A novel histone deacetylase inhibitor exhibits antitumor activity via apoptosis induction, F-actin disruption and gene acetylation in lung cancer - PubMed (original) (raw)
A novel histone deacetylase inhibitor exhibits antitumor activity via apoptosis induction, F-actin disruption and gene acetylation in lung cancer
Yen-An Tang et al. PLoS One. 2010.
Abstract
Background: Lung cancer is the leading cause of cancer mortality worldwide, yet the therapeutic strategy for advanced non-small cell lung cancer (NSCLC) is limitedly effective. In addition, validated histone deacetylase (HDAC) inhibitors for the treatment of solid tumors remain to be developed. Here, we propose a novel HDAC inhibitor, OSU-HDAC-44, as a chemotherapeutic drug for NSCLC.
Methodology/principal findings: The cytotoxicity effect of OSU-HDAC-44 was examined in three human NSCLC cell lines including A549 (p53 wild-type), H1299 (p53 null), and CL1-1 (p53 mutant). The antiproliferative mechanisms of OSU-HDAC-44 were investigated by flow cytometric cell cycle analysis, apoptosis assays and genome-wide chromatin-immunoprecipitation-on-chip (ChIP-on-chip) analysis. Mice with established A549 tumor xenograft were treated with OSU-HDAC-44 or vehicle control and were used to evaluate effects on tumor growth, cytokinesis inhibition and apoptosis. OSU-HDAC-44 was a pan-HDAC inhibitor and exhibits 3-4 times more effectiveness than suberoylanilide hydroxamic acid (SAHA) in suppressing cell viability in various NSCLC cell lines. Upon OSU-HDAC-44 treatment, cytokinesis was inhibited and subsequently led to mitochondria-mediated apoptosis. The cytokinesis inhibition resulted from OSU-HDAC-44-mediated degradation of mitosis and cytokinesis regulators Auroroa B and survivin. The deregulation of F-actin dynamics induced by OSU-HDAC-44 was associated with reduction in RhoA activity resulting from srGAP1 induction. ChIP-on-chip analysis revealed that OSU-HDAC-44 induced chromatin loosening and facilitated transcription of genes involved in crucial signaling pathways such as apoptosis, axon guidance and protein ubiquitination. Finally, OSU-HDAC-44 efficiently inhibited A549 xenograft tumor growth and induced acetylation of histone and non-histone proteins and apoptosis in vivo.
Conclusions/significance: OSU-HDAC-44 significantly suppresses tumor growth via induction of cytokinesis defect and intrinsic apoptosis in preclinical models of NSCLC. Our data provide compelling evidence that OSU-HDAC-44 is a potent HDAC targeted inhibitor and can be tested for NSCLC chemotherapy.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
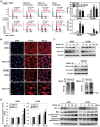
Figure 1. Chemical structure, molecular docking analysis, and the effect of OSU-HDAC-44 on cell viability.
(A) Chemical structure of OSU-HDAC-44 and SAHA. (B) Molecular docking analysis of OSU-HDAC-44 and SAHA. The structures of OSU-HDAC-44 and SAHA were calculated and the docking mode on catalytic domain of HDAC8 was predicted using the docking program GOLD 4.0.1. (C) Dose-dependent effects of OSU-HDAC-44 (left) and SAHA (right) on cell viability in IMR90, H1299, A549 and CL1-1 cells. Cells were treated with 0.5–10 µM of OSU-HDAC-44 or SAHA for 48 h, and cell viability was assessed by trypan blue exclusion assay. (D) OSU-HDAC-44 synergized with cisplatin to suppress cell proliferation. Cells were exposed to cisplatin (Cis) alone for 4 h, OSU-HDAC-44 (HDAC-44) alone for 48 h, or pretreated with OSU-HDAC-44 for 48 h before cisplatin treatment for 4 h, and then drug were withdrew and cells were incubated with drug-free media for additional 48 h. Cell viability was assessed by trypan blue exclusion assay. CL1-1 cells were treated with 4.4 µM cisplatin or 0.3 µM OSU-HDAC-44. A549 cells were treated with 1.6 µM cisplatin or 0.2 µM OSU-HDAC-44. Data represent mean ± SEM from three independent experiments. * P<0.05; ** P<0.01; *** P<0.001.
Figure 2. OSU-HDAC-44 induces cytokinesis inhibition and subsequently leads to intrinsic apoptosis.
(A) The effects of OSU-HDAC-44 on cell cycle distribution in A549 and H1299 cells. Cells were treated with 2.5 µM OSU-HDAC-44 or 5 µM SAHA for indicated times and assessed by flow cytometry. Left, results from one representative experiment are shown. Right, the mean percentage of G2/M and sub-G1 fraction population is plotted in the histogram. (B) The bi-nucleated cells and dysregulation of F-actin induced by OSU-HSAC-44. Cells were treated with 2.5 µM OSU-HDAC-44 for 48 h, and then fixed and stained with DAPI (DNA) and phalloidin (F-actin). Asterisk pointed to the bi-nucleus. Scale bars: 30 µm. (C) OSU-HDAC-44 induced degradation of Aurora B and survivin via 26S proteasome pathway. Upper, time-dependent decreases in Aurora B and survivin protein levels after 2.5 µM OSU-HDAC-44 treatment. Middle, A549 cells were treated with 2.5 µM OSU-HDAC-44 in the presence or absence of MG132 for 24 h. Lower, A549 cells were treated with 2.5 µM OSU-HDAC-44 for 24 h and cell lysate was subjected to IP assay using anti-Aurora B or anti-survivin specific antibodies and blotted with anti-ubiquitination antibody (Anti-Ub). (D) Caspase activity assay (left) and Western blot analyses (Right) confirmed that OSU-HDAC-44 induced intrinsic apoptosis pathway. Cells were treated with 2.5 µM OSU-HDAC-44 for indicated times and the subjected to caspase activity assay and Western blot analyses. Data represent mean ± SEM from three independent experiments. * P<0.05; ** P<0.01.
Figure 3. Effect of OSU-HDAC-44 on the biomarkers associated with broad inhibition on numerous HDACs.
Dose-dependent effects (A) and time-dependent effects (B) of OSU-HDAC-44 on the histone and non-histone proteins. Ac-H3, acetylated histone H3; Ac-H4, acetylated histone H4; Ac-p53, acetylated p53; p53, total p53. (C) OSU-HDAC-44 suppressed activities of class I (HDAC1 and HDAC8), class II (HDAC4 and HDAC6), and class IV (HDAC11) HDACs. Different HDAC isotypes were immunoprecipitated from H1299 nuclear extract by specific antibodies, and then subjected to in vitro HDAC inhibition assay as described in Materials and Methods section. Data represent mean ± SEM from three independent experiments. ** P<0.01; *** P<0.001.
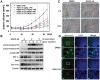
Figure 4. OSU-HDAC-44 decreased RhoA activity via srGAP1 induction, leading to F-actin dysregulation.
(A) Chromatin-immunoprecipitation-PCR analyses confirmed that treatment with 2.5 µM OSU-HDAC-44 for 2 h induced acetylation of histone H3 (H3K9K14Ac) in the promoter region of srGAP1, NR4A1 and FOXO4 genes. (B) OSU-HDAC-44 increased the mRNA levels of srGAP1, NR4A1 and FOXO4 genes using real-time RT-PCR analyses. Cells were treated with 2.5 µM OSU-HDAC-44 for 24 h and total RNA was extracted for the real-time RT-PCR analyses. Data represent mean ± SEM from three independent experiments. *P<0.05. (C) Immunoprecipitation assay indicated that increased interaction between srGAP1 and RhoA was induced by OSU-HDAC-44. A549 cells were treated or untreated with 2.5 µM OSU-HDAC-44 for 24 h and subjected to IP-Western analyses. (D) si-srGAP1 abrogated the OSU-HDAC-44-induced decrease in RhoA activity (upper) and rescued the normal structure of F-actin after OSU-HDAC-44 treatment (lower). A549 cells transfected with srGAP1 siRNA were treated with 2.5 µM OSU-HDAC-44 for 24 h and subjected to RhoA activation assay and immunofluorescence analyses. Scale bars: 30 µm.
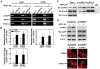
Figure 5. OSU-HDAC-44 effectively induced apoptosis and inhibited A549 xenograft growth.
(A) Mice bearing the established A549 tumors (∼50 mm3) were injected intraperitoneally with 7.5, 15 or 30 mg/kg of OSU-HDAC-44 or 1.5 mg/kg of TSA 3 days/week for three weeks. Eight mice per group were used in the xenograft experiment. The tumor volumes of mice were measured. Points, mean; error bars, 95% confidence intervals. P values were for comparisons with vehicle control (*P<.05; **P<.01; ***P<.001). (B–D) Mice bearing established (about 100∼200 mm3) A549 tumors were injected intraperitoneally with a single dose of OSU-HDAC-44 at 60 mg/kg. After treatment for the indicated time, tumors were harvested and subjected to Western blot or immunohistochemistry analyses. (B) Tumors from two representative mice of each time point (a–f) were harvested and subjected to Western blot analyses using the indicated antibodies. (C) Immunohistochemistry analyses were performed using antibody against cleaved-form of caspase 3 (brownish color). Original magnification ×200. (D) Fluorescence immunohistochemistry analyses were performed using antibody against Aurora B and DAPI (DNA). The images (d–f and j–l, scale bars: 10 µm) were magnified from framed ones (a–c and g–I, scale bars: 30 µm).
Figure 6. The body weight, H&E staining of major organs, and hematological biochemistry examinations of tested animals.
(A) OSU-HDAC-44 treatments did not cause significant body weight loss of tested animals. (B) H&E staining of paraffin-embedded, 5 µm thick sections of the heart, liver, lung and kidney from OSU-HDAC-44-treated and untreated mice with A549 xenografts. There were no apparent histopathologic differences between these tissues sections (original magnification ×200). (C) Hematological biochemistry tests including GOT, GPT, albumin, BUN, creatinine, and WBC were examined and the results showed no significant differences between DMSO and OSU-HDAC-44 treatment.
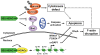
Figure 7. The antitumor activity of OSU-HDAC-44 via cytokinese defect, F-actin disruption, apoptosis induction, and gene acetylation.
OSU-HDAC-44 is a novel pan-HDAC inhibitor that exhibits a broad spectrum of antitumor activities in NSCLC cell and xenograft models, which involves histone acetylation-dependent activation of gene transcription in nucleus. For example, re-expression of NR4A1 and FOXO4 along with caspase activation induces intrinsic apoptosis. In addition, RhoA/F-actin motility control is inhibited by srGAP1 resulting from activation by OSU-HDAC-44. OSU-HDAC-44 also induces post-translational down-regulation of mitotic regulators, Aurora B and survivin leading to cytokinese defect and apoptosis.
Similar articles
- Mitochondrial apoptosis and FAK signaling disruption by a novel histone deacetylase inhibitor, HTPB, in antitumor and antimetastatic mouse models.
Shieh JM, Wei TT, Tang YA, Huang SM, Wen WL, Chen MY, Cheng HC, Salunke SB, Chen CS, Lin P, Chen CT, Wang YC. Shieh JM, et al. PLoS One. 2012;7(1):e30240. doi: 10.1371/journal.pone.0030240. Epub 2012 Jan 18. PLoS One. 2012. PMID: 22279574 Free PMC article. - Efficacy of a novel histone deacetylase inhibitor in murine models of hepatocellular carcinoma.
Lu YS, Kashida Y, Kulp SK, Wang YC, Wang D, Hung JH, Tang M, Lin ZZ, Chen TJ, Cheng AL, Chen CS. Lu YS, et al. Hepatology. 2007 Oct;46(4):1119-30. doi: 10.1002/hep.21804. Hepatology. 2007. PMID: 17654699 - Superior efficacy of co-treatment with the dual PI3K/mTOR inhibitor BEZ235 and histone deacetylase inhibitor Trichostatin A against NSCLC.
Piao J, Chen L, Quan T, Li L, Quan C, Piao Y, Jin T, Lin Z. Piao J, et al. Oncotarget. 2016 Sep 13;7(37):60169-60180. doi: 10.18632/oncotarget.11109. Oncotarget. 2016. PMID: 27507059 Free PMC article. - MicroRNAs in non-small cell lung cancer: Gene regulation, impact on cancer cellular processes, and therapeutic potential.
Petrek H, Yu AM. Petrek H, et al. Pharmacol Res Perspect. 2019 Dec;7(6):e00528. doi: 10.1002/prp2.528. Pharmacol Res Perspect. 2019. PMID: 31859460 Free PMC article. Review. - Histone Deacetylase Inhibitors: A Promising Therapeutic Alternative for Endometrial Carcinoma.
Psilopatis I, Pergaris A, Giaginis C, Theocharis S. Psilopatis I, et al. Dis Markers. 2021 Nov 12;2021:7850688. doi: 10.1155/2021/7850688. eCollection 2021. Dis Markers. 2021. PMID: 34804263 Free PMC article. Review.
Cited by
- Targeting the E2F1/Rb/HDAC1 axis with the small molecule HR488B effectively inhibits colorectal cancer growth.
Duan N, Hu X, Qiu H, Zhou R, Li Y, Lu W, Zhu Y, Shen S, Wu W, Yang F, Liu N. Duan N, et al. Cell Death Dis. 2023 Dec 7;14(12):801. doi: 10.1038/s41419-023-06205-0. Cell Death Dis. 2023. PMID: 38062013 Free PMC article. - The histone deacetylase inhibitor trichostatin a decreases lymphangiogenesis by inducing apoptosis and cell cycle arrest via p21-dependent pathways.
Hrgovic I, Doll M, Kleemann J, Wang XF, Zoeller N, Pinter A, Kippenberger S, Kaufmann R, Meissner M. Hrgovic I, et al. BMC Cancer. 2016 Sep 30;16(1):763. doi: 10.1186/s12885-016-2807-y. BMC Cancer. 2016. PMID: 27716272 Free PMC article. - Differential responsiveness of MET inhibition in non-small-cell lung cancer with altered CBL.
Tan YC, Mirzapoiazova T, Won BM, Zhu L, Srivastava MK, Vokes EE, Husain AN, Batra SK, Sharma S, Salgia R. Tan YC, et al. Sci Rep. 2017 Aug 23;7(1):9192. doi: 10.1038/s41598-017-09078-4. Sci Rep. 2017. PMID: 28835699 Free PMC article. - Epigenetic therapy in lung cancer.
Liu SV, Fabbri M, Gitlitz BJ, Laird-Offringa IA. Liu SV, et al. Front Oncol. 2013 May 30;3:135. doi: 10.3389/fonc.2013.00135. eCollection 2013. Front Oncol. 2013. PMID: 23755372 Free PMC article. - Functional Roles of Acetylated Histone Marks at Mouse Meiotic Recombination Hot Spots.
Getun IV, Wu Z, Fallahi M, Ouizem S, Liu Q, Li W, Costi R, Roush WR, Cleveland JL, Bois PRJ. Getun IV, et al. Mol Cell Biol. 2017 Jan 19;37(3):e00942-15. doi: 10.1128/MCB.00942-15. Print 2017 Feb 1. Mol Cell Biol. 2017. PMID: 27821479 Free PMC article.
References
- Danesi R, de Braud F, Fogli S, de Pas TM, Di Paolo A, et al. Pharmacogenetics of anticancer drug sensitivity in non-small cell lung cancer. Pharmacol Rev. 2003;55:57–103. - PubMed
- Yang P, Allen MS, Aubry MC, Wampfler JA, Marks RS, et al. Clinical features of 5,628 primary lung cancer patients: experience at Mayo Clinic from 1997 to 2003. Chest. 2005;128:452–462. - PubMed
- Pfister DG, Johnson DH, Azzoli CG, Sause W, Smith TJ, et al. American Society of Clinical Oncology treatment of unresectable non-small-cell lung cancer guideline: update 2003. J Clin Oncol. 2004;22:330–353. - PubMed
- Stinchcombe TE, Socinski MA. Current treatments for advanced stage non-small cell lung cancer. Proc Am Thorac Soc. 2009;6:233–241. - PubMed
- Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–2550. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous