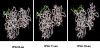Blood-brain barrier permeability and tPA-mediated neurotoxicity - PubMed (original) (raw)
Blood-brain barrier permeability and tPA-mediated neurotoxicity
Rami Abu Fanne et al. Neuropharmacology. 2010 Jun.
Abstract
Tissue type plasminogen activator (tPA) can induce neuronal apoptosis, disrupt the blood-brain barrier (BBB), and promote dilation of the cerebral vasculature. The timing, sequence and contributions of these and other deleterious effects of tPA and their contribution to post-ischemic brain damage after stroke, have not been fully elucidated. To dissociate the effects of tPA on BBB permeability, cerebral vasodilation and protease-dependent pathways, we developed several tPA mutants and PAI-1 derived peptides constructed by computerized homology modeling of tPA. Our data show that intravenous administration of human tPA to rats increases BBB permeability through a non-catalytic process that is associated with reversible neurotoxicity, brain damage, mortality and contributes significantly to its brief therapeutic window. Furthermore, our data show that inhibiting the effect of tPA on BBB function without affecting its catalytic activity, improves outcome and significantly extends its therapeutic window in mechanical as well as in thromboembolic models of stroke.
(c) 2009 Elsevier Ltd. All rights reserved.
Figures
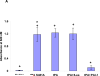
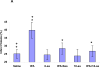
Figure 1. Regulation of BBB integrity by human rtPA and PAI-1. Panel A. Role of the catalytic activity of rtPA in BBB permeability
BBB permeability was measured using Evans blue(Adelson et al. 1998). Anesthetized rats were injected IV with 100 μl of saline or saline containing either WT-rtPA or catalytically inactive rtPA-Ser481Ala (1 mg/kg each) alone or together with PAI-1 or the PA-1 derived peptide Ac-EEIIMD-amide (1 mg/kg). Five minutes later, the rats were given an IV injection of 2% Evans blue (EB) in saline. One hour after injection of the dye, organs were cleared of blood by transcardiac perfusion. The brains were removed, weighed, homogenized in N,N dimethylformamide and centrifuged. Extruded dye was quantified by absorbance at 620 nm. Data are expressed as absorbance per gram of tissue. The mean ± SEM of data from 6 animals/group is shown. Panel B. Effect of PAI-1 derived peptides on rtPA-induced BBB permeability. Three PAI-1 derived peptides of varying length derived from the core sequence EEIIMD, as described in the text (6-aa, 11-aa or 18-aa) were injected alone (1mg/kg) or together with rtPA (1 mg/kg). Permeability was measured as described in the legend to Panel A. The mean ± SEM of data from 8-9 animals/group is shown. Statistical significance was set at P <0.05 using t-test (+) or ANOVA test(*) in this and in each Figure that follows.
Figure 1. Regulation of BBB integrity by human rtPA and PAI-1. Panel A. Role of the catalytic activity of rtPA in BBB permeability
BBB permeability was measured using Evans blue(Adelson et al. 1998). Anesthetized rats were injected IV with 100 μl of saline or saline containing either WT-rtPA or catalytically inactive rtPA-Ser481Ala (1 mg/kg each) alone or together with PAI-1 or the PA-1 derived peptide Ac-EEIIMD-amide (1 mg/kg). Five minutes later, the rats were given an IV injection of 2% Evans blue (EB) in saline. One hour after injection of the dye, organs were cleared of blood by transcardiac perfusion. The brains were removed, weighed, homogenized in N,N dimethylformamide and centrifuged. Extruded dye was quantified by absorbance at 620 nm. Data are expressed as absorbance per gram of tissue. The mean ± SEM of data from 6 animals/group is shown. Panel B. Effect of PAI-1 derived peptides on rtPA-induced BBB permeability. Three PAI-1 derived peptides of varying length derived from the core sequence EEIIMD, as described in the text (6-aa, 11-aa or 18-aa) were injected alone (1mg/kg) or together with rtPA (1 mg/kg). Permeability was measured as described in the legend to Panel A. The mean ± SEM of data from 8-9 animals/group is shown. Statistical significance was set at P <0.05 using t-test (+) or ANOVA test(*) in this and in each Figure that follows.
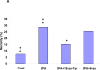
Figure 2. Panel A. Effect of PAI-1 derived peptides on tPA induced vasodilation
Contraction of isolated aortic rings was induced by adding increasing concentrations of phenylephrine (PE), as described in(Haj-Yejia et al. 2000; Nassar et al. 2004). The EC50 was determined in the absence or presence of rtPA (1 nM) alone or in the presence of the 6 (6-aa), 11 (11-aa), 18-aa (18-aa) PAI-1 derived peptide or the 18-aa scrambled (18-aa Sc) peptide (100 nM each). The mean ± SEM of data from 3 experiments is shown. Panel B. Effect of PAI-1 derived peptides on tPA induced plasminogen activation. The capacity of tPA to activate plasminogen was tested in absence or presence of PAI-1 or the PAI-1 derived peptides 6 (6-aa), 11 (11-aa), 18-aa (18-aa) or the 18-aa scrambled (18-aa Sc) peptide. The mean ± SD of data from 3 experiments is shown. Panel C. Effect of rtPA and PAI-1 derived peptides on extravasation of radiolabeled fibrinogen. Anesthetized rats were injected IV with 100 μl of saline or saline containing either WT-rtPA (1 mg/kg each) alone or together with PAI-1 or the PA-1 derived peptide Ac-EEIIMD-amide (1 mg/kg), 18-aa Tyr or scrambled 18-aa peptide. Five minutes later, the rats were given an IV injection of 125I-labled fibrinogen (0.5 mg/ml, specific activity of 300,000 cpm/mL) in saline. One hour after injection of the 125I-fibrinogen, organs were cleared of blood by transcardiac perfusion. The brains were removed, weighed and homogenized in N,N dimethylformamide. Radioactivity was quantified Using γ-counter. Data are expressed as cpm per gram of tissue. The mean ± SEM of data from 6 animals/group is shown.
Figure 2. Panel A. Effect of PAI-1 derived peptides on tPA induced vasodilation
Contraction of isolated aortic rings was induced by adding increasing concentrations of phenylephrine (PE), as described in(Haj-Yejia et al. 2000; Nassar et al. 2004). The EC50 was determined in the absence or presence of rtPA (1 nM) alone or in the presence of the 6 (6-aa), 11 (11-aa), 18-aa (18-aa) PAI-1 derived peptide or the 18-aa scrambled (18-aa Sc) peptide (100 nM each). The mean ± SEM of data from 3 experiments is shown. Panel B. Effect of PAI-1 derived peptides on tPA induced plasminogen activation. The capacity of tPA to activate plasminogen was tested in absence or presence of PAI-1 or the PAI-1 derived peptides 6 (6-aa), 11 (11-aa), 18-aa (18-aa) or the 18-aa scrambled (18-aa Sc) peptide. The mean ± SD of data from 3 experiments is shown. Panel C. Effect of rtPA and PAI-1 derived peptides on extravasation of radiolabeled fibrinogen. Anesthetized rats were injected IV with 100 μl of saline or saline containing either WT-rtPA (1 mg/kg each) alone or together with PAI-1 or the PA-1 derived peptide Ac-EEIIMD-amide (1 mg/kg), 18-aa Tyr or scrambled 18-aa peptide. Five minutes later, the rats were given an IV injection of 125I-labled fibrinogen (0.5 mg/ml, specific activity of 300,000 cpm/mL) in saline. One hour after injection of the 125I-fibrinogen, organs were cleared of blood by transcardiac perfusion. The brains were removed, weighed and homogenized in N,N dimethylformamide. Radioactivity was quantified Using γ-counter. Data are expressed as cpm per gram of tissue. The mean ± SEM of data from 6 animals/group is shown.
Figure 2. Panel A. Effect of PAI-1 derived peptides on tPA induced vasodilation
Contraction of isolated aortic rings was induced by adding increasing concentrations of phenylephrine (PE), as described in(Haj-Yejia et al. 2000; Nassar et al. 2004). The EC50 was determined in the absence or presence of rtPA (1 nM) alone or in the presence of the 6 (6-aa), 11 (11-aa), 18-aa (18-aa) PAI-1 derived peptide or the 18-aa scrambled (18-aa Sc) peptide (100 nM each). The mean ± SEM of data from 3 experiments is shown. Panel B. Effect of PAI-1 derived peptides on tPA induced plasminogen activation. The capacity of tPA to activate plasminogen was tested in absence or presence of PAI-1 or the PAI-1 derived peptides 6 (6-aa), 11 (11-aa), 18-aa (18-aa) or the 18-aa scrambled (18-aa Sc) peptide. The mean ± SD of data from 3 experiments is shown. Panel C. Effect of rtPA and PAI-1 derived peptides on extravasation of radiolabeled fibrinogen. Anesthetized rats were injected IV with 100 μl of saline or saline containing either WT-rtPA (1 mg/kg each) alone or together with PAI-1 or the PA-1 derived peptide Ac-EEIIMD-amide (1 mg/kg), 18-aa Tyr or scrambled 18-aa peptide. Five minutes later, the rats were given an IV injection of 125I-labled fibrinogen (0.5 mg/ml, specific activity of 300,000 cpm/mL) in saline. One hour after injection of the 125I-fibrinogen, organs were cleared of blood by transcardiac perfusion. The brains were removed, weighed and homogenized in N,N dimethylformamide. Radioactivity was quantified Using γ-counter. Data are expressed as cpm per gram of tissue. The mean ± SEM of data from 6 animals/group is shown.
Figure 3. Representation of energetically favorable rtPA/PAI-1 derived peptide-peptide complexes
The peptides adopt an extended conformation upon binding to the previously described PAI-docking site in tPA. Most of the hydrophobic residues of the 18-aa peptide are buried within the hydrophobic surface regions of the enzyme.
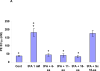
Figure 4. Panel A: Inhibition of the binding of PAI-1 to rtPA by PAI-1–derived peptides
The binding of PAI-1 (1 nM) to immobilized rtPA was measured in the absence (cont.) or presence of increasing concentrations of each of the 3 PAI-1 derived peptides. Equimolar concentrations of rtPA (tPA) served as the positive control to determine 100% inhibition. One of 3 such experiments with equivalent results is shown. Panel B. Energetically favorable conformation of the complex between rtPA (in blue) and the 18-aa peptide (in red) showing that the aromatic rings of Phe383 (in white) are directed outward toward the solvent.
Figure 4. Panel A: Inhibition of the binding of PAI-1 to rtPA by PAI-1–derived peptides
The binding of PAI-1 (1 nM) to immobilized rtPA was measured in the absence (cont.) or presence of increasing concentrations of each of the 3 PAI-1 derived peptides. Equimolar concentrations of rtPA (tPA) served as the positive control to determine 100% inhibition. One of 3 such experiments with equivalent results is shown. Panel B. Energetically favorable conformation of the complex between rtPA (in blue) and the 18-aa peptide (in red) showing that the aromatic rings of Phe383 (in white) are directed outward toward the solvent.
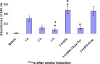
Figure 5. Inhibition of post ischemic rtPA-induced brain damage by PA-1 derived peptides
The MCA of rats was occluded with an intraluminal filament as described in Methods. The thread was withdrawn 1 hour later. One (Panel 5A), two (Panel 5B) or three (Panel 5C) hours after withdrawal of the thread, rtPA (6 mg/kg) alone or together with the PAI-1 derived peptides 6-aa or 18-aaTyr was injected IV (1mg/kg each). Controls were injected with saline. Twenty-four hours later, brain infarct size and volumes were measured, as described in Methods. The mean ± SEM of data from 15 animals/group is shown. The appearance of representative sections is shown in Panel 5D.
Figure 5. Inhibition of post ischemic rtPA-induced brain damage by PA-1 derived peptides
The MCA of rats was occluded with an intraluminal filament as described in Methods. The thread was withdrawn 1 hour later. One (Panel 5A), two (Panel 5B) or three (Panel 5C) hours after withdrawal of the thread, rtPA (6 mg/kg) alone or together with the PAI-1 derived peptides 6-aa or 18-aaTyr was injected IV (1mg/kg each). Controls were injected with saline. Twenty-four hours later, brain infarct size and volumes were measured, as described in Methods. The mean ± SEM of data from 15 animals/group is shown. The appearance of representative sections is shown in Panel 5D.
Figure 5. Inhibition of post ischemic rtPA-induced brain damage by PA-1 derived peptides
The MCA of rats was occluded with an intraluminal filament as described in Methods. The thread was withdrawn 1 hour later. One (Panel 5A), two (Panel 5B) or three (Panel 5C) hours after withdrawal of the thread, rtPA (6 mg/kg) alone or together with the PAI-1 derived peptides 6-aa or 18-aaTyr was injected IV (1mg/kg each). Controls were injected with saline. Twenty-four hours later, brain infarct size and volumes were measured, as described in Methods. The mean ± SEM of data from 15 animals/group is shown. The appearance of representative sections is shown in Panel 5D.
Figure 5. Inhibition of post ischemic rtPA-induced brain damage by PA-1 derived peptides
The MCA of rats was occluded with an intraluminal filament as described in Methods. The thread was withdrawn 1 hour later. One (Panel 5A), two (Panel 5B) or three (Panel 5C) hours after withdrawal of the thread, rtPA (6 mg/kg) alone or together with the PAI-1 derived peptides 6-aa or 18-aaTyr was injected IV (1mg/kg each). Controls were injected with saline. Twenty-four hours later, brain infarct size and volumes were measured, as described in Methods. The mean ± SEM of data from 15 animals/group is shown. The appearance of representative sections is shown in Panel 5D.
Figure 6. Effect of PAI-derived peptides on mortality and neurological recovery. Panel A. Inhibition of post ischemia rtPA-induced mortality
Animals were treated as described in the legend to Figure 5. Death was recorded. Animals were included in the study if they survived up to the initiation of treatment with saline (Cont) or with rtPA or rtPA together with the 6- or 18-aa-Tyr PAI-1 derived peptides. The number of deaths in each group was recorded. The mean from 16 animals/group is shown. Panel B: Preservation of neurological function. Animals were treated as described in the legend to Figure 5. The neurological score was measured 24 hours after induction of ischemia in animals treated with saline (Cont), rtPA or rtPA together with 18-aa-Tyr, as described in Methods. The mean ± SEM of data from 12 animals/group is shown.
Figure 6. Effect of PAI-derived peptides on mortality and neurological recovery. Panel A. Inhibition of post ischemia rtPA-induced mortality
Animals were treated as described in the legend to Figure 5. Death was recorded. Animals were included in the study if they survived up to the initiation of treatment with saline (Cont) or with rtPA or rtPA together with the 6- or 18-aa-Tyr PAI-1 derived peptides. The number of deaths in each group was recorded. The mean from 16 animals/group is shown. Panel B: Preservation of neurological function. Animals were treated as described in the legend to Figure 5. The neurological score was measured 24 hours after induction of ischemia in animals treated with saline (Cont), rtPA or rtPA together with 18-aa-Tyr, as described in Methods. The mean ± SEM of data from 12 animals/group is shown.
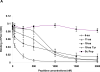
Figure 7. Time course of BBB permeability post ischemia
BBB permeability was measured in untreated animals using Evans blue as described in the legend to Figure 1, before and 1, 2 and 3 hours after induction of brain ischemia by mechanical occlusion. In second set of animals, three hours after induction of brain ischemia, BBB permeability was measured after rtPA was given alone or together with the 6- or 18-aa-Tyr PAI-1 derived peptides. The mean ± SEM of data from 12 animals/group is shown.
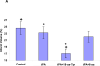
Figure 8. Neurological outcome four hours after thromboembolic stroke in rats treated with rtPA and PAI-1 derived peptides
Four hours after induction of thromboembolic stroke, rats were given saline (Cont), rtPA (6 mg/kg) alone or together with 18-aa-Tyr or 6-aa PAI-1 derive peptides (1 mg/kg each). Twenty four hours later, infarct volume (Panels 8A and 8B) and assessment of neurological function, expressed as the NSS (Panel 8C) were determined as described in Methods. The mean ± SEM of data from 12 animals/group is shown.
Figure 8. Neurological outcome four hours after thromboembolic stroke in rats treated with rtPA and PAI-1 derived peptides
Four hours after induction of thromboembolic stroke, rats were given saline (Cont), rtPA (6 mg/kg) alone or together with 18-aa-Tyr or 6-aa PAI-1 derive peptides (1 mg/kg each). Twenty four hours later, infarct volume (Panels 8A and 8B) and assessment of neurological function, expressed as the NSS (Panel 8C) were determined as described in Methods. The mean ± SEM of data from 12 animals/group is shown.
Figure 8. Neurological outcome four hours after thromboembolic stroke in rats treated with rtPA and PAI-1 derived peptides
Four hours after induction of thromboembolic stroke, rats were given saline (Cont), rtPA (6 mg/kg) alone or together with 18-aa-Tyr or 6-aa PAI-1 derive peptides (1 mg/kg each). Twenty four hours later, infarct volume (Panels 8A and 8B) and assessment of neurological function, expressed as the NSS (Panel 8C) were determined as described in Methods. The mean ± SEM of data from 12 animals/group is shown.
Similar articles
- Ascorbic Acid Reduces the Adverse Effects of Delayed Administration of Tissue Plasminogen Activator in a Rat Stroke Model.
Allahtavakoli M, Amin F, Esmaeeli-Nadimi A, Shamsizadeh A, Kazemi-Arababadi M, Kennedy D. Allahtavakoli M, et al. Basic Clin Pharmacol Toxicol. 2015 Nov;117(5):335-9. doi: 10.1111/bcpt.12413. Epub 2015 May 9. Basic Clin Pharmacol Toxicol. 2015. PMID: 25899606 - Effects of tissue plasminogen activator timing on blood-brain barrier permeability and hemorrhagic transformation in rats with transient ischemic stroke.
Zhang Y, Wang Y, Zuo Z, Wang Z, Roy J, Hou Q, Tong E, Hoffmann A, Sperberg E, Bredno J, Berr SS, Xie M, Lee K, Wintermark M. Zhang Y, et al. J Neurol Sci. 2014 Dec 15;347(1-2):148-54. doi: 10.1016/j.jns.2014.09.036. Epub 2014 Sep 28. J Neurol Sci. 2014. PMID: 25292413 - MRI evaluation of BBB disruption after adjuvant AcSDKP treatment of stroke with tPA in rat.
Ding G, Zhang Z, Chopp M, Li L, Zhang L, Li Q, Wei M, Jiang Q. Ding G, et al. Neuroscience. 2014 Jun 20;271:1-8. doi: 10.1016/j.neuroscience.2014.04.025. Epub 2014 Apr 24. Neuroscience. 2014. PMID: 24769225 Free PMC article. - The neurotoxicity of tissue plasminogen activator?
Kaur J, Zhao Z, Klein GM, Lo EH, Buchan AM. Kaur J, et al. J Cereb Blood Flow Metab. 2004 Sep;24(9):945-63. doi: 10.1097/01.WCB.0000137868.50767.E8. J Cereb Blood Flow Metab. 2004. PMID: 15356416 Review. - New progress in the approaches for blood-brain barrier protection in acute ischemic stroke.
Li Y, Zhong W, Jiang Z, Tang X. Li Y, et al. Brain Res Bull. 2019 Jan;144:46-57. doi: 10.1016/j.brainresbull.2018.11.006. Epub 2018 Nov 15. Brain Res Bull. 2019. PMID: 30448453 Review.
Cited by
- Deficiency in Neuroserpin Exacerbates CoCl2 Induced Hypoxic Injury in the Zebrafish Model by Increased Oxidative Stress.
Han S, Zhang D, Dong Q, Wang X, Wang L. Han S, et al. Front Pharmacol. 2021 Mar 2;12:632662. doi: 10.3389/fphar.2021.632662. eCollection 2021. Front Pharmacol. 2021. PMID: 33737878 Free PMC article. - Review of evidence implicating the plasminogen activator system in blood-brain barrier dysfunction associated with Alzheimer's disease.
Tang MY, Gorin FA, Lein PJ. Tang MY, et al. Ageing Neurodegener Dis. 2022;2:2. doi: 10.20517/and.2022.05. Epub 2022 Jan 29. Ageing Neurodegener Dis. 2022. PMID: 35156107 Free PMC article. - The Role of Extracellular Matrix in Human Neurodegenerative Diseases.
Pintér P, Alpár A. Pintér P, et al. Int J Mol Sci. 2022 Sep 21;23(19):11085. doi: 10.3390/ijms231911085. Int J Mol Sci. 2022. PMID: 36232390 Free PMC article. Review. - Rosmarinus officinalis L. (rosemary) as therapeutic and prophylactic agent.
de Oliveira JR, Camargo SEA, de Oliveira LD. de Oliveira JR, et al. J Biomed Sci. 2019 Jan 9;26(1):5. doi: 10.1186/s12929-019-0499-8. J Biomed Sci. 2019. PMID: 30621719 Free PMC article. Review.
References
- Abola EE, Sussman JL, Prilusky J, Manning NO. Protein Data Bank archives of three-dimensional macromolecular structures. Methods Enzymol. 1997;227:556–571. - PubMed
- Adelson PF, Whalen MJ, Kochanek PM. Blood brain barrier permeability and acute inflammation in two models of traumatic brain injury in the immature rat: a preliminary report. Acta Neurochir Suppl. 1998;71:104–106. al. e. - PubMed
- Akkawi S, Nassar T, Tarshis M, Cines BC, Higazi AA-R. LRP and avB3 mediate tPA-activation of smooth muscle cells. Am J Physiol Heart Circ Physiol. 2006;291:H1351–1359. - PubMed
- Alafuzoff I, Adolfsson R, Grundke-Iqbal I, Winblad B. Blood-brain barrier in Alzheimer dementia and in non-demented elderly. An immunocytochemical study. Acta Neuropathol. 1987;73:160–166. - PubMed
- Armstead W, Cines D, Higazi AA-R. Plasminogen activators contribute to age dependent impairment of NMDA cerebrovasodilation after brain injury. Develop Brain Res. 2005;156:139–146. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HL076406/HL/NHLBI NIH HHS/United States
- P01 HL076406/HL/NHLBI NIH HHS/United States
- NS53410/NS/NINDS NIH HHS/United States
- HD5735501/HD/NICHD NIH HHS/United States
- HL077760/HL/NHLBI NIH HHS/United States
- R01 NS053410/NS/NINDS NIH HHS/United States
- R01 HL077760/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous