Photodynamic therapy-generated vaccine for cancer therapy - PubMed (original) (raw)
Photodynamic therapy-generated vaccine for cancer therapy
Mladen Korbelik et al. Cancer Immunol Immunother. 2006 Aug.
Abstract
A target tumor-derived whole cancer cell therapeutic vaccine was developed based on an in vitro pre-treatment by photodynamic therapy (PDT) and was investigated using a poorly immunogenic tumor model. The vaccine was produced by incubating in vitro expanded mouse squamous cell carcinoma SCCVII cells for 1 h with photosensitizer benzoporphyrin derivative (BPD), then exposing to light (690 nm, 1 J/cm2) and finally to a lethal X-ray dose. Treatment of established subcutaneous SCCVII tumors growing in syngeneic C3H/HeN mice with 2 x 10(7) PDT-vaccine cells per mouse by a peritumoral injection produced a significant therapeutic effect, including growth retardation, regression and cures. Tumor specificity of this PDT-generated vaccine was demonstrated by its ineffectiveness when prepared from a mismatched tumor cell line. Vaccine cells retrieved from the treatment site at 1 h postinjection were intermixed with dendritic cells (DC), exhibited heat shock protein 70 on their surface, and were opsonized by complement C3. Tumor-draining lymph nodes treated by the PDT-vaccine contained dramatically increased numbers of DC as well as B and T lymphocytes (with enlarged memory phenotype fraction in the latter), while high levels of surface-bound C3 were detectable on DC and to a lesser extent on B cells. The PDT-vaccine produced no therapeutic benefit against tumors growing in C3-deficient hosts. It is suggested that surface expression of heat shock proteins and complement opsonization are the two unique features of PDT-treated cells securing avid immune recognition of vaccinated tumor and the development of a strong and effective antitumor adaptive immune response.
Figures
Fig. 1
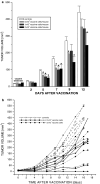
The effect of PDT-generated vaccine on growth of established SCCVII tumors. In vitro cultured SCCVII cells were incubated with BPD, either 0.4 μg/ml (vaccine-1) or 20 μg/ml (vaccine-2), in serum-free medium for 1 h at 37°C, then treated by light (690 nm; 1 J/cm2), followed by the exposure to X-rays (60 Gy). Mice bearing subcutaneously growing SCCVII tumors received each 1×107 of these vaccine cells by peritumoral injection. Response to the vaccine treatment was determined by subsequent tumor size measurement. Also shown is the growth of control untreated tumors, and tumors in control groups injected with 1×107 X-ray-only-treated or lysed SCCVII cells. Omitted for clarity are the day 13 data points for lysed and X-rayed cells (583±77 and 828±153 mm3, respectively). Each treatment group consisted of six mice. Bars are SD. *Significant difference compared to the untreated controls at day 13 postvaccination (P<0.05)
Fig. 2
The impact of cell number used in PDT-generated vaccine on its effectiveness in treating subcutaneous SCCVII tumors. The vaccine was generated as described for the vaccine-1 in Fig. 1. The cell number used per vaccination by peritumoral injection of mice bearing SCCVII tumors was varied in different treatment groups from 5×106 to 5×107. The postvaccination tumor growth is shown either as a means of tumor size (+ SD) per treatment groups (_N_=6) receiving 0 (nonvaccinated controls), 5×106, 1×107, or 2×107 vaccine cells, or b tumor size values for individual tumors receiving 0 (controls), 2×107 or 5×107 vaccine cells. *Significant difference compared to the nonvaccinated controls (P<0.05)
Fig. 3
The effect of PDT-vaccines generated from SCCVII or FsaR tumor cells on growth of SCCVII tumors. The vaccine was generated from either SCCVII or FsaR cells as described for the vaccine-1 in Fig. 1, and 2×107 cells were injected peritumorally per SCCVII tumor-bearing mouse. The growth of tumors after SCCVII cell-based vaccine or mis-matched (FsaR cell-based) vaccine and nonvaccinated control tumors is depicted by showing means of tumor size per treatment group. Bars are SD; _N_=6. *Significant difference compared to the nonvaccinated controls (P<0.05)
Fig. 4
Size of immune cell populations found in lymph nodes draining vaccinated or nonvaccinated SCCVII tumors. Four days after PDT-vaccine treatment as described for the vaccine-1 in Fig. 1 using 2×107 cells per vaccination by peritumoral injection, mice bearing vaccinated SCCVII tumors were sacrificed and inguinal lymph node cells were collected. Antibody staining of these cells followed by flow cytometry enabled their classification and the determination of total number of cells per lymph node for major immune cell populations. The data obtained with lymph node cells collected from SCCVII tumor-bearing control (nonvaccinated) mice are shown for comparison. The insert depicts the levels of T-lymphocytes bearing memory phenotype (CD44+CD45RB–). Bars are SD; _N_=4. The values for all populations in the vaccine group are significantly higher than the values for the equivalent populations in the no vaccine group (P<0.05)
Fig. 5
Sequestration of dendritic cells to the site of PDT-vaccine injection. The mice with SCCVII tumors were sacrificed 1 hour after receiving PDT vaccine cells (2×107 SCCVII cells treated by the vaccine-1 protocol described for Fig. 1) or the same number of X-ray-only-treated SCCVII cells. The cell samples retrieved from the vaccine injection site were stained with FITC-conjugated DEC205 antibody for flow cytometry-based identification of dendritic cells. The vaccine cells were identified by BPD fluorescence. Pre-biotinylation (as described in Materials and Methods) of SCCVII cells used in X-ray-only protocol enabled their identification by subsequent staining with a chromophore-conjugated streptavidin. Dot plot graphs of representative samples are shown
Fig. 6
Complement opsonization of administered PDT-vaccine cells and the role of HSP70. Following the same protocol for PDT-vaccine generation using SCCVII cells and the retrieval from the vaccination site at 1 hour after vaccination of SCCVII tumor-bearing mice as described for Fig. 5, the collected cells were stained with goat anti-mouse C3 antibody or rat anti-mouse C3/C3b/iC3b/C3c antibody. a C3-associated fluorescence intensity in cells retrieved in PDT-vaccine and X-ray-only treatment groups, and b dot plot graph of C3/C3b/iC3b/C3c fluorescence in a representative sample from the PDT-vaccine group. c the impact of co-administration with the PDT-vaccine of HSP70-blocking antibody (40 μg/mouse) or its isotype control immunoglobulin on C3-associated fluorescence on the retrieved cells. The insert in this graph shows the levels of surface HSP70 expression (following staining with anti-HSP70 antibody) on the retrieved cells. Bars are SD; _N_=4. *Significant difference compared to X-ray-only group (P<0.05); **Significant difference compared to PDT vaccine group with no anti-HSP70 treatment (P<0.05)
Fig. 7
Presence of bound C3 on cells in tumor-draining lymph nodes after PDT-vaccine treatment. The vaccine, generated as described for the vaccine-1 in Fig. 1, was given to SCCVII tumor-bearing mice (2×107 vaccine cells per mouse peritumorally). The mice were sacrificed 20 h later, tumor-draining lymph nodes excised and the collected cells stained with antibodies against mouse C3, CD3 (T cells), CD19 (B-cells) and DEC205 (DCs) for flow cytometry analysis. The same was done with tumor-draining lymph node cells obtained from untreated SCCVII tumor-bearing mice (controls). The results are presented as values relative to those in corresponding populations of the control group. Bars are SD; _N_=4. *Significant difference compared to the same population in the control group
Similar articles
- Photodynamic therapy-generated vaccines: relevance of tumour cell death expression.
Korbelik M, Stott B, Sun J. Korbelik M, et al. Br J Cancer. 2007 Nov 19;97(10):1381-7. doi: 10.1038/sj.bjc.6604059. Epub 2007 Oct 30. Br J Cancer. 2007. PMID: 17971767 Free PMC article. - Photodynamic therapy-generated cancer vaccine elicits acute phase and hormonal response in treated mice.
Korbelik M, Merchant S. Korbelik M, et al. Cancer Immunol Immunother. 2012 Sep;61(9):1387-94. doi: 10.1007/s00262-012-1206-8. Epub 2012 Jan 24. Cancer Immunol Immunother. 2012. PMID: 22270715 Free PMC article. - Antitumor Effects of DC Vaccine With ALA-PDT-Induced Immunogenic Apoptotic Cells for Skin Squamous Cell Carcinoma in Mice.
Zhang H, Wang P, Wang X, Shi L, Fan Z, Zhang G, Yang D, Bahavar CF, Zhou F, Chen WR, Wang X. Zhang H, et al. Technol Cancer Res Treat. 2018 Jan 1;17:1533033818785275. doi: 10.1177/1533033818785275. Technol Cancer Res Treat. 2018. PMID: 30025490 Free PMC article. - Improvement of DC vaccine with ALA-PDT induced immunogenic apoptotic cells for skin squamous cell carcinoma.
Ji J, Fan Z, Zhou F, Wang X, Shi L, Zhang H, Wang P, Yang D, Zhang L, Chen WR, Wang X. Ji J, et al. Oncotarget. 2015 Jul 10;6(19):17135-46. doi: 10.18632/oncotarget.3529. Oncotarget. 2015. PMID: 25915530 Free PMC article. - Stimulation of dendritic cells by DAMPs in ALA-PDT treated SCC tumor cells.
Wang X, Ji J, Zhang H, Fan Z, Zhang L, Shi L, Zhou F, Chen WR, Wang H, Wang X. Wang X, et al. Oncotarget. 2015 Dec 29;6(42):44688-702. doi: 10.18632/oncotarget.5975. Oncotarget. 2015. PMID: 26625309 Free PMC article.
Cited by
- The impact of photodynamic therapy on immune system in cancer - an update.
Dudzik T, Domański I, Makuch S. Dudzik T, et al. Front Immunol. 2024 Feb 28;15:1335920. doi: 10.3389/fimmu.2024.1335920. eCollection 2024. Front Immunol. 2024. PMID: 38481994 Free PMC article. Review. - Trial watch: an update of clinical advances in photodynamic therapy and its immunoadjuvant properties for cancer treatment.
Penetra M, Arnaut LG, Gomes-da-Silva LC. Penetra M, et al. Oncoimmunology. 2023 Jun 18;12(1):2226535. doi: 10.1080/2162402X.2023.2226535. eCollection 2023. Oncoimmunology. 2023. PMID: 37346450 Free PMC article. - Current Challenges and Opportunities of Photodynamic Therapy against Cancer.
Huis In 't Veld RV, Heuts J, Ma S, Cruz LJ, Ossendorp FA, Jager MJ. Huis In 't Veld RV, et al. Pharmaceutics. 2023 Jan 18;15(2):330. doi: 10.3390/pharmaceutics15020330. Pharmaceutics. 2023. PMID: 36839652 Free PMC article. Review. - The "Light Knife" for Gastric Cancer: Photodynamic Therapy.
Wang H, Ewetse MP, Ma C, Pu W, Xu B, He P, Wang Y, Zhu J, Chen H. Wang H, et al. Pharmaceutics. 2022 Dec 28;15(1):101. doi: 10.3390/pharmaceutics15010101. Pharmaceutics. 2022. PMID: 36678730 Free PMC article. Review. - Insight into the Prospects for Tumor Therapy Based on Photodynamic Immunotherapy.
Cheng X, Wei Y, Jiang X, Wang C, Liu M, Yan J, Zhang L, Zhou Y. Cheng X, et al. Pharmaceuticals (Basel). 2022 Nov 4;15(11):1359. doi: 10.3390/ph15111359. Pharmaceuticals (Basel). 2022. PMID: 36355531 Free PMC article. Review.
References
- Bohana-Kashtan O, Ziporen L, Donin L, Kraus S, Fishelson Z. Cell signals transduced by complement. 2004;41:583–597. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous