Visceral leishmaniasis in mice devoid of tumor necrosis factor and response to treatment - PubMed (original) (raw)
Visceral leishmaniasis in mice devoid of tumor necrosis factor and response to treatment
H W Murray et al. Infect Immun. 2000 Nov.
Abstract
Tumor necrosis factor (TNF)-deficient mice were challenged with Leishmania donovani to characterize TNF in the response of visceral intracellular infection to antileishmanial chemotherapy. In wild-type controls (i) liver infection peaked at week 2 and resolved, (ii) discrete liver granulomas developed at weeks 2 to 4 and involuted, and (iii) leishmanicidal responses to antimony (Sb), amphotericin B (AmB), and miltefosine were intact. In TNF knockout (KO) mice (i) initial liver infection was unrestrained, plateaued, and then declined somewhat by week 6, (ii) an absent early granulomatous reaction abruptly accelerated with striking tissue inflammation, widespread hepatic necrosis, and 100% mortality by week 10, and (iii) while the initial response to AmB and miltefosine was intact, killing induced by Sb therapy was reduced by >50%. Although initial AmB treatment during weeks 2 to 3 killed 98% of liver parasites, 75% of AmB-treated KO mice subsequently relapsed and died by week 12; however, additional maintenance AmB preserved long-term survival. These results for a model of visceral infection indicate that endogenous TNF is required early on to control intracellular L. donovani, support granuloma development, and mediate optimal initial effects of Sb and prevent relapse after ordinarily curative AmB treatment. A compensatory, TNF-independent antileishmanial mechanism developed in TNF KO mice; however, its effect was uncontrolled fatal inflammation. Chemotherapeutic elimination of the parasite stimulus reversed the hyperinflammatory response and preserved survival.
Figures
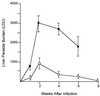
FIG. 1
Course of L. donovani infection in livers of TNF KO (solid circles) and WT control mice (open circles). Results are from four experiments and indicate means ± standard errors of the means for 9 to 14 mice per group at weeks 2, 4, and 6 and for 3 mice per group at weeks 1 and 8. Observations of TNF KO mice ended at week 6 since 50% had died by week 8 (see Fig. 3). Differences in mean LDUs for KO versus WT mice were significant (P < 0.05) at weeks 2 and 4 but not at week 1 or week 6 (P < 0.05).
FIG. 2
Liver histologic response to L. donovani 2 to 4 weeks after infection in WT controls (a) and TNF KO mice (b to f). (a and b) Week 2. WT mice (a) show normal developing granulomas (arrow) at sites of parasitized Kupffer cells; in contrast, in KO mice (b), there is no cellular response at parasitized foci (arrows). (c) Week 3. Rapid granuloma development in KO mice. (d and e) Week 4. KO mice show destructive inflammation with few recognizable granulomas, widespread areas of necrosis (N), and abundant cellular debris (d). Necrotic areas in panel d are ringed by partially preserved tissue containing large masses of intracellular amastigotes (arrows), also shown in panel e. (f) Week 3. Near absence of inflammatory changes in KO mice treated with AmB during the previous week. Magnifications, ×315 (a, b, and f), ×200 (c and d), and ×500 (e).
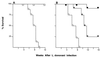
FIG. 3
Survival after L. donovani infection in untreated (A) and AmB-treated mice (B). (A) Results are from two experiments in which a total of 18 WT controls (squares) and 20 TNF KO mice (circles) were infected but were not given antileishmanial treatment. (B) Results are from a single experiment in which infected TNF KO mice were (i) left untreated (open circles; n = 12 mice), (ii) given three AmB injections during weeks 2 to 3 only (hatched circles; n = 8), or (iii) treated similarly with AmB during weeks 2 to 3 and then given once-weekly AmB during weeks 4 to 7 (filled circles; n = 8).
Similar articles
- Roles of endogenous gamma interferon and macrophage microbicidal mechanisms in host response to chemotherapy in experimental visceral leishmaniasis.
Murray HW, Delph-Etienne S. Murray HW, et al. Infect Immun. 2000 Jan;68(1):288-93. doi: 10.1128/IAI.68.1.288-293.2000. Infect Immun. 2000. PMID: 10603400 Free PMC article. - Multiple host defense defects in failure of C57BL/6 ep/ep (pale ear) mice to resolve visceral Leishmania donovani infection.
Murray HW, Hariprashad J, McDermott DF, Stoeckle MY. Murray HW, et al. Infect Immun. 1996 Jan;64(1):161-6. doi: 10.1128/iai.64.1.161-166.1996. Infect Immun. 1996. PMID: 8557335 Free PMC article. - Gamma Interferon-Regulated Chemokines in Leishmania donovani Infection in the Liver.
Murray HW, Luster AD, Zheng H, Ma X. Murray HW, et al. Infect Immun. 2016 Dec 29;85(1):e00824-16. doi: 10.1128/IAI.00824-16. Print 2017 Jan. Infect Immun. 2016. PMID: 27795366 Free PMC article. - Tissue granuloma structure-function in experimental visceral leishmaniasis.
Murray HW. Murray HW. Int J Exp Pathol. 2001 Oct;82(5):249-67. doi: 10.1046/j.1365-2613.2001.00199.x. Int J Exp Pathol. 2001. PMID: 11703536 Free PMC article. Review. - Amphotericin B: A drug of choice for Visceral Leishmaniasis.
Kumari S, Kumar V, Tiwari RK, Ravidas V, Pandey K, Kumar A. Kumari S, et al. Acta Trop. 2022 Nov;235:106661. doi: 10.1016/j.actatropica.2022.106661. Epub 2022 Aug 20. Acta Trop. 2022. PMID: 35998680 Review.
Cited by
- Innate biosignature of treatment failure in human cutaneous leishmaniasis.
Gómez MA, Belew AT, Vargas D, Giraldo-Parra L, Rebellón-Sanchez D, Alexander T, Sayed NE. Gómez MA, et al. Res Sq [Preprint]. 2024 May 2:rs.3.rs-4271873. doi: 10.21203/rs.3.rs-4271873/v1. Res Sq. 2024. PMID: 38746226 Free PMC article. Preprint. - Immunosuppressants alter the immune response associated with Glucantime® treatment for Leishmania infantum infection in a mouse model.
Bernardo L, Solana JC, Sánchez C, Torres A, Reyes-Cruz EY, Carrillo E, Moreno J. Bernardo L, et al. Front Immunol. 2023 Dec 1;14:1285943. doi: 10.3389/fimmu.2023.1285943. eCollection 2023. Front Immunol. 2023. PMID: 38106411 Free PMC article. - Ly6G deficiency alters the dynamics of neutrophil recruitment and pathogen capture during Leishmania major skin infection.
Kleinholz CL, Riek-Burchardt M, Seiß EA, Amore J, Gintschel P, Philipsen L, Bousso P, Relja B, Schraven B, Handschuh J, Mohr J, Müller AJ. Kleinholz CL, et al. Sci Rep. 2021 Jul 23;11(1):15071. doi: 10.1038/s41598-021-94425-9. Sci Rep. 2021. PMID: 34302006 Free PMC article. - Miltefosine enhances infectivity of a miltefosine-resistant Leishmania infantum strain by attenuating its innate immune recognition.
Bulté D, Van Bockstal L, Dirkx L, Van den Kerkhof M, De Trez C, Timmermans JP, Hendrickx S, Maes L, Caljon G. Bulté D, et al. PLoS Negl Trop Dis. 2021 Jul 22;15(7):e0009622. doi: 10.1371/journal.pntd.0009622. eCollection 2021 Jul. PLoS Negl Trop Dis. 2021. PMID: 34292975 Free PMC article. - Potential biomarkers of immune protection in human leishmaniasis.
Rostami MN, Khamesipour A. Rostami MN, et al. Med Microbiol Immunol. 2021 Jun;210(2-3):81-100. doi: 10.1007/s00430-021-00703-8. Epub 2021 May 2. Med Microbiol Immunol. 2021. PMID: 33934238 Free PMC article. Review.
References
- Bean A G D, Roach D R, Briscoe H, France M P, Korner H, Sedgwick J D, Britton W J. Structural deficiencies in granuloma formation in TNF gene-targeted mice underlie the heightened susceptibility to aerosol Mycobacterium tuberculosis infection, which is not compensated for by lymphotoxin. J Immunol. 1999;162:3504–3511. - PubMed
- Beutler B, Grau G E. Tumor necrosis factor in the pathogenesis of infectious diseases. Crit Care Med. 1993;21:S423–S435. - PubMed
- Daftarian P M, Kumar A, Kryworuchko M, Diaz-Mitoma F. IL-10 production is enhanced in human T cells by IL-12 and IL-6 and in monocytes by tumor necrosis factor-α. J Immunol. 1996;157:12–20. - PubMed
- Deckert-Schluter M, Bluethmann H, Rang A, Hof H, Schluter D. Crucial role of TNF receptor type 1 (p55), but not of TNF receptor type 2 (p75) in murine toxoplasmosis. J Immunol. 1998;160:3427–3436. - PubMed
- Dustin M L, Rothlein R, Bhan A K, Dinarello C A, Springer T A. Induction by IL-1 and interferon-γ: tissue distribution, biochemistry, and function of a natural adherence molecule (ICAM-1) J Immunol. 1986;137:245–254. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials