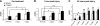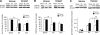Histone deacetylase inhibitor ITF2357 is neuroprotective, improves functional recovery, and induces glial apoptosis following experimental traumatic brain injury - PubMed (original) (raw)
Histone deacetylase inhibitor ITF2357 is neuroprotective, improves functional recovery, and induces glial apoptosis following experimental traumatic brain injury
Na'ama A Shein et al. FASEB J. 2009 Dec.
Abstract
Despite efforts aimed at developing novel therapeutics for traumatic brain injury (TBI), no specific pharmacological agent is currently clinically available. Here, we show that the pan-histone deacetylase (HDAC) inhibitor ITF2357, a compound shown to be safe and effective in humans, improves functional recovery and attenuates tissue damage when administered as late as 24 h postinjury. Using a well-characterized, clinically relevant mouse model of closed head injury (CHI), we demonstrate that a single dose of ITF2357 administered 24 h postinjury improves neurobehavioral recovery from d 6 up to 14 d postinjury (improved neurological score vs. vehicle; P< or =0.05), and that this functional benefit is accompanied by decreased neuronal degeneration, reduced lesion volume (22% reduction vs. vehicle; P< or =0.01), and is preceded by increased acetylated histone H3 levels and attenuation of injury-induced decreases in cytoprotective heat-shock protein 70 kDa and phosphorylated Akt. Moreover, reduced glial accumulation and activation were observed 3 d postinjury, and total p53 levels at the area of injury and caspase-3 immunoreactivity within microglia/macrophages at the trauma area were elevated, suggesting enhanced clearance of these cells via apoptosis following treatment. Hence, our findings underscore the relevance of HDAC inhibitors for ameliorating trauma-induced functional deficits and warrant consideration of applying ITF2357 for this indication.
Figures
Figure 1.
Postinjury administration of ITF2357 improves neurobehavioral recovery after CHI. ITF2357 was administered either 30 min prior to injury (A) or as postinjury treatment given at 1 h or 24 h after impact (B, C). Neurobehavioral status was assessed using the Neurological Severity Score (NSS), such that an increase in ΔNSS reflects faster recovery. Whereas pretreatment with ITF2357 only tended to improve recovery, a significant beneficial effect was observed in mice administered with the agent after injury as compared to matched vehicle-treated controls. Following administration of ITF2357 at 1 h postinjury, recovery over time was accelerated (B). When the compound was administered at 24 h after injury (C), functional benefit was evident from 6 d after trauma and up to 2 wk postinjury (_n_≥9 mice/group). Values are means ±
se
. *P ≤ 0.05, **P ≤ 0.01; Kruskal-Wallis rank test with Dunn’s post hoc test.
Figure 2.
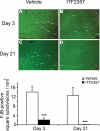
FluoroJade-B histochemistry. Fluoro-Jade B (FJB)-positive cells and processes (arrows) within the hippocampus of the injured hemisphere of vehicle-treated (A, C) or ITF2357-treated (B, D) mice on d 3 (A, B) and d 21 (C, D) postinjury. Data are means ±
se
(FJB-positive square subdivisions/mm2); n = 4 mice/group. ***P ≤ 0.001 Mann-Whitney U test.
Figure 3.
ITF2357 reduces TBI-induced lesion size. Lesion volume was evaluated 40 h postinjury (16 h after vehicle or ITF2357 injection) using TTC staining. A) Representative stains. B) Lesion volume was reduced in ITF2357-treated mice as compared to vehicle controls. Values are means ±
se
; n = 7 mice/group. **P ≤ 0.01; Student’s t test.
Figure 4.
Effects of ITF2357 on levels of AC-H3 (A), HSP70 (B), and p53 (C). ITF2357 was given 24 h after injury, and mice were sacrificed 6 or 24 h following injection (i.e., 30 or 48 h postinjury, respectively). Protein levels within ipsilateral frontal cortical segments were evaluated using Western immunoblotting. Optical density values relative to β-actin within the same sample were obtained using TINA software. Data are means ±
se
; n = 4 mice/group. *P ≤ 0.05, **P ≤ 0.01; ANOVA with Bonferroni post hoc test.
Figure 5.
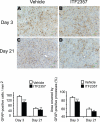
GFAP histochemistry. GFAP immunohistochemistry is shown at the striatum of the injured hemisphere in vehicle-treated (A, C) or ITF2357-treated (B, D) animals on d 3 (A, B) and 21 (C, D) following traumatic injury. Numbers of activated astrocytes and the area covered by their processes were reduced in ITF2357-treated mice on d 3 after trauma. On d 21 postinjury, astrocytes were present at lower numbers in animals given ITF2357 as compared to controls, but surface areas did not differ between groups. Data are means ±
se
; n = 4 mice/group
.
**P ≤ 0.01, ***P ≤ 0.001; Mann-Whitney U test.
Figure 6.
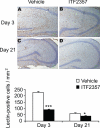
Lectin immunoreactivity. Activated microglia/macrophages within the parahippocampal area of vehicle-treated (A, C) and ITF2357-treated (B, D) animals at 3 d (A, B) and 21 d (C, D) after traumatic injury. Data are presented as means ±
se
; n = 4 mice/group. *P ≤ 0.05, ***P ≤ 0.001; Mann-Whitney U test.
Figure 7.
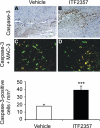
Caspase-3 labeling. Active caspase-3 immunohistochemistry at the area of injury in vehicle-treated (A) and ITF2357-treated (B) animals on d 3 post-trauma. Double immunohistochemistry for active caspase-3 (apoptosis; red fluorescence) and MAC-3 (macrophages/activated microglia; green fluorescence) at the area of trauma in vehicle-treagted (C) and ITF2357-treated (D) animals. Coexpression of both markers was identified using confocal microscopy, suggesting that apoptotic cells were macrophages/activated microglia (yellow cells). Data are means ±
se
; n = 4 mice/group. ***P ≤ 0.001; Mann-Whitney U test.
Similar articles
- Histone deacetylase inhibitors as therapeutic agents for acute central nervous system injuries.
Shein NA, Shohami E. Shein NA, et al. Mol Med. 2011 May-Jun;17(5-6):448-56. doi: 10.2119/molmed.2011.00038. Epub 2011 Jan 25. Mol Med. 2011. PMID: 21274503 Free PMC article. Review. - Histone deacetylase inhibitors exhibit anti-inflammatory and neuroprotective effects in a rat permanent ischemic model of stroke: multiple mechanisms of action.
Kim HJ, Rowe M, Ren M, Hong JS, Chen PS, Chuang DM. Kim HJ, et al. J Pharmacol Exp Ther. 2007 Jun;321(3):892-901. doi: 10.1124/jpet.107.120188. Epub 2007 Mar 19. J Pharmacol Exp Ther. 2007. PMID: 17371805 - [ITF-2357 on inhibition myeloid leukemic cell lines cells proliferation in vitro and its mechanism].
Yu WJ, Wang L, You LS, Mei C, Ma QL, Jin J. Yu WJ, et al. Zhonghua Xue Ye Xue Za Zhi. 2012 May;33(5):366-70. Zhonghua Xue Ye Xue Za Zhi. 2012. PMID: 22781793 Chinese. - Enhancement of Autophagy by Histone Deacetylase Inhibitor Trichostatin A Ameliorates Neuronal Apoptosis After Subarachnoid Hemorrhage in Rats.
Shao A, Wang Z, Wu H, Dong X, Li Y, Tu S, Tang J, Zhao M, Zhang J, Hong Y. Shao A, et al. Mol Neurobiol. 2016 Jan;53(1):18-27. doi: 10.1007/s12035-014-8986-0. Epub 2014 Nov 18. Mol Neurobiol. 2016. PMID: 25399954 - Neuroprotective profile of enoxaparin, a low molecular weight heparin, in in vivo models of cerebral ischemia or traumatic brain injury in rats: a review.
Stutzmann JM, Mary V, Wahl F, Grosjean-Piot O, Uzan A, Pratt J. Stutzmann JM, et al. CNS Drug Rev. 2002 Spring;8(1):1-30. doi: 10.1111/j.1527-3458.2002.tb00213.x. CNS Drug Rev. 2002. PMID: 12070524 Free PMC article. Review.
Cited by
- Omega-3 polyunsaturated fatty acid supplementation improves neurologic recovery and attenuates white matter injury after experimental traumatic brain injury.
Pu H, Guo Y, Zhang W, Huang L, Wang G, Liou AK, Zhang J, Zhang P, Leak RK, Wang Y, Chen J, Gao Y. Pu H, et al. J Cereb Blood Flow Metab. 2013 Sep;33(9):1474-84. doi: 10.1038/jcbfm.2013.108. Epub 2013 Jun 26. J Cereb Blood Flow Metab. 2013. PMID: 23801244 Free PMC article. - Trichostatin A, a Histone Deacetylase Inhibitor, Alleviates Eosinophilic Meningitis Induced by Angiostrongylus cantonensis Infection in Mice.
Zhang Y, Xie H, Tang W, Zeng X, Lin Y, Xu L, Xiao L, Xu J, Wu Z, Yuan D. Zhang Y, et al. Front Microbiol. 2019 Oct 4;10:2280. doi: 10.3389/fmicb.2019.02280. eCollection 2019. Front Microbiol. 2019. PMID: 31636619 Free PMC article. - Lysine deacetylases are produced in pancreatic beta cells and are differentially regulated by proinflammatory cytokines.
Lundh M, Christensen DP, Rasmussen DN, Mascagni P, Dinarello CA, Billestrup N, Grunnet LG, Mandrup-Poulsen T. Lundh M, et al. Diabetologia. 2010 Dec;53(12):2569-78. doi: 10.1007/s00125-010-1892-8. Epub 2010 Sep 28. Diabetologia. 2010. PMID: 20878317 - Microglia: A Potential Drug Target for Traumatic Axonal Injury.
Huang X, You W, Zhu Y, Xu K, Yang X, Wen L. Huang X, et al. Neural Plast. 2021 May 20;2021:5554824. doi: 10.1155/2021/5554824. eCollection 2021. Neural Plast. 2021. PMID: 34093701 Free PMC article. Review. - Posttrauma cotreatment with lithium and valproate: reduction of lesion volume, attenuation of blood-brain barrier disruption, and improvement in motor coordination in mice with traumatic brain injury.
Yu F, Wang Z, Tanaka M, Chiu CT, Leeds P, Zhang Y, Chuang DM. Yu F, et al. J Neurosurg. 2013 Sep;119(3):766-73. doi: 10.3171/2013.6.JNS13135. Epub 2013 Jul 12. J Neurosurg. 2013. PMID: 23848820 Free PMC article.
References
- Jenuwein T, Allis C D. Translating the histone code. Science. 2001;293:1074–1080. - PubMed
- Hahnen E, Hauke J, Trankle C, Eyupoglu I Y, Wirth B, Blumcke I. Histone deacetylase inhibitors: possible implications for neurodegenerative disorders. Expert Opin Investig Drugs. 2008;17:169–184. - PubMed
- Jin K, Mao X O, Simon R P, Greenberg D A. Cyclic AMP response element binding protein (CREB) and CREB binding protein (CBP) in global cerebral ischemia. J Mol Neurosci. 2001;16:49–56. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous