Oestrogen inhibits resveratrol-induced post-translational modification of p53 and apoptosis in breast cancer cells - PubMed (original) (raw)
Oestrogen inhibits resveratrol-induced post-translational modification of p53 and apoptosis in breast cancer cells
S Zhang et al. Br J Cancer. 2004.
Abstract
Resveratrol, a naturally occurring stilbene, induced apoptosis in human breast cancer MCF-7 cells. The mechanism of this effect was dependent on mitogen-activated protein kinase (MAPK, ERK1/2) activation and was associated with serine phosphorylation and acetylation of p53. Treatment of MCF-7 cells with resveratrol in the presence of 17beta-oestradiol (E(2)) further enhanced MAPK activation, but E(2) blocked resveratrol-induced apoptosis, as measured by nucleosome ELISA and DNA fragmentation assays. E(2) inhibited resveratrol-stimulated phosphorylation of serines 15, 20 and 392 of p53 and acetylation of p53 in a concentration- and time-dependent manner. These effects of E(2) on resveratrol action were blocked by ICI 182,780 (ICI), an inhibitor of the nuclear oestrogen receptor-alpha (ER). ICI 182,780 did not block the actions of resveratrol, alone. Electrophoretic mobility studies of p53 binding to DNA and of p21 expression indicated that E(2) inhibited resveratrol-induced, p53-directed transcriptional activity. These results suggest that E(2) inhibits p53-dependent apoptosis in MCF-7 cells by interfering with post-translational modifications of p53 which are essential for p53-dependent DNA binding and consequent stimulation of apoptotic pathways. These studies provide insight into the complex pathways by which apoptosis is induced by resveratrol in E(2)-depleted and -repleted environments.
Figures
Figure 1
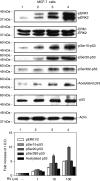
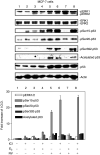
Resveratrol treatment of MCF-7 cells leads to phosphorylation (activation) of MAPK, serine phosphorylation of p53 and acetylation of p53 in MCF-7 breast cancer cells. Cells were treated with 1–100 μ
M
resveratrol (RV) for 4 h. Nuclear proteins were separated by electrophoresis, and immunoblots performed with selected antibodies. Mitogen-activated protein kinase activation, shown as appearance in nuclei of nuclear phosphorylated ERK1 and ERK2 (pERK1 and 2), and phosphorylation of serines 15, 20 and 392 of p53, as well as acetylation of p53, were induced by resveratrol in a concentration-dependent manner (lanes 2–4). Increases in phosphorylation of ERK1/2 and acetylation and phosphorylation of p53 with 1–100 μ
M
RV were each significant at P<0.001 by one-way analysis of variance in three similar experiments. The graph below shows the changes in phosphorylation of ERK1/2 and p53, and acetylation of p53, normalized to a value of 1 in control samples, in three experiments. Means ± s.e.m. are shown. Immunoblots of total nuclear ERKs 1 and 2 (second panel from top) are consistent with activation (phosphorylation) and nuclear accumulation of these proteins, and a slight increase in total nuclear p53 is consistent with the resveratrol effect which we have previously described (Lin et al, 2002; Shih et al, 2002). Actin immunoblots serve as loading controls in this and subsequent figures. Immunoblots in all figures are representative of three or more experiments. Molecular weights of the proteins shown are as follows: pERK1, 44 kDa; pERK2, 42 kDa; p53, 53 kDa; actin, 43 kDa. (I.O.D., integrated optical density).
Figure 2
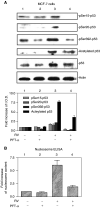
Activation of p53 and apoptosis induced by resveratrol is inhibited by pifithrin-α in MCF-7 cells. (A) Cells were treated with 10 μ
M
resveratrol (RV) for 4 h in the presence or absence of 20 μ
M
pifithrin-α (PFT-α), a specific p53 inhibitor. As shown by immunoblots of nuclear fractions from a representative experiment above, and the accompanying graph below, resveratrol caused nuclear accumulation, serine phosphorylation and acetylation of p53 (P<0.05 comparing lanes 1 and 3 for each parameter). Treatment with PFT-α resulted in decreased phosphorylation and acetylation of p53 (P<0.05 for all parameters, comparing lanes 3 and 4). Molecular weight markers in this figure are not shown, but are similar to those shown in Figure 1. (B) Cells were treated with 10 μ
M
resveratrol for 24 h in the presence or absence of 20 μ
M
PFT-α. Apoptosis, shown by an increase in nucleosome content determined by ELISA, occurred with resveratrol treatment (P<0.001, comparing lanes 1 and 3. Pifithrin-α had no effect alone, but significantly blocked resveratrol-induced apoptosis (comparing lanes 3 and 4, P<0.005). Means±s.e.m. of six experiments are shown.
Figure 3
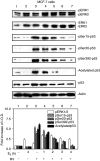
E2 inhibition of resveratrol-induced activation of MAPK and p53 is time dependent. MCF-7 cells were treated with E2 (10−9
M
) alone for 4 h or for different time periods (0–4 h) with resveratrol (RV, 10 μ
M
, 4 h), after which nuclear fractions were prepared from each sample. This series of immunoblots, representative of three experiments, indicates that minimal activation of ERK1/2 by resveratrol (top panel, lane 3) was enhanced by 1–2 h incubation with E2 (lanes 4 and 5). A longer incubation with E2 caused a time-dependent reduction in ERK1/2 phosphorylation (lanes 4–7, P<0.05). Resveratrol induced phosphorylation of serines 15, 20 and 392 of p53 (pSer15-, pSer20- and pSer392-p53), and acetylation of p53 (lane 3 in each panel). However, these effects of resveratrol on p53 post-translational modification were progressively inhibited by coincubation with E2 for 1–4 h. The reductions in resveratrol-induced serine-15, serine-20, and serine-392 phosphorylation and acetylation of p53 by E2 were significant at P<0.003 or less. Molecular weight markers in this figure are not shown, but are similar to those shown in Figure 1.
Figure 4
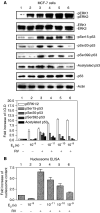
E2 inhibits resveratrol-induced p53 phosphorylation and acetylation, and subsequent apoptosis, in a concentration-dependent manner. (A) MCF-7 cells were treated with E2 (10−11–10−9
M
) with or without resveratrol (RV, 10 μ
M
) for 4 h. Immunoblots of nuclear fractions from this representative experiment show that resveratrol-induced MAPK activation (pERK1/2, top panel, lane 3) was enhanced by 10−11–10−9
M
E2 (lanes 3–6; P<0.005). However, resveratrol-induced ser15, ser20 and ser392 phosphorylation of p53, as well as acetylation of p53, was inhibited by E2 in a dose-dependent manner (P<0.001, lanes 3–6 for all these parameters), with the most marked inhibition occurring at 10−9
M
E2 (lane 6). Molecular weight markers in this figure are not shown, but are similar to those shown in Figure 1. (B) This graph shows the extent of apoptosis, measured by nucleosome ELISA, in MCF-7 cells treated with E2 (10−11–10−9
M
) with or without resveratrol (RV, 10 μ
M
) for 24 h. Resveratrol alone caused apoptosis (lane 3, P<0.001). E2 alone did not induce apoptosis, but the hormone inhibited nucleosome formation induced by resveratrol in a dose-dependent manner (P<0.001). Means±s.e.m. of five experiments are shown.
Figure 5
ICI 182,780 (ICI) inhibits the effects of E2 on resveratrol-induced activation of MAPK and p53. MCF-7 cells were pretreated with 3 n
M
ICI or diluent for 30 min, after which they were treated with 10−9
M
E2 and/or 10 μ
M
resveratrol for 4 h along with continued 3 n
M
ICI or diluent as in the pretreatment period. Results shown are representative of three experiments. Immunoblots of nuclear fractions show that ICI minimally inhibited E2-induced MAPK activation (comparing lanes 3 and 4), but did not inhibit resveratrol-induced MAPK activation (lanes 5 and 6). ICI 182,780 did enhance resveratrol-induced ser15-p53 phosphorylation, although this effect was not statistically significant. The additive effect on MAPK activation of E2 and resveratrol was reduced by ICI (P<0.05, lane 7 vs lane 8), and the inhibitory effect of E2 on resveratrol-induced serine phosphorylation and acetylation of p53 was partially reversed by ICI treatment (P<0.02 for each parameter, comparing lanes 7 and 8). Molecular weight markers in this figure are not shown, but are similar to those shown in Figure 1.
Figure 6
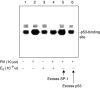
E2 inhibits resveratrol-induced p53 binding to a relevant oligonucleotide in an EMSA. The assay was carried out with nuclear fractions prepared after 4 h treatment of cells with 10 μ
M
resveratrol, 10−9
M
E2, or both agents. p53 binding to the radiolabelled oligonucleotide was seen (lane 2). E2 inhibited p53 binding (lane 4 compared with lane 2). Excess unlabelled oligonucleotide (p53-oligo) decreased p53 binding to the labelled form (lane 6), whereas there was no change in p53 binding with an excess of unlabelled nonspecific SP-1 oligonucleotide (lane 5). This figure is representative of three similar experiments.
Figure 7
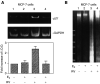
E2 inhibits p53-inducible p21 expression and apoptosis caused by resveratrol. (A) MCF-7 cells were treated with 10 μ
M
resveratrol in the presence or absence of 10−9
M
E2 for 24 h. Based on measurement of p21 and GAPDH cDNA band densities (upper and lower images, respectively), and correction of p21 densities for the levels of GAPDH, a 2.5-fold increase in p21 cDNA abundance was seen with resveratrol treatment (lane 3 compared with lane 1). This increase in p21 expression was significant (P<0.025) by analysis of variance. E2, 10−9
M
, blocked p21 transcription induced by resveratrol (lane 4 compared with lane 3, P<0.05), but had no effect in the absence of resveratrol. (B) MCF-7 cells were treated with 10 μ
M
resveratrol in the presence or absence of 10−9
M
E2 for 24 h. Resveratrol caused DNA fragmentation, indicating apoptosis as shown in this representative figure (lane 3). This effect of resveratrol was inhibited by E2 (lane 4).
Similar articles
- Resveratrol induced serine phosphorylation of p53 causes apoptosis in a mutant p53 prostate cancer cell line.
Lin HY, Shih A, Davis FB, Tang HY, Martino LJ, Bennett JA, Davis PJ. Lin HY, et al. J Urol. 2002 Aug;168(2):748-55. J Urol. 2002. PMID: 12131363 - Mechanisms of dihydrotestosterone action on resveratrol-induced anti-proliferation in breast cancer cells with different ERα status.
Chin YT, Yang SH, Chang TC, Changou CA, Lai HY, Fu E, HuangFu WC, Davis PJ, Lin HY, Liu LF. Chin YT, et al. Oncotarget. 2015 Nov 3;6(34):35866-79. doi: 10.18632/oncotarget.5482. Oncotarget. 2015. PMID: 26456774 Free PMC article. - Resveratrol-induced cyclooxygenase-2 facilitates p53-dependent apoptosis in human breast cancer cells.
Tang HY, Shih A, Cao HJ, Davis FB, Davis PJ, Lin HY. Tang HY, et al. Mol Cancer Ther. 2006 Aug;5(8):2034-42. doi: 10.1158/1535-7163.MCT-06-0216. Mol Cancer Ther. 2006. PMID: 16928824 - Resveratrol and apoptosis.
Lin HY, Tang HY, Davis FB, Davis PJ. Lin HY, et al. Ann N Y Acad Sci. 2011 Jan;1215:79-88. doi: 10.1111/j.1749-6632.2010.05846.x. Ann N Y Acad Sci. 2011. PMID: 21261644 Review. - Molecular mechanism of the chemopreventive effect of resveratrol.
Dong Z. Dong Z. Mutat Res. 2003 Feb-Mar;523-524:145-50. doi: 10.1016/s0027-5107(02)00330-5. Mutat Res. 2003. PMID: 12628512 Review.
Cited by
- Low-dose valproic acid enhances radiosensitivity of prostate cancer through acetylated p53-dependent modulation of mitochondrial membrane potential and apoptosis.
Chen X, Wong JY, Wong P, Radany EH. Chen X, et al. Mol Cancer Res. 2011 Apr;9(4):448-61. doi: 10.1158/1541-7786.MCR-10-0471. Epub 2011 Feb 8. Mol Cancer Res. 2011. PMID: 21303901 Free PMC article. - Therapeutic potentials of resveratrol in combination with radiotherapy and chemotherapy during glioblastoma treatment: a mechanistic review.
Arabzadeh A, Mortezazadeh T, Aryafar T, Gharepapagh E, Majdaeen M, Farhood B. Arabzadeh A, et al. Cancer Cell Int. 2021 Jul 21;21(1):391. doi: 10.1186/s12935-021-02099-0. Cancer Cell Int. 2021. PMID: 34289841 Free PMC article. Review. - Cancer Chemoprevention by Resveratrol: The p53 Tumor Suppressor Protein as a Promising Molecular Target.
Ferraz da Costa DC, Fialho E, Silva JL. Ferraz da Costa DC, et al. Molecules. 2017 Jun 18;22(6):1014. doi: 10.3390/molecules22061014. Molecules. 2017. PMID: 28629161 Free PMC article. Review. - The Role of Resveratrol in Cancer Therapy.
Ko JH, Sethi G, Um JY, Shanmugam MK, Arfuso F, Kumar AP, Bishayee A, Ahn KS. Ko JH, et al. Int J Mol Sci. 2017 Dec 1;18(12):2589. doi: 10.3390/ijms18122589. Int J Mol Sci. 2017. PMID: 29194365 Free PMC article. Review. - A Systematic Review of the Potential Chemoprotective Effects of Resveratrol on Doxorubicin-Induced Cardiotoxicity: Focus on the Antioxidant, Antiapoptotic, and Anti-Inflammatory Activities.
Hu LF, Lan HR, Li XM, Jin KT. Hu LF, et al. Oxid Med Cell Longev. 2021 Aug 22;2021:2951697. doi: 10.1155/2021/2951697. eCollection 2021. Oxid Med Cell Longev. 2021. PMID: 34471463 Free PMC article.
References
- Agarwal ML, Taylor WR, Chernov MV, Chernova OB, Stark GR (1998) The p53 network. J Biol Chem 273: 1–4 - PubMed
- Ashcroft M, Vousden KH (1999) Regulation of p53 stability. Oncogene 18: 7637–7643 - PubMed
- Berlev NA, Liu L, Chehab NH, Mansfield K, Harris KG, Halazonetis TD, Berger SL (2001) Acetylation of p53 activates transcription through recruitment of coactivators/histone acetytransferases. Mol Cell 8: 1243–1254 - PubMed
- Bhat KP, Lantvit D, Christov K, Mehta RG, Moon RC, Pezzuto JM (2001) Estrogenic and antiestrogenic properties of resveratrol in mammary tumor models. Cancer Res 61: 7456–7463 - PubMed
- Bowers JL, Tyulmenkov VV, Jernigan SC, Klinge CM (2000) Resveratrol acts as a mixed agonist/antagonist for estrogen receptors α and β. Endocrinology 141: 3657–3667 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous