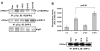Dopamine regulates phosphorylation of VEGF receptor 2 by engaging Src-homology-2-domain-containing protein tyrosine phosphatase 2 - PubMed (original) (raw)
. 2009 Sep 15;122(Pt 18):3385-92.
doi: 10.1242/jcs.053124. Epub 2009 Aug 25.
Affiliations
- PMID: 19706677
- PMCID: PMC2736867
- DOI: 10.1242/jcs.053124
Dopamine regulates phosphorylation of VEGF receptor 2 by engaging Src-homology-2-domain-containing protein tyrosine phosphatase 2
Sutapa Sinha et al. J Cell Sci. 2009.
Abstract
Vascular endothelial growth factor (VEGF)-induced receptor phosphorylation is the crucial step for initiating downstream signaling pathways that lead to angiogenesis or related pathophysiological outcomes. Our previous studies have shown that the neurotransmitter dopamine could inhibit VEGF-induced phosphorylation of VEGF receptor 2 (VEGFR-2), endothelial cell proliferation, migration, microvascular permeability, and thus, angiogenesis. In this study, we address the mechanism by which VEGFR-2 phosphorylation is regulated by dopamine. Here, we demonstrate that D2 dopamine receptor (D2DR) colocalizes with VEGFR-2 at the cell surface. Dopamine pretreatment increases the translocation and colocalization of Src-homology-2-domain-containing protein tyrosine phosphatase (SHP-2) with D2DR at the cell surface. Dopamine administration leads to increased VEGF-induced phosphorylation of SHP-2 and this increased phosphorylation parallels the increased phosphatase activity of SHP-2. Active SHP-2 then dephosphorylates VEGFR-2 at Y951, Y996 and Y1059, but not Y1175. We also observe that SHP-2 knockdown impairs the dopamine-regulated inhibition of VEGF-induced phosphorylation of VEGFR-2 and, subsequently, Src phosphorylation and migration. Our data establish a novel role for SHP-2 phosphatase in the dopamine-mediated regulation of VEGFR-2 phosphorylation.
Figures
Fig. 1.
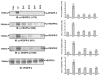
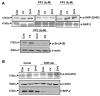
Effect of dopamine and quinpirole pretreatment on VEGF-induced specific tyrosine phosphorylation of VEGFR-2. A significant decrease in VEGF-induced phosphorylation of VEGFR-2 at Y1175, Y996, Y951 and Y1059 is seen in HUVECs pretreated with either dopamine (10 μM) or quinpirole (10 μM) for 15 minutes before VEGF (10 ng/ml) treatment. Con, HUVECs without any VEGF or dopamine treatment; +V, HUVECs treated with only VEGF (10 ng/ml) for 10 minutes; D-V, HUVECs pretreated with only 10 μM dopamine for 15 minutes; D+V, HUVECs pretreated with 10 μM dopamine for 15 minutes and then treated with VEGF (10 ng/ml) for 10 minutes; Q-V, HUVECs treated with only 10 μM quinpirole for 15 minutes; Q+V, HUVECs pretreated with 10 μM quinpirole for 15 minutes and then treated with VEGF (10 ng/ml) for 10 minutes. Total VEGFR-2 was used as the loading control. Results from the blots are summarized graphically on the right. Fold change for each treatment in the blots is quantified by NIH image densitometry and is shown in the bar graph. The figures are representative of three separate experiments with similar results.
Fig. 2.
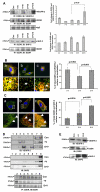
(A) D2DR coprecipitates with VEGFR-2 and SHP-2. Serum-starved (0.1% serum, for 24 hours) HUVECs were pretreated with dopamine (10 μM) for 15 minutes before VEGF (10 ng/ml) stimulation and then the lysates were immunoprecipitated with D2DR antibody and immunoblotted with antibodies against VEGFR-2 and SHP-2. Con, HUVECs without VEGF or dopamine treatment; +V5, HUVECs treated with only VEGF (10 ng/ml) for 5 minutes; +V10, HUVECs treated with only VEGF (10 ng/ml) for 10 minutes; D+V5, HUVEC pretreated with 10 μM dopamine for 15 minutes and then treated with VEGF (10 ng/ml) for 5 minutes; D+V10, HUVECs pretreated with 10 μM dopamine for 15 minutes and then treated with VEGF (10 ng/ml) for 10 minutes. The figures are representative of three separate experiments with similar results. Results from the blots are summarized graphically on the right. (B) D2DR colocalizes with VEGFR-2. Serum-starved HUVECs were pretreated with dopamine for 15 minutes and then stimulated with VEGF (10 ng/ml) for 10 minutes and stained with D2DR (green) and VEGFR-2 (red) antibodies. The D2DR localizes with VEGFR-2 at the cell surface. The intensity of complex formation is shown in the lower chamber (arrow). (a) VEGFR-2 and D2DR colocalize at the cell surface without any VEGF or dopamine treatment. (b) Colocalization of VEGFR-2 and D2DR decreases with VEGF stimulation. (c) Pretreatment with dopamine followed by VEGF induction results in an increase in the colocalization of D2DR and VEGFR-2 at the cell surface. The figures are representative of three separate experiments with similar results. Quantification of surface colocalization is shown in bar graph on the right (mean ± s.d.). (C) D2DR colocalizes with SHP-2. Serum-starved HUVECs were pretreated with dopamine for 15 minutes and then stimulated with VEGF (10 ng/ml) for 10 minutes and stained with D2DR (green) and SHP-2 (red) antibodies. The intensity of complex formation is shown in the lower chamber (arrowhead). (a) Intense D2DR and minor SHP-2 staining were observed at the cell surface without any VEGF or dopamine treatment. (b) Upon VEGF induction, SHP-2 translocated more from the cytosol to the cell surface and localized with D2DR. (c) Pretreatment with dopamine, followed by VEGF induction, leads to a marked increase in colocalization of D2DR with SHP-2 at the cell surface. The figures are representative of three separate experiments with similar results. Quantification of surface colocalization is shown in bar graph on the right (mean ± s.d.). (D) Cofractionation of VEGFR-2 and SHP-2 in the light density membrane fraction of HUVECs. Cell light density membrane fractions were purified using the hyperosmotic carbonate method. Equal volumes of each fraction were separated by SDS-PAGE electrophoresis, immunoblotted, and tested for VEGFR-2 and SHP-2. Presence of D2DR was monitored by immunoprecipitation with an anti-D2DR antibody. Dopamine pretreatment causes increased colocalization of VEGFR-2 and SHP-2 to the low-density domain. (E) Effect of dopamine treatment on biotinylated VEGFR-2 and D2DR. Serum-starved HUVECs were pretreated with dopamine for 15 minutes, then stimulated with VEGF (10 ng/ml) for 10 minutes, and then subjected to cell surface biotinylation following the manufacturer's instructions. After VEGF induction, less biotinylated VEGFR-2 is detected on the cell surface. However, with dopamine pretreatment, increased levels of VEGFR-2 and decreased levels of D2DR are found. From the biotinylation experiment, cell-surface-bound proteins were also recorded. VEGF induction increases surface recruitment of SHP-2 protein and dopamine pretreatment significantly enhances this localization.
Fig. 3.
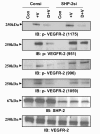
Effect of dopamine on VEGFR-2 phosphorylation in SHP-2-knockdown cells. HUVECs were transfected with a scrambled control (Consi) or SHP-2 siRNA (SHP-2si) using Oligofectamine. After 48 hours, cells were serum-starved and pretreated with dopamine for 15 minutes and then stimulated with VEGF for 10 minutes. In SHP-2-knockdown cells, dopamine cannot inhibit the VEGF-induced phosphorylation of VEGFR-2 at Y951, Y996 and Y1059, but successfully blocks Y1175 phosphorylation. Total VEGFR-2 was used as the loading control. The figures are representative of three separate experiments with similar results.
Fig. 4.
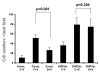
Effect of dopamine on SHP-2 phosphorylation and phosphatase activity. (A) Dopamine leads to an increase in VEGF-induced tyrosine phosphorylation of SHP-2. Serum-starved HUVEC were pretreated with dopamine (10 μM) for 15 minutes before VEGF (10 ng/ml) treatment for 5 or 10 minutes. Cell lysates were then immunoprecipitated with the Tyr-P antibody and immunoblotted with antibodies against VEGFR-2 and SHP-2. The figures are representative of three separate experiments with similar results. (B) SHP-2 phosphatase assay using pNPP. Serum-starved HUVECs were pretreated with 10 μM dopamine for 15 minutes followed by treatment with VEGF (10 ng/ml) for 10 minutes. Lysates were then immunoprecipitated with antibody against SHP-2. One half was run on a gel and the other half used for the phosphatase assay. SHP-2 phosphatase activity is markedly increased (_P_=0.01) after dopamine pretreatment, when compared with VEGF treatment alone. Data represent an average of three independent determinations (± s.d.) normalized against the negative control.
Fig. 5.
(A) Effect of PP2 and PP3 on SHP-2 Y542 phosphorylation. Serum-starved HUVECs were pretreated with PP2 or PP3 (5 μM) for 1 hour before treatment with either 10 μM dopamine or VEGF (10 ng/ml). PP2, a Src kinase inhibitor, significantly inhibits phosphorylation of SHP-2 at Y542. Total SHP-2 was used as a loading control. The phosphorylation of Src at Y418 is significantly inhibited by treatment with 5 μM PP2. The figures are representative of three separate experiments with similar results. (B) Effect of dopamine on Src phosphorylation at Y418 after SHP-2 knockdown. 48 hours after siRNA transfection, HUVECs were serum starved and pretreated with dopamine for 15 minutes, followed by VEGF stimulation for 10 minutes. Dopamine pretreatment does not inhibit Src phosphorylation at Y418 in the absence of SHP-2. β-actin was used as a loading control. The figures are representative of three separate experiments with similar results.
Fig. 6.
Effect of dopamine on migration in SHP-2-knockdown HUVECs. Cells were transfected with the scrambled control or SHP-2 siRNA using Oligofectamine. After 48 hours, cells were serum starved and a migration experiment was carried out as described in the Materials and Methods. After SHP-2 knockdown, dopamine does not inhibit the VEGF-induced HUVEC migration. Data represent an average of three independent determinations (± s.d.), each in triplicate.
Fig. 7.
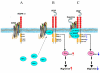
Model for dopamine-mediated regulation of VEGFR-2 phosphorylation by recruiting SHP-2. (A) In untreated HUVECs, D2DR and VEGFR-2 remain associated with each other. (B) Upon VEGF induction, SHP-2 translocates from the cytosol to the cell surface and becomes associated with both VEGFR-2 and D2DR. However, VEGF treatment promotes the dissociation of VEGFR-2 from D2DR to subsequently induce VEGFR-2 phosphorylation and downstream signaling. (C) Dopamine pretreatment, followed by VEGF stimulation for 10 minutes, leads to an increase in the association of D2DR with VEGFR-2. Dopamine treatment also induces an increased association between SHP-2 and D2DR at the cell surface and stimulates the phosphorylation of SHP-2 and its phosphatase activity. Active SHP-2 then inhibits the phosphorylation of VEGFR-2 at Y951, Y996 and Y1059, but not at Y1175. Decreased phosphorylation of VEGFR-2 at Y951 leads to a subsequent decrease in Src phosphorylation at Y418 and its kinase activity, effectively blocking VEGF-induced migration.
Similar articles
- Selective inhibition of vascular endothelial growth factor receptor-2 (VEGFR-2) identifies a central role for VEGFR-2 in human aortic endothelial cell responses to VEGF.
Endo A, Fukuhara S, Masuda M, Ohmori T, Mochizuki N. Endo A, et al. J Recept Signal Transduct Res. 2003;23(2-3):239-54. doi: 10.1081/rrs-120025567. J Recept Signal Transduct Res. 2003. PMID: 14626450 - Gold nanoparticles inhibit vascular endothelial growth factor-induced angiogenesis and vascular permeability via Src dependent pathway in retinal endothelial cells.
Kalishwaralal K, Sheikpranbabu S, BarathManiKanth S, Haribalaganesh R, Ramkumarpandian S, Gurunathan S. Kalishwaralal K, et al. Angiogenesis. 2011 Mar;14(1):29-45. doi: 10.1007/s10456-010-9193-x. Angiogenesis. 2011. PMID: 21061058 Retracted. - Signal transduction via vascular endothelial growth factor (VEGF) receptors and their roles in atherogenesis.
Matsumoto T, Mugishima H. Matsumoto T, et al. J Atheroscler Thromb. 2006 Jun;13(3):130-5. doi: 10.5551/jat.13.130. J Atheroscler Thromb. 2006. PMID: 16835467 Review. - [Research progress of protein tyrosine phosphatase SHP-2].
Cai HK, Deng YC. Cai HK, et al. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2012 Sep;41(5):581-5. doi: 10.3785/j.issn.1008-9292.2012.05.019. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2012. PMID: 23086654 Review. Chinese.
Cited by
- Spiperone Stimulates Regeneration in Pulmonary Endothelium Damaged by Cigarette Smoke and Lipopolysaccharide.
Skurikhin E, Pershina O, Zhukova M, Widera D, Pan E, Pakhomova A, Krupin V, Ermakova N, Skurikhina V, Sandrikina L, Morozov S, Kubatiev A, Dygai A. Skurikhin E, et al. Int J Chron Obstruct Pulmon Dis. 2021 Dec 30;16:3575-3591. doi: 10.2147/COPD.S336410. eCollection 2021. Int J Chron Obstruct Pulmon Dis. 2021. PMID: 35002229 Free PMC article. - Dopamine signaling from ganglion cells directs layer-specific angiogenesis in the retina.
Liang JH, Akhanov V, Ho A, Tawfik M, D'Souza SP, Cameron MA, Lang RA, Samuel MA. Liang JH, et al. Curr Biol. 2023 Sep 25;33(18):3821-3834.e5. doi: 10.1016/j.cub.2023.07.040. Epub 2023 Aug 11. Curr Biol. 2023. PMID: 37572663 Free PMC article. - β₂-glycoprotein I inhibits VEGF-induced endothelial cell growth and migration via suppressing phosphorylation of VEGFR2, ERK1/2, and Akt.
Chiu WC, Lin JY, Lee TS, You LR, Chiang AN. Chiu WC, et al. Mol Cell Biochem. 2013 Jan;372(1-2):9-15. doi: 10.1007/s11010-012-1440-6. Epub 2012 Sep 6. Mol Cell Biochem. 2013. PMID: 22956423 - The Inhibitory Effect of Selected D2 Dopaminergic Receptor Agonists on VEGF-Dependent Neovascularization in Zebrafish Larvae: Potential New Therapy in Ophthalmic Diseases.
Kasica N, Święch A, Saładziak K, Mackiewicz J, Osęka M. Kasica N, et al. Cells. 2022 Apr 2;11(7):1202. doi: 10.3390/cells11071202. Cells. 2022. PMID: 35406766 Free PMC article. - Surface Modification of Calcium Silicate via Mussel-Inspired Polydopamine and Effective Adsorption of Extracellular Matrix to Promote Osteogenesis Differentiation for Bone Tissue Engineering.
Kao CT, Chen YJ, Ng HY, Lee AK, Huang TH, Lin TF, Hsu TT. Kao CT, et al. Materials (Basel). 2018 Sep 9;11(9):1664. doi: 10.3390/ma11091664. Materials (Basel). 2018. PMID: 30205589 Free PMC article.
References
- Basu, S. and Dasgupta, P. S. (2000). Role of dopamine in malignant tumor growth. Endocrine 12, 237-241. - PubMed
- Basu, S., Nagy, J. A., Pal, S., Vasile, E., Eckelhoefer, I. A., Bliss, V. S., Manseau, E. J., Dasgupta, P. S., Dvorak, H. F. and Mukhopadhyay, D. (2001). The neurotransmitter dopamine inhibits angiogenesis induced by vascular permeability factor/vascular endothelial growth factor. Nat. Med. 7, 569-574. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous