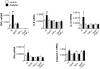Vascular cellular adhesion molecule-1 (VCAM-1) expression in mice retinal vessels is affected by both hyperglycemia and hyperlipidemia - PubMed (original) (raw)
Vascular cellular adhesion molecule-1 (VCAM-1) expression in mice retinal vessels is affected by both hyperglycemia and hyperlipidemia
Carin Gustavsson et al. PLoS One. 2010.
Abstract
Background: Inflammation has been proposed to be important in the pathogenesis of diabetic retinopathy. An early feature of inflammation is the release of cytokines leading to increased expression of endothelial activation markers such as vascular cellular adhesion molecule-1 (VCAM-1). Here we investigated the impact of diabetes and dyslipidemia on VCAM-1 expression in mouse retinal vessels, as well as the potential role of tumor necrosis factor-α (TNFα).
Methodology/principal findings: Expression of VCAM-1 was examined by confocal immunofluorescence microscopy in vessels of wild type (wt), hyperlipidemic (ApoE(-/-)) and TNFα deficient (TNFα(-/-), ApoE(-/-)/TNFα(-/-)) mice. Eight weeks of streptozotocin-induced diabetes resulted in increased VCAM-1 in wt mice, predominantly in small vessels (<10 µm). Diabetic wt mice had higher total retinal TNFα, IL-6 and IL-1β mRNA than controls; as well as higher soluble VCAM-1 (sVCAM-1) in plasma. Lack of TNFα increased higher basal VCAM-1 protein and sVCAM-1, but failed to up-regulate IL-6 and IL-1β mRNA and VCAM-1 protein in response to diabetes. Basal VCAM-1 expression was higher in ApoE(-/-) than in wt mice and both VCAM-1 mRNA and protein levels were further increased by high fat diet. These changes correlated to plasma cholesterol, LDL- and HDL-cholesterol, but not to triglycerides levels. Diabetes, despite further increasing plasma cholesterol in ApoE(-/-) mice, had no effects on VCAM-1 protein expression or on sVCAM-1. However, it increased ICAM-1 mRNA expression in retinal vessels, which correlated to plasma triglycerides.
Conclusions/significance: Hyperglycemia triggers an inflammatory response in the retina of normolipidemic mice and up-regulation of VCAM-1 in retinal vessels. Hypercholesterolemia effectively promotes VCAM-1 expression without evident stimulation of inflammation. Diabetes-induced endothelial activation in ApoE(-/-) mice seems driven by elevated plasma triglycerides but not by cholesterol. Results also suggest a complex role for TNFα in the regulation of VCAM-1 expression, being protective under basal conditions but pro-inflammatory in response to diabetes.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
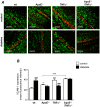
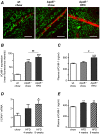
Figure 1. Effects of diabetes on VCAM-1 protein expression in retinal vessels.
(A) Confocal immunofluorescence microscopy images showing VCAM-1 staining (red) in retinal whole-mounts from control non-diabetic and diabetic wild type (wt), ApoE−/−, TNFα−/− and ApoE−/−/TNFα−/− mice. The DNA-binding dye SYTOX (green) was used for nuclear localization. Bars = 50 µm. Measurements were performed 8 weeks after the first STZ-injection, when mice were 30 weeks of age. (B) Summarized data from experiments in A showing mean fluorescence intensity of VCAM-1 in the different groups. White bars represent control and black bars diabetic mice. Two-way analysis of variance (for the effects of diabetes and genotype) revealed significant interactions between factors. Bonferroni posttests yielded *p<0.05 and ***p<0.001 for comparisons between ApoE−/− and TNFα−/−, respectively vs. wt control; ***p<0.001 for comparison between ApoE−/− and ApoE−/−/TNFα−/−; and ### p<0.001 for comparisons between control and diabetes of the same genotype.
Figure 2. The effect of diabetes on VCAM-1 expression is dependent on retinal vessel size.
Summarized data from confocal immunofluorescence microscopy experiments showing mean fluorescence intensity of VCAM-1 in retinal vessels divided into quartiles depending on vessel diameter (<10 µm, 10–15 µm, 16–23 µm and >23 µm). Each graph represents the expression-diameter relationship in the different genotypes. White bars are for control and grey bars for diabetes. The two first bars in each graph (in white and black, for control and diabetes respectively) show the same data as in Figure 1, but are displayed here as references. *p<0.05 and ***p<0.001 indicate differences between control and diabetes of the same size and genotype.
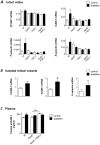
Figure 3. Changes in the expression of vascular adhesion markers in retinal vessels are difficult to resolve if measured in total retinal RNA.
(A) Expression of VCAM-1, ICAM-1, P- and E-selectin mRNA was measured by real time RT-PCR in intact retinas of control (white bars) and diabetic (black bars) wt, ApoE−/−, TNFα−/− and ApoE−/−/TNFα−/− mice. No statistically significant differences were seen between genotypes or between retinas from control and diabetic mice.Results are normalized to the expression of the housekeeping control cyclophilin B. (B) VCAM-1, ICAM-1 and E-selectin mRNA were measured in isolated retinal vessels from control (white) and diabetic (black) ApoE−/− mice. Values are normalized to the expression of cyclophilin B and GAPDH. *p<0.05 indicates difference to the control group. (C) Mean sVCAM-1 concentrations in plasma (ng/ml), measured by ELISA are depicted for control (white bars) and diabetic (black bars) wt, ApoE−/− and ApoE−/−/TNFα−/− mice. *p<0.05 vs. wt control and ***p<0.001 for differences between non-diabetic ApoE−/− and ApoE−/−/TNFα−/− mice. For A–C, measurements were performed 8 weeks after the first STZ-injection, when mice were 30 weeks of age.
Figure 4. Diabetes enhances the expression of inflammatory cytokines in the retina of wt mice but not of ApoE- or TNFα deficient mice.
Expression of _TNF_α, IL-6, IL-1β, IFNγ and caspase-1 mRNA was measured by real time RT-PCR in intact retinas of control (white bars) and diabetic (black bars) wt, ApoE−/−, TNFα−/− and ApoE−/−/TNFα−/− mice. Values are normalized to the expression of cyclophilin B. *p<0.05 and **p<0.01 indicates differences to control wt mice.
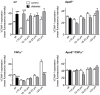
Figure 5. High fat diet (HFD) increases VCAM-1 expression in retinal vessels.
(A) Confocal immunofluorescence microscopy images showing VCAM-1 expression (red) and nuclei stained by SYTOX (green) in retinal whole-mounts from wt mice fed normal chow diet and from ApoE−/− mice fed normal chow or 4 weeks of HFD. Bars = 50 µm. Measurements were performed when mice were 17 weeks of age. (B) Summarized data from experiments as in A, showing increased mean fluorescence intensity of VCAM-1 in ApoE−/− (light gray bar) when compared to wt controls (white bar; ***p<0.001) and further increased VCAM-1 expression in response to HFD (dark gray bar; ## p<0.01). (C) Mean sVCAM-1 concentrations in plasma from the same mice used in A and B. ***p<0.001 vs. wt chow and # p<0.05 for differences between ApoE−/− groups (chow vs. HFD). (D) Expression of VCAM-1 mRNA was studied by real time RT-PCR in normolipidemic FVBN mice. Expression was enhanced by 4 or 8 weeks of HFD (plain and patterned gray bars, respectively) when compared to mice fed regular chow diet (white bars). The effects were significant after 8 weeks (*p<0.05). Values are normalized to the expression of cyclophilin B and GAPDH. (E) Mean sVCAM-1 concentrations in plasma from the same mice used in D. **p<0.01 vs. wt chow.
Similar articles
- [Expressions of atherosclerosis-related genes in aorta in young apoE/LDLR double knockout mice].
Dai XD, Yin M, Jing W, DU HQ, Ye HY, Shang YJ, Zhang L, Zou YY, Qu ZP, Pan J. Dai XD, et al. Sheng Li Xue Bao. 2008 Feb 25;60(1):43-50. Sheng Li Xue Bao. 2008. PMID: 18288357 Chinese. - [Protection of huanglian jiedu decoction on systemic and vascular immune responses of high fat induced apoE(-/-) mice].
Ma YL, Wang BB, Han JY, Li R, Zhang WM, Li T, Chen B, Su J, Wang XB, Zeng H. Ma YL, et al. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2013 Nov;33(11):1520-5. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2013. PMID: 24483114 Chinese. - CD40 Expressed in Endothelial Cells Promotes Upregulation of ICAM-1 But Not Pro-Inflammatory Cytokines, NOS2 and P2X7 in the Diabetic Retina.
Yu JS, Daw J, Portillo JC, Subauste CS. Yu JS, et al. Invest Ophthalmol Vis Sci. 2021 Sep 2;62(12):22. doi: 10.1167/iovs.62.12.22. Invest Ophthalmol Vis Sci. 2021. PMID: 34546322 Free PMC article. - The role of VCAM-1 in diabetic retinopathy: A systematic review and meta-analysis.
Xu Y, Hou H, Zhao L. Xu Y, et al. J Diabetes Complications. 2023 Jan;37(1):108380. doi: 10.1016/j.jdiacomp.2022.108380. Epub 2022 Dec 5. J Diabetes Complications. 2023. PMID: 36525905 Review. - VCAM-1 as a predictor biomarker in cardiovascular disease.
Troncoso MF, Ortiz-Quintero J, Garrido-Moreno V, Sanhueza-Olivares F, Guerrero-Moncayo A, Chiong M, Castro PF, García L, Gabrielli L, Corbalán R, Garrido-Olivares L, Lavandero S. Troncoso MF, et al. Biochim Biophys Acta Mol Basis Dis. 2021 Sep 1;1867(9):166170. doi: 10.1016/j.bbadis.2021.166170. Epub 2021 May 14. Biochim Biophys Acta Mol Basis Dis. 2021. PMID: 34000374 Review.
Cited by
- miR-15a/16 reduces retinal leukostasis through decreased pro-inflammatory signaling.
Ye EA, Liu L, Jiang Y, Jan J, Gaddipati S, Suvas S, Steinle JJ. Ye EA, et al. J Neuroinflammation. 2016 Dec 8;13(1):305. doi: 10.1186/s12974-016-0771-8. J Neuroinflammation. 2016. PMID: 27931222 Free PMC article. - Danhong Huayu Koufuye Prevents Diabetic Retinopathy in Streptozotocin-Induced Diabetic Rats via Antioxidation and Anti-Inflammation.
Chen W, Yao X, Zhou C, Zhang Z, Gui G, Lin B. Chen W, et al. Mediators Inflamm. 2017;2017:3059763. doi: 10.1155/2017/3059763. Epub 2017 May 30. Mediators Inflamm. 2017. PMID: 28638179 Free PMC article. - Novel drugs and their targets in the potential treatment of diabetic retinopathy.
Nawaz MI, Abouammoh M, Khan HA, Alhomida AS, Alfaran MF, Ola MS. Nawaz MI, et al. Med Sci Monit. 2013 Apr 26;19:300-8. doi: 10.12659/MSM.883895. Med Sci Monit. 2013. PMID: 23619778 Free PMC article. Review. - Increased inflammation in atherosclerotic lesions of diabetic Akita-LDLr⁻/⁻ mice compared to nondiabetic LDLr⁻/⁻ mice.
Engelbertsen D, To F, Dunér P, Kotova O, Söderberg I, Alm R, Gomez MF, Nilsson J, Bengtsson E. Engelbertsen D, et al. Exp Diabetes Res. 2012;2012:176162. doi: 10.1155/2012/176162. Epub 2012 Nov 28. Exp Diabetes Res. 2012. PMID: 23243415 Free PMC article. - Diabetic Endothelial Cell Glycogen Synthase Kinase 3β Activation Induces VCAM1 Ectodomain Shedding.
Brishti MA, Raghavan S, Lamar K, Singh UP, Collier DM, Leo MD. Brishti MA, et al. Int J Mol Sci. 2023 Sep 14;24(18):14105. doi: 10.3390/ijms241814105. Int J Mol Sci. 2023. PMID: 37762417 Free PMC article.
References
- Hartge MM, Unger T, Kintscher U. The endothelium and vascular inflammation in diabetes. Diab Vasc Dis Res. 2007;4:84–88. - PubMed
- Antonetti DA, Barber AJ, Bronson SK, Freeman WM, Gardner TW, et al. Diabetic Retinopathy: Seeing Beyond Glucose-Induced Microvascular Disease. Diabetes. 2006;55:2401–2411. - PubMed
- Khalfaoui T, Lizard G, Ouertani-Meddeb A. Adhesion molecules (ICAM-1 and VCAM-1) and diabetic retinopathy in type 2 diabetes. J Mol Histol. 2008:243–249. 2008/01/01 ed. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous