TP53 mutations in human cancers: origins, consequences, and clinical use - PubMed (original) (raw)
Review
TP53 mutations in human cancers: origins, consequences, and clinical use
Magali Olivier et al. Cold Spring Harb Perspect Biol. 2010 Jan.
Abstract
Somatic mutations in the TP53 gene are one of the most frequent alterations in human cancers, and germline mutations are the underlying cause of Li-Fraumeni syndrome, which predisposes to a wide spectrum of early-onset cancers. Most mutations are single-base substitutions distributed throughout the coding sequence. Their diverse types and positions may inform on the nature of mutagenic mechanisms involved in cancer etiology. TP53 mutations are also potential prognostic and predictive markers, as well as targets for pharmacological intervention. All mutations found in human cancers are compiled in the IARC TP53 Database (http://www-p53.iarc.fr/). A human TP53 knockin mouse model (Hupki mouse) provides an experimental model to study mutagenesis in the context of a human TP53 sequence. Here, we summarize current knowledge on TP53 gene variations observed in human cancers and populations, and current clinical applications derived from this knowledge.
Figures
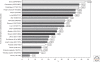
Figure 1.
TP53 mutations prevalence in sporadic cancers. The proportion of tumors with somatic TP53 mutations is indicated. Data from IARC TP53 Database (R13, November 2008)(Petitjean et al. 2007b).
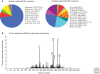
Figure 2.
Type of somatic TP53 mutations in human cancers. (A) Pie charts showing the proportion of the different types of TP53 somatic mutations found in all human cancers. (B) Histogram displaying the position of somatic point mutations in the coding sequence of the TP53 gene. Data from the IARC TP53 Database (R13, November 2008)(Petitjean et al. 2007b).
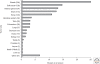
Figure 3.
Geographic distribution of germline TP53 mutations. Number of TP53 germline mutation carrier families in each world region. Data from the IARC TP53 Database (R13, November 2008) (Petitjean et al. 2007b).
Figure 4.
Tumor spectrum in individuals with a germline TP53 mutation. The proportion of specific tumor types among all tumors reported in confirmed TP53 germline mutation carriers is indicated. Data from IARC TP53 Germline Database (R13, November 2008,
http://www-p53.iarc.fr/Germline.html
).
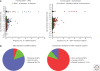
Figure 5.
Functional impact of somatic TP53 mutations in human cancers. (A) Scatter plots showing the frequency (x-axis) of single amino-acid substitutions in relation to their effect (left panel) or functional impacts on transactivation (right panel), and expected substitution rates (_y_-axis in log). Each point represents a single amino-acid substitution that is shaped and colored according to the mutation effect or functional impact. (B) Pie charts displaying the proportion of all somatic single amino-acid substitutions according to their effect (left panel) or functional impacts on transactivation (right panel). Data from the IARC TP53 Database (R13, November 2008)(Petitjean et al. 2007b).
Similar articles
- Applications of the human p53 knock-in (Hupki) mouse model for human carcinogen testing.
Besaratinia A, Pfeifer GP. Besaratinia A, et al. FASEB J. 2010 Aug;24(8):2612-9. doi: 10.1096/fj.10-157263. Epub 2010 Apr 6. FASEB J. 2010. PMID: 20371617 Free PMC article. Review. - TP53 mutations in human cancers: functional selection and impact on cancer prognosis and outcomes.
Petitjean A, Achatz MI, Borresen-Dale AL, Hainaut P, Olivier M. Petitjean A, et al. Oncogene. 2007 Apr 2;26(15):2157-65. doi: 10.1038/sj.onc.1210302. Oncogene. 2007. PMID: 17401424 Review. - Unveiling the methylation status of CpG dinucleotides in the substituted segment of the human p53 knock-in (Hupki) mouse genome.
Kim SI, Hollstein M, Pfeifer GP, Besaratinia A. Kim SI, et al. Mol Carcinog. 2010 Dec;49(12):999-1006. doi: 10.1002/mc.20683. Mol Carcinog. 2010. PMID: 20945503 Free PMC article. - Linking environmental carcinogen exposure to TP53 mutations in human tumours using the human TP53 knock-in (Hupki) mouse model.
Kucab JE, Phillips DH, Arlt VM. Kucab JE, et al. FEBS J. 2010 Jun;277(12):2567-83. doi: 10.1111/j.1742-464X.2010.07676.x. FEBS J. 2010. PMID: 20553493 Review. - TP53 Variations in Human Cancers: New Lessons from the IARC TP53 Database and Genomics Data.
Bouaoun L, Sonkin D, Ardin M, Hollstein M, Byrnes G, Zavadil J, Olivier M. Bouaoun L, et al. Hum Mutat. 2016 Sep;37(9):865-76. doi: 10.1002/humu.23035. Epub 2016 Jul 8. Hum Mutat. 2016. PMID: 27328919
Cited by
- A recent update on the connection between dietary phytochemicals and skin cancer: emerging understanding of the molecular mechanism.
Singh H, Mishra AK, Mohanto S, Kumar A, Mishra A, Amin R, Darwin CR, Emran TB. Singh H, et al. Ann Med Surg (Lond). 2024 Aug 7;86(10):5877-5913. doi: 10.1097/MS9.0000000000002392. eCollection 2024 Oct. Ann Med Surg (Lond). 2024. PMID: 39359831 Free PMC article. Review. - The prognostic role of p53 and its correlation with CDK9 in urothelial carcinoma.
Borowczak J, Szczerbowski K, Maniewski M, Zdrenka M, Słupski P, Andrusewicz H, Łysik-Miśkurka J, Rutkiewicz P, Bodnar M, Szylberg Ł. Borowczak J, et al. Clin Transl Oncol. 2023 Mar;25(3):830-840. doi: 10.1007/s12094-022-02994-6. Epub 2022 Nov 14. Clin Transl Oncol. 2023. PMID: 36374405 Free PMC article. - Recent advances and challenges of rare variant association analysis in the biobank sequencing era.
Chen W, Coombes BJ, Larson NB. Chen W, et al. Front Genet. 2022 Oct 6;13:1014947. doi: 10.3389/fgene.2022.1014947. eCollection 2022. Front Genet. 2022. PMID: 36276986 Free PMC article. Review. - Pharmacological targeting of glucose-6-phosphate dehydrogenase in human erythrocytes by Bay 11-7082, parthenolide and dimethyl fumarate.
Ghashghaeinia M, Giustarini D, Koralkova P, Köberle M, Alzoubi K, Bissinger R, Hosseinzadeh Z, Dreischer P, Bernhardt I, Lang F, Toulany M, Wieder T, Mojzikova R, Rossi R, Mrowietz U. Ghashghaeinia M, et al. Sci Rep. 2016 Jun 29;6:28754. doi: 10.1038/srep28754. Sci Rep. 2016. PMID: 27353740 Free PMC article. - Impact of concurrent genomic alterations in epidermal growth factor receptor (EGFR)-mutated lung cancer.
Gini B, Thomas N, Blakely CM. Gini B, et al. J Thorac Dis. 2020 May;12(5):2883-2895. doi: 10.21037/jtd.2020.03.78. J Thorac Dis. 2020. PMID: 32642201 Free PMC article. Review.
References
- Aas T, Borresen AL, Geisler S, Smith-Sorensen B, Johnsen H, Varhaug JE, Akslen LA, Lonning PE 1996. Specific P53 mutations are associated with de novo resistance to doxorubicin in breast cancer patients. Nat Med 2:811–814 - PubMed
- Ambs S, Hussain SP, Marrogi AJ, Harris CC 1999. Cancer-prone oxyradical overload disease. IARC Sci Publ 150:295–302 - PubMed
- Arlt VM, Stiborova M, vom BJ, Simoes ML, Lord GM, Nortier JL, Hollstein M, Phillips DH, Schmeiser HH 2007. Aristolochic acid mutagenesis: Molecular clues to the aetiology of Balkan endemic nephropathy-associated urothelial cancer. Carcinogenesis 28:2253–2261 - PubMed
- Barker N, Ridgway RA, van Es JH, van de Wetering M, Begthel H, van den Born M, Danenberg E, Clarke AR, Sansom OJ, Clevers H 2009. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature 457:608–611 - PubMed
- Bergamaschi D, Gasco M, Hiller L, Sullivan A, Syed N, Trigiante G, Yulug I, Merlano M, Numico G, Comino A, et al.2003. p53 polymorphism influences response in cancer chemotherapy via modulation of p73-dependent apoptosis. Cancer Cell 3:387–402 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous