The Salmonella enterica serotype typhimurium effector proteins SipA, SopA, SopB, SopD, and SopE2 act in concert to induce diarrhea in calves - PubMed (original) (raw)
The Salmonella enterica serotype typhimurium effector proteins SipA, SopA, SopB, SopD, and SopE2 act in concert to induce diarrhea in calves
Shuping Zhang et al. Infect Immun. 2002 Jul.
Abstract
Salmonella enterica serotype Typhimurium requires a functional type III secretion system encoded by Salmonella pathogenicity island 1 (SPI1) to cause diarrhea. We investigated the role of genes encoding secreted target proteins of the SPI1-associated type III secretion system for enteropathogenicity in calves. Salmonella serotype Typhimurium strains having mutations in sptP, avrA, sspH1, or slrP induced fluid secretion in the bovine ligated ileal loop model at levels similar to that of the wild type. In contrast, mutations in sipA, sopA, sopB, sopD, or sopE2 significantly reduced fluid accumulation in bovine ligated ileal loops at 8 h postinfection. A strain carrying mutations in sipA, sopA, sopB, sopD, and sopE2 (sipA sopABDE2 mutant) caused the same level of fluid accumulation in bovine ligated ileal loops as a strain carrying a mutation in sipB, a SPI1 gene required for the translocation of effector proteins into host cells. A positive correlation was observed between the severity of histopathological lesions detected in the ileal mucosa and the levels of fluid accumulation induced by the different mutants. After oral infection of calves, the Salmonella serotype Typhimurium sipAsopABDE2 mutant caused only mild diarrhea and was more strongly attenuated than strains having only single mutations. These data demonstrate that SipA, SopA, SopB, SopD, and SopE2 are major virulence factors responsible for diarrhea during Salmonella serotype Typhimurium infection of calves.
Figures
FIG. 1.
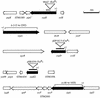
Mutations in effector genes (solid arrows) which are required for fluid accumulation in calves. The open arrows indicate the positions of genes in the surrounding DNA region. Numbers indicate the positions of insertions or deletions relative to the first nucleotide (+1) in the open reading frame. The bars indicate internal fragments of sopD and sopE2 that were cloned into suicide vectors to inactivate these genes.
FIG. 2.
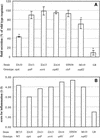
Ability of Salmonella serotype Typhimurium strains carrying mutations in SPI1 effector genes to induce secretory and inflammatory changes in bovine ligated ileal loops at 8 h postinfection. (A) Data for fluid accumulation in loops shown as percentages of the fluid secretion elicited by the wild type (ATCC 14028 for M119 and IR715 for ZA10, ZA11, ZA13, ZA14, and STN39). The 100% line indicates the amount of fluid accumulation elicited by the Salmonella serotype Typhimurium wild type. An asterisks indicates that the fluid accumulation was significantly lower than the wild-type response (P < 0.05). (B) Inflammatory responses scored on a scale from 1 to 5 according the criteria described in Materials and Methods. WT, wild type.
FIG. 3.
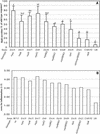
Ability of Salmonella serotype Typhimurium mutants carrying multiple mutations in SPI1 effector genes to induce secretory and inflammatory changes in bovine ligated ileal loops at 8 h postinfection. (A) Data for fluid accumulation in loops shown as percentages of the fluid secretion elicited by the wild type (IR715). The 100% line indicates the amount of fluid accumulation elicited by the Salmonella serotype Typhimurium wild type. All mutants shown elicited significantly less fluid accumulation than the wild type (IR715) (P < 0.05). The results are means from experiments performed with three animals, in which each strain was tested in two loops/animal. The bars indicate means ± standard deviations. The same letter above two bars indicates that the amounts of fluid accumulation elicited by the mutants are not significantly different. Different letters above two bars indicate that the amounts of fluid accumulation elicited by the mutants are significantly different (P < 0.05). (B) Inflammatory changes in infected loops assessed by microscopic examination of hematoxylin- and eosin-stained thin sections of infected tissue. wt, wild type.
FIG. 4.
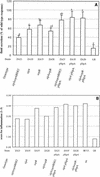
In vivo complementation in bovine ligated ileal loops at 8 h postinfection of strains carrying a sipA deletion with the cloned sipA gene. (A) Data for fluid accumulation in loops shown as percentages of the fluid secretion elicited by the wild type (IR715). The results are means from three experiments in which each strain was tested in at least two loops/animal. The bars indicate means ± standard deviations. The same letter above two bars indicates that the amounts of fluid accumulation elicited by the mutants are not significantly different. Different letters above two bars indicate that the amounts of fluid accumulation elicited by the mutants are significantly different (P < 0.05). (B) Inflammatory changes in infected loops assessed by microscopic examination of hematoxylin- and eosin-stained thin sections of infected tissue. wt, wild type.
FIG. 5.
Ability of SPI1 mutants to cause diarrhea and lethal morbidity in calves infected orally with a dose of 1010 CFU per animal. Lethal morbidity caused by the wild type or by Salmonella serotype Typhimurium mutants over time is shown in the upper graphs. The lower graphs show the severity of diarrhea over time, which was scored on a scale from 1 to 5, as follows: 1, normal feces; 2, soft feces with loss of distinct conformation; 3, diarrhea, loose feces with reduced solid matter; 4, diarrhea, aqueous feces with markedly reduced or little solid matter, or fibrin; 5, diarrhea, aqueous feces with no solid matter, fibrin and blood.
FIG. 6.
Change in the plasma sodium concentration during oral infection of calves with Salmonella serotype Typhimurium strains. The graph shows the difference in the plasma sodium concentration between a sample collected on day 2 postinfection and a preinfection sample. Solid circles indicate that the plasma sodium concentration on day 2 postinfection was below the normal range. The open circle indicates that the plasma sodium concentration on day 2 postinfection was within the normal range. WT, wild type.
FIG. 7.
Recovery of Salmonella serotype Typhimurium strains from the feces of calves after oral infection with a dose of 1010 CFU per animal. The bars indicate the mean ± standard deviation for four calves. An asterisks indicates that the difference between the wild type (IR715) and an individual mutant was significant. wt, wild type.
FIG. 8.
Representative examples of the gross pathology and histopathology of Peyer's patches and the terminal ileum of calves inoculated orally with 1010 CFU of different Salmonella serotype Typhimurium strains. (A) Severe acute fibrinopurulent necrotizing enteritis with segmental or continuous pseudomembrane formation in a calf infected with wild-type strain IR715 (similar pathological changes were observed in calves infected with the sopA mutant, the sopD mutant, or the sopE2 mutant). Bar = 1 cm. (B) Marked subacute fibrinopurulent necrotizing enteritis often confined to the Peyer's patches of the terminal ileum of a calf infected with strain ZA10 (sipA). Bar = 1 cm. (C) Normal Peyer's patches and ileum of a calf infected with strain ZA21 (sipAsopABDE). Bar = 1 cm. (D to F) Hematoxylin- and eosin-stained sections of Peyer's patches of calves infected with IR715, ZA10 (sipA), and ZA21 (sipAsopABDE), respectively. The short arrows indicate areas of lymphoid depletion; the long arrows indicate various degrees of fibrinopurulent necrotizing ileitis at the mucosal surface. Bars = 200 μm.
Similar articles
- SipA, SopA, SopB, SopD, and SopE2 contribute to Salmonella enterica serotype typhimurium invasion of epithelial cells.
Raffatellu M, Wilson RP, Chessa D, Andrews-Polymenis H, Tran QT, Lawhon S, Khare S, Adams LG, Bäumler AJ. Raffatellu M, et al. Infect Immun. 2005 Jan;73(1):146-54. doi: 10.1128/IAI.73.1.146-154.2005. Infect Immun. 2005. PMID: 15618149 Free PMC article. - Role of SPI-1 secreted effectors in acute bovine response to Salmonella enterica Serovar Typhimurium: a systems biology analysis approach.
Lawhon SD, Khare S, Rossetti CA, Everts RE, Galindo CL, Luciano SA, Figueiredo JF, Nunes JE, Gull T, Davidson GS, Drake KL, Garner HR, Lewin HA, Bäumler AJ, Adams LG. Lawhon SD, et al. PLoS One. 2011;6(11):e26869. doi: 10.1371/journal.pone.0026869. Epub 2011 Nov 11. PLoS One. 2011. PMID: 22096503 Free PMC article. - Host restriction of Salmonella enterica serotype Typhi is not caused by functional alteration of SipA, SopB, or SopD.
Raffatellu M, Sun YH, Wilson RP, Tran QT, Chessa D, Andrews-Polymenis HL, Lawhon SD, Figueiredo JF, Tsolis RM, Adams LG, Bäumler AJ. Raffatellu M, et al. Infect Immun. 2005 Dec;73(12):7817-26. doi: 10.1128/IAI.73.12.7817-7826.2005. Infect Immun. 2005. PMID: 16299271 Free PMC article. - Characterization of effector proteins translocated via the SPI1 type III secretion system of Salmonella typhimurium.
Ehrbar K, Mirold S, Friebel A, Stender S, Hardt WD. Ehrbar K, et al. Int J Med Microbiol. 2002 Feb;291(6-7):479-85. doi: 10.1078/1438-4221-00156. Int J Med Microbiol. 2002. PMID: 11890547 Review. - Molecular pathogenesis of Salmonella enterica serotype typhimurium-induced diarrhea.
Zhang S, Kingsley RA, Santos RL, Andrews-Polymenis H, Raffatellu M, Figueiredo J, Nunes J, Tsolis RM, Adams LG, Bäumler AJ. Zhang S, et al. Infect Immun. 2003 Jan;71(1):1-12. doi: 10.1128/IAI.71.1.1-12.2003. Infect Immun. 2003. PMID: 12496143 Free PMC article. Review. No abstract available.
Cited by
- The frequency and duration of Salmonella-macrophage adhesion events determines infection efficiency.
Achouri S, Wright JA, Evans L, Macleod C, Fraser G, Cicuta P, Bryant CE. Achouri S, et al. Philos Trans R Soc Lond B Biol Sci. 2015 Feb 5;370(1661):20140033. doi: 10.1098/rstb.2014.0033. Philos Trans R Soc Lond B Biol Sci. 2015. PMID: 25533091 Free PMC article. - Diarrhea and colitis in mice require the Salmonella pathogenicity island 2-encoded secretion function but not SifA or Spv effectors.
Fierer J, Okamoto S, Banerjee A, Guiney DG. Fierer J, et al. Infect Immun. 2012 Oct;80(10):3360-70. doi: 10.1128/IAI.00404-12. Epub 2012 Jul 9. Infect Immun. 2012. PMID: 22778101 Free PMC article. - Cooperative interactions between flagellin and SopE2 in the epithelial interleukin-8 response to Salmonella enterica serovar typhimurium infection.
Huang FC, Werne A, Li Q, Galyov EE, Walker WA, Cherayil BJ. Huang FC, et al. Infect Immun. 2004 Sep;72(9):5052-62. doi: 10.1128/IAI.72.9.5052-5062.2004. Infect Immun. 2004. PMID: 15321998 Free PMC article. - The Salmonella translocated effector SopA is targeted to the mitochondria of infected cells.
Layton AN, Brown PJ, Galyov EE. Layton AN, et al. J Bacteriol. 2005 May;187(10):3565-71. doi: 10.1128/JB.187.10.3565-3571.2005. J Bacteriol. 2005. PMID: 15866946 Free PMC article. - Diverse secreted effectors are required for Salmonella persistence in a mouse infection model.
Kidwai AS, Mushamiri I, Niemann GS, Brown RN, Adkins JN, Heffron F. Kidwai AS, et al. PLoS One. 2013 Aug 12;8(8):e70753. doi: 10.1371/journal.pone.0070753. eCollection 2013. PLoS One. 2013. PMID: 23950998 Free PMC article.
References
- Ahmer, B. M., J. van Reeuwijk, P. R. Watson, T. S. Wallis, and F. Heffron. 1999. Salmonella SirA is a global regulator of genes mediating enteropathogenesis. Mol. Microbiol. 31:971-982. - PubMed
- Altmeyer, R. M., J. K. McNern, J. C. Bossio, I. Rosenshine, B. B. Finlay, and J. E. Galán. 1993. Cloning and molecular characterization of a gene involved in Salmonella adherence and invasion of cultured epithelial cells. Mol. Microbiol. 7:89-98. - PubMed
- Bajaj, V., C. Hwang, and C. A. Lee. 1995. hilA is a novel ompR/toxR family member that activates the expression of Salmonella typhimurium invasion genes. Mol. Microbiol. 18:715-727. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AI44170/AI/NIAID NIH HHS/United States
- R01 AI044170/AI/NIAID NIH HHS/United States
- R29 AI040124/AI/NIAID NIH HHS/United States
- R01 AI040124/AI/NIAID NIH HHS/United States
- AI40124/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical