10.1091/mbc.6.12.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Howard J, Hyman AA. Dynamics and mechanics of the microtubule plus end. Nature. 2003;422:753–758. doi: 10.1038/nature01600. [DOI] [PubMed] [Google Scholar]
- 9.Kueh HY, Mitchison TJ. Structural plasticity in actin and tubulin polymer dynamics. Science. 2009;325:960–963. doi: 10.1126/science.1168823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gell C, et al. Microtubule dynamics reconstituted in vitro and imaged by single-molecule fluorescence microscopy. Methods in cell biology. 2010;95:221–245. doi: 10.1016/S0091-679X(10)95013-9. [DOI] [PubMed] [Google Scholar]
- 11.Maurer SP, et al. EB1 Accelerates Two Conformational Transitions Important for Microtubule Maturation and Dynamics. Current biology: CB. 2014;24:372–384. doi: 10.1016/j.cub.2013.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duellberg C, Cade NI, Holmes D, Surrey T. The size of the EB cap determines instantaneous microtubule stability. eLife. 2016;5 doi: 10.7554/eLife.13470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gardner MK, et al. Rapid microtubule self-assembly kinetics. Cell. 2011;146:582–592. doi: 10.1016/j.cell.2011.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Howard J, Hyman AA. Growth, fluctuation and switching at microtubule plus ends. Nature reviews. Molecular cell biology. 2009;10:569–574. doi: 10.1038/nrm2713. [DOI] [PubMed] [Google Scholar]
- 15.Gittes F, Mickey B, Nettleton J, Howard J. Flexural rigidity of microtubules and actin filaments measured from thermal fluctuations in shape. The Journal of cell biology. 1993;120:923–934. doi: 10.1083/jcb.120.4.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gates SN, et al. Science. Vol. 357. American Association for the Advancement of Science; 2017. pp. 273–279. [Google Scholar]
- 17.Zhao M, et al. Nature. Vol. 518. Nature Publishing Group; 2015. pp. 61–67. [Google Scholar]
- 18.Brouhard GJ, Rice LM. The contribution of alphabeta-tubulin curvature to microtubule dynamics. The Journal of cell biology. 2014;207:323–334. doi: 10.1083/jcb.201407095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bieling P, et al. Reconstitution of a microtubule plus-end tracking system in vitro. Nature. 2007;450:1100–1105. doi: 10.1038/nature06386. [DOI] [PubMed] [Google Scholar]
- 20.Mandelkow EM, Mandelkow E, Milligan RA. Microtubule dynamics and microtubule caps: a time-resolved cryo-electron microscopy study. The Journal of cell biology. 1991;114:977–991. doi: 10.1083/jcb.114.5.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chretien D, Fuller SD, Karsenti E. Structure of growing microtubule ends: two-dimensional sheets close into tubes at variable rates. The Journal of cell biology. 1995;129:1311–1328. doi: 10.1083/jcb.129.5.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lodish HF. Molecular cell biology. 8. W.H. Freeman-Macmillan Learning; 2016. [Google Scholar]
- 23.Sept D, McCammon JA. Biophys J. 2001;81:667–674. doi: 10.1016/S0006-3495(01)75731-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang R, Alushin GM, Brown A, Nogales E. Mechanistic Origin of Microtubule Dynamic Instability and Its Modulation by EB Proteins. Cell. 2015;162:849–859. doi: 10.1016/j.cell.2015.07.012. This paper, along with ref. 29, defined the expanded, compacted, and compacted and twisted conformations of αβ-tubulin in the lattice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guesdon A, et al. EB1 interacts with outwardly curved and straight regions of the microtubule lattice. Nature cell biology. 2016;18:1102–1108. doi: 10.1038/ncb3412. [DOI] [PubMed] [Google Scholar]
- 26.Wang HW, Nogales E. Nucleotide-dependent bending flexibility of tubulin regulates microtubule assembly. Nature. 2005;435:911–915. doi: 10.1038/nature03606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zakharov P, et al. Molecular and Mechanical Causes of Microtubule Catastrophe and Aging. Biophysical journal. 2015;109:2574–2591. doi: 10.1016/j.bpj.2015.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.VanBuren V, Cassimeris L, Odde DJ. Mechanochemical model of microtubule structure and self-assembly kinetics. Biophysical journal. 2005;89:2911–2926. doi: 10.1529/biophysj.105.060913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alushin GM, et al. High-resolution microtubule structures reveal the structural transitions in alphabeta-tubulin upon GTP hydrolysis. Cell. 2014;157:1117–1129. doi: 10.1016/j.cell.2014.03.053. This paper, along with ref. 24, defined the expanded, compacted, and compacted and twisted conformations of αβ-tubulin in the lattice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ravelli RB, et al. Insight into tubulin regulation from a complex with colchicine and a stathmin-like domain. Nature. 2004;428:198–202. doi: 10.1038/nature02393. [DOI] [PubMed] [Google Scholar]
- 31.Coue M, Lombillo VA, McIntosh JR. Microtubule depolymerization promotes particle and chromosome movement in vitro. The Journal of cell biology. 1991;112:1165–1175. doi: 10.1083/jcb.112.6.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koshland DE, Mitchison TJ, Kirschner MW. Polewards chromosome movement driven by microtubule depolymerization in vitro. Nature. 1988;331:499–504. doi: 10.1038/331499a0. [DOI] [PubMed] [Google Scholar]
- 33.Buey RM, Diaz JF, Andreu JM. The nucleotide switch of tubulin and microtubule assembly: a polymerization-driven structural change. Biochemistry. 2006;45:5933–5938. doi: 10.1021/bi060334m. [DOI] [PubMed] [Google Scholar]
- 34.Rice LM, Montabana EA, Agard DA. The lattice as allosteric effector: structural studies of alphabeta- and gamma-tubulin clarify the role of GTP in microtubule assembly. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:5378–5383. doi: 10.1073/pnas.0801155105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nawrotek A, Knossow M, Gigant B. The determinants that govern microtubule assembly from the atomic structure of GTP-tubulin. Journal of molecular biology. 2011;412:35–42. doi: 10.1016/j.jmb.2011.07.029. [DOI] [PubMed] [Google Scholar]
- 36.Howes SC, et al. Structural differences between yeast and mammalian microtubules revealed by cryo-EM. The Journal of cell biology. 2017;216:2669–2677. doi: 10.1083/jcb.201612195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.von Loeffelholz O, et al. Nucleotide- and Mal3-dependent changes in fission yeast microtubules suggest a structural plasticity view of dynamics. Nat Commun. 2017;8:2110. doi: 10.1038/s41467-017-02241-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dogterom M, Yurke B. Measurement of the force-velocity relation for growing microtubules. Science. 1997;278:856–860. doi: 10.1126/science.278.5339.856. [DOI] [PubMed] [Google Scholar]
- 39.Holy TE, Dogterom M, Yurke B, Leibler S. Assembly and positioning of microtubule asters in microfabricated chambers. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:6228–6231. doi: 10.1073/pnas.94.12.6228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tolic-Norrelykke IM, Sacconi L, Thon G, Pavone FS. Positioning and elongation of the fission yeast spindle by microtubule-based pushing. Current biology: CB. 2004;14:1181–1186. doi: 10.1016/j.cub.2004.06.029. [DOI] [PubMed] [Google Scholar]
- 41.Tran PT, Marsh L, Doye V, Inoue S, Chang F. A mechanism for nuclear positioning in fission yeast based on microtubule pushing. The Journal of cell biology. 2001;153:397–411. doi: 10.1083/jcb.153.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grishchuk EL, Molodtsov MI, Ataullakhanov FI, McIntosh JR. Force production by disassembling microtubules. Nature. 2005;438:384–388. doi: 10.1038/nature04132. [DOI] [PubMed] [Google Scholar]
- 43.Powers AF, et al. The Ndc80 kinetochore complex forms load-bearing attachments to dynamic microtubule tips via biased diffusion. Cell. 2009;136:865–875. doi: 10.1016/j.cell.2008.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Asbury CL, Gestaut DR, Powers AF, Franck AD, Davis TN. The Dam1 kinetochore complex harnesses microtubule dynamics to produce force and movement. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:9873–9878. doi: 10.1073/pnas.0602249103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Franck AD, et al. Tension applied through the Dam1 complex promotes microtubule elongation providing a direct mechanism for length control in mitosis. Nature cell biology. 2007;9:832–837. doi: 10.1038/ncb1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Volkov VA, et al. Long tethers provide high-force coupling of the Dam1 ring to shortening microtubules. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:7708–7713. doi: 10.1073/pnas.1305821110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Akiyoshi B, et al. Tension directly stabilizes reconstituted kinetochore-microtubule attachments. Nature. 2010;468:576–579. doi: 10.1038/nature09594. This study demonstrated that kinetochore particles purified from yeast form load-bearing attachments to dynamic microtubules, and that tension applied through the kinetochores alters microtubule dynamics. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Trushko A, Schaffer E, Howard J. The growth speed of microtubules with XMAP215-coated beads coupled to their ends is increased by tensile force. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:14670–14675. doi: 10.1073/pnas.1218053110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang PJ, Huffaker TC. Stu2p: A microtubule-binding protein that is an essential component of the yeast spindle pole body. The Journal of cell biology. 1997;139:1271–1280. doi: 10.1083/jcb.139.5.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miller MP, Asbury CL, Biggins S. A TOG Protein Confers Tension Sensitivity to Kinetochore-Microtubule Attachments. Cell. 2016;165:1428–1439. doi: 10.1016/j.cell.2016.04.030. In this work, the authors discovered that the microtubule polymerase Stu2 contributes to kinetochore-microtubule coupling in a tension-dependent way that is independent of polymerase activity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Driver JW, Geyer EA, Bailey ME, Rice LM, Asbury CL. Direct measurement of conformational strain energy in protofilaments curling outward from disassembling microtubule tips. eLife. 2017;6 doi: 10.7554/eLife.28433. In this study, the authors quantified the amount of mechanical work performed by a depolymerizing microtubule. They showed that this work was not changed by a mutation that slows microtubule shrinking by altering the tubulin conformational cycle. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gennerich A, Carter AP, Reck-Peterson SL, Vale RD. Force-induced bidirectional stepping of cytoplasmic dynein. Cell. 2007;131:952–965. doi: 10.1016/j.cell.2007.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Howard J. The movement of kinesin along microtubules. Annu Rev Physiol. 1996;58:703–729. doi: 10.1146/annurev.ph.58.030196.003415. [DOI] [PubMed] [Google Scholar]
- 54.Geyer EA, et al. A mutation uncouples the tubulin conformational and GTPase cycles, revealing allosteric control of microtubule dynamics. eLife. 2015;4:e10113. doi: 10.7554/eLife.10113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Doodhi H, et al. Termination of Protofilament Elongation by Eribulin Induces Lattice Defects that Promote Microtubule Catastrophes. Current biology: CB. 2016;26:1713–1721. doi: 10.1016/j.cub.2016.04.053. [DOI] [PubMed] [Google Scholar]
- 56.Mohan R, et al. End-binding proteins sensitize microtubules to the action of microtubule-targeting agents. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:8900–8905. doi: 10.1073/pnas.1300395110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dixit R, et al. Microtubule plus-end tracking by CLIP-170 requires EB1. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:492–497. doi: 10.1073/pnas.0807614106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Maurer SP, Bieling P, Cope J, Hoenger A, Surrey T. GTPgammaS microtubules mimic the growing microtubule end structure recognized by end-binding proteins (EBs) Proceedings of the National Academy of Sciences of the United States of America. 2011;108:3988–3993. doi: 10.1073/pnas.1014758108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Maurer SP, Fourniol FJ, Bohner G, Moores CA, Surrey T. EBs recognize a nucleotide-dependent structural cap at growing microtubule ends. Cell. 2012;149:371–382. doi: 10.1016/j.cell.2012.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zanic M, Stear JH, Hyman AA, Howard J. EB1 recognizes the nucleotide state of tubulin in the microtubule lattice. PloS one. 2009;4:e7585. doi: 10.1371/journal.pone.0007585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hyman AA, Salser S, Drechsel DN, Unwin N, Mitchison TJ. Role of GTP hydrolysis in microtubule dynamics: information from a slowly hydrolyzable analogue, GMPCPP. Molecular biology of the cell. 1992;3:1155–1167. doi: 10.1091/mbc.3.10.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Belmont LD, Mitchison TJ. Identification of a protein that interacts with tubulin dimers and increases the catastrophe rate of microtubules. Cell. 1996;84:623–631. doi: 10.1016/s0092-8674(00)81037-5. [DOI] [PubMed] [Google Scholar]
- 63.Steinmetz MO, et al. Op18/stathmin caps a kinked protofilament-like tubulin tetramer. The EMBO journal. 2000;19:572–580. doi: 10.1093/emboj/19.4.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mulder AM, et al. A new model for binding of kinesin 13 to curved microtubule protofilaments. The Journal of cell biology. 2009;185:51–57. doi: 10.1083/jcb.200812052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hunter AW, et al. The kinesin-related protein MCAK is a microtubule depolymerase that forms an ATP-hydrolyzing complex at microtubule ends. Molecular cell. 2003;11:445–457. doi: 10.1016/s1097-2765(03)00049-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Helenius J, Brouhard G, Kalaidzidis Y, Diez S, Howard J. The depolymerizing kinesin MCAK uses lattice diffusion to rapidly target microtubule ends. Nature. 2006;441:115–119. doi: 10.1038/nature04736. [DOI] [PubMed] [Google Scholar]
- 67.Desai A, Verma S, Mitchison TJ, Walczak CE. Kin I kinesins are microtubule-destabilizing enzymes. Cell. 1999;96:69–78. doi: 10.1016/s0092-8674(00)80960-5. [DOI] [PubMed] [Google Scholar]
- 68.Gardner MK, Zanic M, Gell C, Bormuth V, Howard J. Depolymerizing kinesins Kip3 and MCAK shape cellular microtubule architecture by differential control of catastrophe. Cell. 2011;147:1092–1103. doi: 10.1016/j.cell.2011.10.037. [DOI] [PubMed] [Google Scholar]
- 69.Walczak CE, Mitchison TJ, Desai A. XKCM1: a Xenopus kinesin-related protein that regulates microtubule dynamics during mitotic spindle assembly. Cell. 1996;84:37–47. doi: 10.1016/s0092-8674(00)80991-5. [DOI] [PubMed] [Google Scholar]
- 70.Varga V, et al. Yeast kinesin-8 depolymerizes microtubules in a length-dependent manner. Nature cell biology. 2006;8:957–962. doi: 10.1038/ncb1462. [DOI] [PubMed] [Google Scholar]
- 71.Gupta ML, Jr, Carvalho P, Roof DM, Pellman D. Plus end-specific depolymerase activity of Kip3, a kinesin-8 protein, explains its role in positioning the yeast mitotic spindle. Nature cell biology. 2006;8:913–923. doi: 10.1038/ncb1457. [DOI] [PubMed] [Google Scholar]
- 72.Varga V, Leduc C, Bormuth V, Diez S, Howard J. Kinesin-8 motors act cooperatively to mediate length-dependent microtubule depolymerization. Cell. 2009;138:1174–1183. doi: 10.1016/j.cell.2009.07.032. [DOI] [PubMed] [Google Scholar]
- 73.Arellano-Santoyo H, et al. A Tubulin Binding Switch Underlies Kip3/Kinesin-8 Depolymerase Activity. Developmental cell. 2017;42:37–51e38. doi: 10.1016/j.devcel.2017.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang W, et al. Insight into microtubule disassembly by kinesin-13s from the structure of Kif2C bound to tubulin. Nat Commun. 2017;8:70. doi: 10.1038/s41467-017-00091-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Asenjo AB, et al. Structural model for tubulin recognition and deformation by kinesin-13 microtubule depolymerases. Cell reports. 2013;3:759–768. doi: 10.1016/j.celrep.2013.01.030. [DOI] [PubMed] [Google Scholar]
- 76.Al-Bassam J, et al. Fission yeast Alp14 is a dose-dependent plus end-tracking microtubule polymerase. Molecular biology of the cell. 2012;23:2878–2890. doi: 10.1091/mbc.E12-03-0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Brouhard GJ, et al. XMAP215 is a processive microtubule polymerase. Cell. 2008;132:79–88. doi: 10.1016/j.cell.2007.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hussmann F, Drummond DR, Peet DR, Martin DS, Cross RA. Alp7/TACC-Alp14/TOG generates long-lived, fast-growing MTs by an unconventional mechanism. Sci Rep. 2016;6:20653. doi: 10.1038/srep20653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Podolski M, Mahamdeh M, Howard J. Stu2, the budding yeast XMAP215/Dis1 homolog, promotes assembly of yeast microtubules by increasing growth rate and decreasing catastrophe frequency. The Journal of biological chemistry. 2014;289:28087–28093. doi: 10.1074/jbc.M114.584300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Matsuo Y, et al. An unconventional interaction between Dis1/TOG and Mal3/EB1 in fission yeast promotes the fidelity of chromosome segregation. Journal of cell science. 2016;129:4592–4606. doi: 10.1242/jcs.197533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ayaz P, et al. A tethered delivery mechanism explains the catalytic action of a microtubule polymerase. eLife. 2014;3:e03069. doi: 10.7554/eLife.03069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ayaz P, Ye X, Huddleston P, Brautigam CA, Rice LM. A TOG:alphabeta-tubulin complex structure reveals conformation-based mechanisms for a microtubule polymerase. Science. 2012;337:857–860. doi: 10.1126/science.1221698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hibbel A, et al. Kinesin Kip2 enhances microtubule growth in vitro through length-dependent feedback on polymerization and catastrophe. eLife. 2015;4 doi: 10.7554/eLife.10542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chen Y, Hancock WO. Kinesin-5 is a microtubule polymerase. Nat Commun. 2015;6:8160. doi: 10.1038/ncomms9160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Guzik-Lendrum S, Rayment I, Gilbert SP. Homodimeric Kinesin-2 KIF3CC Promotes Microtubule Dynamics. Biophysical journal. 2017;113:1845–1857. doi: 10.1016/j.bpj.2017.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sardar HS, Luczak VG, Lopez MM, Lister BC, Gilbert SP. Mitotic kinesin CENP-E promotes microtubule plus-end elongation. Current biology: CB. 2010;20:1648–1653. doi: 10.1016/j.cub.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Akhmanova A, et al. Clasps are CLIP-115 and -170 associating proteins involved in the regional regulation of microtubule dynamics in motile fibroblasts. Cell. 2001;104:923–935. doi: 10.1016/s0092-8674(01)00288-4. [DOI] [PubMed] [Google Scholar]
- 88.Al-Bassam J, et al. CLASP promotes microtubule rescue by recruiting tubulin dimers to the microtubule. Developmental cell. 2010;19:245–258. doi: 10.1016/j.devcel.2010.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.van Riel WE, et al. Kinesin-4 KIF21B is a potent microtubule pausing factor. eLife. 2017;6 doi: 10.7554/eLife.24746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Atherton J, et al. A structural model for microtubule minus-end recognition and protection by CAMSAP proteins. Nature structural & molecular biology. 2017;24:931–943. doi: 10.1038/nsmb.3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Petry S, Vale RD. Microtubule nucleation at the centrosome and beyond. Nature cell biology. 2015;17:1089–1093. doi: 10.1038/ncb3220. [DOI] [PubMed] [Google Scholar]
- 92.Roostalu J, Surrey T. Microtubule nucleation: beyond the template. Nature reviews. Molecular cell biology. 2017;18:702–710. doi: 10.1038/nrm.2017.75. [DOI] [PubMed] [Google Scholar]
- 93.Gardner MK, Zanic M, Howard J. Microtubule catastrophe and rescue. Current opinion in cell biology. 2013;25:14–22. doi: 10.1016/j.ceb.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Oosawa F, Asakura S. Thermodynamics of the polymerization of protein. Academic Press; 1975. [Google Scholar]
- 95.Erickson HP, Pantaloni D. The role of subunit entropy in cooperative assembly. Nucleation of microtubules and other two-dimensional polymers. Biophysical journal. 1981;34:293–309. doi: 10.1016/S0006-3495(81)84850-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Voter WA, Erickson HP. The kinetics of microtubule assembly. Evidence for a two-stage nucleation mechanism. The Journal of biological chemistry. 1984;259:10430–10438. [PubMed] [Google Scholar]
- 97.Kuchnir Fygenson D, Flyvbjerg H, Sneppen K, Libchaber A, Leibler S. Spontaneous nucleation of microtubules. Phys Rev E Stat Phys Plasmas Fluids Relat Interdiscip Topics. 1995;51:5058–5063. doi: 10.1103/physreve.51.5058. [DOI] [PubMed] [Google Scholar]
- 98.Zheng Y, Wong ML, Alberts B, Mitchison T. Nucleation of microtubule assembly by a gamma-tubulin-containing ring complex. Nature. 1995;378:578–583. doi: 10.1038/378578a0. [DOI] [PubMed] [Google Scholar]
- 99.Moritz M, Braunfeld MB, Sedat JW, Alberts B, Agard DA. Microtubule nucleation by gamma-tubulin-containing rings in the centrosome. Nature. 1995;378:638–640. doi: 10.1038/378638a0. [DOI] [PubMed] [Google Scholar]
- 100.Lindeboom JJ, et al. A mechanism for reorientation of cortical microtubule arrays driven by microtubule severing. Science. 2013;342:1245533. doi: 10.1126/science.1245533. [DOI] [PubMed] [Google Scholar]
- 101.Wieczorek M, Bechstedt S, Chaaban S, Brouhard GJ. Microtubule-associated proteins control the kinetics of microtubule nucleation. Nature cell biology. 2015;17:907–916. doi: 10.1038/ncb3188. This study carefully quantified the kinetics of microtubule nucleation on templates like the γ-TuRC and blunt microtubule seeds. It provided evidence for a common obstacle to microtubule nucleation that is related to the lack of a nornal microtubule end structure, and demonstrated that microtubule-associated proteins can regulate the effeciency of microtubule nucleation. [DOI] [PubMed] [Google Scholar]
- 102.Kollman JM, Merdes A, Mourey L, Agard DA. Microtubule nucleation by gamma-tubulin complexes. Nature reviews. Molecular cell biology. 2011;12:709–721. doi: 10.1038/nrm3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Heald R, et al. Self-organization of microtubules into bipolar spindles around artificial chromosomes in Xenopus egg extracts. Nature. 1996;382:420–425. doi: 10.1038/382420a0. [DOI] [PubMed] [Google Scholar]
- 104.Bre MH, Karsenti E. Effects of brain microtubule-associated proteins on microtubule dynamics and the nucleating activity of centrosomes. Cell Motil Cytoskeleton. 1990;15:88–98. doi: 10.1002/cm.970150205. [DOI] [PubMed] [Google Scholar]
- 105.Popov AV, Severin F, Karsenti E. XMAP215 is required for the microtubule-nucleating activity of centrosomes. Current biology: CB. 2002;12:1326–1330. doi: 10.1016/s0960-9822(02)01033-3. [DOI] [PubMed] [Google Scholar]
- 106.Roostalu J, Cade NI, Surrey T. Complementary activities of TPX2 and chTOG constitute an efficient importin-regulated microtubule nucleation module. Nature cell biology. 2015;17:1422–1434. doi: 10.1038/ncb3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wittmann T, Wilm M, Karsenti E, Vernos I. TPX2, A novel xenopus MAP involved in spindle pole organization. The Journal of cell biology. 2000;149:1405–1418. doi: 10.1083/jcb.149.7.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gruss OJ, et al. Ran induces spindle assembly by reversing the inhibitory effect of importin alpha on TPX2 activity. Cell. 2001;104:83–93. doi: 10.1016/s0092-8674(01)00193-3. [DOI] [PubMed] [Google Scholar]
- 109.Gruss OJ, et al. Chromosome-induced microtubule assembly mediated by TPX2 is required for spindle formation in HeLa cells. Nature cell biology. 2002;4:871–879. doi: 10.1038/ncb870. [DOI] [PubMed] [Google Scholar]
- 110.Reid TA, et al. Suppression of microtubule assembly kinetics by the mitotic protein TPX2. Journal of cell science. 2016;129:1319–1328. doi: 10.1242/jcs.178806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Alfaro-Aco R, Thawani A, Petry S. Structural analysis of the role of TPX2 in branching microtubule nucleation. The Journal of cell biology. 2017;216:983–997. doi: 10.1083/jcb.201607060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Petry S, Groen AC, Ishihara K, Mitchison TJ, Vale RD. Branching microtubule nucleation in Xenopus egg extracts mediated by augmin and TPX2. Cell. 2013;152:768–777. doi: 10.1016/j.cell.2012.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhang R, Roostalu J, Surrey T, Nogales E. Structural insight into TPX2-stimulated microtubule assembly. eLife. 2017;6 doi: 10.7554/eLife.30959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Moores CA, et al. Mechanism of microtubule stabilization by doublecortin. Molecular cell. 2004;14:833–839. doi: 10.1016/j.molcel.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 115.Bechstedt S, Lu K, Brouhard GJ. Doublecortin Recognizes the Longitudinal Curvature of the Microtubule End and Lattice. Current biology: CB. 2014 doi: 10.1016/j.cub.2014.08.039. [DOI] [PubMed] [Google Scholar]
- 116.Fourniol FJ, et al. Template-free 13-protofilament microtubule-MAP assembly visualized at 8 A resolution. The Journal of cell biology. 2010;191:463–470. doi: 10.1083/jcb.201007081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bechstedt S, Brouhard GJ. Doublecortin recognizes the 13-protofilament microtubule cooperatively and tracks microtubule ends. Developmental cell. 2012;23:181–192. doi: 10.1016/j.devcel.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lazarus JE, Moughamian AJ, Tokito MK, Holzbaur EL. Dynactin subunit p150(Glued) is a neuron-specific anti-catastrophe factor. PLoS Biol. 2013;11:e1001611. doi: 10.1371/journal.pbio.1001611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Moores CA, et al. Distinct roles of doublecortin modulating the microtubule cytoskeleton. The EMBO journal. 2006;25:4448–4457. doi: 10.1038/sj.emboj.7601335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Leano JB, Rogers SL, Slep KC. A cryptic TOG domain with a distinct architecture underlies CLASP-dependent bipolar spindle formation. Structure. 2013;21:939–950. doi: 10.1016/j.str.2013.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Schaedel L, et al. Microtubules self-repair in response to mechanical stress. Nat Mater. 2015;14:1156–1163. doi: 10.1038/nmat4396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Aumeier C, et al. Self-repair promotes microtubule rescue. Nature cell biology. 2016;18:1054–1064. doi: 10.1038/ncb3406. These papers demonstrated that microtubules ‘soften’ in response to repeated bending stress, that this damage can be repaired through incorporation of fresh tubulin into the damaged site, and that this repair machanism can promote microtubule rescue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Xu Z, et al. Microtubules acquire resistance from mechanical breakage through intralumenal acetylation. Science. 2017;356:328–332. doi: 10.1126/science.aai8764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Portran D, Schaedel L, Xu Z, Thery M, Nachury MV. Tubulin acetylation protects long-lived microtubules against mechanical ageing. Nature cell biology. 2017;19:391–398. doi: 10.1038/ncb3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Janosi IM, Chretien D, Flyvbjerg H. Modeling elastic properties of microtubule tips and walls. Eur Biophys J. 1998;27:501–513. doi: 10.1007/s002490050160. [DOI] [PubMed] [Google Scholar]
- 126.Zanic M, Widlund PO, Hyman AA, Howard J. Synergy between XMAP215 and EB1 increases microtubule growth rates to physiological levels. Nature cell biology. 2013;15:688–693. doi: 10.1038/ncb2744. [DOI] [PubMed] [Google Scholar]
- 127.Nogales E, Whittaker M, Milligan RA, Downing KH. High-resolution model of the microtubule. Cell. 1999;96:79–88. doi: 10.1016/s0092-8674(00)80961-7. [DOI] [PubMed] [Google Scholar]
- 128.Janke C, Bulinski JC. Post-translational regulation of the microtubule cytoskeleton: mechanisms and functions. Nature reviews. Molecular cell biology. 2011;12:773–786. doi: 10.1038/nrm3227. [DOI] [PubMed] [Google Scholar]
- 129.Kuriyama R. In vitro polymerization of flagellar and ciliary outer fiber tubulin into microtubules. J Biochem. 1976;80:153–165. doi: 10.1093/oxfordjournals.jbchem.a131247. [DOI] [PubMed] [Google Scholar]
- 130.Kilmartin JV. Purification of yeast tubulin by self-assembly in vitro. Biochemistry. 1981;20:3629–3633. doi: 10.1021/bi00515a050. [DOI] [PubMed] [Google Scholar]
- 131.Gupta ML, Jr, et al. beta-Tubulin C354 mutations that severely decrease microtubule dynamics do not prevent nuclear migration in yeast. Molecular biology of the cell. 2002;13:2919–2932. doi: 10.1091/mbc.E02-01-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bode CJ, Gupta ML, Suprenant KA, Himes RH. The two alpha-tubulin isotypes in budding yeast have opposing effects on microtubule dynamics in vitro. EMBO Rep. 2003;4:94–99. doi: 10.1038/sj.embor.embor716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gupta ML, Jr, Bode CJ, Georg GI, Himes RH. Understanding tubulin-Taxol interactions: mutations that impart Taxol binding to yeast tubulin. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:6394–6397. doi: 10.1073/pnas.1131967100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Uchimura S, Oguchi Y, Hachikubo Y, Ishiwata S, Muto E. Key residues on microtubule responsible for activation of kinesin ATPase. The EMBO journal. 2010;29:1167–1175. doi: 10.1038/emboj.2010.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Uchimura S, et al. Identification of a strong binding site for kinesin on the microtubule using mutant analysis of tubulin. The EMBO journal. 2006;25:5932–5941. doi: 10.1038/sj.emboj.7601442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Johnson V, Ayaz P, Huddleston P, Rice LM. Design, overexpression, and purification of polymerization-blocked yeast alphabeta-tubulin mutants. Biochemistry. 2011;50:8636–8644. doi: 10.1021/bi2005174. [DOI] [PubMed] [Google Scholar]
- 137.Minoura I, et al. Overexpression, purification, and functional analysis of recombinant human tubulin dimer. FEBS letters. 2013;587:3450–3455. doi: 10.1016/j.febslet.2013.08.032. [DOI] [PubMed] [Google Scholar]
- 138.Ti SC, et al. Mutations in Human Tubulin Proximal to the Kinesin-Binding Site Alter Dynamic Instability at Microtubule Plus- and Minus-Ends. Developmental cell. 2016;37:72–84. doi: 10.1016/j.devcel.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Vemu A, et al. Structure and Dynamics of Single-isoform Recombinant Neuronal Human Tubulin. The Journal of biological chemistry. 2016;291:12907–12915. doi: 10.1074/jbc.C116.731133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Sirajuddin M, Rice LM, Vale RD. Regulation of microtubule motors by tubulin isotypes and post-translational modifications. Nature cell biology. 2014;16:335–344. doi: 10.1038/ncb2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Pamula MC, Ti SC, Kapoor TM. The structured core of human beta tubulin confers isotype-specific polymerization properties. The Journal of cell biology. 2016;213:425–433. doi: 10.1083/jcb.201603050. [DOI] [PMC free article] [PubMed] [Google Scholar]