The c-MYC oncoprotein is a substrate of the acetyltransferases hGCN5/PCAF and TIP60 - PubMed (original) (raw)
Comparative Study
. 2004 Dec;24(24):10826-34.
doi: 10.1128/MCB.24.24.10826-10834.2004.
Yanping Du, Penny G Ard, Charles Phillips, Beth Carella, Chi-Ju Chen, Carrie Rakowski, Chandrima Chatterjee, Paul M Lieberman, William S Lane, Gerd A Blobel, Steven B McMahon
Affiliations
- PMID: 15572685
- PMCID: PMC533976
- DOI: 10.1128/MCB.24.24.10826-10834.2004
Comparative Study
The c-MYC oncoprotein is a substrate of the acetyltransferases hGCN5/PCAF and TIP60
Jagruti H Patel et al. Mol Cell Biol. 2004 Dec.
Abstract
The c-MYC oncoprotein functions as a sequence-specific transcription factor. The ability of c-MYC to activate transcription relies in part on the recruitment of cofactor complexes containing the histone acetyltransferases mammalian GCN5 (mGCN5)/PCAF and TIP60. In addition to acetylating histones, these enzymes have been shown to acetylate other proteins involved in transcription, including sequence-specific transcription factors. This study was initiated in order to determine whether c-MYC is a direct substrate of mGCN5 and TIP60. We report here that mGCN5/PCAF and TIP60 acetylate c-MYC in vivo. By using nanoelectrospray tandem mass spectrometry to examine c-MYC purified from human cells, the major mGCN5-induced acetylation sites have been mapped. Acetylation of c-MYC by either mGCN5/PCAF or TIP60 results in a dramatic increase in protein stability. The data reported here suggest a conserved mechanism by which acetyltransferases regulate c-MYC function by altering its rate of degradation.
Figures
FIG. 1.
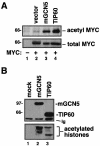
The c-MYC oncoprotein is acetylated in vivo by its cofactors mGCN5 and TIP60. (A) The human lung cancer cell line H1299 was transfected with an expression vector for c-MYC (lanes 2 to 4). Mock transfected cells served as a negative control (lane 1). In addition, transfections included expression vectors for the acetyltransferases mGCN5 and TIP60, as indicated. Twenty-four hours posttransfection, cell lysates were produced, and acetylated proteins were precipitated with a universal antiacetyllysine antibody. Precipitates (top panel) and lysates (bottom panel) were resolved by SDS-PAGE and Western blotted for c-MYC. (B) H1299 cells were transfected with expression vectors for mGCN5 or TIP60, as indicated. Lysates were subjected to immunoprecipitation for the FLAG epitope present on the acetyltransferases. Precipitates were either resolved by SDS-PAGE and Western blotted for the FLAG epitope (top panel) or subjected to an in vitro acetyltransferase assay using purified histones as the substrate (bottom panel). The migration of molecular weight markers is indicated at the left. The migration of the immunoglobulin heavy chain from the immunoprecipitating antibody is indicated (Ig).
FIG. 2.
mGCN5 acetylates the three mammalian MYC proteins, c-MYC, L-MYC, and N-MYC. Expression vectors for FLAG epitope-tagged versions of the mammalian MYC family members c-MYC (lanes 3 and 4), L-MYC (lanes 5 and 6), and N-MYC (lanes 7 and 8) were transfected into H1299 cells. In addition, transfections included an expression vector for mGCN5 (lanes 2, 4, 6, and 8), as indicated. As a negative control, cotransfections including the empty expression vector were also performed (lanes 1, 3, 5, and 7). Lysates were resolved, blotted, and probed for the FLAG epitope common to c-MYC, L-MYC, and N-MYC (middle panel) or for mGCN5 (top panel). Antiacetyllysine immunoprecipitates (i.p.) were also resolved and blotted for FLAG to detect acetylated forms of the MYC family proteins (bottom panel).
FIG. 3.
mGCN5-mediated acetylation of c-MYC does not require the MbII domain (A) H1299 cells were transfected with expression vectors encoding either wild-type c-MYC (wt, lanes 2 and 3) or a mutant lacking amino acids 129 to 145 (ΔMbII, lanes 4 and 5). Transfections also included the expression vector for mGCN5 (lanes 3 and 5), as indicated. After blotting, both lysates (top panel) and antiacetyl- lysine precipitates (bottom panel) were probed for the FLAG epitope present on c-MYC. (B) In parallel with the transfections shown in panel A, H1299 cells were transfected with an expression vector for the c-MYC partner MAX in the presence (lane 2) or absence (lane 1) of the mGCN5 expression vector. Lysates (top panel) and antiacetyl- lysine precipitates (bottom panel) were resolved and blotted for MAX.
FIG. 4.
Mapping of c-MYC acetylation sites in vitro and in vivo. (A) H1299 cells were transfected with expression vectors for FLAG epitope-tagged versions of either wild-type c-MYC (amino acids 1 to 439, lanes 2 and 3) or a truncation mutant encoding only amino acids 1 to 268 (lanes 4 and 5), as indicated. Mock-transfected cells served as a control (lane 1). Lysates and antiacetyllysine immunoprecipitates were resolved and probed for the FLAG epitope common to the wild-type and mutant c-MYC proteins or mGCN5, as indicated. The migration of molecular weight markers is indicated at the left. Ig, immunoglobulin. (B) An in vitro acetylation assay was performed on glutathione _S_-transferase fusion proteins containing either the amino (amino acids 1 to 204) or carboxy (amino acids 200 to 439) terminal (term) region of c-MYC produced and purified from E. coli. The catalytic domain of the mGCN5 paralog PCAF was also produced and purified from E. coli. Purified proteins were assayed in the combinations indicated in an in vitro acetylation assay. The p45 subunit of NFE2, a known substrate for mGCN5/PCAF, was included as a positive control. The migration of molecular weight markers is indicated at the right. (C) FLAG epitope-tagged c-MYC expressed in either the presence or the absence of mGCN5 was purified from transfected H1299 cells. These proteins were subjected to μLC-MS/MS analysis to identify in vivo sites of mGCN5-mediated acetylation. This analysis revealed acetylation at lysine 149 of c-MYC in both the presence and the absence of mGCN5 overexpression. Two additional sites of acetylation were observed in the presence of mGCN5. The first of these lies within the c-MYC NLS at amino acid 323, and the second lies within the LZ domain at amino acid 417, as indicated. The individual peptides recovered for each region are indicated by thick black lines beneath the amino acid sequence of the region. Acetylated lysines within these peptides are indicated in red. Other functional domains of the c-MYC protein are indicated as follows: MbII, the highly conserved MYC homology box II region; B, the basic DNA binding motif; HLH, the helix-loop-helix domain essential for dimerization with MAX. (D) H1299 cells were transfected with the c-MYC expression vector in the presence or absence of mGCN5 expression, as indicated. Cell lysates were generated under denaturing conditions. These lysates and antiacetyllysine immunoprecipitates (i.p.) were blotted and probed for c-MYC. Lysates were also probed for mGCN5 and for β-tubulin.
FIG. 5.
Dimerization with MAX and nuclear localization are not affected by c-MYC acetylation. (A) To assess whether acetylation of c-MYC altered its potential for dimerization with MAX, H1299 cells were transfected with the FLAG c-MYC expression vector (lanes 2 and 3), in the presence (lane 3) or absence (lane 2) of mGCN5, as indicated. Lysates and anti-FLAG immunoprecipitates (i.p.) were resolved by SDS-PAGE and blotted for the FLAG epitope to detect the tagged c-MYC and mGCN5, as indicated. In addition, the blots were probed for endogenous MAX to determine the amount dimerized with c-MYC. To confirm that c-MYC was efficiently acetylated by mGCN5 in this experiment, a portion of the lysates were subjected to immunoprecipitation with antiacetyllysine antisera. After blotting, these precipitates were probed for the FLAG tag to visualize acetylated c-MYC (bottom panel). (B) The effect of acetylation on c-MYC's subcellular localization was assessed in H1299 cells transfected with the FLAG c-MYC expression vector (lanes 2 and 3), in the presence (lane 3) or absence (lane 2) of mGCN5. Whole-cell lysates (top panel) were produced from a portion of the transfected cells, while the remaining cells were subjected to differential extraction of the cytoplasmic (cyto.; left middle panel) and nuclear fractions (right middle panel), as indicated. The presence of c-MYC in the various fractions was detected by probing for the FLAG epitope. To confirm that c-MYC was efficiently acetylated by mGCN5 in this experiment, a portion of the lysates were subjected to immunoprecipitation with antiacetyllysine antisera. These precipitates were blotted and probed for the FLAG tag to visualize acetylated c-MYC (bottom panel). The migration of molecular weight markers is indicated at the left.
FIG. 6.
Acetylation by mGCN5 stabilizes c-MYC. In order to determine the effect of acetylation on the stability of the c-MYC protein, half-life studies were conducted with H1299 cells transfected with the FLAG epitope-tagged c-MYC expression vector. In addition, transfections included vectors encoding either mGCN5 or CBP, as indicated. After transfection, cells were treated with cycloheximide to block further protein synthesis. Cells were harvested at the times indicated, and c-MYC levels were determined by Western blotting. Half-life values were determined by densitometric quantitation of Western blots. Signals for c-MYC levels at each time point were plotted using a logarithmic curve-fit algorithm, and the time point at which c-MYC levels decreased to 50% of their original value was determined and reported as the half-life. Because acetylation dramatically increased the overall steady-state levels of c-MYC, shorter exposures of c-MYC blots from the cells coexpressing mGCN5 or CBP were used for densitometry. Cells transfected with c-MYC in the absence of either acetyltransferase served as the source for determining the c-MYC basal half-life.
FIG. 7.
TIP60 increases c-MYC half-life. To determine the effect of acetylation by TIP60 on the stability of the c-MYC protein, half-life studies were conducted with H1299 cells as described for Fig. 6. Briefly, cells were transfected with the FLAG epitope-tagged c-MYC expression vector, in the presence or absence of TIP60, as indicated. Cell lysates were examined for c-MYC levels, and half-life values were determined as described in the legend to Fig. 6. As a control, one group of cells was treated with the proteasome inhibitor MG-132 to artificially stabilize c-MYC.
FIG. 8.
Mutation of c-MYC acetylation sites inhibits mGCN5-induced stabilization. In experiments (EXPT) identical to those presented in Fig. 6 and 7, wild-type (wt) and acetylation site mutant (K323/417R) versions of c-MYC were compared for their stabilization in response to mGCN5. (A) In two independent experiments, the loss of acetylation inhibited the ability of mGCN5 to stabilize c-MYC from 2.4- to 3.2-fold down to 1.6-fold (data are displayed as half-lives in minutes). (B) Western blots from experiment #2 are shown.
Similar articles
- Dual regulation of c-Myc by p300 via acetylation-dependent control of Myc protein turnover and coactivation of Myc-induced transcription.
Faiola F, Liu X, Lo S, Pan S, Zhang K, Lymar E, Farina A, Martinez E. Faiola F, et al. Mol Cell Biol. 2005 Dec;25(23):10220-34. doi: 10.1128/MCB.25.23.10220-10234.2005. Mol Cell Biol. 2005. PMID: 16287840 Free PMC article. - MYC recruits the TIP60 histone acetyltransferase complex to chromatin.
Frank SR, Parisi T, Taubert S, Fernandez P, Fuchs M, Chan HM, Livingston DM, Amati B. Frank SR, et al. EMBO Rep. 2003 Jun;4(6):575-80. doi: 10.1038/sj.embor.embor861. EMBO Rep. 2003. PMID: 12776177 Free PMC article. - Application of a fluorescent histone acetyltransferase assay to probe the substrate specificity of the human p300/CBP-associated factor.
Trievel RC, Li FY, Marmorstein R. Trievel RC, et al. Anal Biochem. 2000 Dec 15;287(2):319-28. doi: 10.1006/abio.2000.4855. Anal Biochem. 2000. PMID: 11112280 - Acetylation of general transcription factors by histone acetyltransferases.
Imhof A, Yang XJ, Ogryzko VV, Nakatani Y, Wolffe AP, Ge H. Imhof A, et al. Curr Biol. 1997 Sep 1;7(9):689-92. doi: 10.1016/s0960-9822(06)00296-x. Curr Biol. 1997. PMID: 9285713 Review.
Cited by
- Immune regulation by histone deacetylases: a focus on the alteration of FOXP3 activity.
Zhang H, Xiao Y, Zhu Z, Li B, Greene MI. Zhang H, et al. Immunol Cell Biol. 2012 Jan;90(1):95-100. doi: 10.1038/icb.2011.101. Epub 2011 Nov 29. Immunol Cell Biol. 2012. PMID: 22124370 Free PMC article. Review. - Metabolite-derived protein modifications modulating oncogenic signaling.
Liu Y, Vandekeere A, Xu M, Fendt SM, Altea-Manzano P. Liu Y, et al. Front Oncol. 2022 Sep 23;12:988626. doi: 10.3389/fonc.2022.988626. eCollection 2022. Front Oncol. 2022. PMID: 36226054 Free PMC article. Review. - Histone deacetylase inhibitors (HDACIs): multitargeted anticancer agents.
Ververis K, Hiong A, Karagiannis TC, Licciardi PV. Ververis K, et al. Biologics. 2013;7:47-60. doi: 10.2147/BTT.S29965. Epub 2013 Feb 25. Biologics. 2013. PMID: 23459471 Free PMC article. - An H4K16 histone acetyltransferase mediates decondensation of the X chromosome in C. elegans males.
Lau AC, Zhu KP, Brouhard EA, Davis MB, Csankovszki G. Lau AC, et al. Epigenetics Chromatin. 2016 Oct 19;9:44. doi: 10.1186/s13072-016-0097-x. eCollection 2016. Epigenetics Chromatin. 2016. PMID: 27777629 Free PMC article. - Impact of acetylation on tumor metabolism.
Zhao D, Li FL, Cheng ZL, Lei QY. Zhao D, et al. Mol Cell Oncol. 2014 Oct 29;1(3):e963452. doi: 10.4161/23723548.2014.963452. eCollection 2014 Jul-Sep. Mol Cell Oncol. 2014. PMID: 27308346 Free PMC article. Review.
References
- Banerjee, D., and A. Liefshitz. 2001. Potential of the proteasomal inhibitor MG-132 as an anticancer agent, alone and in combination. Anticancer Res. 21:3941-3947. - PubMed
- Bannister, A. J., E. A. Miska, D. Gorlich, and T. Kouzarides. 2000. Acetylation of importin-alpha nuclear import factors by CBP/p300. Curr. Biol. 10:467-470. - PubMed
- Barlev, N. A., L. Liu, N. H. Chehab, K. Mansfield, K. G. Harris, T. D. Halazonetis, and S. L. Berger. 2001. Acetylation of p53 activates transcription through recruitment of coactivators/histone acetyltransferases. Mol. Cell 8:1243-1254. - PubMed
- Blackwell, T. K., L. Kretzner, E. M. Blackwood, R. N. Eisenman, and H. Weintraub. 1990. Sequence-specific DNA binding by the c-Myc protein. Science 250:1149-1151. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- CA 085678/CA/NCI NIH HHS/United States
- R01 DK058044/DK/NIDDK NIH HHS/United States
- DK 58044/DK/NIDDK NIH HHS/United States
- R01 CA098172/CA/NCI NIH HHS/United States
- R37 DK058044/DK/NIDDK NIH HHS/United States
- CA 098172/CA/NCI NIH HHS/United States
- R01 CA090465/CA/NCI NIH HHS/United States
- R01 CA085678/CA/NCI NIH HHS/United States
- CA 090465/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous