Protein tyrosine phosphatase SHP-2: a proto-oncogene product that promotes Ras activation - PubMed (original) (raw)
Review
Protein tyrosine phosphatase SHP-2: a proto-oncogene product that promotes Ras activation
Takashi Matozaki et al. Cancer Sci. 2009 Oct.
Abstract
SHP-2 is a cytoplasmic protein tyrosine phosphatase (PTP) that contains two Src homology 2 (SH2) domains. Although PTPs are generally considered to be negative regulators on the basis of their ability to oppose the effects of protein tyrosine kinases, SHP-2 is unusual in that it promotes the activation of the Ras-MAPK signaling pathway by receptors for various growth factors and cytokines. The molecular basis for the activation of SHP-2 is also unique: In the basal state, the NH(2)-terminal SH2 domain of SHP-2 interacts with the PTP domain, resulting in autoinhibition of PTP activity; the binding of SHP-2 via its SH2 domains to tyrosine-phosphorylated growth factor receptors or docking proteins, however, results in disruption of this intramolecular interaction, leading to exposure of the PTP domain and catalytic activation. Indeed, SHP-2 proteins with artificial mutations in the NH(2)-terminal SH2 domain have been shown to act as dominant active mutants in vitro. Such activating mutations of PTPN11 (human SHP-2 gene) were subsequently identified in individuals with Noonan syndrome, a human developmental disorder that is sometimes associated with juvenile myelomonocytic leukemia. Furthermore, somatic mutations of PTPN11 were found to be associated with pediatric leukemia. SHP-2 is also thought to participate in the development of other malignant disorders, but in a manner independent of such activating mutations. Biochemical and functional studies of SHP-2 and genetic analysis of PTPN11 in human disorders have thus converged to provide new insight into the pathogenesis of cancer as well as potential new targets for cancer treatment.
Figures
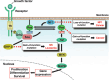
Figure 1
The function of Ras and its deregulation. Ras is an essential component of the signaling pathway that underlies growth factor‐induced cell proliferation, differentiation, or survival. Growth factors stimulate the tyrosine kinase (TK) activity and consequent autophosphorylation of their receptors, resulting in recruitment of the adaptor protein Grb2, which forms a constitutive complex with Sos, a guanine nucleotide exchange factor that catalyzes conversion of the GDP‐bound (inactive) form of Ras to the GTP‐bound (active) form. Activated Ras in turn induces activation of the Raf‐MEK‐MAPK cascade, which leads to changes in gene expression that are required for the induction of cell proliferation, differentiation, or survival. Neurofibromin (encoded by the tumor suppressor gene NF1) shows sequence similarity to the catalytic domain of GTPase‐activating proteins (GAPs) that negatively regulate Ras by increasing its intrinsic GTPase activity. SHP‐2, a cytoplasmic protein tyrosine phosphatase (PTP), promotes the activation of Ras by regulating signaling upstream of Ras. Mutations of Ras genes that result in constitutive activation of the encoded protein (gain‐of‐function mutation) induce activation of the Raf‐MEK‐MAPK cascade in the absence of growth factor stimulation, resulting in cell transformation and development of cancer. Loss of NF1 (loss‐of‐function mutation) also induces constitutive activation of Ras and gives rise to neurofibromatosis type 1 (NF1) as well as to cancer. Mutations of PTPN11 (human SHP‐2 gene) that result in constitutive activation of the encoded phosphatase (gain‐of‐function mutation) promote Ras activation and cause Noonan syndrome (NS) as well as leukemia.
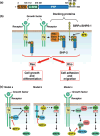
Figure 2
Structure and function of SHP‐2. (a) Domain organization of human SHP‐2. SHP‐2 contains two tandem SH2 domains (N‐SH2 and C‐SH2), a single protein tyrosine phosphatase (PTP) domain, and a COOH‐terminal hydrophobic tail that includes tyrosine phosphorylation sites. The residue numbers of amino acids that delineate the various domains( 51 ) or correspond to the phosphorylation sites are indicated. (b) In response to extracellular stimuli, SHP‐2 binds via its SH2 domains either to autophosphorylated growth factor receptors (such as that for platelet‐derived growth factor [PDGF]) or to docking proteins (such as insulin receptor substrates [IRSs], Grb2‐associated binder proteins [Gabs], fibroblast growth factor receptor substrate [FRS], and signal regulatory protein α[SIRPα; also known as SHP substrate‐1, SHPS‐1]) that are tyrosine‐phosphorylated by activated receptor tyrosine kinases (RTKs) or by Src family kinases (SFKs). Such interactions result in the activation of SHP‐2 and its consequent promotion of Ras activation, leading to cell growth or differentiation. SHP‐2 also participates in the regulation of cell adhesion and migration by controlling the activity of Rho. (c) Models proposed for the activation of Ras by SHP‐2. In model a, SHP‐2 promotes Ras activation by dephosphorylating tyrosine‐phosphorylated sites of growth factor receptors that bind p120 RasGAP (GAP). Dephosphorylation of these sites prevents inhibition of Ras activation by p120 RasGAP. According to model b, SHP‐2 promotes the activation of SFKs by dephosphorylating Cbp/PAG. Dephosphorylation of Cbp prevents the access of Csk (a negative regulator of SFKs) to SFKs, which may promote Ras activation by an as yet unclear mechanism (dotted arrow). In model c, SHP‐2 promotes Ras‐MAPK activation by dephosphorylating Sprouty, a negative regulator of Ras. Tyrosine‐phosphorylated Sprouty binds the Grb2–Sos complex and thereby prevents its interaction with Ras. SHP‐2 dephosphorylates Sprouty in response to growth factor stimulation, thereby preventing its interaction with Grb2.
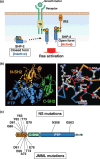
Figure 3
Intramolecular regulation of the protein tyrosine phosphatase (PTP) activity of SHP‐2 and the distribution of SHP‐2 mutations associated with Noonan syndrome (NS) or juvenile myelomonocytic leukemia (JMML). (a) Mechanism for regulation of the PTP activity of SHP‐2. In the basal state, the NH2‐terminal SH2 domain of SHP‐2 interacts with the PTP domain (closed form), resulting in autoinhibition of PTP activity. In response to extracellular stimuli, SHP‐2 binds via its SH2 domains to tyrosine‐phosphorylated growth factor receptors or docking proteins such as insulin receptor substrate (IRS), resulting in its adoption of an open conformation (open form) that is catalytically active. PH, pleckstrin homology domain. (b) A ribbon diagram of the crystal structure of human SHP‐2 is shown in the left panel. The NH2‐ and COOH‐terminal SH2 domains are shown in brown and green, respectively. The PTP domain is shown in blue. The circled region in the left panel is depicted in the right panel, which shows amino acids that participate in the formation of hydrogen bonds (dotted lines) that mediate the interaction of the NH2‐terminal SH2 domain with the PTP domain. Red sphere, oxygen; white sphere, carbon; blue sphere, nitrogen; yellow sphere, sulfur. (c) Distribution of residues of SHP‐2 that are frequently mutated in NS or JMML.
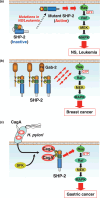
Figure 4
Roles of SHP‐2 in human cancer. (a) Mutations of SHP‐2 associated with Noonan syndrome (NS) or juvenile myelomonocytic leukemia (JMML) disrupt the intramolecular interaction between the NH2‐terminal SH2 domain and the protein tyrosine phosphatase (PTP) domain and thereby result in a loss of autoinhibition of PTP activity. The constitutive activation of SHP‐2 in the absence of growth factor stimulation results in aberrant activation of the Ras‐MAPK pathway, which in turn leads to the development of NS or leukemia. (b) Gab‐2, a pleckstrin homology (PH) domain‐containing docking protein, binds and activates SHP‐2 in response to a variety of cytokines and growth factors. Overexpression of Gab‐2 in human breast cancer may result in hyperactivation of SHP‐2 and consequent aberrant activation of the Ras‐MAPK pathway. (c) CagA in Helicobacter pylori (H. pylori)‐infected gastric epithelial cells undergoes tyrosine phosphorylation by Src family kinases (SFKs). Tyrosine‐phosphorylated CagA serves as a docking protein for SHP‐2 and thereby triggers aberrant activation of SHP‐2 and the Ras‐MAPK pathway and subsequent development of gastric cancer.
Similar articles
- Noonan syndrome-associated SHP2/PTPN11 mutants cause EGF-dependent prolonged GAB1 binding and sustained ERK2/MAPK1 activation.
Fragale A, Tartaglia M, Wu J, Gelb BD. Fragale A, et al. Hum Mutat. 2004 Mar;23(3):267-77. doi: 10.1002/humu.20005. Hum Mutat. 2004. PMID: 14974085 - Mutations in PTPN11 implicate the SHP-2 phosphatase in leukemogenesis.
Loh ML, Vattikuti S, Schubbert S, Reynolds MG, Carlson E, Lieuw KH, Cheng JW, Lee CM, Stokoe D, Bonifas JM, Curtiss NP, Gotlib J, Meshinchi S, Le Beau MM, Emanuel PD, Shannon KM. Loh ML, et al. Blood. 2004 Mar 15;103(6):2325-31. doi: 10.1182/blood-2003-09-3287. Epub 2003 Nov 26. Blood. 2004. PMID: 14644997 - The novel role of the C-terminal region of SHP-2. Involvement of Gab1 and SHP-2 phosphatase activity in Elk-1 activation.
Huang Q, Lerner-Marmarosh N, Che W, Ohta S, Osawa M, Yoshizumi M, Glassman M, Yan C, Berk BC, Abe J. Huang Q, et al. J Biol Chem. 2002 Aug 9;277(32):29330-41. doi: 10.1074/jbc.M112450200. Epub 2002 May 14. J Biol Chem. 2002. PMID: 12011040 - New and Unexpected Biological Functions for the Src-Homology 2 Domain-Containing Phosphatase SHP-2 in the Gastrointestinal Tract.
Coulombe G, Rivard N. Coulombe G, et al. Cell Mol Gastroenterol Hepatol. 2015 Nov 14;2(1):11-21. doi: 10.1016/j.jcmgh.2015.11.001. eCollection 2016 Jan. Cell Mol Gastroenterol Hepatol. 2015. PMID: 28174704 Free PMC article. Review. - The tyrosine phosphatase Shp2 (PTPN11) in cancer.
Chan G, Kalaitzidis D, Neel BG. Chan G, et al. Cancer Metastasis Rev. 2008 Jun;27(2):179-92. doi: 10.1007/s10555-008-9126-y. Cancer Metastasis Rev. 2008. PMID: 18286234 Review.
Cited by
- Different partners, opposite outcomes: a new perspective of the immunobiology of indoleamine 2,3-dioxygenase.
Orabona C, Pallotta MT, Grohmann U. Orabona C, et al. Mol Med. 2012 Jul 18;18(1):834-42. doi: 10.2119/molmed.2012.00029. Mol Med. 2012. PMID: 22481272 Free PMC article. Review. - Shp2 plays an important role in acute cigarette smoke-mediated lung inflammation.
Li FF, Shen J, Shen HJ, Zhang X, Cao R, Zhang Y, Qui Q, Lin XX, Xie YC, Zhang LH, Jia YL, Dong XW, Jiang JX, Bao MJ, Zhang S, Ma WJ, Wu XM, Shen H, Xie QM, Ke Y. Li FF, et al. J Immunol. 2012 Sep 15;189(6):3159-67. doi: 10.4049/jimmunol.1200197. Epub 2012 Aug 13. J Immunol. 2012. PMID: 22891281 Free PMC article. - miR-489 is a tumour-suppressive miRNA target PTPN11 in hypopharyngeal squamous cell carcinoma (HSCC).
Kikkawa N, Hanazawa T, Fujimura L, Nohata N, Suzuki H, Chazono H, Sakurai D, Horiguchi S, Okamoto Y, Seki N. Kikkawa N, et al. Br J Cancer. 2010 Sep 7;103(6):877-84. doi: 10.1038/sj.bjc.6605811. Epub 2010 Aug 10. Br J Cancer. 2010. PMID: 20700123 Free PMC article. - Conformational Changes in the Cytoplasmic Region of KIR3DL1 upon Interaction with SHP-2.
Cheng H, Schwell V, Curtis BR, Fazlieva R, Roder H, Campbell KS. Cheng H, et al. Structure. 2019 Apr 2;27(4):639-650.e2. doi: 10.1016/j.str.2019.01.009. Epub 2019 Feb 14. Structure. 2019. PMID: 30773397 Free PMC article.
References
- Schubbert S, Shannon K, Bollag G. Hyperactive Ras in developmental disorders and cancer. Nat Rev Cancer 2007; 7: 295–308. - PubMed
- Malumbres M, Barbacid M. RAS oncogenes: the first 30 years. Nat Rev Cancer 2003; 3: 459–65. - PubMed
- Egan SE, Weinberg RA. The pathway to signal achievement. Nature 1993; 365: 781–3. - PubMed
- Takai Y, Sasaki T, Matozaki T. Small GTP‐binding proteins. Physiol Rev 2001; 81: 153–208. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous