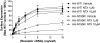The μ-opioid receptor variant N190K is unresponsive to peptide agonists yet can be rescued by small-molecule drugs - PubMed (original) (raw)
The μ-opioid receptor variant N190K is unresponsive to peptide agonists yet can be rescued by small-molecule drugs
Jean-Philippe Fortin et al. Mol Pharmacol. 2010 Nov.
Abstract
The μ-opioid receptor (MOR) plays an important role in modulating analgesia, feeding behavior, and a range of autonomic functions. In the current study, we investigated the degree to which 13 naturally occurring missense mutations affect the pharmacological properties of the human MOR. After expression of each receptor in human embryonic kidney 293 cells, signaling (Gα(i/o)-mediated) induced by peptide agonists was assessed using luciferase reporter gene assays. Multiple mutants (S66F, S147C, R260H, R265C, R265H, and S268P) show a significant reduction in agonist potency. At the N190K variant, agonist-mediated signaling was essentially absent. Enzyme-linked immunosorbent assay, microscopic analysis, and radioligand binding assays revealed that this mutant shows markedly reduced cell-surface expression, whereas all other receptor variants were expressed at normal levels. Surface expression of the N190K variant could be increased by incubation with the alkaloid agonist buprenorphine or with either naltrexone or naloxone, structurally related MOR antagonists. We were surprised to find that both putative antagonists, despite being inactive at the wild-type MOR, triggered a concentration-dependent increase in N190K receptor-mediated signaling. In contrast, peptidic ligands failed to promote expression or rescue function of the N190K mutant. Subsequent analysis of the N190K variant in an ethnically diverse cohort identified this isoform in a subgroup of African Americans. Taken together, our studies reveal that the N190K mutation leads to severe functional alterations and, in parallel, changes the response to established MOR ligands. The extent to which this mutation results in physiological abnormalities or affects drug sensitivity in selected populations (e.g., those with chronic pain or addiction) remains to be investigated.
Figures
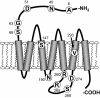
Fig. 1.
A diagram of the MOR illustrating the position of missense mutations within the receptor protein. Respective residues in the wild-type protein are indicated by the single letter code.
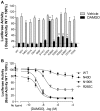
Fig. 2.
Selected MOR missense mutations alter DAMGO-induced signaling. HEK293 cells were transiently transfected with either the WT or a MOR isoform and a CRE6x-Luc reporter gene construct. Twenty-four hours after transfection, cells were incubated for 6 h with or without a saturating concentration of DAMGO (1 μM) (A) or increasing concentrations of DAMGO (B) diluted in serum-free medium supplemented with 0.5 μM forskolin. After stimulation, luciferase activity was quantified as described under Materials and Methods. All activity values were normalized relative to the forskolin-stimulated maximum at the WT MOR (100% activity). Data represent the mean ± S.E.M. from at least four independent experiments, each performed in triplicate. Significance, efficacy of mutant versus wild-type MOR, analysis of variance followed by Dunnett's post test; *, p < 0.05; **, p < 0.01.
Fig. 3.
The N190K MOR missense mutation alters cell-surface expression. Cell-surface expression of HA-tagged MORs increases as a function of cDNA concentration. HEK293 cells were transfected with increasing amounts of plasmid encoding either the wild-type or a mutant HA-tagged MOR. After 48 h, surface expression was measured by ELISA as described under Materials and Methods. Expression data are shown as a percentage of the maximal value observed at the wild-type MOR (transfection of 16 ng of cDNA/well). Each data point represents the mean ± S.E.M. from at least four independent experiments, each performed in triplicate. Significance, surface expression of WT versus mutant MOR; one-way analysis of variance with Dunnett's post test. **, p < 0.01.
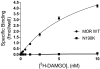
Fig. 4.
Cells expressing the N190K variant display no specific [3H]DAMGO surface binding sites. HEK293 cells plated onto 24-well plates were transiently transfected with either pcDNA1.1 or a plasmid encoding the WT MOR or N190K variant. Forty-eight hours after transfection, cells were washed and incubated for 3 h at room temperature with the indicated concentrations of [3H]DAMGO. Incubations were performed and terminated as described under Materials and Methods. Nonspecific binding, established using HEK293 cells transfected with the pcDNA1.1 plasmid, was subtracted from the total binding values measured at receptor expressing cells. Data represent the mean ± S.E.M. from three independent experiments, each performed in triplicate.
Fig. 5.
Cell surface expression of the N190K mutant is enhanced by the small-molecule antagonist naltrexone. HEK293 cells were transiently transfected with plasmid encoding an HA-tagged version of the WT or N190K MOR. Twenty-four hours later, cells were treated for 18 h with media containing vehicle or increasing concentrations (1 or 10 μM) of naltrexone. The levels of surface expression of each HA-tagged receptor were assessed using ELISA as described under Materials and Methods. Data represent the mean ± S.E.M. from at least four independent experiments, each performed in triplicate. Significance, surface expression of vehicle-treated WT or mutant MOR versus antagonist treated cells; one-way analysis of variance with Dunnett's post test. **,p < 0.01.
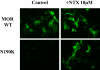
Fig. 6.
Cell surface expression of the N190K mutant can be rescued by the small molecule naltrexone. HEK293 cells were transiently transfected with a plasmid encoding either an HA-tagged WT or N190K MOR. Twenty-four hours later, cells were treated for 18 h with media containing vehicle or 10 μM naltrexone. The levels of surface expression of each HA-tagged receptor were visualized using confocal microscopy as described under Materials and Methods.
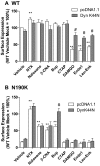
Fig. 7.
Expression of a dominant dynamin mutant (DynK44N) fails to rescue surface expression of the N190K mutant. HEK293 cells were transiently transfected with either plasmid encoding the HA-tagged WT MOR or N190K variant in combination with pcDNA1.1 or the DynK44N-encoding plasmid. DynK44N-rescued agonist-induced internalization of the WT MOR (A), however had no effect on the surface expression of the N190K variant in either the presence or the absence of ligands (B). After 24 h, cells were treated with media containing vehicle or 10 μM ligand. Surface expression levels of the receptors were assessed by ELISA as described under Materials and Methods. Data represent the mean ± S.E.M. from at least four independent experiments, each performed in triplicate. Significance, one-way analysis of variance with Dunnett's post test, surface expression of vehicle versus ligand treated cells, **, p < 0.01; surface expression in the absence versus presence of DynK44N, #, p < 0.01. Bup, buprenorphine; Endo1, endomorphin 1.
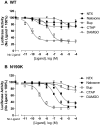
Fig. 8.
Naltrexone, naloxone, and buprenorphine display agonist activity at the N190K variant. HEK293 cells were transiently transfected with either the WT (A) or the N190K (B) MOR isoform and a CRE6x-Luc reporter gene construct. Twenty-four hours after transfection, cells were incubated for 18 h with increasing concentrations of DAMGO, naltrexone, naloxone buprenorphine, or CTAP together with 0.5 μM forskolin. After stimulation, luciferase activity was quantified as described under_Materials and Methods_. DAMGO activates the WT MOR but has minimal if any effect at the N190K variant. Conversely, NTX and naloxone are strong agonists at the N190K variant but show minimal if any activity at the WT MOR. All activity values were normalized relative to the forskolin-stimulated maximum at the WT MOR (= 100%). Data represent the mean ± S.E.M. from at least four independent experiments, each performed in triplicate.
Similar articles
- Different effects of opioid antagonists on mu-, delta-, and kappa-opioid receptors with and without agonist pretreatment.
Wang D, Sun X, Sadee W. Wang D, et al. J Pharmacol Exp Ther. 2007 May;321(2):544-52. doi: 10.1124/jpet.106.118810. Epub 2007 Jan 31. J Pharmacol Exp Ther. 2007. PMID: 17267582 - Neutral antagonist activity of naltrexone and 6beta-naltrexol in naïve and opioid-dependent C6 cells expressing a mu-opioid receptor.
Divin MF, Bradbury FA, Carroll FI, Traynor JR. Divin MF, et al. Br J Pharmacol. 2009 Apr;156(7):1044-53. doi: 10.1111/j.1476-5381.2008.00035.x. Epub 2009 Feb 13. Br J Pharmacol. 2009. PMID: 19220294 Free PMC article. - A single nucleotide polymorphic mutation in the human mu-opioid receptor severely impairs receptor signaling.
Befort K, Filliol D, Decaillot FM, Gaveriaux-Ruff C, Hoehe MR, Kieffer BL. Befort K, et al. J Biol Chem. 2001 Feb 2;276(5):3130-7. doi: 10.1074/jbc.M006352200. Epub 2000 Nov 6. J Biol Chem. 2001. PMID: 11067846 - Ligand-Free Signaling of G-Protein-Coupled Receptors: Relevance to μ Opioid Receptors in Analgesia and Addiction.
Sadee W, McKew JC. Sadee W, et al. Molecules. 2022 Sep 8;27(18):5826. doi: 10.3390/molecules27185826. Molecules. 2022. PMID: 36144565 Free PMC article. Review. - Biased Opioid Antagonists as Modulators of Opioid Dependence: Opportunities to Improve Pain Therapy and Opioid Use Management.
Sadee W, Oberdick J, Wang Z. Sadee W, et al. Molecules. 2020 Sep 11;25(18):4163. doi: 10.3390/molecules25184163. Molecules. 2020. PMID: 32932935 Free PMC article. Review.
Cited by
- Tapentadol shows lower intrinsic efficacy at µ receptor than morphine and oxycodone.
Manandhar P, Connor M, Santiago M. Manandhar P, et al. Pharmacol Res Perspect. 2022 Feb;10(1):e00921. doi: 10.1002/prp2.921. Pharmacol Res Perspect. 2022. PMID: 35084120 Free PMC article. - Convergent Balancing Selection on the Mu-Opioid Receptor in Primates.
Sweeney CG, Rando JM, Panas HN, Miller GM, Platt DM, Vallender EJ. Sweeney CG, et al. Mol Biol Evol. 2017 Jul 1;34(7):1629-1643. doi: 10.1093/molbev/msx105. Mol Biol Evol. 2017. PMID: 28333316 Free PMC article. - A heroin addiction severity-associated intronic single nucleotide polymorphism modulates alternative pre-mRNA splicing of the μ opioid receptor gene OPRM1 via hnRNPH interactions.
Xu J, Lu Z, Xu M, Pan L, Deng Y, Xie X, Liu H, Ding S, Hurd YL, Pasternak GW, Klein RJ, Cartegni L, Zhou W, Pan YX. Xu J, et al. J Neurosci. 2014 Aug 13;34(33):11048-66. doi: 10.1523/JNEUROSCI.3986-13.2014. J Neurosci. 2014. PMID: 25122903 Free PMC article. - Genetic Polymorphisms Affect Mouse and Human Trace Amine-Associated Receptor 1 Function.
Shi X, Walter NA, Harkness JH, Neve KA, Williams RW, Lu L, Belknap JK, Eshleman AJ, Phillips TJ, Janowsky A. Shi X, et al. PLoS One. 2016 Mar 31;11(3):e0152581. doi: 10.1371/journal.pone.0152581. eCollection 2016. PLoS One. 2016. PMID: 27031617 Free PMC article. - Morphine-induced internalization of the L83I mutant of the rat μ-opioid receptor.
Cooke AE, Oldfield S, Krasel C, Mundell SJ, Henderson G, Kelly E. Cooke AE, et al. Br J Pharmacol. 2015 Jan;172(2):593-605. doi: 10.1111/bph.12709. Epub 2014 Jul 1. Br J Pharmacol. 2015. PMID: 24697554 Free PMC article.
References
- Aiyer AN, Kip KE, Mulukutla SR, Marroquin OC, Hipps L, Jr, Reis SE. (2007) Predictors of significant short-term increases in blood pressure in a community-based population. Am J Med 120:960–967 - PubMed
- Al-Fulaij MA, Ren Y, Beinborn M, Kopin AS. (2008) Pharmacological analysis of human D1 AND D2 dopamine receptor missense variants. J Mol Neurosci 34:211–223 - PubMed
- Befort K, Filliol D, Decaillot FM, Gaveriaux-Ruff C, Hoehe MR, Kieffer BL. (2001) A single nucleotide polymorphic mutation in the human mu-opioid receptor severely impairs receptor signaling. J Biol Chem 276:3130–3137 - PubMed
- Beinborn M, Ren Y, Bläker M, Chen C, Kopin AS. (2004) Ligand function at constitutively active receptor mutants is affected by two distinct yet interacting mechanisms. Mol Pharmacol 65:753–760 - PubMed
- Bläker M, Ren Y, Gordon MC, Hsu JE, Beinborn M, Kopin AS. (1998) Mutations within the cholecystokinin-B/gastrin receptor ligand ‘pocket’ interconvert the functions of nonpeptide agonists and antagonists. Mol Pharmacol 54:857–863 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials