Commensal-induced regulatory T cells mediate protection against pathogen-stimulated NF-kappaB activation - PubMed (original) (raw)
Commensal-induced regulatory T cells mediate protection against pathogen-stimulated NF-kappaB activation
Caitlin O'Mahony et al. PLoS Pathog. 2008.
Abstract
Host defence against infection requires a range of innate and adaptive immune responses that may lead to tissue damage. Such immune-mediated pathologies can be controlled with appropriate T regulatory (Treg) activity. The aim of the present study was to determine the influence of gut microbiota composition on Treg cellular activity and NF-kappaB activation associated with infection. Mice consumed the commensal microbe Bifidobacterium infantis 35624 followed by infection with Salmonella typhimurium or injection with LPS. In vivo NF-kappaB activation was quantified using biophotonic imaging. CD4+CD25+Foxp3+ T cell phenotypes and cytokine levels were assessed using flow cytometry while CD4+ T cells were isolated using magnetic beads for adoptive transfer to naïve animals. In vivo imaging revealed profound inhibition of infection and LPS induced NF-kappaB activity that preceded a reduction in S. typhimurium numbers and murine sickness behaviour scores in B. infantis-fed mice. In addition, pro-inflammatory cytokine secretion, T cell proliferation, and dendritic cell co-stimulatory molecule expression were significantly reduced. In contrast, CD4+CD25+Foxp3+ T cell numbers were significantly increased in the mucosa and spleen of mice fed B. infantis. Adoptive transfer of CD4+CD25+ T cells transferred the NF-kappaB inhibitory activity. Consumption of a single commensal micro-organism drives the generation and function of Treg cells which control excessive NF-kappaB activation in vivo. These cellular interactions provide the basis for a more complete understanding of the commensal-host-pathogen trilogue that contribute to host homeostatic mechanisms underpinning protection against aberrant activation of the innate immune system in response to a translocating pathogen or systemic LPS.
Conflict of interest statement
The authors are affiliated with a multi-departmental university campus-based research company (Alimentary Health Ltd.), which investigates host-flora interactions and the therapeutic manipulation of these interactions in various human and animal disorders. The content of this article was neither influenced nor constrained by this fact.
Figures
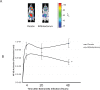
Figure 1. B. infantis attenuates NF-κB activation in vivo in response to S. typhimurium infection.
(A) A representative in vivo image illustrates that NF-κB activation in NF-κBlux transgenic mice four hours following S. typhimurium infection is attenuated when an animal is pre-fed B. infantis; (B) In vivo NF-κB activation in _B. infantis_-fed animals was significant reduced compared to NF-κB activation in placebo-fed controls (n = 6/group). *p<0.05 versus placebo.
Figure 2. B. infantis reduces S. typhimurium disease severity and systemic translocation.
(A) Macroscopic clinical scoring of mice infected with S. typhimurium reveals a significant reduction in B. infantis pre-fed mice disease symptoms 8 days following initial infection (n = 10/group); (B) S. typhimurium numbers were reduced in the spleens and livers of B. infantis fed mice 6 days following infection (n = 18/group). *p<0.05 versus placebo.
Figure 3. NF-κB activation is suppressed in LPS-injected mice when pre-treated with B. infantis.
(A) Whole body imaging of NF-κB_lux_ mice 4 hours following LPS injection reveals suppressed NF-κB activation in the _B. infantis_-fed animals. (B) Organs were removed for imaging purposes and a representative image is illustrated. (C) The mean increase in NF-κB activation following i.p. injection of LPS is significantly less in the ileum, spleen and liver of _B. infantis_-fed mice. *p<0.05 versus placebo, n = 5/group.
Figure 4. Cytokine production is suppressed in _S. typhimurium_-infected mice when pre-treated with B. infantis.
Cytokine release by isolated cells was examined immediately prior to S. typhimurium infection (Day 0) or four days (Day 4) after infection. (A) In vitro cytokine production by anti-CD3/CD28 stimulated Peyer's patch cells is significantly reduced in S. typhimurium infected mice when fed B. infantis with no differences being observed prior to infection; (B) In vitro IFN-γ and IL-10 production by anti-CD3/CD28 stimulated spleenocytes is significantly increased in placebo-fed animals following S. typhimurium infection. However, this increase was not observed in animals consuming B. infantis. TNF-α levels were similar for the two groups; (C) LPS stimulated splenocytes released comparable amounts of cytokine from the two groups of animals prior to infection but four days following Salmonella translocation, LPS stimulated TNF-α, IL-6 and MCP-1 release was significantly less in the _B. infantis_-fed animals. *p<0.05 versus placebo, n = 8/group at each timepoint.
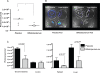
Figure 5. Dendritic cells are less activated in _B. infantis_-fed mice.
(A) The percentage of CD11c+MHC II+ mature dendritic cells staining positive for the co-stimulatory molecule CD80 are reduced in the Peyer's patch of _B. infantis_-fed mice prior to and during S. typhimurium infection. (B) Splenocyte dendritic cell CD80 expression is similar in both groups of un-infected mice but is significantly up-regulated with Salmonella infection only in the placebo group. *p<0.05 versus placebo, n = 6–9 animals per group per timepoint.
Figure 6. Bifidobacterium infantis consumption increases Treg numbers and activity in S. typhimurium infected mice.
(A) There is a significant increase in the percentage of CD4+ cells co-expressing CD25 in the Peyer's patch of animals fed B. infantis, particularly following Salmonella infection; (B) There are also significantly more CD4+CD25+ cells in the spleen of animals fed B. infantis; (C) A representative flow cytometry dot-plot illustrates that the majority of the CD25+ cells within the spleen also stain positive for Foxp3 which is graphed for _B. infantis_-fed animals in (D); (E) CD4+CD25+ T cells suppressed proliferation of naïve CFSE labelled CD4+ cells while depletion of the CD25+ subset cells removed the suppressive effect. *p<0.05 versus placebo.
Figure 7. NF-κB activation & TNF-α secretion in response to LPS is significantly reduced by adoptive transfer of CD4+CD25+ T cells from _B. infantis_-fed animals.
(A) In vivo adoptive transfer of CD4+ NF-κB−/− T cells from _B. infantis_-fed animals resulted in significant attenuation of NF-κB activity following i.p. LPS administration in NF-κBlux+/+ animals compared to mice that received T cells from placebo-fed controls (n = 6/group); (B) A subsequent study performed an identical adoptive transfer experiment except that nuclear NF-κB activation was measured using an ELISA system instead of biophotonic imaging - identical results were observed in that CD4+ T cells from _B. infantis_-fed animals significantly reduced LPS stimulated NF-κB activation (n = 5/group); (C) Removal of the CD25+ subpopulation from the CD4+ cells resulted in loss of the NF-kB suppressive activity while adoptive transfer of the CD25+ population alone replicated the CD4+ suppressive effect; (D) Similarily, the reduction in TNF-α secretion associated with CD4+ T cell transfer was lost when CD25+ were depleted but was replicated with transfer of CD25+ cells alone (n = 5/group). *p<0.05 versus placebo T cells; **p<0.05 versus CD4 T cells.
Similar articles
- Bifidobacterium animalis AHC7 protects against pathogen-induced NF-κB activation in vivo.
O'Mahony D, Murphy S, Boileau T, Park J, O'Brien F, Groeger D, Konieczna P, Ziegler M, Scully P, Shanahan F, Kiely B, O'Mahony L. O'Mahony D, et al. BMC Immunol. 2010 Dec 22;11:63. doi: 10.1186/1471-2172-11-63. BMC Immunol. 2010. PMID: 21176205 Free PMC article. - Functional modulation of human intestinal epithelial cell responses by Bifidobacterium infantis and Lactobacillus salivarius.
O'Hara AM, O'Regan P, Fanning A, O'Mahony C, Macsharry J, Lyons A, Bienenstock J, O'Mahony L, Shanahan F. O'Hara AM, et al. Immunology. 2006 Jun;118(2):202-15. doi: 10.1111/j.1365-2567.2006.02358.x. Immunology. 2006. PMID: 16771855 Free PMC article. - Bifidobacterium infantis suppression of Peyer's patch MIP-1α and MIP-1β secretion during Salmonella infection correlates with increased local CD4+CD25+ T cell numbers.
Scully P, Macsharry J, O'Mahony D, Lyons A, O'Brien F, Murphy S, Shanahan F, O'Mahony L. Scully P, et al. Cell Immunol. 2013 Feb;281(2):134-40. doi: 10.1016/j.cellimm.2013.03.008. Epub 2013 Apr 11. Cell Immunol. 2013. PMID: 23648818 - Regulation of nuclear factor-κB in autoimmunity.
Sun SC, Chang JH, Jin J. Sun SC, et al. Trends Immunol. 2013 Jun;34(6):282-9. doi: 10.1016/j.it.2013.01.004. Epub 2013 Feb 20. Trends Immunol. 2013. PMID: 23434408 Free PMC article. Review. - NF-κB in control of regulatory T cell development, identity, and function.
Hövelmeyer N, Schmidt-Supprian M, Ohnmacht C. Hövelmeyer N, et al. J Mol Med (Berl). 2022 Jul;100(7):985-995. doi: 10.1007/s00109-022-02215-1. Epub 2022 Jun 8. J Mol Med (Berl). 2022. PMID: 35672519 Free PMC article. Review.
Cited by
- Early exposure to food contaminants reshapes maturation of the human brain-gut-microbiota axis.
Sarron E, Pérot M, Barbezier N, Delayre-Orthez C, Gay-Quéheillard J, Anton PM. Sarron E, et al. World J Gastroenterol. 2020 Jun 21;26(23):3145-3169. doi: 10.3748/wjg.v26.i23.3145. World J Gastroenterol. 2020. PMID: 32684732 Free PMC article. Review. - Toll-like receptor-mediated immune responses in intestinal macrophages; implications for mucosal immunity and autoimmune diseases.
Zhou Z, Ding M, Huang L, Gilkeson G, Lang R, Jiang W. Zhou Z, et al. Clin Immunol. 2016 Dec;173:81-86. doi: 10.1016/j.clim.2016.09.005. Epub 2016 Sep 9. Clin Immunol. 2016. PMID: 27620642 Free PMC article. Review. - IBS: An epigenetic perspective.
Dinan TG, Cryan J, Shanahan F, Keeling PW, Quigley EM. Dinan TG, et al. Nat Rev Gastroenterol Hepatol. 2010 Aug;7(8):465-71. doi: 10.1038/nrgastro.2010.99. Epub 2010 Jun 29. Nat Rev Gastroenterol Hepatol. 2010. PMID: 20585338 Review. - Bifidobacterium: Host-Microbiome Interaction and Mechanism of Action in Preventing Common Gut-Microbiota-Associated Complications in Preterm Infants: A Narrative Review.
Sadeghpour Heravi F, Hu H. Sadeghpour Heravi F, et al. Nutrients. 2023 Jan 30;15(3):709. doi: 10.3390/nu15030709. Nutrients. 2023. PMID: 36771414 Free PMC article. Review. - The human microbiome, asthma, and allergy.
Riiser A. Riiser A. Allergy Asthma Clin Immunol. 2015 Dec 10;11:35. doi: 10.1186/s13223-015-0102-0. eCollection 2015. Allergy Asthma Clin Immunol. 2015. PMID: 26664362 Free PMC article. Review.
References
- Servin AL. Antagonistic activities of lactobacilli and bifidobacteria against microbial pathogens. FEMS Microbiol Rev. 2004;28:405–40. - PubMed
- Gill HS. Probiotics to enhance anti-infective defences in the gastrointestinal tract. Best Pract Res Clin Gastroenterol. 2003;17:755–73. - PubMed
- Belkaid Y, Rouse BT. Natural regulatory T cells in infectious disease. Nature Immunol. 2005;6:353–360. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials