Therapeutic efficacy of PUMA for malignant glioma cells regardless of p53 status - PubMed (original) (raw)
Therapeutic efficacy of PUMA for malignant glioma cells regardless of p53 status
Hideaki Ito et al. Hum Gene Ther. 2005 Jun.
Abstract
Replacement of the p53 tumor suppressor gene is a rational approach to the management of malignant gliomas because p53 is frequently mutated or inactivated in these cancers. Major weaknesses of this approach are that malignant gliomas are mixtures of cells with wild-type and mutant p53, and that tumor cells exhibiting wildtype p53 are resistant to p53 gene transfer. An effective alternative is needed to overcome these difficulties. p53-upregulated modulator of apoptosis (PUMA) was identified as a p53-inducible proapoptotic molecule. Our purpose was to elucidate a role for PUMA in p53 gene therapy and to investigate whether PUMA is an efficient substitute for p53 in cancer therapy. We demonstrated that PUMA was upregulated in mutant p53 malignant glioma cells (U373-MG and T98G) undergoing apoptosis but was not upregulated in apoptosis-resistant wild-type p53 malignant glioma cells (U87-MG and D54) after adenoviral transfer of p53. Overexpression of PUMA resulted in massive apoptosis associated with mitochondrial damage and caspase-3 activation in all tumor cells tested. Use of the human telomerase reverse transcriptase (hTERT) promoter system induced apoptosis only in malignant glioma cells with telomerase activity, while sparing normal cells lacking telomerase. The ability of PUMA to induce apoptosis was greater than that of caspase-6 or caspase-8 transfer, using the same system. Moreover, exogenous expression of PUMA under the hTERT promoter system significantly suppressed the growth of subcutaneous U87-MG tumors in nude mice and did not induce apoptosis in surrounding nontumor tissues. These results indicate that PUMA, which is regulated under a tumor-specific expression system such as the hTERT promoter, may be better than p53 as a therapeutic tool for malignant gliomas.
Figures
Fig. 1
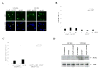
Induction of apoptosis in malignant glioma cells with different p53 status by Ad-p53. A: U373-MG (mutant p53) or U87-MG cells (wild-type p53) were infected with Ad-p53 and/or Ad-GFP at an MOI of 20. After 24 h, tumor cells were fixed and stained with Hoechst 33258. Arrows indicate representative apoptotic nuclei. Bars, 10 μm. B: Percentage of apoptotic cells in U373-MG or U87-MG cells infected with Ad-p53 and/or Ad-GFP at an MOI of 5, 10, or 20 for 24 h. Data shown are the mean ± SD of apoptotic cells among 200 GFP-positive cells chosen at random from three different areas. C: Transcriptional activity of the PUMA promoter. U373-MG or U87-MG cells were infected with Ad-p53 and/or Ad-GFP at an MOI of 20 for 24 h after reporters (4×BS2WT-Luc or 4×BS2MUT-Luc) were transfected. Luciferase activity in each cell line was normalized, with the represented value indicating a percentage of the positive control plasmid (PGL3-control) in each cell line. Data shown are the mean ± SD from three independent experiments. D: Increase in PUMA protein expression by Ad-p53. U373-MG or U87-MG cells were infected with Ad-GFP and/or Ad-p53 at an MOI of 20 for 24 h. Untreated cells were included as a control. Cell lysates were used for Western blots to assess the expression of PUMA. Actin serves as a loading control.
Fig. 2
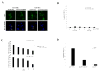
Antitumor effect of PUMA overexpression on malignant glioma cells with different p53 status. A: U373-MG (mutant p53) or U87-MG cells (wild-type p53) were infected with Ad-PUMA or Ad-PUMAΔBH3 at 20 MOI. After 24 h, tumor cells were fixed and stained with Hoechst 33258. Arrows indicate representative apoptotic nuclei. Bars, 10 μm. B: Percentage of apoptotic cells in U373-MG or U87-MG cells by Ad-PUMA or Ad-PUMAΔBH3 at an MOI of 5, 10, or 20 for 24 h. Data shown are the mean ± SD of apoptotic cells among 200 GFP-positive cells chosen at random from three different areas. C: Effect of PUMA overexpression on cell viability. U373-MG and U87-MG cells were seeded at 1 × 104 cells/well in 96-well plates and incubated overnight at 37°C. The cells were infected with Ad-PUMA or Ad-PUMAΔBH3 at an MOI of 5, 10, 20, or 50 for 24 h. Cell proliferation was assessed by a WST-1 assay. The proliferation of the uninfected cells was considered to be 100% for each cell line. Results shown are the mean ± SD of three independent experiments. D: Percentage of apoptotic cells in T98G (mutant p53) or D54 cells (wild-type p53) by Ad-PUMA, Ad-p53 and/or Ad-GFP at an MOI of 20 for 24 h. Data shown are the mean ± SD of apoptotic cells among 200 GFP-positive cells chosen at random from three different areas.
Fig. 3
Bax translocation to the mitochondria by PUMA expression. A: Induction of Bax translocation from cytosol to mitochondria. U373-MG cells were infected with Ad-PUMA or Ad-PUMAΔBH3 at 20 MOI for 24 h. HSP60 and Bax protein were detected by immunofluorescence assay using anti-HSP60 antibody (green fluorescence) and anti-Bax antibody (red fluorescence), respectively. Co-localization of mitochondria and Bax is indicated as orange in the merged picture. Bars, 20 μm. B: Percentage of tumor cells exhibiting Bax translocation after exposure to Ad-PUMA or Ad-PUMAΔBH3 at an MOI of 20 for 24 h. Data shown are the mean ± SD of Bax-translocated cells among more than 100 cells chosen at random from three different areas.
Fig. 4
Mechanisms of PUMA-induced apoptosis. A: Disruption of mitochondrial membrane potential in malignant glioma cells by overexpression of PUMA. Rhodamine 123 was used to determine changes in mitochondrial membrane potential using FACS analysis. After treatment with Ad-PUMA or Ad-PUMAΔBH3 at an MOI of 20 for 24 h, attached and detached U373-MG and U87-MG cells were collected and stained with rhodamine 123. B: Release of cytochrome c into cytosol by Ad-PUMA. U373-MG and U87-MG cells were infected with Ad-PUMA or Ad-PUMAΔBH3 at 20 MOI for 24 h. Cytosolic extracts were used for Western blots to assess release of cytochrome c. Actin serves as a loading control. C: Activation of caspase-3 by Ad-PUMA. Activity of caspase-3 in U373-MG and U87-MG cells infected with Ad-PUMA or Ad-PUMAΔBH3 at an MOI of 20 for 24 h was measured with the CleavaLite™ Caspase-3 activity assay kit. Results shown are the mean ± SD of three independent experiments.
Fig. 5
Induction of apoptosis in malignant glioma cells or normal cells by HA-PUMA or hTERT/HA-PUMA. A: Malignant glioma cells (U373-MG and U87-MG) and normal fibroblasts MRC5 were transfected with the HA-PUMA or hTERT/HA-PUMA vector. The PGL3-control was used as a reporter gene when the hTERT/HA-PUMA construct was transfected into MRC5 cells. After 48 h, immunohistochemical staining using anti-HA or anti-luciferase antibody was performed, followed by Hoechst 33258 staining. Arrows indicate representative apoptotic nuclei. Bars, 10 μm. B: Percentage of apoptotic cells in U373-MG, U87-MG, and MRC5 cells transfected with the HA-PUMA, hTERT/HA-PUMA, hTERT/rev-caspase-6, or hTERT/caspase-8 construct for 48 h. Data shown are the mean ± SD of apoptotic cells among 200 HA-, luciferase-, caspase-6, or caspase-8-positive cells chosen at random from three different areas.
Fig. 6
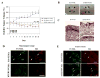
Effect of PUMA expression on subcutaneous tumors in nude mice. A: Tumors from U87-MG cells (1 × 106 cells) were established subcutaneously in nude mice. When the tumors reached a mean volume of 30–50 mm3, the hTERT/HA-PUMA, HA-PUMA or hTERT/luciferase construct (10 μg/20 μl PBS) mixed with Lipofectamine 2000 (2 μl) was administered directly into the tumors daily for 5 days. Tumor volume was determined using calipers up to day 21. 10 mice were used in each treatment group. Five arrows indicate when the mice were treated. Results shown are the mean ± SD of the percentage of change in tumor volume. *_P<_0.001 (on day 21). B: Photographs of subcutaneous U87-MG tumors next day (day 6) after the last injection of HA-PUMA or hTERT/HA-PUMA construct as described above. Arrowheads indicate the border of subcutaneous tumors. Bars, 2 mm. C: Hematoxylin and eosin (HE) staining of subcutaneous U87-MG tumors together with non-tumor tissues collected from the mice treated as described in B. T indicates subcutaneous tumors. Bars, 250 μm. D: HA expression and TUNEL analysis in non-tumor tissues collected from the mice treated as described in B. Arrows indicate representative TUNEL- and HA-positive cells. Bars, 50 μm. E: HA expression and TUNEL analysis in subcutaneous tumors collected from the mice treated as described in B. Arrows indicate representative TUNEL- and HA-positive cells. Bars, 50 μm.
Similar articles
- Caspase-8 gene therapy using the human telomerase reverse transcriptase promoter for malignant glioma cells.
Komata T, Kondo Y, Kanzawa T, Ito H, Hirohata S, Koga S, Sumiyoshi H, Takakura M, Inoue M, Barna BP, Germano IM, Kyo S, Kondo S. Komata T, et al. Hum Gene Ther. 2002 Jun 10;13(9):1015-25. doi: 10.1089/104303402753812421. Hum Gene Ther. 2002. PMID: 12067435 - Treatment of malignant glioma cells with the transfer of constitutively active caspase-6 using the human telomerase catalytic subunit (human telomerase reverse transcriptase) gene promoter.
Komata T, Kondo Y, Kanzawa T, Hirohata S, Koga S, Sumiyoshi H, Srinivasula SM, Barna BP, Germano IM, Takakura M, Inoue M, Alnemri ES, Shay JW, Kyo S, Kondo S. Komata T, et al. Cancer Res. 2001 Aug 1;61(15):5796-802. Cancer Res. 2001. PMID: 11479218 - Combination therapy of malignant glioma cells with 2-5A-antisense telomerase RNA and recombinant adenovirus p53.
Komata T, Kondo Y, Koga S, Ko SC, Chung LW, Kondo S. Komata T, et al. Gene Ther. 2000 Dec;7(24):2071-9. doi: 10.1038/sj.gt.3301327. Gene Ther. 2000. PMID: 11223987 - Adenovirus-mediated p53 gene therapy for human gliomas.
Lang FF, Yung WK, Sawaya R, Tofilon PJ. Lang FF, et al. Neurosurgery. 1999 Nov;45(5):1093-104. doi: 10.1097/00006123-199911000-00016. Neurosurgery. 1999. PMID: 10549925 Review. - Apoptotic genes in cancer therapy.
Opalka B, Dickopp A, Kirch HC. Opalka B, et al. Cells Tissues Organs. 2002;172(2):126-32. doi: 10.1159/000065609. Cells Tissues Organs. 2002. PMID: 12426489 Review.
Cited by
- Landscape of EGFR signaling network in human cancers: biology and therapeutic response in relation to receptor subcellular locations.
Han W, Lo HW. Han W, et al. Cancer Lett. 2012 May 28;318(2):124-34. doi: 10.1016/j.canlet.2012.01.011. Epub 2012 Jan 17. Cancer Lett. 2012. PMID: 22261334 Free PMC article. Review. - Tumor growth inhibitory effect of ADAMTS1 is accompanied by the inhibition of tumor angiogenesis.
Obika M, Ogawa H, Takahashi K, Li J, Hatipoglu OF, Cilek MZ, Miyoshi T, Inagaki J, Ohtsuki T, Kusachi S, Ninomiya Y, Hirohata S. Obika M, et al. Cancer Sci. 2012 Oct;103(10):1889-97. doi: 10.1111/j.1349-7006.2012.02381.x. Epub 2012 Aug 29. Cancer Sci. 2012. PMID: 22776012 Free PMC article. - Gene therapy for brain tumors: basic developments and clinical implementation.
Assi H, Candolfi M, Baker G, Mineharu Y, Lowenstein PR, Castro MG. Assi H, et al. Neurosci Lett. 2012 Oct 11;527(2):71-7. doi: 10.1016/j.neulet.2012.08.003. Epub 2012 Aug 10. Neurosci Lett. 2012. PMID: 22906921 Free PMC article. Review. - Gene therapy for brain cancer: combination therapies provide enhanced efficacy and safety.
Candolfi M, Kroeger KM, Muhammad AK, Yagiz K, Farrokhi C, Pechnick RN, Lowenstein PR, Castro MG. Candolfi M, et al. Curr Gene Ther. 2009 Oct;9(5):409-21. doi: 10.2174/156652309789753301. Curr Gene Ther. 2009. PMID: 19860655 Free PMC article. Review. - PUMA-mediated apoptosis in fibroblast-like synoviocytes does not require p53.
You X, Boyle DL, Hammaker D, Firestein GS. You X, et al. Arthritis Res Ther. 2006;8(6):R157. doi: 10.1186/ar2052. Arthritis Res Ther. 2006. PMID: 17014719 Free PMC article.
References
- Bold RJ, Termuhlen PM, McConkey DJ. Apoptosis, cancer and cancer therapy. Surg Oncol. 1997;6:133–142. - PubMed
- Clayman GL, El-Naggar AK, Lippman SM, Henderson YC, Frederick M, Merritt JA, Zumstein LA, Timmons TM, Liu TJ, Ginsberg L, Roth JA, Hong WK, Bruso P, Goepfert H. Adenovirus-mediated p53 gene transfer in patients with advanced recurrent head and neck squamous cell carcinoma. J Clin Oncol. 1998;16:2221–2232. - PubMed
- Daido S, Kanzawa T, Yamamoto A, Takeuchi H, Kondo Y, Kondo S. Pivotal role of the cell death factor BNIP3 in ceramide-induced autophagic cell death in malignant glioma cells. Cancer Res. 2004;64:4286–4293. - PubMed
- DeMasters BK, Markham N, Lillehei KO, Shroyer KR. Differential telomerase expression in human primary intracranial tumors. Am J Clin Pathol. 1997;107:548–554. - PubMed
- Dewson G, Snowden RT, Almond JB, Dyer MJ, Cohen GM. Conformational change and mitochondrial translocation of Bax accompany proteasome inhibitor–induced apoptosis of chronic lymphocytic leukemic cells. Oncogene. 2003;22:2643–2654. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA088936-03/CA/NCI NIH HHS/United States
- R01 CA088936/CA/NCI NIH HHS/United States
- R01 CA088936-02/CA/NCI NIH HHS/United States
- R01 CA088936-05/CA/NCI NIH HHS/United States
- CA088936/CA/NCI NIH HHS/United States
- R01 CA108558-01/CA/NCI NIH HHS/United States
- R01 CA108558-02/CA/NCI NIH HHS/United States
- R01 CA108558/CA/NCI NIH HHS/United States
- R01 CA088936-04/CA/NCI NIH HHS/United States
- CA108558/CA/NCI NIH HHS/United States
- R01 CA088936-01A1/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous