Berberine induces cell cycle arrest and apoptosis in human gastric carcinoma SNU-5 cell line - PubMed (original) (raw)
Berberine induces cell cycle arrest and apoptosis in human gastric carcinoma SNU-5 cell line
Jing-Pin Lin et al. World J Gastroenterol. 2006.
Abstract
Aim: To investigate the relationship between the inhibited growth (cytotoxic activity) of berberine and apoptotic pathway with its molecular mechanism of action.
Methods: The in vitro cytotoxic techniques were complemented by cell cycle analysis and determination of sub-G1 for apoptosis in human gastric carcinoma SNU-5 cells. Percentage of viable cells, cell cycle, and sub-G1 group (apoptosis) were examined and determined by the flow cytometric methods. The associated proteins for cell cycle arrest and apoptosis were examined by Western blotting.
Results: For SNU-5 cell line, the IC50 was found to be 48 micromol/L of berberine. In SNU-5 cells treated with 25-200 micromol/L berberine, G2/M cell cycle arrest was observed which was associated with a marked increment of the expression of p53, Wee1 and CDk1 proteins and decreased cyclin B. A concentration-dependent decrease of cells in G0/G1 phase and an increase in G2/M phase were detected. In addition, apoptosis detected as sub-G0 cell population in cell cycle measurement was proved in 25-200 micromol/L berberine-treated cells by monitoring the apoptotic pathway. Apoptosis was identified by sub-G0 cell population, and upregulation of Bax, downregulation of Bcl-2, release of Ca2+, decreased the mitochondrial membrane potential and then led to the release of mitochondrial cytochrome C into the cytoplasm and caused the activation of caspase-3, and finally led to the occurrence of apoptosis.
Conclusion: Berberine induces p53 expression and leads to the decrease of the mitochondrial membrane potential, Cytochrome C release and activation of caspase-3 for the induction of apoptosis.
Figures
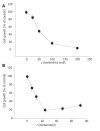
Figure 1
Percentage of viable SNU-5 cells treated with berberine with 24-h incubation. The SNU-5 cells (2×105 cells/well; 12-well plate) were plated in 80% Iscove’s modified Dulbecco’s medium+20% FBS with different concentrations of berberine for 24 h (Panel A) or 100 µmol/L berberine for 6, 12, 24, 48, and 72 h (Panel B). Then the cells were collected by centrifugation and the viable cells were determined by trypan blue exclusion and flow cytometry as described in Materials and Methods. Each point is mean ± SD of three experiments.
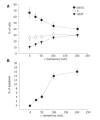
Figure 2
Flow cytometric analysis of the effects of berberine on SNU-5 cell cycle and sub-G1 group. The SNU-5 cells were exposed to various concentrations of berberine for 48 h, and the cells were harvested and analyzed for cell cycle (Panel A: the percent of cells in phase) and sub-G1 group (Panel B: the percent of cells in apoptosis) were analyzed by flow cytometry as described in Materials and methods. Data represents mean ± SD of three experiments.
Figure 3
Changes of levels of CDK1 (A), cyclin B1 (B), Wee1 (C), and Cdc2 (D) in SNU-5 cells after exposure to berberine. SNU-5 cells (5×109/L) were treated with 0, 25, 50, 100, and, 200 μmol/L berberine for 24 h, then cytosolic fraction and total protein were determined as described in Materials and Methods.
Figure 4
Flow cytometric analysis of reactive oxygen species (A) and Ca2+ concentration (B) in human gastric carcinoma SNU-5 cells with 100 µmol/L berberine for various time periods. The SNU-5 cells (5×105 cells/mL) were treated with 100 μmol/L berberine for 0, 0.5, 1, 1.5, 2, 4, 6, and 12 h to detect the changes of ROS and Ca2+ cmcentration. The zero cancentration was defined as control. The percentage of cells stained with DCFH-DA dye was determined by flow cytometry as described in the Materials and Methods section.
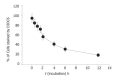
Figure 5
Flow cytometric analysis of mitochondrial membrane potential in human SNU-5 cells with 100 µmol/L berberine for various time periods. The SNU-5 cells (5×105 cells/L) were treated with various concentrations of berberine. The zero concentration was defined as control. The percentage of cells stained with DiOL6 dye, was determined by flow cytometry as described in the Materials and Methods.
Figure 6
Flow cytometric analysis of the effects of berberine induced caspase-3 activity (A) and apoptosis (B). The SNU-5 cells were incubated with 100 µmol/L berberine with or without z-VAD-fmk treatment for determination of caspase-3 activity and apoptosis.
Figure 7
Changes of levels of p53(A), Bcl-2(B), Bax(C), and cytochrome C (D) in SNU-5 cells after exposure to berberine. SNU-5 cells (5×106/mL) were treated with 0, 25, 50, 100, and 200 μmol/L berberine for 24 h, then cytosolic fraction and total protein were determined as described in Materials and Methods. The levels of p53, p21, Bcl-2, Bax, and cytochrome C were determind by Western blotting as described in Materials and Methods.
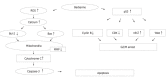
Figure 8
Proposed model of berberine mechanism of action on G2/M arrest and apoptosis in SNU-5 cells. Berberine induced p53 expression that led to the decrease of cyclin B and CDK1 but increase of the expression of cdc25c and Wee1 for G2/M arrest. Berberine induced ROS, Ca2+ production and decreased MMP levels led to cytochrome C release and caspase-3 activity, causing apoptosis in SNU-5 cells.
Similar articles
- Berberine induces apoptosis in human HSC-3 oral cancer cells via simultaneous activation of the death receptor-mediated and mitochondrial pathway.
Lin CC, Yang JS, Chen JT, Fan S, Yu FS, Yang JL, Lu CC, Kao MC, Huang AC, Lu HF, Chung JG. Lin CC, et al. Anticancer Res. 2007 Sep-Oct;27(5A):3371-8. Anticancer Res. 2007. PMID: 17970083 - Involvement of reactive oxygen species and caspase-dependent pathway in berberine-induced cell cycle arrest and apoptosis in C6 rat glioma cells.
Chen TC, Lai KC, Yang JS, Liao CL, Hsia TC, Chen GW, Lin JJ, Lin HJ, Chiu TH, Tang YJ, Chung JG. Chen TC, et al. Int J Oncol. 2009 Jun;34(6):1681-90. doi: 10.3892/ijo_00000299. Int J Oncol. 2009. PMID: 19424587 - Berberine, a natural product, induces G1-phase cell cycle arrest and caspase-3-dependent apoptosis in human prostate carcinoma cells.
Mantena SK, Sharma SD, Katiyar SK. Mantena SK, et al. Mol Cancer Ther. 2006 Feb;5(2):296-308. doi: 10.1158/1535-7163.MCT-05-0448. Mol Cancer Ther. 2006. PMID: 16505103 - Berberine induces G1 arrest and apoptosis in human glioblastoma T98G cells through mitochondrial/caspases pathway.
Eom KS, Hong JM, Youn MJ, So HS, Park R, Kim JM, Kim TY. Eom KS, et al. Biol Pharm Bull. 2008 Apr;31(4):558-62. doi: 10.1248/bpb.31.558. Biol Pharm Bull. 2008. PMID: 18379040
Cited by
- Therapeutic Potential of Berberine in the Treatment of Glioma: Insights into Its Regulatory Mechanisms.
Asemi Z, Behnam M, Pourattar MA, Mirzaei H, Razavi ZS, Tamtaji OR. Asemi Z, et al. Cell Mol Neurobiol. 2021 Aug;41(6):1195-1201. doi: 10.1007/s10571-020-00903-5. Epub 2020 Jun 18. Cell Mol Neurobiol. 2021. PMID: 32557203 Review. - Potential Role of Natural Products to Combat Radiotherapy and Their Future Perspectives.
Akter R, Najda A, Rahman MH, Shah M, Wesołowska S, Hassan SSU, Mubin S, Bibi P, Saeeda S. Akter R, et al. Molecules. 2021 Oct 2;26(19):5997. doi: 10.3390/molecules26195997. Molecules. 2021. PMID: 34641542 Free PMC article. Retracted. Review. - Berberine modulates AP-1 activity to suppress HPV transcription and downstream signaling to induce growth arrest and apoptosis in cervical cancer cells.
Mahata S, Bharti AC, Shukla S, Tyagi A, Husain SA, Das BC. Mahata S, et al. Mol Cancer. 2011 Apr 15;10:39. doi: 10.1186/1476-4598-10-39. Mol Cancer. 2011. PMID: 21496227 Free PMC article. - Mitosis-targeted anti-cancer therapies: where they stand.
Chan KS, Koh CG, Li HY. Chan KS, et al. Cell Death Dis. 2012 Oct 18;3(10):e411. doi: 10.1038/cddis.2012.148. Cell Death Dis. 2012. PMID: 23076219 Free PMC article. Review. - The Effect of Dark Septate Endophytic Fungi on Mahonia oiwakensis.
Lin LC, Tan YL, Lin WR, Ku KL, Ho ST. Lin LC, et al. Plants (Basel). 2021 Aug 20;10(8):1723. doi: 10.3390/plants10081723. Plants (Basel). 2021. PMID: 34451768 Free PMC article.
References
- Ghosh AK, Bhattacharyya FK, Ghosh DK. Leishmania donovani: amastigote inhibition and mode of action of berberine. Exp Parasitol. 1985;60:404–413. - PubMed
- HANO K, MIMURA F, OKU S, OKU K, KANI S, HAGIHARA A, OKAMURA T, NISHIYAMA T, MIYAZAKI S, HONDA F. [Pharmacological studies on metabolism of cancer tissues. XIII. pharmacological studies on carcinostatic effects of some plant components and their derivatives. I] Gan. 1957;48:443–445. - PubMed
- Hoshi A, Ikekawa T, Ikeda Y, Shirakawa S, Iigo M. Antitumor activity of berberrubine derivatives. Gann. 1976;67:321–325. - PubMed
- Zhang RX, Dougherty DV, Rosenblum ML. Laboratory studies of berberine used alone and in combination with 1,3-bis(2-chloroethyl)-1-nitrosourea to treat malignant brain tumors. Chin Med J (Engl) 1990;103:658–665. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous