Direct activation of TACE-mediated ectodomain shedding by p38 MAP kinase regulates EGF receptor-dependent cell proliferation - PubMed (original) (raw)
Direct activation of TACE-mediated ectodomain shedding by p38 MAP kinase regulates EGF receptor-dependent cell proliferation
Pinglong Xu et al. Mol Cell. 2010.
Abstract
Inflammatory stimuli activate ectodomain shedding of TNF-alpha, L-selectin, and other transmembrane proteins. We show that p38 MAP kinase, which is activated in response to inflammatory or stress signals, directly activates TACE, a membrane-associated metalloprotease that is also known as ADAM17 and effects shedding in response to growth factors and Erk MAP kinase activation. p38alpha MAP kinase interacts with the cytoplasmic domain of TACE and phosphorylates it on Thr(735), which is required for TACE-mediated ectodomain shedding. Activation of TACE by p38 MAP kinase results in the release of TGF-alpha family ligands, which activate EGF receptor signaling, leading to enhanced cell proliferation. Conversely, depletion of p38alpha MAP kinase activity suppresses EGF receptor signaling and downstream Erk MAP kinase signaling, as well as autocrine EGF receptor-dependent proliferation. Autocrine EGF receptor activation through TACE-mediated ectodomain shedding intimately links inflammation and cancer progression and may play a role in stress and conditions that relate to p38 MAP kinase activation.
Copyright 2010 Elsevier Inc. All rights reserved.
Figures
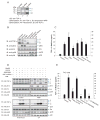
Figure 1
Activation of the p38 MAP kinase pathway induces ectodomain shedding of TGF-α. (A), Cα cells express transmembrane TGF-α forms I, II and III, as shown by immunoblotting of cell lysates. Forms II and III are visualized by cell surface protein biotinylation. (B) and (C), Cα cells were treated as indicated, and ectodomain shedding of TGF-α was examined by immunoblotting of cell lysates for transmembrane TGF-α (B, upper panel), or quantifying TGF-α released into the medium by ELISA (C). In (B) the middle and lower panels show activation of Erk (anti-pErk) or p38 MAPK (anti-pp38) versus total Erk or p38 MAPK. Error bars show the standard deviations based on triplicate values of each data set. *, P < 0.01, compared with control. (D), Effects of pathway inhibitors on TGF-α shedding. Cα cells were treated with PMA, anisomycin or UV light without or with the indicated inhibitor. Immunoblotting of cell lysates shows transmembrane TGF-α, and activated Erk (anti-pErk) or p38 MAPK (anti-pp38) versus total Erk or p38 MAPK. (E), Ectodomain shedding of TGF-α in T4-2 cells treated with PMA, anisomycin or IL-1β without or with U0126 or SB203580. TGF-α released into the medium was quantified by ELISA. *, P < 0.001, compared with control. **, P < 0.01, compared with corresponding PMA, anisomycin or IL-1β stimulation.
Figure 2
p38α MAP kinase mediates anisomycin- and UV light-induced ectodomain shedding. (A), Cα cells were treated with anisomycin, UV light or PMA, and increasing doses of SB203580, and shedding was visualized by immunoblotting for transmembrane TGF-α. The lower panels show p38 MAPK activation using anti-phospho-p38 MAPK immunoblotting. (B) and (C), Cα cells were transfected with p38α MAPK siRNA or control siRNA. Ecdodomain shedding of TGF-α was induced by PMA, anisomycin or UV light, and evaluated by immunoblotting of cell lysates for transmembrane TGF-α (B), or ELISA of TGF-α released in the medium (C). Immunoblotting for p38 MAPK or phospho-p38 MAPK revealed the p38α MAPK activation. *, P < 0.01, compared with control. **, P < 0.01, compared with corresponding control siRNA transfection. (D), Cα cells were treated with PMA, anisomycin or UV light, without or with cycloheximide or brefeldin A. Shedding was assessed by immunoblotting of cell lysates for transmembrane TGF-α. (E), Ectodomain shedding was induced with PMA or anisomycin without or with MNTmPyP, PTIO or L-NIL, and evaluated by immunoblotting of cell lysates for TGF-α.
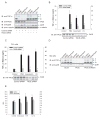
Figure 3
TACE effects TGF-α release in response to p38 MAP kinase activation. (A) and (B), Cα cells were transfected with TACE siRNA or control siRNA, stimulated with PMA, anisomycin or UV light, and shedding of TGF-α was evaluated by immunoblotting cell lysates for transmembrane TGF-α (A), or by ELISA of TGF-α in the medium (B). Immunoblotting cell lysates with anti-TACE or anti-phospho-p38 MAPK antibodies showed silencing of TACE expression, or activation of p38 MAPK in response to anisomycin or UV light. *, P < 0.05, compared with corresponding transfection with control siRNA. (C), T4-2 cells, transfected with TACE siRNA or control siRNA, were treated with PMA, anisomycin or UV light, and TGF-α released in the medium was quantified by ELISA. Silencing of TACE expression was apparent by immunoblotting of cell lysates for TACE (lower panel). *, P < 0.05, compared with corresponding transfection with control siRNA. (D), M2 cells, lacking active TACE, were transfected to express TGF-α and TACE or inactive E406A TACE mutant. Shedding of TGF-α was visualized by immunoblotting of cell lysates for transmembrane TGF-α. The lower panel shows p38 MAPK activation, assessed by immunoblotting for phospho-p38 MAPK. (E), In vitro proteolytic activity of TACE immunoprecipitated from T4-2 cells that were treated with anisomycin, LPS or IL-1β, in the absence or presence of SB203580. *, P < 0.05, compared with DMSO control.
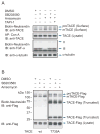
Figure 4
p38α MAP kinase interacts with and phosphorylates TACE. (A), Cα cells were transfected to expresss Myc-tagged TACE with Flag-tagged p38α or p38β MAPK or inactive p38α-AF or p38β-AF. Cell lysates were subjected to anti-Myc immunoprecipitation, and anti-Flag immunoblotting. TACE and p38 MAPK expression were shown by immunoblotting of cell lysates. (B), TACE, TACE (T735A) mutant, p38α MAPK, p38α-AF and activated MKK6 (MKK6-E) were generated by in vitro transcription and translation. p38α MAPK was then activated through phosphorylation by MKK6-E. Phosphorylated p38α MAPK and TACE were purified using anti-Flag beads, and incubated with 32P-γATP in a kinase assay. The lower panels show immunoblotting of the proteins in the kinase assays. TACE is smaller than in vivo expressed TACE due to lack of glycosylation. (C), In vitro kinase assays as in (B) were performed with purified proteins from transfected Cα cells, with SB203580 added to inhibit p38α MAPK. (D), Cα cells were transfected to express TACE with p38α or p38β MAPK, or p38α-AF or p38β-AF, with or without MKK6-E. Cells were treated for 30 min or not with anisomycin, lysed, and TACE and p38 MAPK proteins were purified using anti-Flag beads and incubated with 32P-γATP in a kinase assay. The proteins were separated by SDS-PAGE, and autoradiography detected 32P-labelled TACE. (E), Cα cells and Cα cells expressing TACE or T735A TACE were cultured in 32P-orthophosphate, treated with anisomycin, with or without SB203580, and lysed. Immunoprecipitated, 32P-labelled TACE was visualized by SDS-PAGE and autoradiography. Anti-Flag immunoblotting showed the immunoprecipitated TACE-Flag. (F), T4–2 cells, transfected or not with control siRNA or p38α MAPK siRNA, were treated or not with SB203580, anisomycin or IL-1β for 30 min, lysed and subjected to anti-TACE immunoprecipitation and immunoblotting with anti-phosphoThr-Pro (anti-pTP) antibody to reveal phosphorylated TACE, or with anti-TACE antibody to reveal endogenous TACE. The lower panel shows p38 MAPK levels, assessed by immunoblotting. (G), Untransfected T4-2 cells were treated or not with IL-1β or anisomycin for 15 min, lysed and subjected to anti-TACE or or IgG (Mock) immunoprecipitation, followed by anti-p38 MAPK immunoblotting. Immunoblotting shows endogenous TACE and p38 MAPK in cell lysates. Immunoprecipitated TACE was visualized by immunoblotting, and immunoprecipitate supernatants were adsorbed to Con A-Sepharose followed by immunoblotting using anti-TACE antibody.
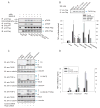
Figure 5
Phosphorylation of TACE at Thr735 is required for activation of shedding by p38 MAP kinase. (A), Wild-type or mutant TACE were expressed in Cα cells, purified using anti-Flag beads, incubated with 32P-γATP and p38α MAPK, which was separately co-expressed with MKK6-E and purified using anti-Flag beads. Phosphorylated TACE was visualized by autoradiography (upper panel), or immunoblotting with anti-pTP antibody (middle panel). Anti-Flag immunoblotting revealed the levels of TACE and p38α MAPK. (B), M2 cells, transfected to express TGF-α and low level wild-type or mutant TACE, were treated with anisomycin or SB203580, or additionally transfected to express p38α-AF. Ectodomain shedding was evaluated by measuring TGF-α in the medium. TACE was visualized by anti-Flag immunoblotting of proteins precipitated with anti-Flag M2 beads (upper panel). *, P < 0.01, compared with wild-type TACE control. **, P < 0.01, compared with wild-type TACE or wild-type TACE with anisomycin. (C) and (D), Cα cells and Cα cells expressing human wild-type or T735A TACE were transfected with control or TACE siRNA to silence endogenous TACE expression. Ectodomain shedding was induced with PMA or anisomycin, and evaluated by immunoblotting for transmembrane TGF-α (C) or measuring TGF-α in the medium (D). TACE was visualized by immunoblotting; siRNA-mediated silencing of endogenous TACE could only be revealed in Cα cells that do not express human TACE. *, P < 0.01, compared with cells expressing wild-type TACE.
Figure 6
Activation of p38 MAP kinase enhances TACE levels at the cell surface. (A), Cα cells were treated with anisomycin, without or with SB203580 or TAPI-1. Pro-TACE and active TACE (mTACE) were detected at the cell surface by immunoblotting cell surface biotinylated proteins with anti-TACE antibody, and total glycosylated TACE in the cell lysate was shown by immunoblotting concanavalin A-adsorbed proteins. Cell surface biotinylation detected TGF-α forms II and III at the cell surface, and correlated the increased cell surface mTACE level with increased shedding of TGF-α. (B), Cell surface TACE, detected as in (A), in Cα cells stably expressing wild-type or T735A TACE, and treated with SB203580 or anisomycin. Pro-TACE and mTACE were detected, and truncated TACE forms were at the surface of cells expressing T735A but not wild-type TACE. The lower panel shows the total TACE in cell lysates.
Figure 7
p38 MAP kinase signaling stimulates T4-2 cell proliferation by TACE-mediated shedding of EGF receptor ligands. (A) and (B), Effects of TAPI-1, the EGFR inhibitor AG1478, MEK inhibitor PD98059, SB203580, or negative control SB202474 on the proliferation of T4-2 cells. BrdU incorporation was measured by ELISA and expressed relative to untreated control. *, P < 0.01, compared with DMSO control. (C) and (D), Effect of IL-1β on proliferation of cells transfected with p38α MAPK, TACE or control siRNA, or treated with neutralizing antibodies to TGF-α and amphiregulin (AR), or the EGFR, or rabbit IgG. BrdU incorporation was measured by ELISA and expressed relative to untreated control. *, P < 0.01, compared with control. **, P < 0.001, compared with IL-1β-treated cells transfected with control siRNA, or treated with IgG. (E) and (F), T4-2 cells, transfected or not with p38α MAPK, TACE or control siRNA, were treated for 60 (E) or 30 min (F) with IL-1β or anisomycin, without or with neutralizing antibodies to TGF-α and amphiregulin (AR), or the EGFR, or rabbit IgG. TGF-α released in the medium was quantified by ELISA (E). In (F), anti-EGFR immunoprecipitates were blotted with anti-phosphoTyr (anti-PY) to show EGFR activation, or with anti-EGFR for EGFR expression. Endogenous p38 MAPK and TACE were visualized by immunoblotting of cell lysates or Con A-adsorbed proteins. *, P < 0.001, compared with control siRNA. **, P < 0.001, compared with IL-1β stimulation of cells transfected with control siRNA, or treated with IgG. (G), T4-2 cells were transfected with TACE, p38α MAPK or control siRNA. Immunoblotting of cell lysates showed downregulation of endogenous TACE or p38α MAPK expression in siRNA-transfected T4-2 cells. EGF receptor activation and expression are shown as in (F). Erk MAPK activation was shown by immunoblotting for phospho-Erk MAPK (pErk) and total Erk MAPK. The right panel shows the proliferation with or without 5 ng/ml TGF-α or EGF. *, P < 0.01, compared with untreated cells transfected with control siRNA. **, P < 0.01, compared with untreated cells transfected with TACE or p38α MAPK siRNA.
Similar articles
- TACE activation by MAPK-mediated regulation of cell surface dimerization and TIMP3 association.
Xu P, Liu J, Sakaki-Yumoto M, Derynck R. Xu P, et al. Sci Signal. 2012 May 1;5(222):ra34. doi: 10.1126/scisignal.2002689. Sci Signal. 2012. PMID: 22550340 Free PMC article. - Ectodomain shedding of pro-TGF-alpha is required for COX-2 induction and cell survival in renal medullary cells exposed to osmotic stress.
Küper C, Bartels H, Fraek ML, Beck FX, Neuhofer W. Küper C, et al. Am J Physiol Cell Physiol. 2007 Dec;293(6):C1971-82. doi: 10.1152/ajpcell.00404.2007. Epub 2007 Oct 17. Am J Physiol Cell Physiol. 2007. PMID: 17942633 - TACE/ADAM17 processing of EGFR ligands indicates a role as a physiological convertase.
Lee DC, Sunnarborg SW, Hinkle CL, Myers TJ, Stevenson MY, Russell WE, Castner BJ, Gerhart MJ, Paxton RJ, Black RA, Chang A, Jackson LF. Lee DC, et al. Ann N Y Acad Sci. 2003 May;995:22-38. doi: 10.1111/j.1749-6632.2003.tb03207.x. Ann N Y Acad Sci. 2003. PMID: 12814936 Review.
Cited by
- TIMP3 Modulates GHR Abundance and GH Sensitivity.
Zhang Y, Wang X, Loesch K, May LA, Davis GE, Jiang J, Frank SJ. Zhang Y, et al. Mol Endocrinol. 2016 Jun;30(6):587-99. doi: 10.1210/me.2015-1302. Epub 2016 Apr 13. Mol Endocrinol. 2016. PMID: 27075707 Free PMC article. - Effects of Prometryn Exposure on Hepatopancreas Oxidative Stress and Intestinal Flora in Eriocheir sinensis (Crustacea: Decapoda).
Huang P, Cao L, Du J, Gao J, Zhang Y, Sun Y, Li Q, Nie Z, Xu G. Huang P, et al. Antioxidants (Basel). 2023 Aug 2;12(8):1548. doi: 10.3390/antiox12081548. Antioxidants (Basel). 2023. PMID: 37627543 Free PMC article. - TNF-α converting enzyme-mediated ErbB4 transactivation by TNF promotes colonic epithelial cell survival.
Hilliard VC, Frey MR, Dempsey PJ, Peek RM Jr, Polk DB. Hilliard VC, et al. Am J Physiol Gastrointest Liver Physiol. 2011 Aug;301(2):G338-46. doi: 10.1152/ajpgi.00057.2011. Epub 2011 May 26. Am J Physiol Gastrointest Liver Physiol. 2011. PMID: 21617117 Free PMC article. - A Lactobacillus rhamnosus GG-derived soluble protein, p40, stimulates ligand release from intestinal epithelial cells to transactivate epidermal growth factor receptor.
Yan F, Liu L, Dempsey PJ, Tsai YH, Raines EW, Wilson CL, Cao H, Cao Z, Liu L, Polk DB. Yan F, et al. J Biol Chem. 2013 Oct 18;288(42):30742-30751. doi: 10.1074/jbc.M113.492397. Epub 2013 Sep 16. J Biol Chem. 2013. PMID: 24043629 Free PMC article. - Activation of Toll-like Receptor 2 (TLR2) induces Interleukin-6 trans-signaling.
Flynn CM, Garbers Y, Lokau J, Wesch D, Schulte DM, Laudes M, Lieb W, Aparicio-Siegmund S, Garbers C. Flynn CM, et al. Sci Rep. 2019 May 13;9(1):7306. doi: 10.1038/s41598-019-43617-5. Sci Rep. 2019. PMID: 31086276 Free PMC article.
References
- Beutler B, Krochin N, Milsark IW, Luedke C, Cerami A. Control of cachectin (tumor necrosis factor) synthesis: mechanisms of endotoxin resistance. Science. 1986;232:977–980. - PubMed
- Blobel CP. ADAMs: key components in EGFR signalling and development. Nat Rev Mol Cell Biol. 2005;6:32–43. - PubMed
- Bringman TS, Lindquist PB, Derynck R. Different transforming growth factor-α species are derived from a glycosylated and palmitoylated transmembrane precursor. Cell. 1987;48:429–440. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous