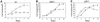Deregulated beta-catenin induces a p53- and ARF-dependent growth arrest and cooperates with Ras in transformation - PubMed (original) (raw)
Deregulated beta-catenin induces a p53- and ARF-dependent growth arrest and cooperates with Ras in transformation
A Damalas et al. EMBO J. 2001.
Abstract
Aberrant activation of beta-catenin contributes to the onset of a variety of tumors. We report that a tumor-derived beta-catenin mutant induces accumulation and activation of the p53 tumor suppressor protein. Induction is mediated through ARF, an alternative reading frame product of the INK4A tumor suppressor locus, in a manner partially dependent on the transcription factor E2F1. In wild-type mouse embryo fibroblasts, mutant beta-catenin inhibits cell proliferation and imposes a senescence-like phenotype. This does not occur in cells lacking either ARF or p53, where deregulated beta-catenin actually overrides density-dependent growth inhibition and cooperates with activated Ras in transformation. Thus, the oncogenic activity of deregulated beta-catenin is curtailed by concurrent activation of the p53 pathway, thereby providing a protective mechanism against cancer. When the p53 pathway is impaired, deregulated beta-catenin is free to manifest its oncogenic features. This can occur not only by p53 mutations, but also by ablation of ARF expression, as observed frequently in early stages of colorectal carcinogenesis.
Figures
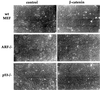
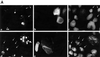
Fig. 1. Induction of p53 and ARF by deregulated β-catenin.(A) Comparison of β-catenin levels in different cell types. Cell extracts were prepared from CRC lines (SW48, HCT116, SW480) and from fibroblasts infected with recombinant retroviruses encoding either β-catenin S33Y (wtMEF + β-cat) or puromycin resistance only (wtMEF). Four micrograms of protein of each sample was subjected to western blot analysis with a monoclonal antibody directed against β-catenin (Sigma). (B) Low passage MEFs, derived from either wt or p53-null animals, were infected with recombinant retroviruses encoding either HA-tagged S33Y tumor-derived mutant β-catenin (β) or puromycin resistance only (v). Cells were trypsinized and replated 48 h later. Cells were re-fed the next day with medium containing 0.1% serum, and harvested after another 48 h. Twenty micrograms of protein of each sample was subjected to sequential western blot analysis with antibodies directed against p53, p19ARF and α-tubulin as a control for equal loading. (C) Low passage p53-null MEFs, infected with either β-catenin (β) or control retrovirus (v), were transiently transfected with a firefly luciferase reporter plasmid driven by the murine ARF promoter, either alone or together with a plasmid expressing dominant negative TCF4 (DN-TCF4). Transfections were done in triplicate. Luciferase activity was normalized for Renilla luciferase readings in the same extracts (see Materials and methods). (D) wt MEFs infected with a retrovirus encoding HA-tagged β-catenin S33Y (a–c) or with pBabe-puro control retrovirus (d–f) were fixed and stained with p19ARF-specific antiserum (a,d), B23 antiserum to visualize nucleoli (b,e) and DAPI to visualize nuclei (c,f). (E) wt MEFs were infected and processed essentially as in (B), except that blots were probed sequentially for p53, p21Waf1 and α-tubulin. Identical samples, run in parallel lanes of the same gel, were probed for p19ARF. (F) wt MEFs were infected and processed as in (B), except that the cells were maintained in medium containing 10% serum throughout the experiment.
Fig. 1. Induction of p53 and ARF by deregulated β-catenin.(A) Comparison of β-catenin levels in different cell types. Cell extracts were prepared from CRC lines (SW48, HCT116, SW480) and from fibroblasts infected with recombinant retroviruses encoding either β-catenin S33Y (wtMEF + β-cat) or puromycin resistance only (wtMEF). Four micrograms of protein of each sample was subjected to western blot analysis with a monoclonal antibody directed against β-catenin (Sigma). (B) Low passage MEFs, derived from either wt or p53-null animals, were infected with recombinant retroviruses encoding either HA-tagged S33Y tumor-derived mutant β-catenin (β) or puromycin resistance only (v). Cells were trypsinized and replated 48 h later. Cells were re-fed the next day with medium containing 0.1% serum, and harvested after another 48 h. Twenty micrograms of protein of each sample was subjected to sequential western blot analysis with antibodies directed against p53, p19ARF and α-tubulin as a control for equal loading. (C) Low passage p53-null MEFs, infected with either β-catenin (β) or control retrovirus (v), were transiently transfected with a firefly luciferase reporter plasmid driven by the murine ARF promoter, either alone or together with a plasmid expressing dominant negative TCF4 (DN-TCF4). Transfections were done in triplicate. Luciferase activity was normalized for Renilla luciferase readings in the same extracts (see Materials and methods). (D) wt MEFs infected with a retrovirus encoding HA-tagged β-catenin S33Y (a–c) or with pBabe-puro control retrovirus (d–f) were fixed and stained with p19ARF-specific antiserum (a,d), B23 antiserum to visualize nucleoli (b,e) and DAPI to visualize nuclei (c,f). (E) wt MEFs were infected and processed essentially as in (B), except that blots were probed sequentially for p53, p21Waf1 and α-tubulin. Identical samples, run in parallel lanes of the same gel, were probed for p19ARF. (F) wt MEFs were infected and processed as in (B), except that the cells were maintained in medium containing 10% serum throughout the experiment.
Fig. 1. Induction of p53 and ARF by deregulated β-catenin.(A) Comparison of β-catenin levels in different cell types. Cell extracts were prepared from CRC lines (SW48, HCT116, SW480) and from fibroblasts infected with recombinant retroviruses encoding either β-catenin S33Y (wtMEF + β-cat) or puromycin resistance only (wtMEF). Four micrograms of protein of each sample was subjected to western blot analysis with a monoclonal antibody directed against β-catenin (Sigma). (B) Low passage MEFs, derived from either wt or p53-null animals, were infected with recombinant retroviruses encoding either HA-tagged S33Y tumor-derived mutant β-catenin (β) or puromycin resistance only (v). Cells were trypsinized and replated 48 h later. Cells were re-fed the next day with medium containing 0.1% serum, and harvested after another 48 h. Twenty micrograms of protein of each sample was subjected to sequential western blot analysis with antibodies directed against p53, p19ARF and α-tubulin as a control for equal loading. (C) Low passage p53-null MEFs, infected with either β-catenin (β) or control retrovirus (v), were transiently transfected with a firefly luciferase reporter plasmid driven by the murine ARF promoter, either alone or together with a plasmid expressing dominant negative TCF4 (DN-TCF4). Transfections were done in triplicate. Luciferase activity was normalized for Renilla luciferase readings in the same extracts (see Materials and methods). (D) wt MEFs infected with a retrovirus encoding HA-tagged β-catenin S33Y (a–c) or with pBabe-puro control retrovirus (d–f) were fixed and stained with p19ARF-specific antiserum (a,d), B23 antiserum to visualize nucleoli (b,e) and DAPI to visualize nuclei (c,f). (E) wt MEFs were infected and processed essentially as in (B), except that blots were probed sequentially for p53, p21Waf1 and α-tubulin. Identical samples, run in parallel lanes of the same gel, were probed for p19ARF. (F) wt MEFs were infected and processed as in (B), except that the cells were maintained in medium containing 10% serum throughout the experiment.
Fig. 2. Induction of ARF and p53 by LiCl. Early passage wt MEFs were plated at a density of 2 × 105/10-cm dish. Fourteen hours later, LiCl was added to the indicated final concentration, for an additional 48 h. Cells were then harvested and processed as in Figure 1.
Fig. 3. ARF is required for accumulation and activation of p53 in response to oncogenic β-catenin. Early passage MEFs, derived from wt or ARF-deficient (ARF–/–) mice, were infected as in Figure 1B. Protein extracts (20 µg total protein/lane) were subjected to western blot analysis essentially as in Figure 1B, except that HA-tag specific antibodies were employed to visualize the mutant β-catenin (HA-mβ-catenin).
Fig. 4. Involvement of E2F1 in the induction of the ARF–p53 pathway by β-catenin. (A) Induction of ARF and p53 in E2F1-null cells. MEFs from wt and E2F1-null mice were infected and analyzed as in Figure 3. Band intensities were determined by densitometry (NIH-Image). Numbers below lanes indicate the fold induction of the respective protein by mutant β-catenin, calculated relative to the parallel control virus-infected wt MEF sample, after normalizing for α-tubulin. (B) Western blot analysis of cyclin D1 protein in wt MEFs infected with either control (v) or mutant β-catenin (β) retrovirus. Infection and protein analysis were as in Figure 1B.
Fig. 5. Overexpression of mutant β-catenin elicits a senescence-like phenotype dependent on ARF and p53. Early passage MEFs, derived from wt, ARF-null or p53-null mice, were infected with control retrovirus or retrovirus encoding HA-tagged β-catenin S33Y. Cells were trypsinized and replated 48 h later, and after an additional day fresh medium containing 10% serum was added. Cultures were maintained in the same medium for 6 days. Phase contrast photographs were taken at a magnification of 100×.
Fig. 6. Deregulated β-catenin inhibits cell proliferation in an ARF- and p53-dependent manner. Cultures of wt, ARF-null and p53-null MEFs were infected with control retrovirus (circles) or retrovirus encoding HA-tagged β-catenin S33Y (squares) and processed as in Figure 5. Infected cells were replated in 10-cm dishes, at a seeding density of 2 × 105 cells/dish. Triplicate cultures were counted at the indicated number of days after replating. The SE is indicated.
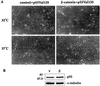
Fig. 7. wt p53 activity is required for induction of a senescence-like phenotype by deregulated β-catenin. Early passage p53–/– MEFs were infected with control retrovirus or retrovirus encoding HA-tagged β-catenin S33Y. Forty-eight hours later cells were trypsinized, replated and subjected to a second round of infection with a retrovirus encoding the ts p53 mutant p53Val135. After 48 h fresh medium containing 10% serum was added, and some of the cultures were transferred to 32°C while the others were left at 37.5°C. (A) Phase contrast photographs taken 6 days after plating (magnification = 100×). (B) Western blot analysis of p53 protein levels in cultures maintained at 32°C and extracted 4 days after replating.
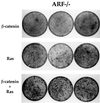
Fig. 8. Oncogenic β-catenin cooperates with Ras in transformation of ARF-null MEFs. Early passage ARF–/– MEFs (1.5 × 105 cells/10-cm dish) were infected with either control retrovirus or retrovirus encoding β-catenin S33Y. Two days later the cultures were trypsinized and replated again at 1.5 × 105 cells/10-cm dish, and subjected to a second round of infection with a retrovirus encoding the Val12 mutant H-Ras. Cultures were fixed 8–9 days later and stained with Giemsa stain. Triplicate dishes are shown. Where only a single oncogene is indicated, the cultures were actually also subjected to a second infection with control retrovirus to maintain the infection history of each culture equal.
Fig. 9. ARF is induced by constitutive β-catenin signaling in SW480 CRC cells. (A) SW480 cells were plated at a density of 5 × 105 cells/well in a 6-well plate. Two days later, cultures were transiently transfected with either myc-tagged axin (5 µg) (a–c) or a combination of GFP (50 ng) plus DN-TCF4 (5 µg) (d–f). Twenty-four hours later cells were fixed. Endogenous p14ARF (a,c) was visualized by staining with polyclonal anti-p14ARF antibodies. Cells positive for transfected axin were visualized by staining with a monoclonal antibody directed against the myc tag (b). Putative DN-TCF4 transfectants were identified by GFP fluorescence (e). DAPI staining was employed to visualize nuclei (c,f). (B) Quantitative analysis of the experiment shown in (A). The left and right panels depict the data for SW480 cultures transfected with either axin or DN-TCF+GFP, respectively. (–) and (+) relate to the cell subpopulations staining negative or positive for the transfected protein (myc-tagged axin and GFP, respectively; GFP is expected to mark DN-TCF positive cells). In each case, the number relates to the percentage of cells within the given subpopulation where prominent nucleolar ARF staining was easily discernable. (C) SW480 cells were transiently transfected with a luciferase reporter driven by the mouse p19ARF promoter plus either control vector (v) or plasmids expressing DN-TCF4 or axin. Transfections were done in triplicate. Luciferase activity was normalized for Renilla luciferase readings in the same extracts (see Materials and methods).
Fig. 9. ARF is induced by constitutive β-catenin signaling in SW480 CRC cells. (A) SW480 cells were plated at a density of 5 × 105 cells/well in a 6-well plate. Two days later, cultures were transiently transfected with either myc-tagged axin (5 µg) (a–c) or a combination of GFP (50 ng) plus DN-TCF4 (5 µg) (d–f). Twenty-four hours later cells were fixed. Endogenous p14ARF (a,c) was visualized by staining with polyclonal anti-p14ARF antibodies. Cells positive for transfected axin were visualized by staining with a monoclonal antibody directed against the myc tag (b). Putative DN-TCF4 transfectants were identified by GFP fluorescence (e). DAPI staining was employed to visualize nuclei (c,f). (B) Quantitative analysis of the experiment shown in (A). The left and right panels depict the data for SW480 cultures transfected with either axin or DN-TCF+GFP, respectively. (–) and (+) relate to the cell subpopulations staining negative or positive for the transfected protein (myc-tagged axin and GFP, respectively; GFP is expected to mark DN-TCF positive cells). In each case, the number relates to the percentage of cells within the given subpopulation where prominent nucleolar ARF staining was easily discernable. (C) SW480 cells were transiently transfected with a luciferase reporter driven by the mouse p19ARF promoter plus either control vector (v) or plasmids expressing DN-TCF4 or axin. Transfections were done in triplicate. Luciferase activity was normalized for Renilla luciferase readings in the same extracts (see Materials and methods).
Fig. 9. ARF is induced by constitutive β-catenin signaling in SW480 CRC cells. (A) SW480 cells were plated at a density of 5 × 105 cells/well in a 6-well plate. Two days later, cultures were transiently transfected with either myc-tagged axin (5 µg) (a–c) or a combination of GFP (50 ng) plus DN-TCF4 (5 µg) (d–f). Twenty-four hours later cells were fixed. Endogenous p14ARF (a,c) was visualized by staining with polyclonal anti-p14ARF antibodies. Cells positive for transfected axin were visualized by staining with a monoclonal antibody directed against the myc tag (b). Putative DN-TCF4 transfectants were identified by GFP fluorescence (e). DAPI staining was employed to visualize nuclei (c,f). (B) Quantitative analysis of the experiment shown in (A). The left and right panels depict the data for SW480 cultures transfected with either axin or DN-TCF+GFP, respectively. (–) and (+) relate to the cell subpopulations staining negative or positive for the transfected protein (myc-tagged axin and GFP, respectively; GFP is expected to mark DN-TCF positive cells). In each case, the number relates to the percentage of cells within the given subpopulation where prominent nucleolar ARF staining was easily discernable. (C) SW480 cells were transiently transfected with a luciferase reporter driven by the mouse p19ARF promoter plus either control vector (v) or plasmids expressing DN-TCF4 or axin. Transfections were done in triplicate. Luciferase activity was normalized for Renilla luciferase readings in the same extracts (see Materials and methods).
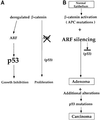
Fig. 10. Schematic model depicting the relationship between deregulated β-catenin and the ARF–p53 pathway in MEFs and during carcinogenesis. In MEFs, ablation of ARF prevents the activation of p53 by deregulated β-catenin and spares the cells from p53-mediated growth inhibition (this study). In carcinogenesis, it is proposed that ARF silencing by promoter hypermethylation or by other mechanisms enables emerging tumor cells to benefit from the oncogenic activities of deregulated β-catenin while avoiding the inhibitory consequences of p53 activation. Additional genetic alterations, occurring at later stages of carcinogenesis, eventually generate a selective pressure for mutation of the p53 gene and lead to full malignancy. See Discussion for further details.
Similar articles
- Activation of ARF by oncogenic stress in mouse fibroblasts is independent of E2F1 and E2F2.
Palmero I, Murga M, Zubiaga A, Serrano M. Palmero I, et al. Oncogene. 2002 May 2;21(19):2939-47. doi: 10.1038/sj.onc.1205371. Oncogene. 2002. PMID: 12082524 - C/EBPbeta cooperates with RB:E2F to implement Ras(V12)-induced cellular senescence.
Sebastian T, Malik R, Thomas S, Sage J, Johnson PF. Sebastian T, et al. EMBO J. 2005 Sep 21;24(18):3301-12. doi: 10.1038/sj.emboj.7600789. Epub 2005 Aug 18. EMBO J. 2005. PMID: 16107878 Free PMC article. - Human ARF binds E2F1 and inhibits its transcriptional activity.
Eymin B, Karayan L, Séité P, Brambilla C, Brambilla E, Larsen CJ, Gazzéri S. Eymin B, et al. Oncogene. 2001 Mar 1;20(9):1033-41. doi: 10.1038/sj.onc.1204220. Oncogene. 2001. PMID: 11314038 - p53-Dependent and -independent functions of the Arf tumor suppressor.
Sherr CJ, Bertwistle D, DEN Besten W, Kuo ML, Sugimoto M, Tago K, Williams RT, Zindy F, Roussel MF. Sherr CJ, et al. Cold Spring Harb Symp Quant Biol. 2005;70:129-37. doi: 10.1101/sqb.2005.70.004. Cold Spring Harb Symp Quant Biol. 2005. PMID: 16869746 Review. - Dynamics of ARF regulation that control senescence and cancer.
Ko A, Han SY, Song J. Ko A, et al. BMB Rep. 2016 Nov;49(11):598-606. doi: 10.5483/bmbrep.2016.49.11.120. BMB Rep. 2016. PMID: 27470213 Free PMC article. Review.
Cited by
- β-Catenin C-terminal signals suppress p53 and are essential for artery formation.
Riascos-Bernal DF, Chinnasamy P, Cao LL, Dunaway CM, Valenta T, Basler K, Sibinga NE. Riascos-Bernal DF, et al. Nat Commun. 2016 Aug 8;7:12389. doi: 10.1038/ncomms12389. Nat Commun. 2016. PMID: 27499244 Free PMC article. - Increased expression of prothymosin-α, independently or combined with TP53, correlates with poor prognosis in colorectal cancer.
Zhang M, Cui F, Lu S, Lu H, Jiang T, Chen J, Zhang X, Jin Y, Peng Z, Tang H. Zhang M, et al. Int J Clin Exp Pathol. 2014 Jul 15;7(8):4867-76. eCollection 2014. Int J Clin Exp Pathol. 2014. PMID: 25197357 Free PMC article. - Pak4 induces premature senescence via a pathway requiring p16INK4/p19ARF and mitogen-activated protein kinase signaling.
Cammarano MS, Nekrasova T, Noel B, Minden A. Cammarano MS, et al. Mol Cell Biol. 2005 Nov;25(21):9532-42. doi: 10.1128/MCB.25.21.9532-9542.2005. Mol Cell Biol. 2005. PMID: 16227603 Free PMC article. - Homozygous deletion of glycogen synthase kinase 3beta bypasses senescence allowing Ras transformation of primary murine fibroblasts.
Liu S, Fang X, Hall H, Yu S, Smith D, Lu Z, Fang D, Liu J, Stephens LC, Woodgett JR, Mills GB. Liu S, et al. Proc Natl Acad Sci U S A. 2008 Apr 1;105(13):5248-53. doi: 10.1073/pnas.0704242105. Epub 2008 Mar 26. Proc Natl Acad Sci U S A. 2008. PMID: 18367674 Free PMC article. - Novel ARF/p53-independent senescence pathways in cancer repression.
Chan CH, Gao Y, Moten A, Lin HK. Chan CH, et al. J Mol Med (Berl). 2011 Sep;89(9):857-67. doi: 10.1007/s00109-011-0766-y. Epub 2011 May 19. J Mol Med (Berl). 2011. PMID: 21594579 Free PMC article. Review.
References
- Bates S., Phillips,A.C., Clark,P.A., Stott,F., Peters,G., Ludwig,R.L. and Vousden,K.H. (1998) p14ARF links the tumour suppressors RB and p53. Nature, 395, 124–125. - PubMed
- Behrens J., von Kries,J.P., Kuhl,M., Bruhn,L., Wedlich,D., Grosschedl, R. and Birchmeier,W. (1996) Functional interaction of β-catenin with the transcription factor LEF-1. Nature, 382, 638–642. - PubMed
- Behrens J., Jerchow,B.A., Wurtele,M., Grimm,J., Asbrand,C., Wirtz,R., Kuhl,M., Wedlich,D. and Birchmeier,W. (1998) Functional interaction of an axin homolog, conductin, with β-catenin, APC and GSK3β. Science, 280, 596–599. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous